Low-Temperature Enhancement-Mode Amorphous Oxide Thin-Film Transistors in Solution Process Using a Low-Pressure Annealing
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
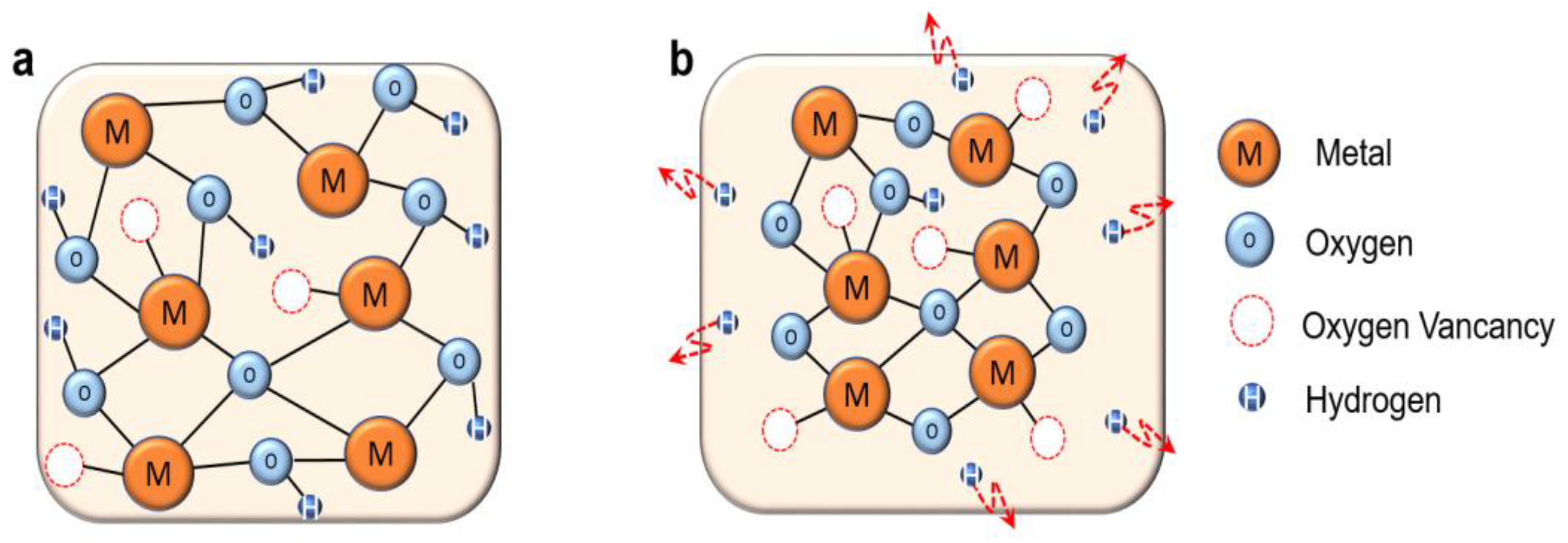
3.1. Effect of LPA on Atomic Bonding State of InOx
3.2. Physical Properties of InOx Semiconductor Thin Films
3.3. Electrical Characteristics of LPA InOx TFTs
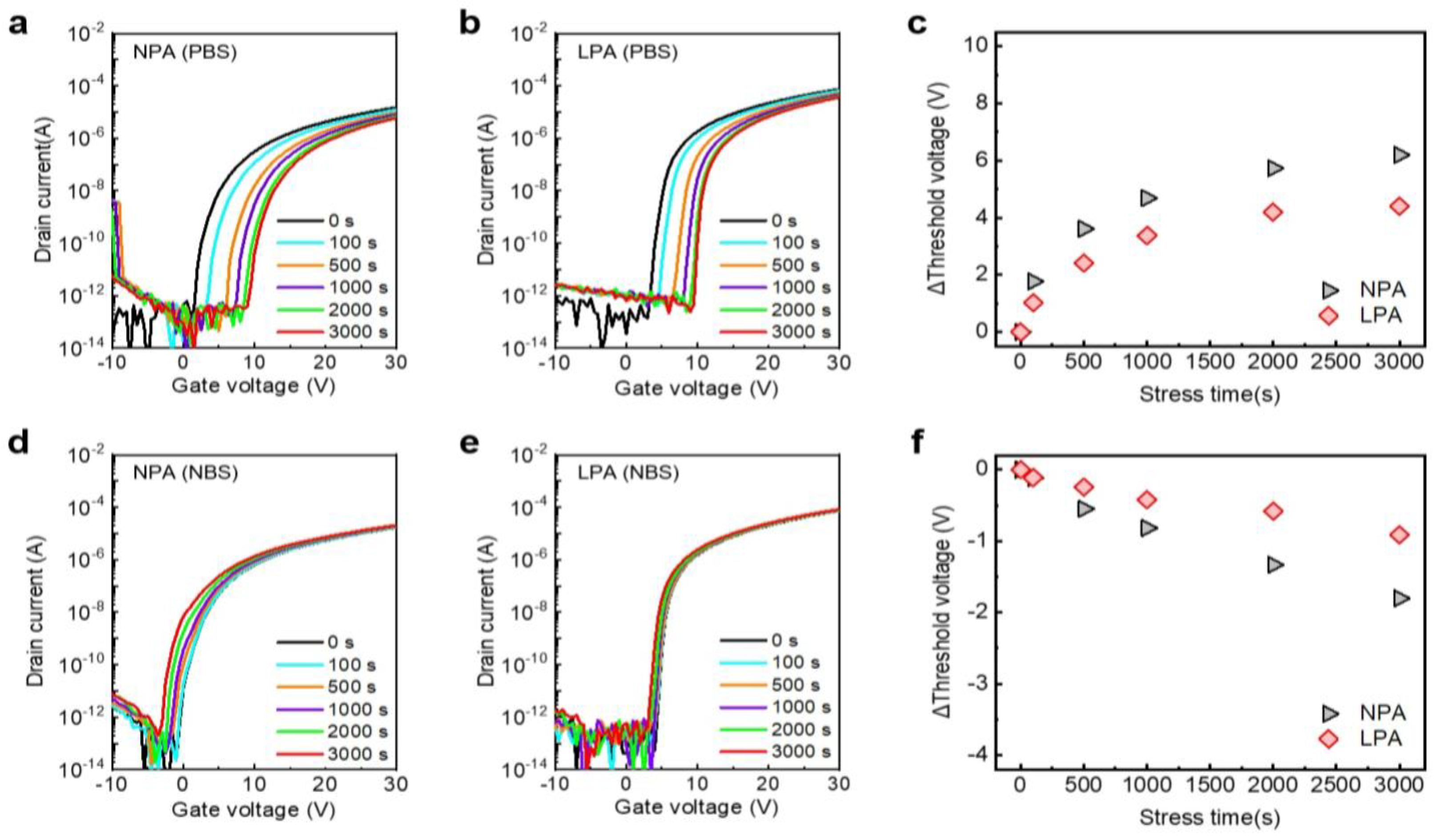
3.4. Bias Stress-Induced Instability of LPA InOx TFTs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fortunato, E.; Barquinha, P.; Martins, R. Oxide semiconductor thin-film transistors: A review of recent advances. Adv. Mater. 2012, 24, 2945–2986. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Marks, T.J.; Facchetti, A. Metal oxides for optoelectronic applications. Nat. Mater. 2016, 15, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Si, M.; Lin, Z.; Sun, X.; Wang, H.; Ye, P.D. Scaled indium oxide transistors fabricated using atomic layer deposition. Nat. Electron. 2022, 5, 164–170. [Google Scholar] [CrossRef]
- Park, J.S.; Maeng, W.J.; Kim, H.S.; Park, J.S. Review of recent developments in amorphous oxide semiconductor thin-film transistor devices. Thin Solid Film. 2012, 520, 1679–1693. [Google Scholar] [CrossRef]
- Xu, W.; Li, H.; Xu, J.-B.; Wang, L. Recent Advances of Solution-Processed Metal Oxide Thin-Film Transistors. ACS Appl. Mater. Interfaces 2018, 10, 25878–250901. [Google Scholar] [CrossRef]
- Park, W.P.; Kang, B.H.; Kim, H.J. A Review of Low-Temperature Solution-Processed Metal Oxide Thin-Film Transistors for Flexible Electronics. Adv. Funct. Mater. 2020, 30, 1904632. [Google Scholar] [CrossRef]
