Recent Developments on the Catalytic and Biosensing Applications of Porous Nanomaterials
Abstract
1. Introduction
2. Properties and Characterization of Porous NMs
3. Catalytic Applications of Porous NMs
3.1. Silica and Silica-Supported Catalysts
3.1.1. Functionalized Mesoporous Silica Nanocatalyst
3.1.2. Porous Silica Supported Metal-Doped Catalysts
3.1.3. Porous Phyllosilicate Nanocatalysts
- (1)
- H2O + CH4 → CO + 3H2
- (2)
- CO2 + CH4 → 2CO + 2H2
- (3)
- O2 + 2CH4 → 2CO + 4H2
3.2. Metal-Oxide- and Phosphate-Based Catalysts
3.3. Pure Organic and Organic–Inorganic Hybrid Nanocatalysts
3.4. Composite-Nanomaterials-Based Catalyst
3.5. Porous Carbon-Based Nanocatalyst
3.6. Porous Metal-Based Catalyst
4. Applications of Porous NMs in Biosensing
4.1. Colorimetric Biosensing
4.2. Porous NMs in Fluorescence Biosensing
4.3. Electrochemical Biosensing Using Porous NMs
4.4. Carbon-Based NMs for Biosensors
4.5. Non Carbon-Based NMs for Biosensors
5. Summary and Future Prospect
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Feynman, R. There’s plenty of room at the bottom. Eng. Sci. 1960, 23, 22–36. [Google Scholar]
- Khan, I.; Khalid, S.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Khin, M.M.; Nair, A.S.; Babu, V.J.; Murugan, R.; Ramakrishna, S. A review on nanomaterials for environmental remediation. Energy Environ. Sci. 2012, 5, 8075–8109. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- De, M.; Ghosh, P.S.; Rotello, V.M. Applications of nanoparticles in biology. Adv. Mater. 2008, 20, 4225–4241. [Google Scholar] [CrossRef]
- Sharma, N.; Ojha, H.; Bharadwaj, A.; Pathak, D.P.; Sharma, R.S. Preparation and catalytic applications of nanomaterials: A review. RSC Adv. 2015, 5, 53381–53403. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Ko, C.H.; Ryoo, R. Characterization of the Porous Structure of SBA-15. Chem. Mater. 2000, 12, 1961–1968. [Google Scholar] [CrossRef]
- Bhanja, P.; Bhaumik, A. Porous nanomaterials as green catalyst for the conversion of biomass to bioenergy. Fuel 2016, 185, 432–441. [Google Scholar] [CrossRef]
- Shinde, S.P.; Suryawanshi, S.P.; Patil, K.K.; Belekar, V.M.; Sankpal, S.A.; Delekar, S.D.; Jadhav, S.A. A brief overview of recent progress in porous silica as catalyst supports. J. Compos. Sci. 2021, 5, 75. [Google Scholar] [CrossRef]
- Joseph, T.M.; Mahapatra, D.K.; Esmaeili, A.; Piszczyk, L.; Hasanin, M.S.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a unique position in medicine. Nanomaterials 2023, 13, 574. [Google Scholar] [CrossRef]
- Duan, L.; Wang, C.; Zhang, W.; Ma, B.; Deng, Y.; Li, W.; Zhao, D. Interfacial Assembly and Applications of Functional Mesoporous Materials. Chem. Rev. 2021, 121, 14349–14429. [Google Scholar] [CrossRef]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of Mesoporous Silica Nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Li, Z.; Dai, S. Mesoporous Carbon Materials: Synthesis and Modification. Angew. Chem. Int. Ed. 2008, 47, 3696–3717. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liu, J.; Li, C.; Li, Y.; Tade, M.O.; Dai, S.; Yamauchi, Y. Synthesis of Nitrogen-Doped Mesoporous Carbon Spheres with Extra-Large Pores through Assembly of Diblock Copolymer Micelles. Angew. Chem. Int. Ed. 2014, 54, 588–593. [Google Scholar] [CrossRef]
