Laser-Ablative Synthesis of Silicon–Iron Composite Nanoparticles for Theranostic Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. NP Synthesis
2.3. NP Characterization
3. Results
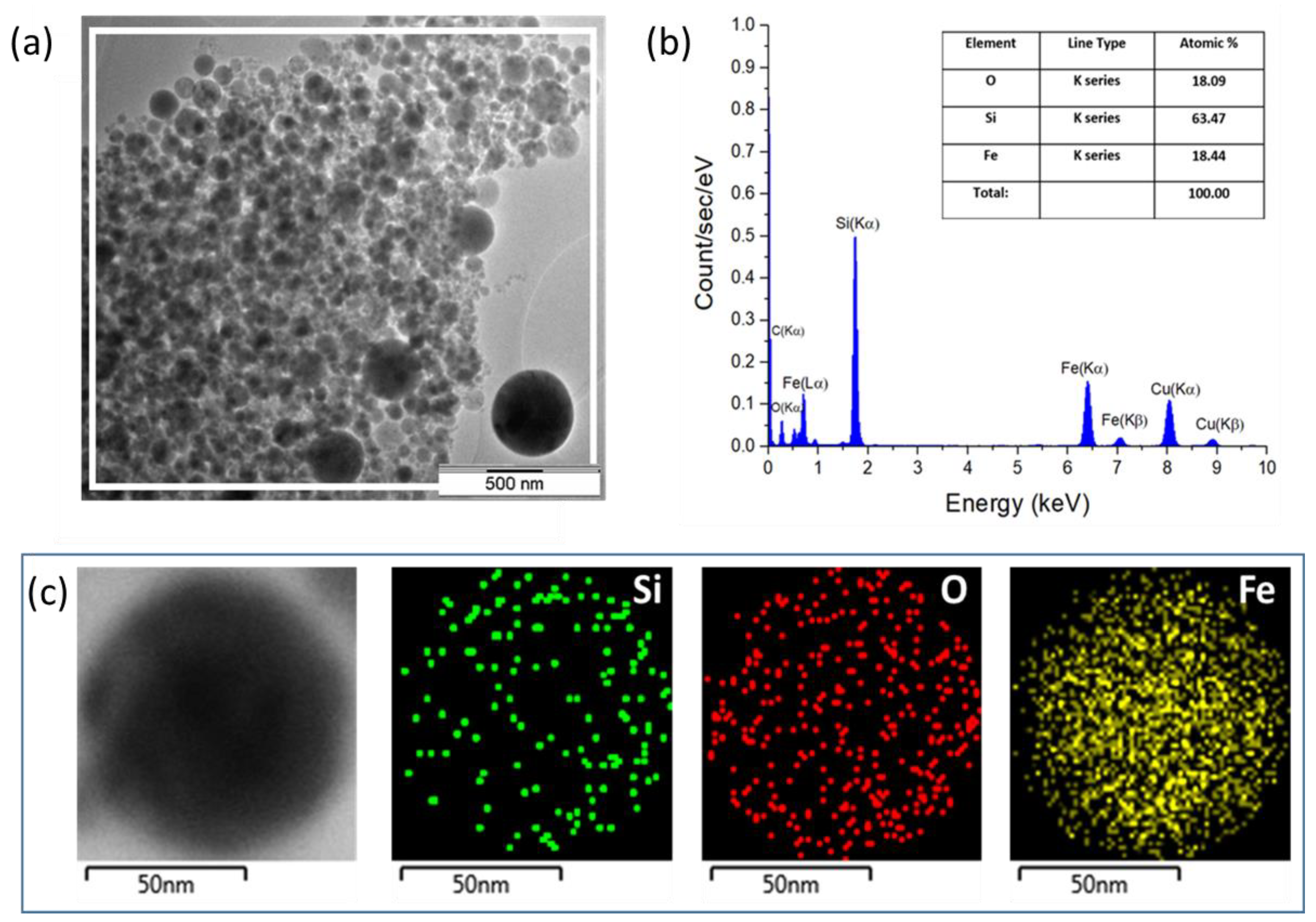
3.1. Size and Morphology of NPs
3.2. Composition and Structure of NPs
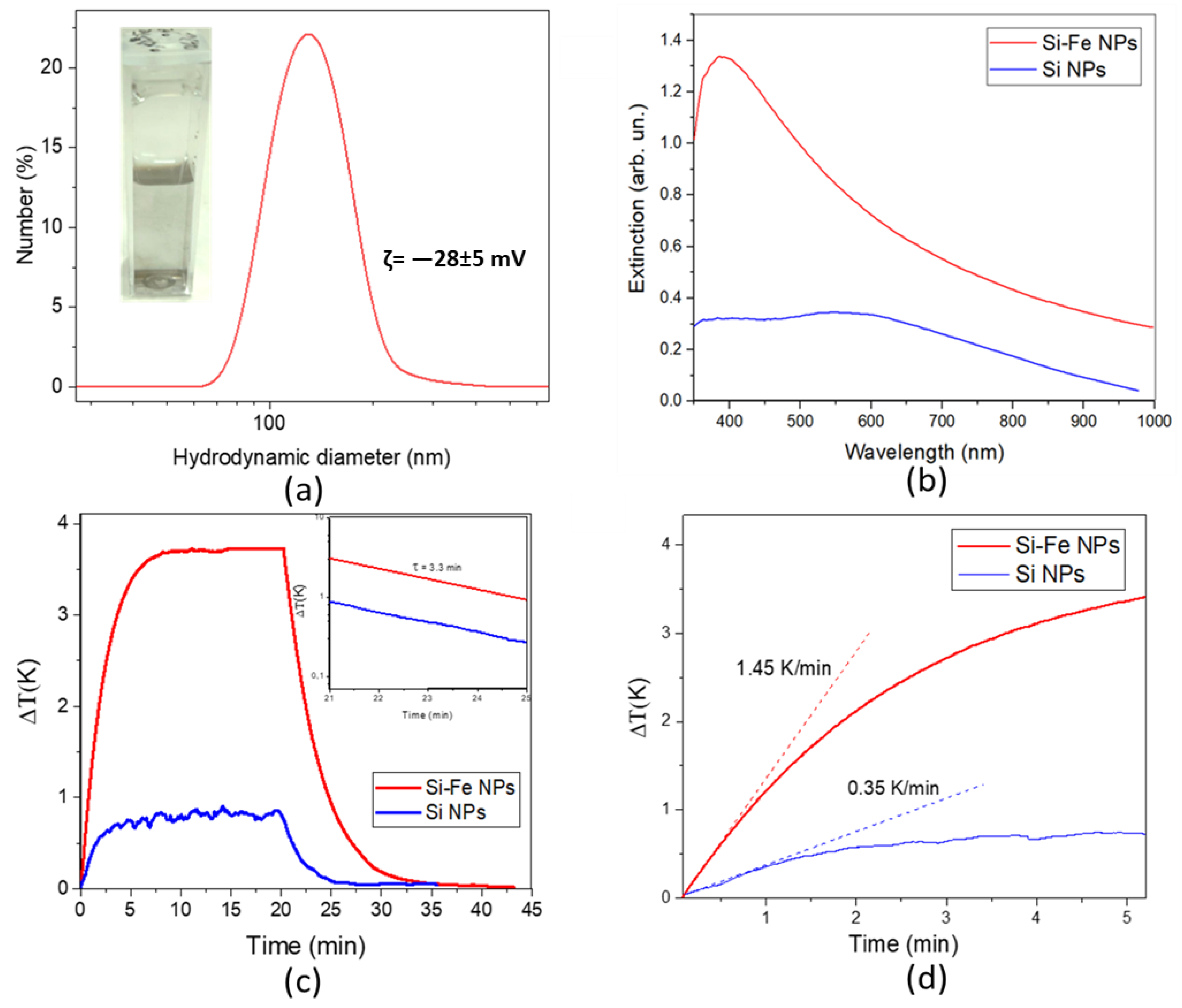
3.3. Optical and Photothermal Properties
3.4. MRI Contrasting Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xue, Y.; Gao, Y.; Meng, F.; Luo, L. Recent progress of nanotechnology-based theranostic systems in cancer treatments. Cancer Biol. Med. 2021, 18, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lee, D.; Kim, S.; Kim, H.-H.; Jeong, S.; Kim, J. Contrast Agents for Photoacoustic Imaging: A Review Focusing on the Wavelength Range. Biosensors 2022, 12, 594. [Google Scholar] [CrossRef] [PubMed]
- West, J.L.; Halas, N.J. Engineered nanomaterials for biophotonics applications: Improving sensing, imaging, and therapeutics. Annu. Rev. Biomed. Eng. 2003, 5, 285–292. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, C.S.; Yoon, H. Nanoparticulate Photoluminescent Probes for Bioimaging: Small Molecules and Polymers. Int. J. Mol. Sci. 2022, 23, 4949. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, G.; Metrangolo, P.; Dichiarante, V. Photoluminescent nanocluster-based probes for bioimaging applications. Photochem. Photobiol. Sci. 2022, 21, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, K.; Follen, M.; Aaron, J.; Pavlova, I.; Malpica, A.; Lotan, R.; Richards-Kortum, R. Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res. 2003, 63, 1999–2004. [Google Scholar] [PubMed]
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; García-Martín, M.L. Magnetic Nanoparticles as MRI Contrast Agents. Top. Curr. Chem. 2020, 378, 40. [Google Scholar] [CrossRef]
- Myrovali, E.; Papadopoulos, K.; Charalampous, G.; Kesapidou, P.; Vourlias, G.; Kehagias, T.; Angelakeris, M.; Wiedwald, U. Toward the Separation of Different Heating Mechanisms in Magnetic Particle Hyperthermia. ACS Omega 2023, 8, 12955–12967. [Google Scholar] [CrossRef]
- Park, J.-H.; Gu, L.; Von Maltzahn, G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat. Mater. 2009, 8, 331–336. [Google Scholar] [CrossRef]
- Baati, T.; Al-Kattan, A.; Esteve, M.-A.; Njim, L.; Ryabchikov, Y.; Chaspoul, F.; Hammami, M.; Sentis, M.; Kabashin, A.V.; Braguer, D. Ultrapure laser-synthesized Si-based nanomaterials for biomedical applications: In vivo assessment of safety and biodistribution. Sci. Rep. 2016, 6, 25400. [Google Scholar] [CrossRef] [PubMed]