- Fukuda, K.; Someya, T. Recent Progress in the Development of Printed Thin-Film Transistors and Circuits with High-Resolution Printing Technology. Adv. Mater. 2016, 29, 1524–4095. [Google Scholar] [CrossRef]
- Ahn, B.D.; Jeon, H.J.; Sheng, J.; Park, J.; Park, J. A review on the recent developments of solution processes for oxide thin film transistors. Semicond. Sci. Technol. 2015, 30, 268–1242. [Google Scholar] [CrossRef]
- Kamiya, T.; Hosno, H. Material characteristics and applications of transparent amorphous oxide semiconductors. NPG Asia Mater. 2010, 2, 15–22. [Google Scholar] [CrossRef]
- Kim, H.S.; Byrne, P.D.; Facchetti, A.; Marks, T.J. High Performance Solution-Processed Indium Oxide Thin-Film Transistors. J. Am. Chem. Soc. 2008, 130, 12580–12581. [Google Scholar] [CrossRef]
- Choi, S.; Park, J.; Hwang, S.H.; Kim, C.; Kim, Y.-S.; Oh, S.; Baeck, J.H.; Bae, J.U.; Noh, J.; Lee, S.W.; et al. Excessive Oxygen Peroxide Model-Based Analysis of Positive-Bias-Stress and Negative-Bias-Illumination-Stress Instabilities in Self-Aligned Top-Gate Coplanar In–Ga–Zn–O Thin-Film Transistors. Adv. Electron. Mater. 2022, 8, 2101062. [Google Scholar] [CrossRef]
- Sohna, Y.; Moon, G.; Choi, K.; Kim, Y.S.; Park, K.C. Effects of TFT mobility variation in the threshold voltage compensation circuit of the OLED display. J. Inf. Disp. 2016, 18, 25–30. [Google Scholar] [CrossRef]
- Buchholz, D.B.; Ma, Q.; Alducin, D.; Ponce, A.; Yacaman, M.J.; Khanal, R.; Medvedeva, J.E.; Chang, R. The Structure and Properties of Amorphous Indium Oxide. ACS Appl. Mater. Interfaces 2014, 26, 5401–5411. [Google Scholar] [CrossRef]
