Assessing the In Vitro Digestion of Lactoferrin-Curcumin Nanoparticles Using the Realistic Gastric Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lf–Curcumin Nanoparticles Preparation
2.3. The In Vitro RGM
2.4. In Vitro Digestion Protocol
2.5. Dynamic Light Scattering
2.6. Lf Intrinsic Fluorescence
2.7. Confocal Scanning Laser Microscopy
2.8. Real-Time Analyses of Lf Nanoparticles during In Vitro Gastric Digestion
2.9. Statistical Analysis
3. Results and Discussion
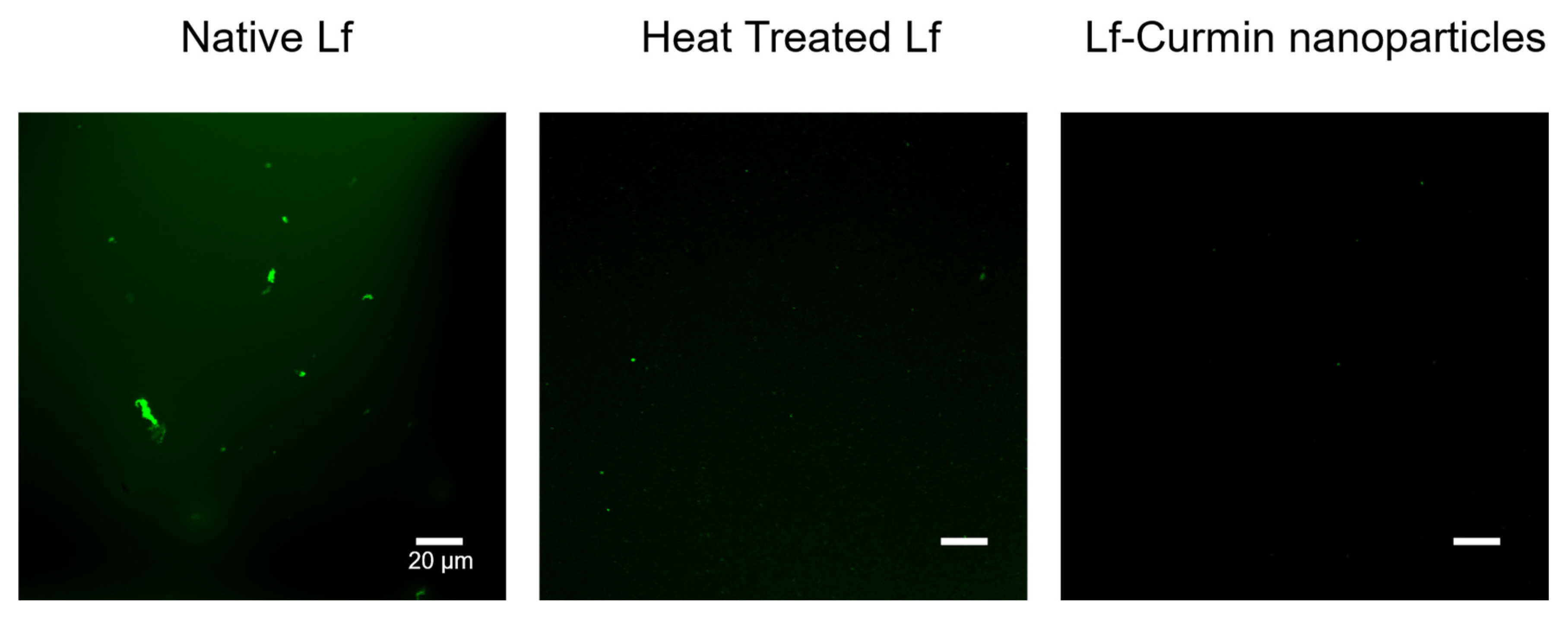
3.1. Lf–Curcumin Nanoparticles’ Characterization
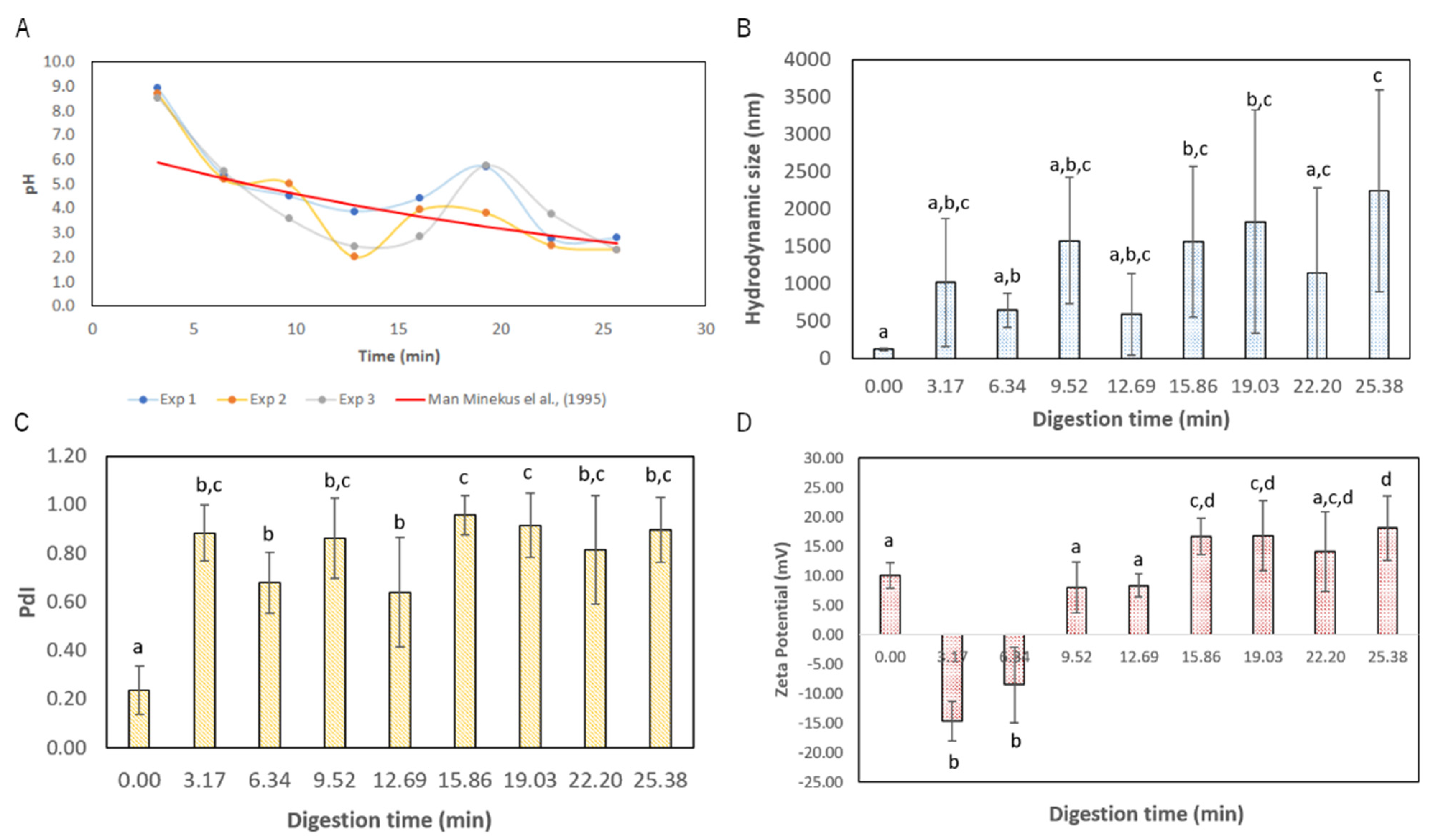
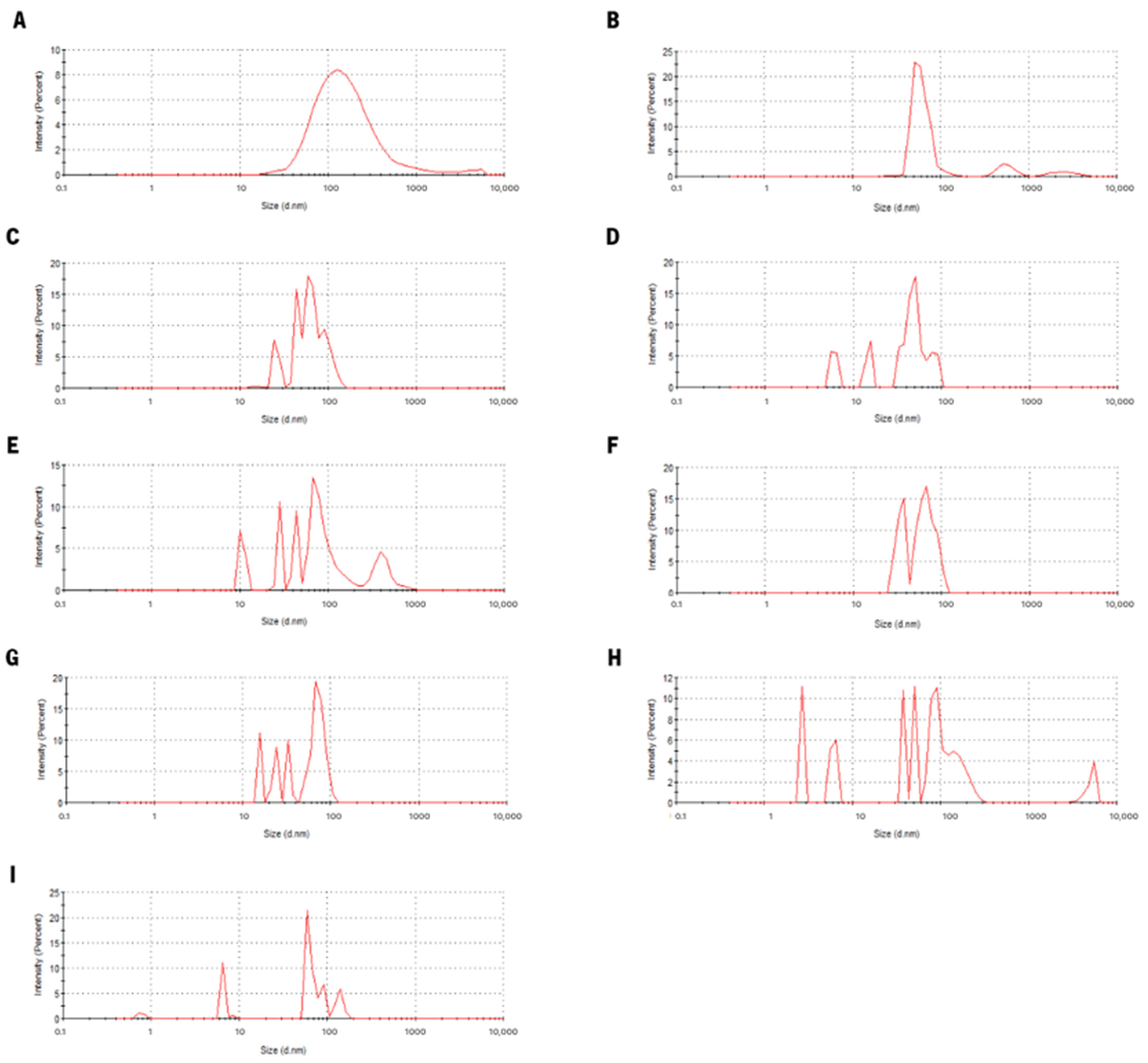
3.2. Lf–Curcumin Nanoparticles’ Behaviour during In Vitro Gastric Digestion
3.3. Curcumin Release during the In Vitro Digestion Process
3.4. In Situ Assessment of Protein Nanoparticles’ Hydrolysis Using UV-VIS-SWNIR Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jafari, S.M.; Katouzian, I.; Akhavan, S. Safety and regulatory issues of nanocapsules. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Elsevier: Amsterdam, The Netherlands, 2017; pp. 545–590. [Google Scholar] [CrossRef]