- Luo, W.; Zhao, T.; Li, Y.; Wei, J.; Xu, P.; Li, X.; Wang, Y.; Zhang, W.; Elzatahry, A.A.; Alghamdi, A.; et al. A Micelle Fusion-Aggregation Assembly Approach to Mesoporous Carbon Materials with Rich Active Sites for Ultrasensitive Ammonia Sensing. J. Am. Chem. Soc. 2016, 138, 12586–12595. [Google Scholar] [CrossRef]
- Tang, J.; Liu, J.; Salunkhe, R.R.; Wang, T.; Yamauchi, Y. Nitrogen-Doped Hollow Carbon Spheres with Large Mesoporous Shells Engineered from Diblock Copolymer Micelles. Chem. Commun. 2016, 52, 505–508. [Google Scholar] [CrossRef]
- Wang, Y.; Arandiyan, H.; Scott, J.; Bagheri, A.; Dai, H.; Amal, R. Recent advances in ordered meso/microporous metal oxides for heterogeneous catalysis: A review. J. Mater. Chem. A 2017, 5, 8825–8846. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, H.; Zhou, Y.; Zhang, L.; Wu, Z.; Yang, S.; Shi, H.; Zhu, Q.; Chen, Y.; Dai, S. Mesoporous MnCeOx solid solutions for low temperature and selective oxidation of hydrocarbons. Nat. Commun. 2015, 6, 8446. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, J.H.; Lee, Y.; Cho, E.-B.; Jang, Y.J. Boosting solar-driven N2 to NH3 conversion using defect-engineered TiO2/CuO heterojunction photocatalyst. Appl. Surf. Sci. 2023, 620, 156812. [Google Scholar] [CrossRef]
- Ren, Y.; Ma, Z.; Bruce, P.G. Ordered mesoporous metal oxides: Synthesis and applications. Chem. Soc. Rev. 2012, 41, 4909–4927. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Chowdhury, A.; Bhunia, K.; Ghosh, A.; Chakraborty, D.; Das, M.; Kayal, U.; Modak, A.; Pradhan DBhaumik, A. Ni (II) and Cu (II) Grafted Porphyrin-Pyrene based Conjugated Microporous Polymers as Bifunctional Electrocatalysts for Overall Water Splitting. Electrochim. Acta 2023, 459, 142553. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, G.; She, P.; Zhong, G.; Yan, W.; Guan, B.Y.; Yamauchi, Y. Mesoporous Nanoarchitectures for Electrochemical Energy Conversion and Storage. Adv. Mater. 2020, 32, 2004654. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Peeters, E.; Makshina, E.V.; Parvulescu, V.I.; Sels, B.F. Advances in Porous and Nanoscale Catalysts for Viable Biomass Conversion. Chem. Soc. Rev. 2019, 48, 2366–2421. [Google Scholar] [CrossRef]
- Alsudairy, Z.; Brown, N.; Campbell, A.; Ambus, A.; Brown, B.; Smith-Petty, K.; Li, X. Covalent organic frameworks in heterogeneous catalysis: Recent advances and future perspective. Mater. Chem. Front. 2023, in press. [Google Scholar] [CrossRef]
- Luque, R.; Ahmad, A.; Tariq, S.; Mubashir, M.; Javed, M.S.; Rajendran, S.; Varma, R.S.; Ali, A.; Xia, C. Functionalized interconnected porous materials for heterogeneous catalysis, energy conversion and storage applications: Recent advances and future perspectives. Mater. Today 2023, in press. [Google Scholar] [CrossRef]
- Banerjee, S.; Anayah, R.I.; Gerke, C.S.; Sara Thoi, V. From Molecules to Porous Materials: Integrating Discrete Electrocatalytic Active Sites into Extended Frameworks. ACS Cent. Sci. 2020, 6, 1671–1684. [Google Scholar] [CrossRef]
- Kang, Y.; Tang, Y.; Zhu, L.; Jiang, B.; Xu, X.; Guselnikova, O.; Li, H.; Asahi, T.; Yamauchi, Y. Porous Nanoarchitectures of Nonprecious Metal Borides: From Controlled Synthesis to Heterogeneous Catalyst Applications. ACS Catal. 2022, 12, 14773–14793. [Google Scholar] [CrossRef]