- Babu, S.H.; Kaleemulla, S.; Madlhusudhana, N.; Krishnamoorthi, C. Indium oxide: A transparent, conducting ferromagnetic semiconductor for spintronic applications. J. Magn. Magn. Mater. 2016, 416, 66–74. [Google Scholar] [CrossRef]
- Kim, D.-K.; Seo, K.H.; Kwon, D.H.; Jeon, S.H.; Hwang, Y.J.; Wang, Z.; Park, J.H.; Lee, S.H.; Jang, J.W.; Kang, I.M.; et al. Viable strategy to minimize trap states of patterned oxide thin films for both exceptional electrical performance and uniformity in sol–gel processed transistors. J. Chem. Eng. 2022, 441, 66–74. [Google Scholar] [CrossRef]
- Kim, Y.H.; Heo, J.S.; Kim, T.H.; Park, S.J.; Yoon, M.H.; Kim, J.W.; Oh, M.S.; Yi, G.R.; Noh, Y.Y.; Park, S.K. Flexible metal-oxide devices made by room-temperature photochemical activation of sol–gel films. Nature 2012, 489, 128–132. [Google Scholar] [CrossRef]
- Heo, J.S.; Jo, J.W.; Kang, J.; Jeong, C.Y.; Jeong, H.Y.; Kim, S.K.; Kim, K.; Kwon, H.I.; Kim, J.Y.; Kim, Y.H.; et al. Water-Mediated Photochemical Treatments for Low-Temperature Passivation of Metal-Oxide Thin-Film Transistors. ACS Appl. Mater. Interfaces 2016, 8, 10403–10412. [Google Scholar] [CrossRef]
- Yeom, H.-I.; Ko, J.B.; Mun, G.; Park, S.-H.K. High mobility polycrystalline indium oxide thin-film transistors by means of plasma-enhanced atomic layer deposition. J. Mater. Chem. C 2016, 4, 6873–6880. [Google Scholar] [CrossRef]