- Kharin, A.Y.; Lysenko, V.V.; Rogov, A.; Ryabchikov, Y.V.; Geloen, A.; Tishchenko, I.; Marty, O.; Sennikov, P.G.; Kornev, R.A.; Zavestovskaya, I.N. Bi-Modal Nonlinear Optical Contrast from Si Nanoparticles for Cancer Theranostics. Adv. Opt. Mater. 2019, 7, 1801728. [Google Scholar] [CrossRef]
- Timoshenko, V.Y.; Kudryavtsev, A.; Osminkina, L.A.; Vorontsov, A.; Ryabchikov, Y.V.; Belogorokhov, I.; Kovalev, D.; Kashkarov, P. Silicon nanocrystals as photosensitizers of active oxygen for biomedical applications. Jetp. Lett. 2006, 83, 423–426. [Google Scholar] [CrossRef]
- Lee, C.; Kim, H.; Hong, C.; Kim, M.; Hong, S.; Lee, D.; Lee, W.I. Porous silicon as an agent for cancer thermotherapy based on near-infrared light irradiation. J. Mater. Chem. 2008, 18, 4790–4795. [Google Scholar] [CrossRef]
- Oleshchenko, V.; Kharin, A.Y.; Alykova, A.; Karpukhina, O.; Karpov, N.; Popov, A.; Bezotosnyi, V.; Klimentov, S.; Zavestovskaya, I.; Kabashin, A. Localized infrared radiation-induced hyperthermia sensitized by laser-ablated silicon nanoparticles for phototherapy applications. Appl. Surf. Sci. 2020, 516, 145661. [Google Scholar] [CrossRef]
- Tamarov, K.P.; Osminkina, L.A.; Zinovyev, S.V.; Maximova, K.A.; Kargina, J.V.; Gongalsky, M.B.; Ryabchikov, Y.; Al-Kattan, A.; Sviridov, A.P.; Sentis, M. Radio frequency radiation-induced hyperthermia using Si nanoparticle-based sensitizers for mild cancer therapy. Sci. Rep. 2014, 4, 7034. [Google Scholar] [CrossRef]
- Sangaiya, P.; Jayaprakash, R. A review on iron oxide nanoparticles and their biomedical applications. J. Supercond. Nov. Magn. 2018, 31, 3397–3413. [Google Scholar] [CrossRef]
- Stepien, G.; Moros, M.; Pérez-Hernández, M.; Monge, M.; Gutiérrez, L.; Fratila, R.M.; de las Heras, M.; Menao Guillen, S.; Puente Lanzarote, J.J.; Solans, C. Effect of surface chemistry and associated protein corona on the long-term biodegradation of iron oxide nanoparticles in vivo. ACS Appl. Mater. Interfaces 2018, 10, 4548–4560. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, M.; Zeng, J.; Huo, L.; Liu, K.; Wei, R.; Ni, K.; Gao, J. Recent advances in engineering iron oxide nanoparticles for effective magnetic resonance imaging. Bioact. Mater. 2022, 12, 214–245. [Google Scholar] [CrossRef]