- Madalena, D.A.; Pereira, R.N.; Vicente, A.A.; Ramos, Ó.L. New Insights on Bio-Based Micro- and Nanosystems in Food. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 708–714. [Google Scholar] [CrossRef]
- Bourbon, A.I.; Martins, J.T.; Pinheiro, A.C.; Madalena, D.A.; Marques, A.; Nunes, R.; Vicente, A.A. Nanoparticles of lactoferrin for encapsulation of food ingredients. Biopolym. Nanostructures Food Encapsulation Purp. 2019, 1, 147–168. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Gonçalves, R.F.; A Madalena, D.; A Vicente, A. Towards the understanding of the behavior of bio-based nanostructures during in vitro digestion. Curr. Opin. Food Sci. 2017, 15, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Araújo, J.F.; Bourbon, A.; Simões, L.; Vicente, A.A.; Coutinho, P.J.G.; Ramos, Ó.L. Physicochemical characterisation and release behaviour of curcumin-loaded lactoferrin nanohydrogels into food simulants. Food Funct. 2020, 11, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.F.S.; Martins, J.T.; Abrunhosa, L.; Vicente, A.A.; Pinheiro, A.C. Nanoemulsions for Enhancement of Curcumin Bioavailability and Their Safety Evaluation: Effect of Emulsifier Type. Nanomaterials 2021, 11, 815. [Google Scholar] [CrossRef]