- Socratous, J.; Kulbinder, K.; Vaynzof, Y.; Sadhanala, A.; Brow, A.; Sepe, A.; Steiner, U.; Sirringhaus, H. Electronic Structure of Low-Temperature Solution-Processed Amorphous Metal Oxide Semiconductors for Thin-Film Transistor Applications. Adv. Funct. Mater. 2015, 25, 1873–1885. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, G.; Liu, A.; Song, H.; Hou, Y.; Shinb, B.; Shan, F. Low-Temperature Fabrication of High Performance Indium Oxide Thin Film Transistors Condensation annealing temp. RSC Adv. 2015, 5, 37807–37813. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, H.; Gong, J.; Sun, C.; Jiang, X. Role of oxygen desorption during vacuum annealing in the improvement of electrical properties of aluminum doped zinc oxide films synthesized by sol gel method. J. Appl. Phys. 2007, 102, 102–107. [Google Scholar] [CrossRef]
- Xie, J.Z.; Murarka, S.P.; Guo, X.S.; Lanford, W.A. Stability of hydrogen in silicon nitride films deposited by low-pressure and plasma enhanced chemical vapor deposition techniques. J. Vac. Sci. Technol. B Mater. 1989, 25, 150–152. [Google Scholar] [CrossRef]
- Wang, H.; Li, P.; Chen, Z.; Yang, B.; Wei, B.; Fu, C.; Ding, X.; Zhang, J. Physico-Chemical Origins of Electrical Characteristics and Instabilities in Solution-Processed ZnSnO Thin-Film Transistors. Coatings 2022, 12, 1534. [Google Scholar] [CrossRef]
- Jeon, J.W.; Um, J.G.; Lee, S.; Jang, J. Control of O-H bonds at a-IGZO/SiO2 interface by long time thermal annealing for highly stable oxide TFT. AIP Adv. 2017, 7, 125110. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Ning, H.; Lu, K.; Zhang, H.; Zhang, X.; Yao, R.; Fang, Z.; Lu, X.; Peng, J. Induced nano-scale self-formedmetal-oxide interlayer in amorphous silicon tin oxide thin flm transistors. Sci. Rep. 2018, 7, 4160. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.M.; Jung, J.; Rim, Y.S.; Kim, D.L.; Kim, H.J. Improvement in Negative Bias Stress Stability of Solution-Processed Amorphous In–Ga–Zn–O Thin-Film Transistors Using Hydrogen Peroxide. ACS Appl. Mater. Interfaces 2014, 6, 3371–3377. [Google Scholar] [CrossRef]
- Rim, Y.S.; Jeong, W.H.; Kim, D.L.; Lim, H.S.; Kim, K.M.; Kim, H.J. Simultaneous modification of pyrolysis and densification for low-temperature solution-processed flexible oxide thin-film transistors. J. Mater. Chem. 2012, 22, 12491–12497. [Google Scholar] [CrossRef]
- Arulkumar, S.; Parthiban, S.; Kwon, J.Y.; Uraoka, Y.; Bermundo, J.P.S.; Mukherjee, A.; Das, B. High mobility silicon indium oxide thin-film transistor fabrication by sputtering process. Vacuum 2022, 199, 110963. [Google Scholar] [CrossRef]
- Park, W.T.; Son, I.Y.; Park, H.W.; Chung, K.B.; Xu, Y.; Lee, T.; Noh, Y.Y. Facile Routes to Improve Performance of Solution-Processed Amorphous Metal Oxide Thin Film Transistors by Water Vapor Annealing. ACS Appl. Mater. Interfaces 2015, 24, 13289–13294. [Google Scholar] [CrossRef]
- Chang, J.; Chang, K.L.; Chi, C.; Zhang, J.; Wu, J. Water induced zinc oxide thin film formation and its transistor performance. J. Mater. Chem. C 2014, 2, 5397–5403. [Google Scholar] [CrossRef]
- Faber, H.; Das, S.; Lin, Y.-H.; Pliatsikas, N.; Zhao, K.; Kehagias, T.; Dimitrakopulos, G.; Amassian, A.; Patsalas, P.A.; Anthopoulos, D.A. Heterojunction oxide thin-film transistors with unprecedented electron mobility grown from solution. Sci. Adv. 2017, 3, e1602640. [Google Scholar] [CrossRef] [PubMed]