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron oxide based nanoparticles for multimodal imaging and magnetoresponsive therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Atkins, T.M.; Muthuswamy, E.; Kamali, S.; Tu, C.; Louie, A.Y.; Kauzlarich, S.M. Development of iron-doped silicon nanoparticles as bimodal imaging agents. ACS Nano. 2012, 6, 5596–5604. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kinsella, J.M.; Ananda, S.; Andrew, J.S.; Grondek, J.F.; Chien, M.P.; Scadeng, M.; Gianneschi, N.C.; Ruoslahti, E.; Sailor, M.J. Enhanced magnetic resonance contrast of Fe3O4 nanoparticles trapped in a porous silicon nanoparticle host. Adv. Maters. 2011, 23, H248–H253. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, H.; Doostmohammadi, M. Nanoparticle Synthesis, Applications, and Toxicity. In Applications of Nanobiotechnology; IntechOpen: London, UK, 2019. [Google Scholar]
- Kargina, Y.V.; Perepukhov, A.M.; Kharin, A.Y.; Zvereva, E.A.; Koshelev, A.V.; Zinovyev, S.V.; Maximychev, A.V.; Alykova, A.F.; Sharonova, N.V.; Zubov, V.P. Silicon Nanoparticles Prepared by Plasma-Assisted Ablative Synthesis: Physical Properties and Potential Biomedical Applications. Phys. Status Solidi. A 2019, 216, 1800897. [Google Scholar] [CrossRef]
- Kargina, Y.V.; Sobolev, A.V.; Kozlyakova, E.S.; Vasiliev, A.N.; Kharin, A.Y.; Sharonova, N.V.; Perepukhov, A.M.; Stavitskaya, A.V.; Ischenko, A.A.; Timoshenko, V.Y. Composite silicon-iron nanoparticles: Physical properties and potential application in MRI contrasting. J. Nanoparticle Res. 2022, 24, 115. [Google Scholar] [CrossRef]
- Rafieepour, A.; Azari, M.R.; Peirovi, H. Investigation of the effect of magnetite iron oxide particles size on cytotoxicity in A549 cell line. Toxicol. Ind. Health 2019, 35, 703–713. [Google Scholar] [CrossRef]
- Zhang, D.; Gokce, B.; Barcikowski, S. Laser synthesis and processing of colloids: Fundamentals and applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Meunier, M. Synthesis of colloidal nanoparticles during femtosecond laser ablation of gold in water. J. Appl. Phys. 2003, 94, 7941–7943. [Google Scholar] [CrossRef]
- Popov, A.A.; Tselikov, G.; Dumas, N.; Berard, C.; Metwally, K.; Jones, N.; Al-Kattan, A.; Larrat, B.; Braguer, D.; Mensah, S. Laser-synthesized TiN nanoparticles as promising plasmonic alternative for biomedical applications. Sci. Rep. 2019, 9, 1194. [Google Scholar] [CrossRef]