- Chakraborty, D.; Bej, S.; Sahoo, S.; Chongdar, S.; Ghosh, A.; Banerjee, P.; Bhaumik, A. Novel NanoporousTi-Phosphonate Metal–Organic Framework for Selective Sensing of 2,4,6-Trinitrophenol and a Promising Electrode in an Energy Storage Device. ACS Sustain. Chem. Eng. 2021, 9, 14224–14237. [Google Scholar] [CrossRef]
- Chakraborty, D.; Ghorai, A.; Chowdhury, A.; Banerjee, S.; Bhaumik, A. A Tetradentate Phosphonate Ligand-Based Ni-MOF as a Support for Designing High-Performance Proton-Conducting Materials. Chem.-Asian J. 2021, 16, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, J.; Zhao, D. Mesoporous Materials for Energy Conversion and Storage Devices. Nat. Rev. Mater. 2016, 1, 16023. [Google Scholar] [CrossRef]
- Chakraborty, D.; Ghorai, A.; Bhanja, P.; Banerjee, S.; Bhaumik, A. High Proton Conductivity in a Charge Carrier-Induced Ni(II) Metal–Organic Framework. New J. Chem. 2022, 46, 1867–1876. [Google Scholar] [CrossRef]
- Chakraborty, D.; Dam, T.; Modak, A.; Pant, K.K.; Chandra, B.K.; Majee, A.; Ghosh, A.; Bhaumik, A. A novel crystalline nanoporous iron phosphonate-based metal–organic framework as an efficient anode material for lithium ion batteries. New J. Chem. 2021, 45, 15458–15468. [Google Scholar] [CrossRef]
- Wattanakit, C.; Côme, Y.B.S.; Lapeyre, V.; Bopp, P.A.; Heim, M.; Yadnum, S.; Nokbin, S.; Warakulwit, C.; Limtrakul, J.; Kuhn, A. Enantioselective Recognition at Mesoporous Chiral Metal Surfaces. Nat. Commun. 2014, 5, 3325. [Google Scholar] [CrossRef]
- Robby, A.I.; Park, S.Y. Recyclable Metal Nanoparticle-Immobilized Polymer Dot on Montmorillonite for Alkaline Phosphatase-Based Colorimetric Sensor with Photothermal Ablation of Bacteria. Anal. Chim. Acta 2019, 1082, 152–164. [Google Scholar] [CrossRef]
- Fang, Y.; Li, C.; Bo, J.; Henzie, J.; Yamauchi, Y.; Asahi, T. Chiral Sensing with Mesoporous Pd@Pt Nanoparticles. ChemElectroChem. 2017, 4, 1832–1835. [Google Scholar] [CrossRef]
- Karunakaran, G.; Cho, E.-B.; Kumar, G.S.; Kolesnikov, E.; Govindaraj, S.K.; Mariyappan, K.; Boobalan, S. CTAB enabled microwave-hydrothermal assisted mesoporous Zn-doped hydroxyapatite nanorods synthesis using bio-waste Nodipecten nodosus scallop for biomedical implant applications. Environ. Res. 2023, 216, 114683. [Google Scholar] [CrossRef]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Huang, H.; Cao, X.; Chen, X.; Cao, D. Porous Organic Polymers as a Platform for Sensing Applications. Chem. Soc. Rev. 2022, 51, 2031–2080. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Xu, R.; Wang, H.; Zhang, Y.; Wei, Q. Progress and Prospects of Electrochemiluminescence Biosensors Based on Porous Nanomaterials. Biosensors 2022, 12, 508. [Google Scholar] [CrossRef]
- Souza, J.E.d.S.; de Oliveira, G.P.; Alexandre, J.Y.N.H.; Neto, J.G.L.; Sales, M.B.; Junior, P.G.d.S.; de Oliveira, A.L.B.; de Souza, M.C.M.; dos Santos, J.C.S. A Comprehensive Review on the Use of Metal–Organic Frameworks (MOFs) Coupled with Enzymes as Biosensors. Electrochem 2022, 3, 89–113. [Google Scholar] [CrossRef]
- Aggarwal, V.; Solanki, S.; Malhotra, B.D. Applications of metal–organic framework-based bioelectrodes. Chem. Sci. 2022, 13, 8727–8743. [Google Scholar] [CrossRef] [PubMed]