- Ting, C.-C.; Fan, H.-Y.; Tsai, M.-K.; Li, W.-Y.; Yong, H.-E.; Lin, Y.-F. Improvement of electrical characteristics in the solution-processed nanocrystalline indium oxide thin-film transistors depending on yttrium doping concentration. Mater. Sci. Appl. 2013, 211, 800–810. [Google Scholar] [CrossRef]
- Kim, G.H.; Ahn, D.B.; Shin, H.S.; Jeong, W.H.; Kim, H.J.; Kim, H.J. Effect of indium composition ratio on solution-processed nanocrystalline InGaZnO thin film transistors. Appl. Phys. Lett. 2009, 94, 233501. [Google Scholar] [CrossRef]
- Lee, J.S.; Song, S.M.; Kim, Y.H.; Kwon, J.Y.; Han, M.K. Effects of O2 plasma treatment on low temperature solution-processed zinc tin oxide thin film transistors. Mater. Sci. Appl. 2013, 210, 1745–1749. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Chou, C.-H.; Chen, Y.-T.; Jhang, W.-C. A Study of Solution-Processed Zinc–Tin-Oxide Semiconductors for Thin-Film Transistors. IEEE Trans. Electron. Devices 2019, 66, 2631–2636. [Google Scholar] [CrossRef]
- Sabri, M.M.; Jung, J.; Yoon, D.H.; Yoon, S.; Tak, Y.J.; Kim, H.J. Hydroxyl radical-assisted decomposition and oxidation in solution-processed indium oxide thin-film transistors. J. Mater. Chem. C 2015, 3, 7499–7505. [Google Scholar] [CrossRef]
- Hwang, Y.-J.; Kim, D.-K.; Jeon, S.-H.; Park, J.; Lee, S.-H.; Jang, J.; Kang, I.M.; Bae, J.H. Importance of Structural Relaxation on the Electrical Characteristics and Bias Stability of Solution-Processed ZnSnO Thin-Film Transistors. Nanomaterials 2022, 12, 3097. [Google Scholar] [CrossRef]
- Kim, H.J.; Je, S.Y.; Won, J.Y.; Baek, H.J.; Jeon, J.K. Effect of antimony doping on the low-temperature performance of solu-tion-processed indium oxide thin film transistors. Phys. Status Solidi 2014, 8, 924–927. [Google Scholar]