- Pastukhov, A.I.; Belyaev, I.B.; Bulmahn, J.C.; Zelepukin, I.V.; Popov, A.A.; Zavestovskaya, I.N.; Klimentov, S.M.; Deyev, S.M.; Prasad, P.N.; Kabashin, A.V. Laser-ablative aqueous synthesis and characterization of elemental boron nanoparticles for biomedical applications. Sci. Rep. 2022, 12, 9129. [Google Scholar] [CrossRef]
- Al-Kattan, A.; Tselikov, G.; Metwally, K.; Popov, A.A.; Mensah, S.; Kabashin, A.V. Laser ablation-assisted synthesis of plasmonic SiAu core-satellite nanocomposites for biomedical applications. Nanomaterials 2021, 11, 592. [Google Scholar] [CrossRef]
- Popov, A.A.; Swiatkowska-Warkocka, Z.; Marszalek, M.; Tselikov, G.; Zelepukin, I.V.; Al-Kattan, A.; Deyev, S.M.; Klimentov, S.M.; Itina, T.E.; Kabashin, A.V. Laser-Ablative Synthesis of Ultrapure Magneto-Plasmonic Core-Satellite Nanocomposites for Biomedical Applications. Nanomaterials 2022, 12, 649. [Google Scholar] [CrossRef] [PubMed]
- Kögler, M.; Ryabchikov, Y.V.; Uusitalo, S.; Popov, A.; Popov, A.; Tselikov, G.; Välimaa, A.-L.; Al-Kattan, A.; Hiltunen, J.; Laitinen, R.; et al. Bare laser-synthesized Au-based nanoparticles as nondisturbing surface-enhanced Raman scattering probes for bacteria identification. J. Biophotonics 2018, 11, e201700225. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.A.; Tikhonowski, G.V.; Shakhov, P.V.; Popova-Kuznetsova, E.A.; Tselikov, G.I.; Romanov, R.I.; Markeev, A.M.; Klimentov, S.M.; Kabashin, A.V. Synthesis of Titanium Nitride Nanoparticles by Pulsed Laser Ablation in Different Aqueous and Organic Solutions. Nanomaterials 2022, 12, 1672. [Google Scholar] [CrossRef] [PubMed]
- Richardson, H.H.; Carlson, M.T.; Tandler, P.J.; Hernandez, P.; Govorov, A.O. Experimental and Theoretical Studies of Light-to-Heat Conversion and Collective Heating Effects in Metal NanoparticleSolutions. Nano Lett. 2009, 9, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Smith, D.A.; Pinchuk, A. Size-Dependent Photothermal Conversion Efficiencies of Plasmonically Heated Gold Nanoparticles. J. Phys. Chem. 2013, 117, 27073–27080. [Google Scholar] [CrossRef]
- Alykova, A.F.; Yakunin, V.G.; Timoshenko, V.Y.; Zavestovskaya, I.N. Optical methods of silicon nanoparticle diagnostics for applications in biomedicine. J. Biomed. Photonics Eng. 2019, 5, 020304. [Google Scholar] [CrossRef]
- Testa-Anta, M.; Ramos-Docampo, M.A.; Comesaña-Hermo, M.; Rivas-Murias, B.; Salgueirino, V. Raman spectroscopy to unravel the magnetic properties of iron oxide nanocrystals for bio-related applications. Nanoscale Adv. 2019, 1, 2086–2103. [Google Scholar] [CrossRef]