- Dourandish, Z.; Tajik, S.; Beitollahi, H.; Jahani, P.M.; Nejad, F.G.; Sheikhshoaie, I.; Di Bartolomeo, A. A Comprehensive Review of Metal-Organic Framework: Synthesis, Characterization, and Investigation of Their Application in Electrochemical Biosensors for Biomedical Analysis. Sensors 2022, 22, 2238. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Musib, D.; Saha, R.; Das, A.; Raza, M.K.; Ramu, V.; Chongdar, S.; Sarkar, K.; Bhaumik, A. Highly stable tetradentate phosphonate-based green fluorescent Cu-MOF for anticancer therapy and antibacterial activity. Mater. Today Chem. 2022, 24, 100882. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous silica nanoparticles: A comprehensive review on synthesis and recent advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef]
- Han, X.; Yang, S.; Schröder, M. Porous Metal-Organic Frameworks as Emerging Sorbents for Clean Air. Nat. Rev. Chem. 2019, 3, 108–118. [Google Scholar] [CrossRef]
- Perovic, M.; Qin, Q.; Oschatz, M. From Molecular Precursors to Nanoparticles-Tailoring the Adsorption Properties of Porous Carbon Materials by Controlled Chemical Functionalization. Adv. Funct. Mater. 2020, 30, 1908371. [Google Scholar] [CrossRef]
- Stein, A.; Wang, Z.; Fierke, M.A. Functionalization of Porous Carbon Materials with Designed Pore Architecture. Adv. Mater. 2009, 21, 265–293. [Google Scholar] [CrossRef]
- Baleizão, C.; Gigante, B.; Garcia, H.; Corma, A. Vanadyl Salen Complexes Covalently Anchored to Single-Wall Carbon Nanotubes as Heterogeneous Catalysts for the Cyanosilylation of Aldehydes. J. Catal. 2004, 221, 77–84. [Google Scholar] [CrossRef]
- Pal, N. Nanoporous metal oxide composite materials: A journey from the past, present to future. Adv. Colloid Interface Sci. 2020, 280, 102156. [Google Scholar] [CrossRef]
- Rizvi, M.; Gerengi, H.; Gupta, P. Functionalization of nanomaterials: Synthesis and characterization. functionalized nanomaterials for corrosion mitigation: Synthesis, characterization, and applications. ACS Symp. Ser. Am. Chem. Soc. 2022, 1418, 67–85. [Google Scholar]
- Pal, N.; Sunwoo, Y.; Park, J.-S.; Kim, T.; Cho, E.-B. Newly designed mesoporous silica and organosilica nanostructures based on pentablock copolymer templates in weakly acidic media. Nanomaterials 2021, 11, 2522. [Google Scholar] [CrossRef]
- Xie, W.; Li, J. Magnetic solid catalysts for sustainable and cleaner biodiesel production: A comprehensive review. Renew. Sustain. Energy Rev. 2023, 171, 113017. [Google Scholar] [CrossRef]
- Xie, W.; Gao, C.; Li, J. Sustainable biodiesel production from low-quantity oils utilizing H6PV3MoW8O40 supported on magnetic Fe3O4/ZIF-8 composites. Renew. Energy 2021, 168, 927–937. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H. Grafting copolymerization of dual acidic ionic liquid on core-shell structured magnetic silica: A magnetically recyclable Brönsted acid catalyst for biodiesel production by one-pot transformation of low-quality oils. Fuel 2021, 283, 118893. [Google Scholar] [CrossRef]
- Xie, W.; Han, Y.; Wang, H. Magnetic Fe3O4/MCM-41 composite-supported sodium silicate as heterogeneous catalysts for biodiesel production. Renew. Energy 2018, 125, 675–681. [Google Scholar] [CrossRef]
- Pal, N.; Bhaumik, A. Soft templating strategies for the synthesis of mesoporous materials: Inorganic, organic–inorganic hybrid and purely organic solids. Adv. Colloid Interface Sci. 2013, 189–190, 21–41. [Google Scholar] [CrossRef]
- Rana, S.; Velázquez, J.J.; Jonnalagadda, S.B. Eco-friendly synthesis of organo-functionalized mesoporous silica for the condensation reaction. Catalysts 2022, 12, 1212. [Google Scholar] [CrossRef]