- Jeong, J.K. Photo-bias instability of metal oxide thin film transistors for advanced active matrix displays. J. Meter. Res. 2013, 28, 16. [Google Scholar] [CrossRef]
- Janotti, A.; Walle, C.G.V. Oxygen vacancies in ZnO. Appl. Phys. Lett. 2005, 87, 122102. [Google Scholar] [CrossRef]
- Ide, K.; Nomura, K.; Hosono, H.; Kamiya, T. Electronic Defects in Amorphous Oxide Semiconductors: A Review. Phys. Status Solidi A 2019, 216, 1800372. [Google Scholar] [CrossRef]
- Choi, S.; Jang, J.; Kang, H.; Baeck, J.H.; Bae, J.U.; Park, K.S.; Yoon, S.H.; Kang, I.B.; Kim, D.M.; Choi, S.J.; et al. Systematic Decomposition of the Positive Bias Stress Instability in Self-Aligned Coplanar InGaZnO Thin-Film Transistors. IEEE Elec. Dev. Lett. 2017, 38, 580–583. [Google Scholar] [CrossRef]
- Yang, G.W.; Park, J.; Choi, S.; Kim, C.; Kim, D.M.; Choi, S.-J.; Bae, J.H.; Cho, H.; Kim, D.H. Total Subgap Range Density of States-Based Analysis of the Effect of Oxygen Flow Rate on the Bias Stress Instabilities in a-IGZO TFTs. IEEE Trans. Electron. Devices 2022, 69, 166–173. [Google Scholar] [CrossRef]

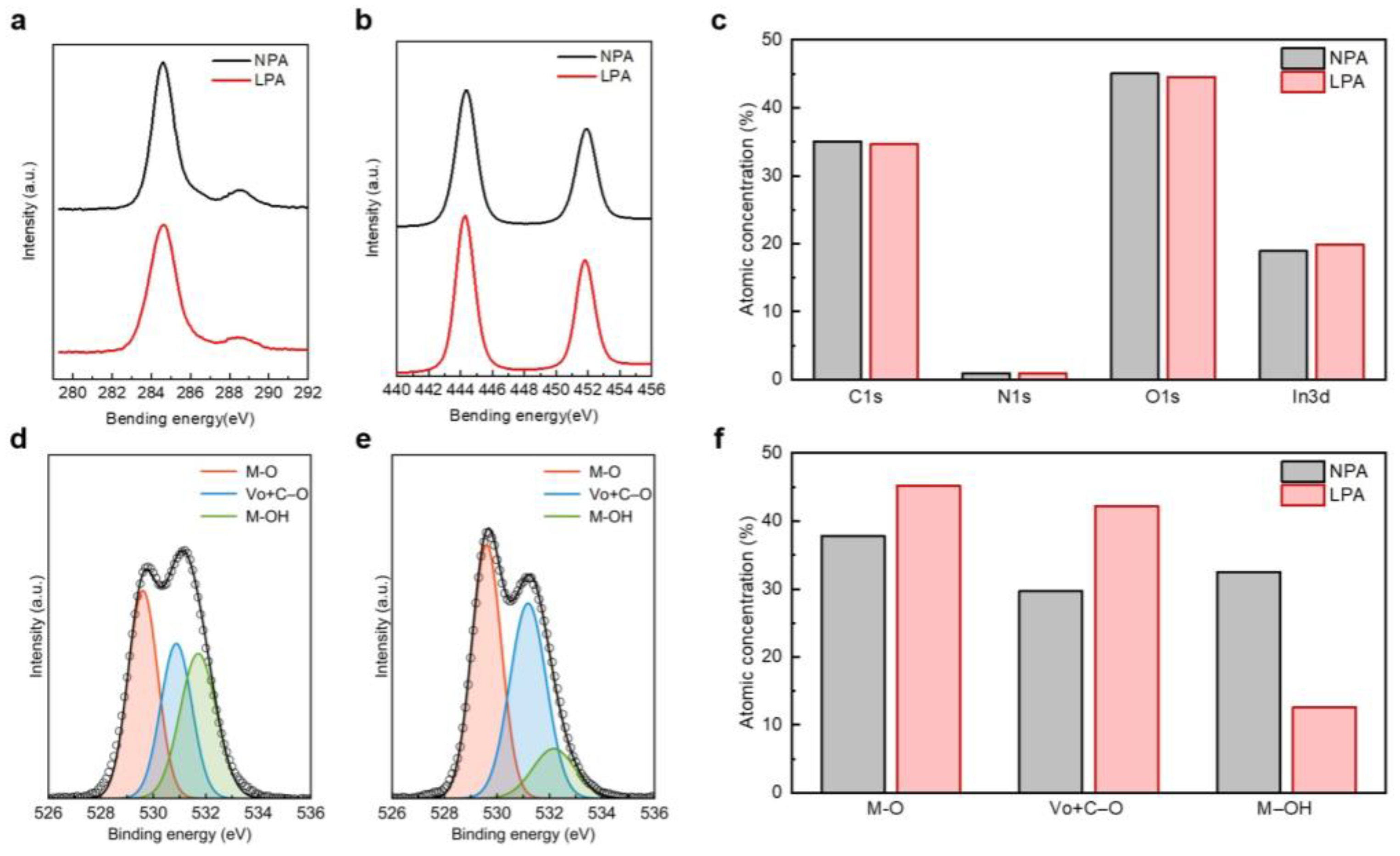
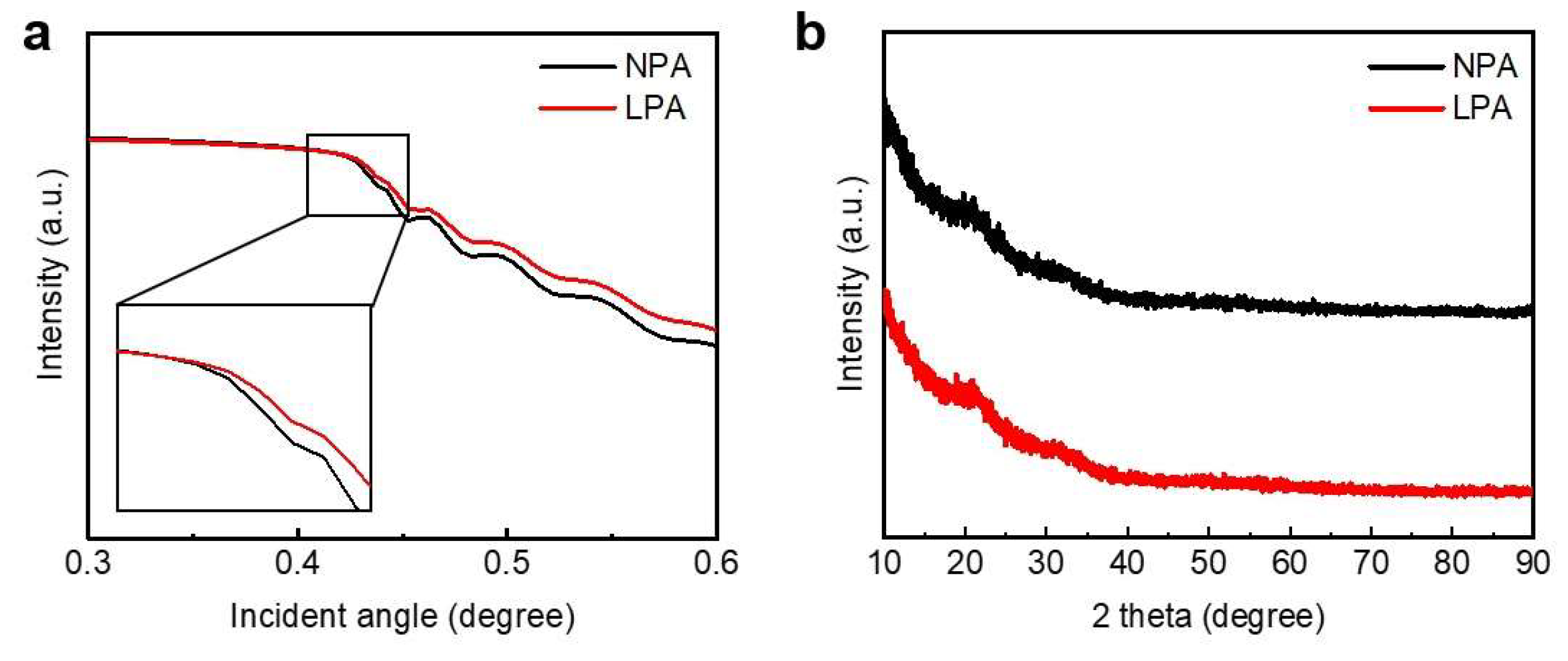
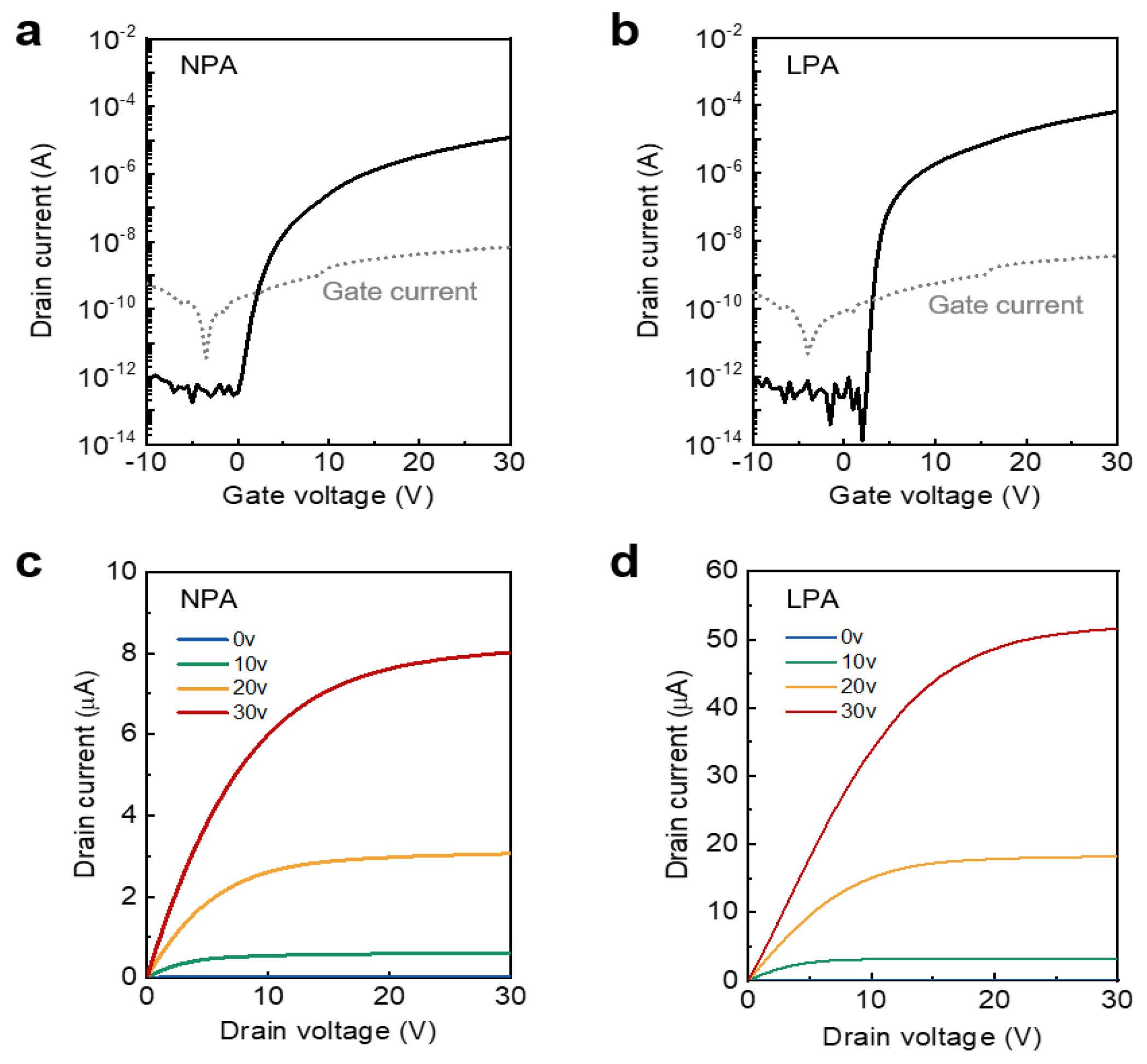
| Semiconductor | Dielectric | T (°C) | Vth (V) | μFE (cm2 V−1 s−1) | SS (V dec−1) | Ion/Off | Reference |
|---|---|---|---|---|---|---|---|
| InOx | SiO2 | 500 | 6.74 | 0.95 | 0.24 | 1.55 × 105 | [32] |
| InGaZnO | SiO2 | 450 | −5.09 | 1.25 | 1.05 | 5.2 × 103 | [33] |
| ZnSnO | SiO2 | 350 | 10.7 | 0.58 | - | ~1 × 106 | [34] |