- Kuznetsov, Y.M.; Dorokhin, M.V.; Nezhdanov, A.V.; Zdoroveichev, D.A.; Lesnikov, V.P.; . Ved, M.V. Formation of the β-FeSi2 phase by pulsed laser deposition. J. Phys. Conf. Ser. 2021, 1851, 012007. [Google Scholar] [CrossRef]
- Miki, T.; Matsui, Y.; Matsubara, K.; Kishimoto, K. Electron paramagnetic resonance of defects in β-iron disilicide ceramics. J. Appl. Phys. 1994, 75, 1693–1698. [Google Scholar] [CrossRef]
- Helms, C.R.; Poindexter, E.H. The silicon-silicon dioxide system: Its microstructure and imperfections. Rep. Prog. Phys. 1994, 57, 191. [Google Scholar] [CrossRef]
- Pereira, R.N.; Rowe, D.J.; Anthony, R.J.; Kortshagen, U. Oxidation of freestanding silicon nanocrystals probed with electron spin resonance of interfacial dangling bonds. Phys. Rev. B 2011, 83, 155327. [Google Scholar] [CrossRef]
- Tian, Q.; Jiang, F.; Zou, R.; Liu, Q.; Chen, Z.; Zhu, M.; Yang, S.; Wang, J. Hydrophilic Cu9S5 Nanocrystals: A Photothermal Agent with a 25.7% Heat Conversion Efficiency for Photothermal Ablation of Cancer Cells in Vivo. ACS Nano 2011, 5, 9761–9771. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, X.; Yan, L.; Zhou, L.; Tian, G.; Wenyan, Y.; Liming, W.; Ying, L.; Zhongbo, H.; Gu, Z.; et al. Bismuth Sulfide Nanorods as a Precision Nanomedicine for in Vivo Multimodal Imaging-Guided Photothermal Therapy of Tumor. ACS Nano 2015, 9, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.T.; Yufu, H.; Yuxuan, L.; Feng, L.; Xiaomei, W.; Yuqi, L.; Jie, L.; Yuanyua, J.; Yu, W. Gadolinium-Chelated Conjugated Polymer-Based Nanotheranostics for Photoacoustic/Magnetic Resonance/NIR-II Fluorescence Imaging-Guided Cancer Photothermal Therapy. Theranostics 2019, 9, 4168–4181. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, Y.; Shen, J.; Wei, J.; Yu, C.; Kong, B.; Liu, W.; Yang, H.; Yang, S.; Wang, W. Iron/iron oxide core/shell nanoparticles for magnetic targeting MRI and near-infrared photothermal therapy. Biomaterials 2014, 35, 7470–7478. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, M.K.; Toma, S.H.; Rodrigues, S.F.; Borges Shimada, A.L.; Loiola, R.A.; Cervantes Rodríguez, H.J.; Oliveira, P.V.; Luz, M.S.; Rabbani, S.R.; Toma, H.E.; et al. Ultrasmall cationic superparamagnetic iron oxide nanoparticles as nontoxic and efficient MRI contrast agent and magnetic-targeting tool. Int. J. Nanomedicine 2015, 10, 4731. [Google Scholar] [CrossRef]