- Pal, N.; Lee, J.-H.; Cho, E.-B. Recent trends in morphology-controlled synthesis and application of mesoporous silica nanoparticles. Nanomaterials 2020, 10, 2122. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Y.; Yang, Q. Organo-functionalized silica hollow nanospheres: Synthesis and catalytic application. J. Mater. Chem. A 2013, 1, 1525–1535. [Google Scholar] [CrossRef]
- Chen, H.-T.; Huh, S.; Wiench, J.W.; Pruski, M.; Victor, S.-Y.L. Dialkylaminopyridine-functionalized mesoporous silica nanosphere as an efficient and highly stable heterogeneous nucleophilic catalyst. J. Am. Chem. Soc. 2005, 127, 13305–13311. [Google Scholar] [CrossRef] [PubMed]
- Shylesh, S.; Wagner, A.; Seifert, A.; Ernst, S.; Thiel, W.R. Cooperative acid–base effects with functionalized mesoporous silica nanoparticles: Applications in carbon-carbon bond-formation reactions. Chem. Eur. J. 2009, 15, 7052–7062. [Google Scholar] [CrossRef]
- Peng, W.-H.; Lee, Y.-Y.; Wu, C.; Wu, K.C.-W. Acid-base bi-functionalized, large-pored mesoporous silica nanoparticles for cooperative catalysis of one-pot cellulose-to-HMF conversion. J. Mater. Chem. 2012, 22, 23181–23185. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Thomas, I.L. Alternative Energy Technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef]
- Turner, J.A. Sustainable Hydrogen Production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, J.; Wang, C.; Zhai, T.-T.; Bao, W.-J.; Xu, J.-J.; Xia, X.-H.; Chen, H.-Y. Hot Electron of Au Nanorods Activates the Electrocatalysis of Hydrogen Evolution on MoS2 Nanosheets. J. Am. Chem. Soc. 2015, 137, 7365–7370. [Google Scholar] [CrossRef]
- Xie, J.F.; Gao, L.; Jiang, H.L.; Zhang, X.D.; Lei, F.C.; Hao, P.; Tang, B.; Xie, Y. Platinum Nanocrystals Decorated on Defect-Rich MoS2 Nanosheets for pH-Universal Hydrogen Evolution Reaction. Cryst. Growth Des. 2019, 19, 60–65. [Google Scholar] [CrossRef]
- Li, D.; Zong, Z.; Tang, Z.; Liu, Z.; Chen, S.; Tian, Y.; Wang, X. Total Water Splitting Catalyzed by Co@Ir Core-Shell Nanoparticles Encapsulated in Nitrogen-Doped Porous Carbon Derived from Metal-Organic Frameworks. ACS Sustain. Chem. Eng. 2018, 6, 5105–5114. [Google Scholar] [CrossRef]
- Kung, C.W.; Mondloch, J.E.; Wang, T.C.; Bury, W.; Hoffeditz, W.; Klahr, B.M.; Klet, R.C.; Pellin, M.J.; Farha, O.K.; Hupp, J.T. Metal-Organic Framework Thin Films as Platforms for Atomic Layer Deposition of Cobalt Ions to Enable Electrocatalytic Water Oxidation. ACS Appl. Mater. Interfaces 2015, 7, 28223–28230. [Google Scholar] [CrossRef]
- Qiu, C.; Jiang, J.; Ai, L.H. when layered nickel-cobalt silicate hydroxide nanosheets meet carbon nanotubes: A synergetic coaxial nanocable structure for enhanced electrocatalytic water oxidation. ACS Appl. Mater. Interfaces 2016, 8, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Z.; Bai, G.; Wang, Q.; Zhang, J.; Wang, W.; Zhou, L.; Yi, J.; Ma, Z. Nickel-iron layered silicate nanomembrane as efficient electrocatalyst for oxygen evolution reaction in alkaline media. Fuel 2023, 332, 126209. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, X.; Ai, L. Natural reed leaves derived nickel-cobalt silicate hydroxides with phosphate modification enabling efficient oxygen evolution electrocatalysis. Colloids Surf. A Physicochem. Eng. Asp. 2023, 667, 131370. [Google Scholar] [CrossRef]