- Furlund, C.; Ulleberg, E.; Devold, T.; Flengsrud, R.; Jacobsen, M.; Sekse, C.; Holm, H.; Vegarud, G. Identification of lactoferrin peptides generated by digestion with human gastrointestinal enzymes. J. Dairy Sci. 2013, 96, 75–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef]
- Bokkhim, H.; Bansal, N.; Grøndahl, L.; Bhandari, B. In-vitro digestion of different forms of bovine lactoferrin encapsulated in alginate micro-gel particles. Food Hydrocoll. 2016, 52, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Mulet-Cabero, A.-I.; Egger, L.; Portmann, R.; Ménard, O.; Marze, S.; Minekus, M.; Le Feunteun, S.; Sarkar, A.; Grundy, M.M.-L.; Carrière, F.; et al. A standardised semi-dynamic in vitro digestion method suitable for food—An international consensus. Food Funct. 2020, 11, 1702–1720. [Google Scholar] [CrossRef] [Green Version]
- Bourbon, A.I.; Cerqueira, M.A.; Vicente, A.A. Encapsulation and controlled release of bioactive compounds in lactoferrin-glycomacropeptide nanohydrogels: Curcumin and caffeine as model compounds. J. Food Eng. 2016, 180, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Ferrua, M.J.; Singh, R.P. Understanding