- Rohrer, M.; Bauer, H.; Mintorovitch, J.; Requardt, M.; Weinmann, H.-J. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Investig. Radiol. 2005, 40, 715. [Google Scholar] [CrossRef] [PubMed]
- Sharonova, N.V.; Svirshchevskaya, E.V.; Popov, A.A.; Karpov, N.V.; Tikhonovskiy, G.V.; Zakharkiv, A.Y.; Sizova, S.V.; Timoshenko, V.Y.; Klimentov, S.M.; Oleinikov, V.A. Interaction of SiFe Nanoparticles with Epithelial and Lymphoid Cells. Russ. J. Bioorg. Chem. 2020, 46, 1198–1206. [Google Scholar] [CrossRef]



| Type of NPs | Photothermal Conversion Efficiency, % | Relaxivity, mM−1s−1 | Ref. |
|---|---|---|---|
| Si-Fe, laser ablated | 33–43 | 1740 (r2) | This study |
| Si, laser ablated | 10–14 | 51 (r2) | |
| Si-Fe from Ar plasma jets | 53–820 (r2) | [24,25] | |
| Au | 60–80 (at 532 nm) | [36] | |
| CuS | 25.7 | [43] | |
| Gd-doped polymeric | 26 | 11 (r1) | [44,45] |
| SPIO | 20 | 35–200 (r2) | [46,47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bubnov, A.A.; Belov, V.S.; Kargina, Y.V.; Tikhonowski, G.V.; Popov, A.A.; Kharin, A.Y.; Shestakov, M.V.; Perepukhov, A.M.; Syuy, A.V.; Volkov, V.S.; et al. Laser-Ablative Synthesis of Silicon–Iron Composite Nanoparticles for Theranostic Applications. Nanomaterials 2023, 13, 2256. https://doi.org/10.3390/nano13152256
Bubnov AA, Belov VS, Kargina YV, Tikhonowski GV, Popov AA, Kharin AY, Shestakov MV, Perepukhov AM, Syuy AV, Volkov VS, et al. Laser-Ablative Synthesis of Silicon–Iron Composite Nanoparticles for Theranostic Applications. Nanomaterials. 2023; 13(15):2256. https://doi.org/10.3390/nano13152256
Chicago/Turabian StyleBubnov, Alexander A., Vladimir S. Belov, Yulia V. Kargina, Gleb V. Tikhonowski, Anton A. Popov, Alexander Yu. Kharin, Mikhail V. Shestakov, Alexander M. Perepukhov, Alexander V. Syuy, Valentyn S. Volkov, and et al. 2023. "Laser-Ablative Synthesis of Silicon–Iron Composite Nanoparticles for Theranostic Applications" Nanomaterials 13, no. 15: 2256. https://doi.org/10.3390/nano13152256
APA StyleBubnov, A. A., Belov, V. S., Kargina, Y. V., Tikhonowski, G. V., Popov, A. A., Kharin, A. Y., Shestakov, M. V., Perepukhov, A. M., Syuy, A. V., Volkov, V. S., Khovaylo, V. V., Klimentov, S. M., Kabashin, A. V., & Timoshenko, V. Y. (2023). Laser-Ablative Synthesis of Silicon–Iron Composite Nanoparticles for Theranostic Applications. Nanomaterials, 13(15), 2256. https://doi.org/10.3390/nano13152256