- Mu, Y.; Zhang, Y.; Pei, X.; Dong, X.; Kou, Z.; Cui, M.; Meng, C. Dispersed FeOx nanoparticles decorated with Co2SiO4 hollow spheres for enhanced oxygen evolution reaction. J. Colloid Interface Sci. 2022, 611, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Zhang, Y.; Mu, Y.; Cui, M.; Tian, F.; Meng, C. Cobalt oxide decorated three-dimensional amorphous carbon/cobalt silicate composite derived from bamboo leaves enables the enhanced oxygen evolution reaction. Chem. Eng. Sci. 2022, 251, 117490. [Google Scholar] [CrossRef]
- Pei, X.; Yi, S.; Zhao, Y.; Mu, Y.; Yu, Y.; Cui, M.; Meng, C.; Huang, C.; Zhang, Y. Nickel oxide nanoparticles dispersed on biomass-derived amorphous carbon/cobalt silicate support accelerate the oxygen evolution reaction. J. Colloid Interface Sci. 2022, 616, 476–487. [Google Scholar] [CrossRef]
- Qiu, C.; Ai, L.; Jiang, J. Layered Phosphate-Incorporated Nickel–Cobalt Hydrosilicates for Highly Efficient Oxygen Evolution Electrocatalysis. ACS Sustain. Chem. Eng. 2018, 6, 4492–4498. [Google Scholar] [CrossRef]
- Mu, Y.; Zhang, Y.; Feng, Z.; Dong, X.; Jing, X.; Pei, X.; Zhao, Y.; Kou, Z.; Meng, C. Bifunctional electrocatalyst junction engineering: CoP nanoparticles in-situ anchored on Co3(Si2O5)2(OH)2 nanosheets for highly efficient water split-ting. Chem. Eng. J. 2023, 460, 141709. [Google Scholar] [CrossRef]
- Pal, N.; Cho, E.-B.; Kim, D. Synthesis of ordered mesoporous silica/ceria-silica composites and their high catalytic performance for solvent-free oxidation of benzyl alcohol at room temperature. RSC Adv. 2014, 4, 9213–9222. [Google Scholar] [CrossRef]
- Pal, N.; Cho, E.-B.; Kim, D.; Gunathilake, C.; Jaroniec, M. Catalytic activity of CeIVO2/Ce2IIIO3-silica mesoporous composite materials for oxidation and esterification reactions. Chem. Eng. J. 2015, 262, 1116–1125. [Google Scholar] [CrossRef]
- Xua, C.; Deb, S.; Baluc, A.M.; Ojedad, M.; Luque, R. Mechanochemical synthesis of advanced nanomaterials for catalytic applications. Chem. Commun. 2015, 51, 6698–6713. [Google Scholar] [CrossRef] [PubMed]
- Awoke, Y.; Chebude, Y.; Díaz, I. Ti-PMO materials as selective catalysts for the epoxidation of cyclohexene and vernonia oil. Catal. Today 2022, 390–391, 246–257. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, P.; Wai, P.T.; Gu, Q.; Zhang, W. Recent progress in application of molybdenum-based catalysts for epoxidation of alkenes. Catalysts 2019, 9, 31. [Google Scholar] [CrossRef]
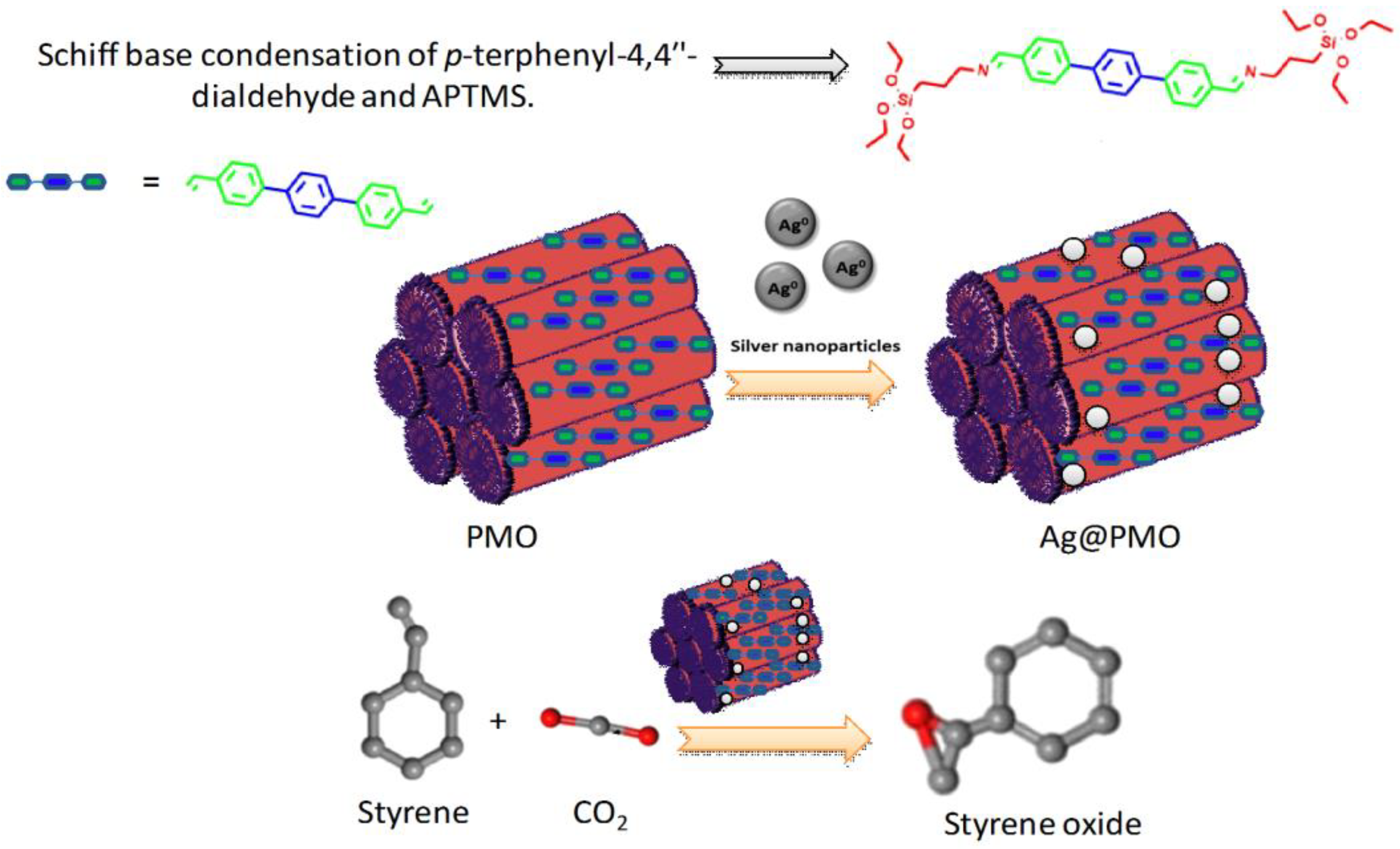
- Chatterjee, S.; Das, S.; Bhanja, P.; Erakula, N.E.S.; Thapa, R.; Ruidas, S.; Chongdar, S.; Ray, S.; Bhaumik, A. Ag nanoparticles immobilized over highly porous crystalline organosilica for epoxidation of styrene using CO2 as oxidant. J. CO2 Util. 2022, 55, 101843. [Google Scholar] [CrossRef]
- Kankala, R.K.; Zhang, H.; Liu, C.-G.; Kanubaddi, K.R.; Lee, C.-H.; Wang, S.-B.; Cui, W.; Santos, H.A.; Lin, L. Metal species-encapsulated mesoporous silica nanoparticles: Current advancements and latest breakthroughs. Adv. Funct. Mater. 2019, 29, 1902652. [Google Scholar] [CrossRef]
- Baykal, U.K.A. Fabrication and characterization of Fe3O4@APTES@PAMAM-Ag highly active and recyclable magnetic nanocatalyst: Catalytic reduction of 4-nitrophenol. Mater. Res. Bull. 2014, 60, 79–87. [Google Scholar]