the fluid dynamics of gastric digestion using computational modeling. Procedia Food Sci. 2012, 1, 1465–1472. [Google Scholar] [CrossRef] [Green Version]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Möller, M.; Denicola, A. Protein tryptophan accessibility studied by fluorescence quenching. Biochem. Mol. Biol. Educ. 2002, 30, 175–178. [Google Scholar] [CrossRef]
- Liang, L.; Qi, C.; Wang, X.; Jin, Q.; McClements, D.J. Influence of Homogenization and Thermal Processing on the Gastrointestinal Fate of Bovine Milk Fat: In Vitro Digestion Study. J. Agric. Food Chem. 2017, 65, 11109–11117. [Google Scholar] [CrossRef]
- Gonçalves, R.F.; Martins, J.T.; Abrunhosa, L.; Baixinho, J.; Matias, A.A.; Vicente, A.A.; Pinheiro, A.C. Lipid-based nanostructures as a strategy to enhance curcumin bioaccessibility: Behavior under digestion and cytotoxicity assessment. Food Res. Int. 2021, 143, 110278. [Google Scholar] [CrossRef] [PubMed]
- Bollimpelli, V.S.; Kumar, P.; Kumari, S.; Kondapi, A.K. Neuroprotective effect of curcumin-loaded lactoferrin nano particles against rotenone induced neurotoxicity. Neurochem. Int. 2016, 95, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.P.; More, M.P.; Karande, K.P.; Chitalkar, R.V.; Patil, P.O.; Deshmukh, P.K. Optimization of desolvation process for fabrication of lactoferrin nanoparticles using quality by design approach. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1101–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahdar, A.; Amini, N.; Askari, F.; Susan, M.A.B.H. Dynamic light scattering: A useful technique to characterize nanoparticles. J. Nanoanalysis 2019, 6, 80–89. [Google Scholar]
- Herriott, R.M.; Desreux, V.; Northrop, J.H. Electrophoresis of Pepsin. J. Gen. Physiol. 1940, 23, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Andreeva, N.S.; James, M.N.G. Why Does Pepsin Have a Negative Charge at Very Low pH? An Analysis of Conserved Charged Residues in Aspartic Proteinases BT—Structure and Function of the Aspartic Proteinases: Genetics, Structures, and Mechanisms. In Structure and Function of the Aspartic Proteinases; Dunn, B.M., Ed.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 39–45. [Google Scholar] [CrossRef]
- Bourbon, A.; Pinheiro, A.C.; Cerqueira, M.A.; Vicente, A.A. In vitro digestion of lactoferrin-glycomacropeptide nanohydrogels incorporating bioactive compounds: Effect of a chitosan coating. Food Hydrocoll. 2018, 84, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Madalena, D.; Fernandes, J.-M.; Avelar, Z.; Gonçalves, R.F.; Ramos, L.; Vicente, A.A.; Pinheiro, A.C. Emerging challenges in assessing bio-based nanosystems’ behaviour under in vitro digestion focused on food applications—A critical view and future perspectives. Food Res. Int. 2022, 157, 111417. [Google Scholar] [CrossRef]
- Du, X.; Jing, H.; Wang, L.; Huang, X.; Mo, L.; Bai, X.; Wang, H. pH-shifting formation of goat milk casein nanoparticles from insoluble peptide aggregates and encapsulation of curcumin for enhanced dispersibility and bioactivity. LWT 2022, 154, 112753. [Google Scholar] [CrossRef]
- Peng, H.; Gan, Z.; Xiong, H.; Luo, M.; Yu, N.; Wen, T.; Wang, R.; Li, Y. Self-Assembly of Protein Nanoparticles from Rice Bran Waste and Their Use as Delivery System for Curcumin. ACS Sustain. Chem. Eng. 2017, 5, 6605–6614. [Google Scholar] [CrossRef]