| ZnSnO | SiO2 | 600 | - | 1.12 | 0.39 | 3.0 × 107 | [35] |
| InOx | SiO2 | 240 | - | 1.57 | 0.45 | 3.9 × 107 | [36] |
| ZnSnO | SiO2 | 500 | −0.95 | 2.41 | 1.25 | 3.9 × 106 | [37] |
| InSbO | SiO2 | 300 | 1.9 | 4.60 | 0.29 | 3 × 107 | [38] |
| InOx | SiO2 | 200 | 4.40 | 0.81 | 0.28 | 4.91 × 109 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, W.; Park, J.-H.; Eun, J.-S.; Lee, J.; Na, J.-H.; Lee, S.-H.; Jang, J.; Kang, I.M.; Kim, D.-K.; Bae, J.-H. Low-Temperature Enhancement-Mode Amorphous Oxide Thin-Film Transistors in Solution Process Using a Low-Pressure Annealing. Nanomaterials 2023, 13, 2231. https://doi.org/10.3390/nano13152231
Park W, Park J-H, Eun J-S, Lee J, Na J-H, Lee S-H, Jang J, Kang IM, Kim D-K, Bae J-H. Low-Temperature Enhancement-Mode Amorphous Oxide Thin-Film Transistors in Solution Process Using a Low-Pressure Annealing. Nanomaterials. 2023; 13(15):2231. https://doi.org/10.3390/nano13152231
Chicago/Turabian StylePark, Won, Jun-Hyeong Park, Jun-Su Eun, Jinuk Lee, Jeong-Hyeon Na, Sin-Hyung Lee, Jaewon Jang, In Man Kang, Do-Kyung Kim, and Jin-Hyuk Bae. 2023. "Low-Temperature Enhancement-Mode Amorphous Oxide Thin-Film Transistors in Solution Process Using a Low-Pressure Annealing" Nanomaterials 13, no. 15: 2231. https://doi.org/10.3390/nano13152231
APA StylePark, W., Park, J.-H., Eun, J.-S., Lee, J., Na, J.-H., Lee, S.-H., Jang, J., Kang, I. M., Kim, D.-K., & Bae, J.-H. (2023). Low-Temperature Enhancement-Mode Amorphous Oxide Thin-Film Transistors in Solution Process Using a Low-Pressure Annealing. Nanomaterials, 13(15), 2231. https://doi.org/10.3390/nano13152231