- Diacon, A.; Rusen, E.; Trifan, A.; Șomoghi, R.; Tutunaru, O.; Crăciun, G.; Busuioc, C.; Voicu, G. Preparation of metal and metal oxide doped silica hollow spheres and the evaluation of their catalytic performance. Colloid Polym. Sci. 2020, 298, 1401–1410. [Google Scholar] [CrossRef]
- Maity, A.; Polshettiwar, V. Dendritic fibrous nanosilica for catalysis, energy harvesting, carbon dioxide mitigation, drug delivery, and sensing. ChemSusChem 2017, 10, 3866–3913. [Google Scholar] [CrossRef]
- Chandrashekhar, V.G.; Senthamarai, T.; Kadam, G.A.; Malina, O.; Kašlík, J.; Radek, Z.; Gawande, M.B.; Jagadeesh, R.V.; Beller, M. Silica-supported Fe/Fe-O nanoparticles for the catalytic hydrogenation of nitriles to amines in the presence of aluminium additives. Nat. Catal. 2022, 5, 20–29. [Google Scholar] [CrossRef]
- Snoussi, Y.; Bastide, Y.; Abderrabba, M.; Chehimi, M.M. Sonochemical synthesis of Fe3O4@NH2-mesoporous silica@Polypyrrole/Pd: A core/double shell nanocomposite for catalytic applications. Ultrason. Sonochem. 2018, 41, 551–561. [Google Scholar] [CrossRef]
- Xie, W.; Zang, X. Covalent Immobilization of Lipase onto Aminopropyl-Functionalized Hydroxyapatite-Encapsulated-γ-Fe2O3 Nanoparticles: A Magnetic Biocatalyst for Interesterification of Soybean Oil. Food Chem. 2017, 227, 397–403. [Google Scholar] [CrossRef]
- Xie, W.; Huang, M. Immobilization of Candida Rugosa Lipase onto Graphene Oxide Fe3O4 Nanocomposite: Characterization and Application for Biodiesel Production. Energy Convers. Manag. 2018, 159, 42–53. [Google Scholar] [CrossRef]
- Xie, W.; Zang, X. Immobilized lipase on core–shell structured Fe3O4–MCM-41 nanocomposites as a magnetically recyclable biocatalyst for interesterification of soybean oil and lard. Food Chem. 2016, 194, 1283–1292. [Google Scholar] [CrossRef]
- Campelo, J.M.; Luna, D.; Luque, R.; Marinas, J.M.; Romero, A.A. Sustainable Preparation of Supported Metal Nanoparticles and Their Applications in Catalysis. ChemSusChem 2009, 2, 18–45. [Google Scholar] [CrossRef]
- Ciotonea, C.; Dragoi, B.; Ungureanu, A.; Chirieac, A.; Petit, S.; Royer, S.; Dumitriu, E. Nanosized transition metals in controlled environments of phyllosilicate-mesoporous silica composites as highly thermostable and active catalysts. Chem. Comm. 2013, 49, 7665–7667. [Google Scholar] [CrossRef] [PubMed]
- Duarte, H.A.; Lourenço, M.P.; Heine, T.; Guimarães, L. Clay Mineral Nanotubes: Stability, Structure and Properties. In Stoichiometry and Materials Science—When Numbers Matter, 1st ed.; Innocenti, A., Kamarulzaman, N., Eds.; Intech Open: London, UK, 2012. [Google Scholar]
- Pal, N.; Jo, J.W.; Narsimulu, D.; Cho, E.B.; Yu, J.S. Hierarchical multi-metal-doped mesoporous NiO-silica nanoparticles towards a viable platform for Li-ion battery electrode application. Korean J. Chem. Eng. 2022, 39, 1959–1967. [Google Scholar] [CrossRef]
- Sekhar, S.C.; Lee, J.H.; Cho, E.B.; Yu, J.S. Unveiling redox-boosted mesoporous Co@NiO-SiO2 hybrid composite with hetero-morphologies as an electrode candidate for durable hybrid supercapacitors. J. Mater. Res. Technol. 2021, 13, 1899–1907. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Q. Synthesis and regeneration of Ni-phyllosilicate catalysts using a versatile double-accelerator method: A comprehensive study. ACS Catal. 2021, 20, 12570–12584. [Google Scholar] [CrossRef]