- Beltrán, J.D.; Sandoval-Cuellar, C.E.; Bauer, K.; Quintanilla-Carvajal, M.X. In-vitro digestion of high-oleic palm oil nanoliposomes prepared with unpurified soy lecithin: Physical stability and nano-liposome digestibility. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 578, 123603. [Google Scholar] [CrossRef]
- Simões, L.; Martins, J.T.; Pinheiro, A.C.; Vicente, A.A.; Ramos, Ó.L. β-lactoglobulin micro- and nanostructures as bioactive compounds vehicle: In vitro studies. Food Res. Int. 2020, 131, 108979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
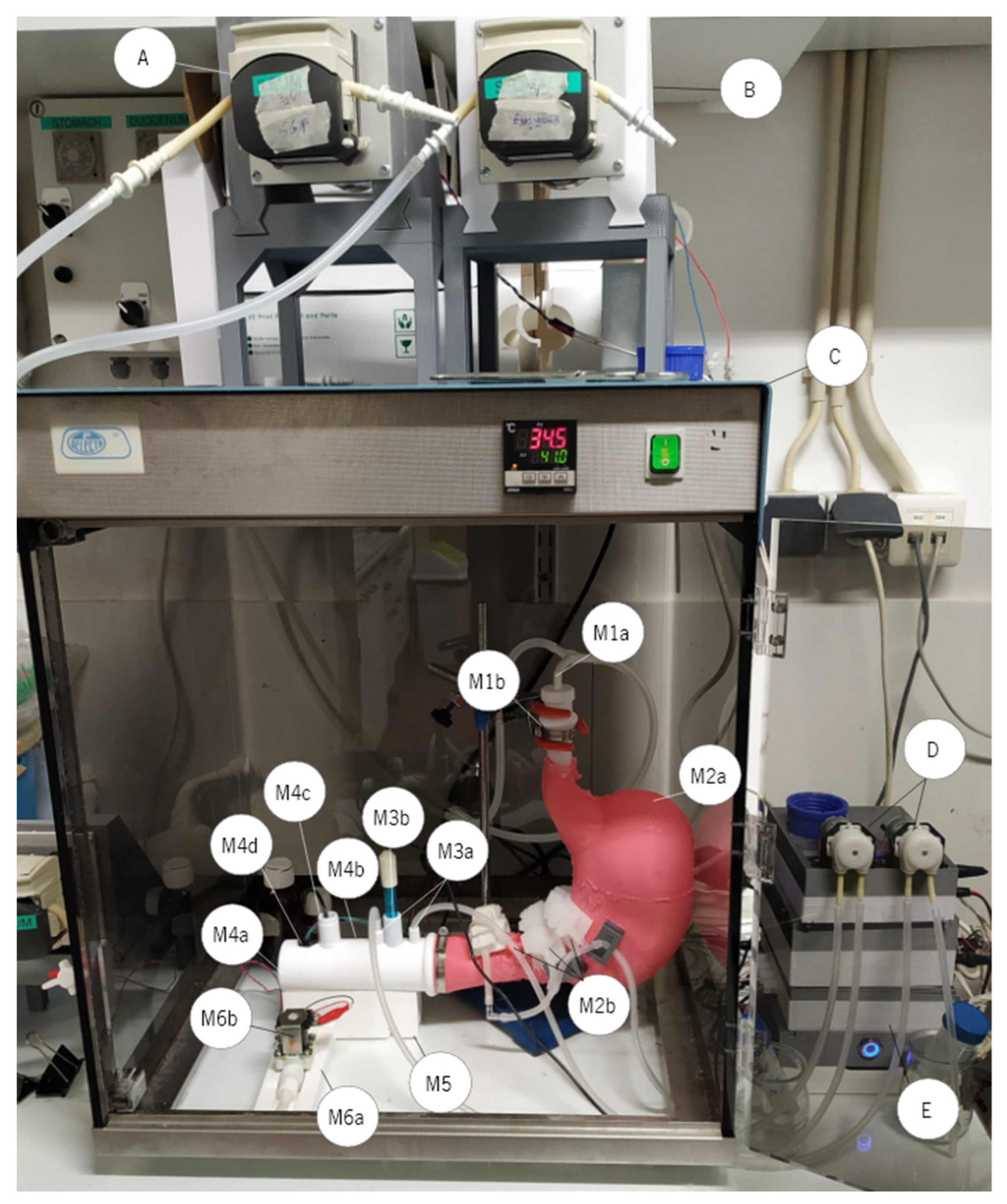
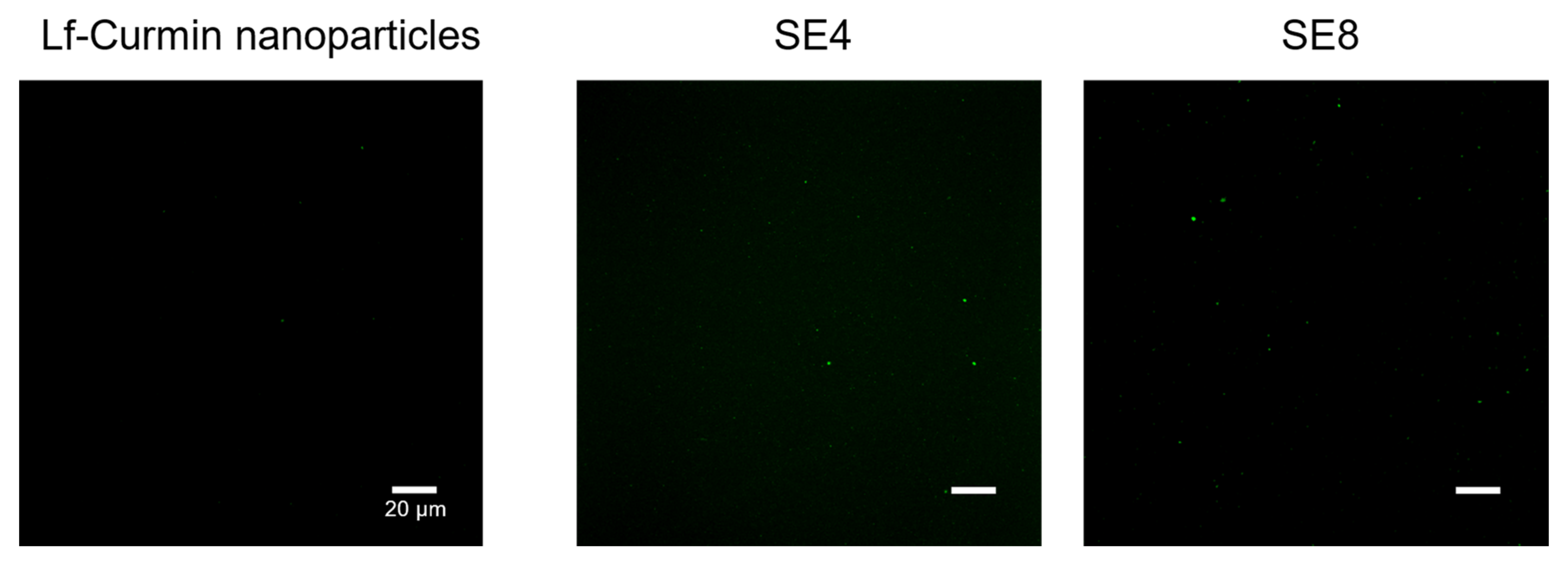
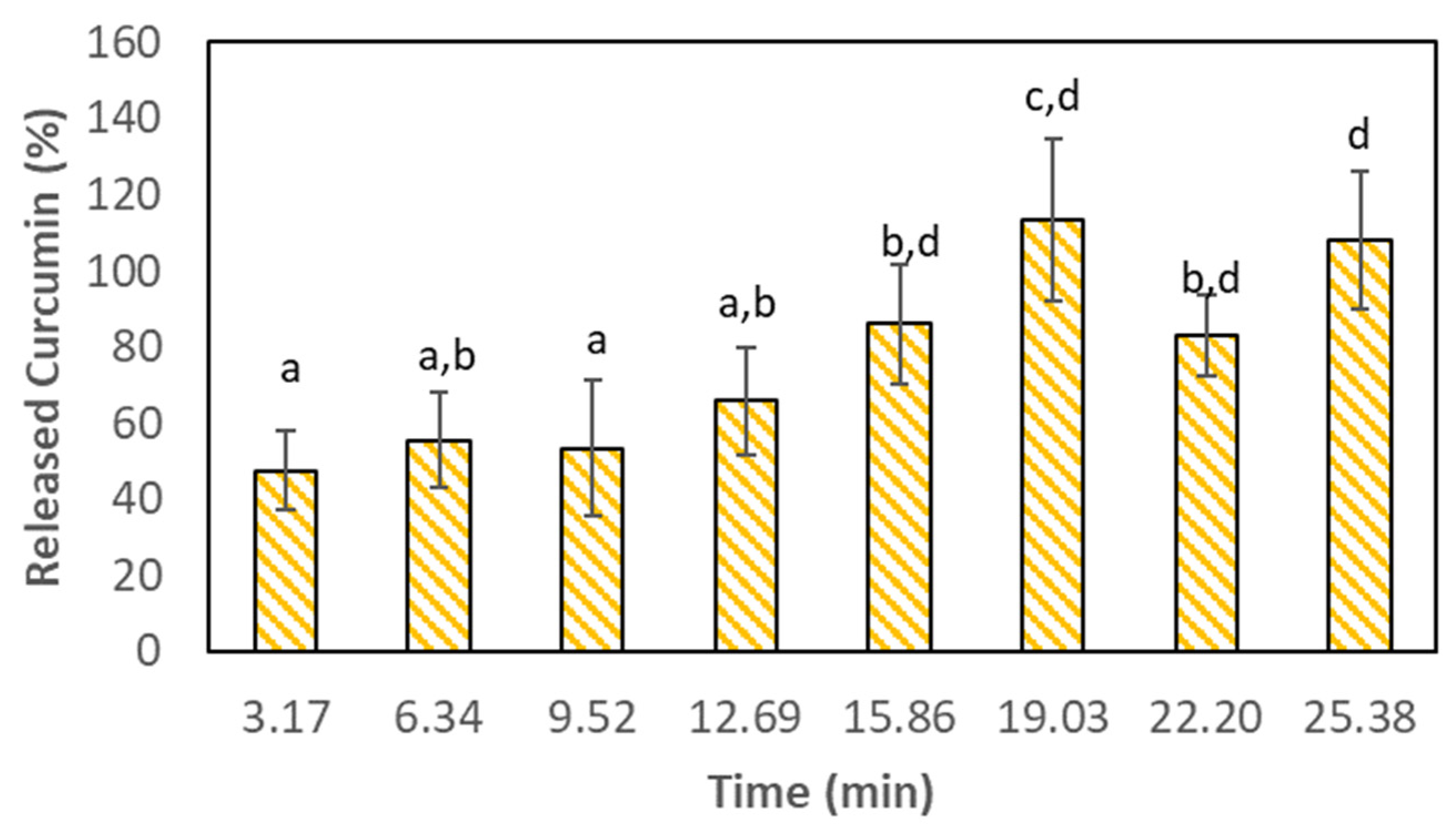
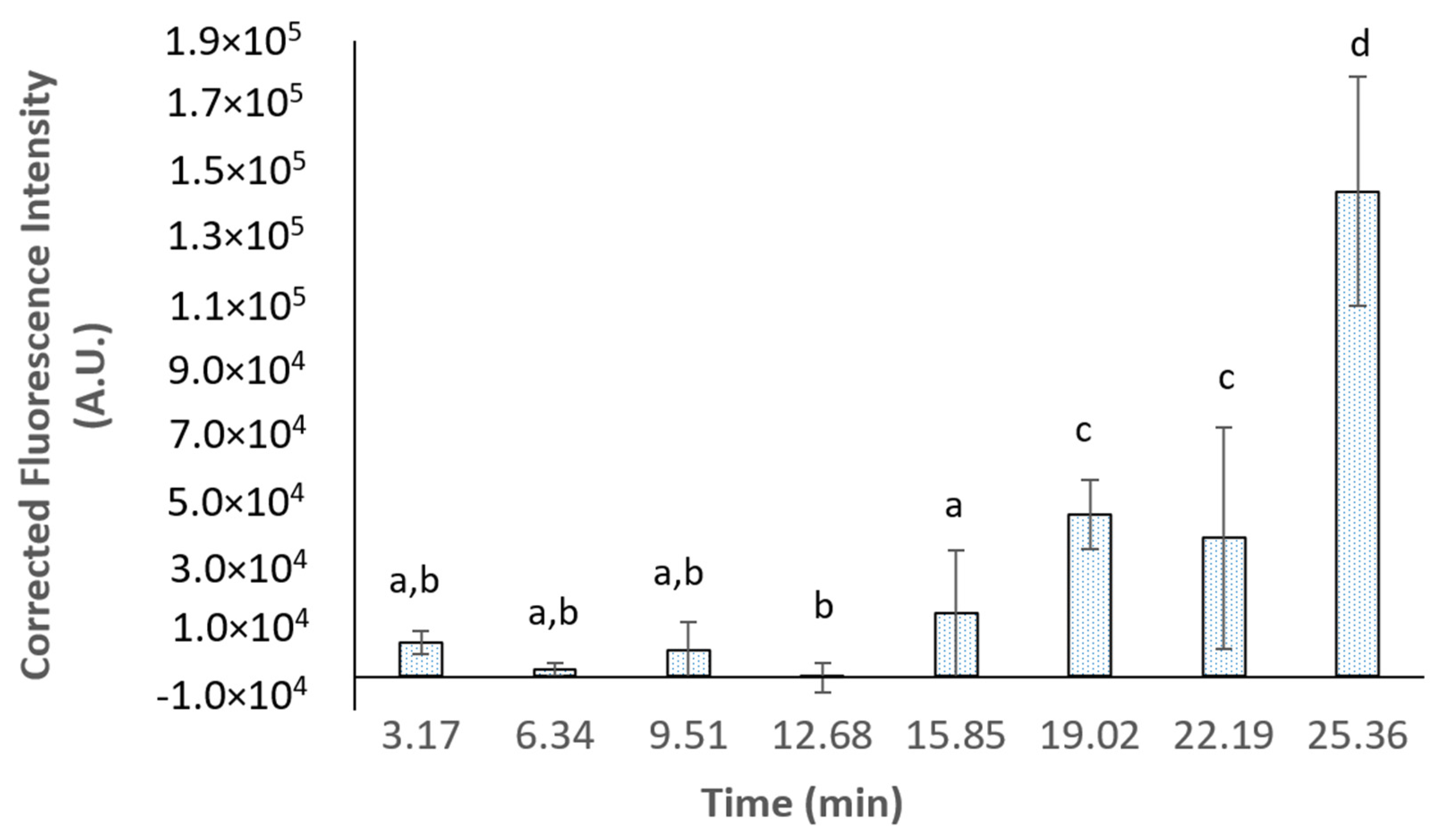
| Fluid | Oral Phase | Gastric Phase | SGF Flow (mL·min−1) | Enzymes Flow (mL·min−1) | SE Rate (mL·min−1) | Number of SE |
|---|---|---|---|---|---|---|
| Electrolyte solution (mL) | 6.40 (SSF) | 76.40 (SGF) | 3.26 | 0.37 | 8.09 | 8 |
| 0.3 M CaCl2 (µL) | 40.00 | 54.00 | ||||
| Water (mL) | 1.56 | 18.39 |
| Nanoparticle | Hydrodynamic Size (nm) | PdI | Zeta Potential (mV) | AE (%) |
|---|---|---|---|---|
| Lf–Curcumin | 118.97 ± 12.45 | 0.24 ± 0.10 | 10.04 ± 2.16 | 82.10 ± 0.04 |
| SE1 | SE2 | SE3 | |
|---|---|---|---|
| Free NH2 groups (mg·mL−1) | 0.0681 ± 0.0290 a | 0.1225 ± 0.0002 b | 0.2222 ± 0.1389 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madalena, D.A.; Araújo, J.F.; Ramos, Ó.L.; Vicente, A.A.; Pinheiro, A.C. Assessing the In Vitro Digestion of Lactoferrin-Curcumin Nanoparticles Using the Realistic Gastric Model. Nanomaterials 2023, 13, 2237. https://doi.org/10.3390/nano13152237
Madalena DA, Araújo JF, Ramos ÓL, Vicente AA, Pinheiro AC. Assessing the In Vitro Digestion of Lactoferrin-Curcumin Nanoparticles Using the Realistic Gastric Model. Nanomaterials. 2023; 13(15):2237. https://doi.org/10.3390/nano13152237
Chicago/Turabian StyleMadalena, Daniel A., João F. Araújo, Óscar L. Ramos, António A. Vicente, and Ana C. Pinheiro. 2023. "Assessing the In Vitro Digestion of Lactoferrin-Curcumin Nanoparticles Using the Realistic Gastric Model" Nanomaterials 13, no. 15: 2237. https://doi.org/10.3390/nano13152237
APA StyleMadalena, D. A., Araújo, J. F., Ramos, Ó. L., Vicente, A. A., & Pinheiro, A. C. (2023). Assessing the In Vitro Digestion of Lactoferrin-Curcumin Nanoparticles Using the Realistic Gastric Model. Nanomaterials, 13(15), 2237. https://doi.org/10.3390/nano13152237