- Lee, J.-H.; Cho, E.-B. High hydrothermal stability of mesoporous Ni-phyllosilicate spherical particles. Appl. Surf. Sci. 2022, 590, 153114. [Google Scholar] [CrossRef]
- Sivaiah, M.V.; Petit, S.; Beaufort, M.F.; Eyidi, D.; Barrault, J.; Batiot-Dupeyrat, C.; Valange, S. Nickel based catalysts derived from hydrothermally synthesized 1:1 and 2:1 phyllosilicates as precursors for carbon dioxide reforming of methane. Microporous Mesoporous Mater. 2011, 140, 69–80. [Google Scholar] [CrossRef]
- Yang, M.; Jin, P.; Fan, Y.; Huang, C.; Zhang, N.; Weng, W.; Chen, M.; Wan, H. Ammonia-assisted synthesis towards a phyllosilicate derived highly-dispersed and long-lived Ni/SiO2 catalyst. Catal. Sci. Technol. 2015, 5, 5095–5099. [Google Scholar] [CrossRef]
- Ang, M.L.; Oemar, U.; Saw, E.T.; Mo, L.; Kathiraser, Y.; Chia, B.H.; Kawi, S. Highly active Ni/xNa/CeO2 catalyst for the water-gas shift reaction: Effect of sodium on methane suppression. ACS Catal. 2014, 4, 3237–3248. [Google Scholar] [CrossRef]
- Ashok, J.; Wai, M.H.; Kawi, S. Nickel-based catalysts for high temperature water gas shift reaction-methane suppression. ChemCatChem 2018, 10, 3927–3942. [Google Scholar] [CrossRef]
- Bian, Z.; Li, Z.; Ashok, J.; Kawi, S. A highly active and stable Ni-Mg phyllosilicate nanotubular catalyst for ultra high temperature water-gas shift reaction. Chem. Commun. 2015, 51, 16324–16326. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Cho, E.-B.; Jung, S.C.; Kim, S.C.; Park, Y. Hydrocarbons production from m-cresol as a lignin model compound over nickel silicate catalysts. J. Nanosci. Nanotechnol. 2020, 20, 5738–5741. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Liu, Q. Highly efficient Ni-phyllosilicate catalyst with surface and interface confinement for CO2 and CO methanation. Ind. Eng. Chem. Res. 2021, 60, 6981–6992. [Google Scholar] [CrossRef]
- Giorgianni, G.; Mebrahtu, C.; Perathoner, S.; Centi, G.; Abate, S. Hydrogenation of dimethyl oxalate to ethylene glycol on Cu/SiO2 catalysts prepared by a deposition-decomposition method: Optimization of the operating conditions and pre-reduction procedure. Catal. Today 2022, 390, 343–353. [Google Scholar] [CrossRef]
- Gong, J.; Yue, H.; Zhao, Y.; Zhao, S.; Zhao, L.; Lv, J.; Wang, S.; Ma, X. Synthesis of ethanol via syngas on Cu/SiO2 catalysts with balanced Cu0-Cu+ sites. J. Am. Chem. Soc. 2012, 134, 13922–13925. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Xu, Z.N.; Peng, S.Y.; Zhang, M.J.; Lu, G.; Chen, Q.S.; Chen, Y.; Guo, G.C. High-performance and long-lived Cu/SiO2 nanocatalyst for CO2 hydrogenation. ACS Catal. 2015, 5, 4255–4259. [Google Scholar] [CrossRef]
- Park, J.C.; Kang, S.W.; Kim, J.-C.; Kwon, J.I.; Jang, S.; Rhim, G.B.; Kim, M.; Chun, D.H.; Lee, H.-T.; Jung, H.; et al. Synthesis of Co/SiO2 hybrid nanocatalyst via twisted Co3Si2O5(OH)4 nanosheets for high-temperature Fischer-Tropsch reaction. Nano Res. 2017, 10, 1044–1055. [Google Scholar] [CrossRef]
- Sarkar, S.; Sarkar, S.; Patra, A.K. Boat-, cuboid-, and dumbbell-shaped hierarchical morphology of Cerium (IV) hydroxidophosphate materials for oxidative coupling reaction. ChemNanoMat 2022, 8, 202200294. [Google Scholar] [CrossRef]
- Ren, Y.; Ma, Z.; Qian, L.; Dai, S.; He, H.; Bruce, P.G. Ordered crystalline mesoporous oxides as catalysts for CO oxidation. Catal. Lett. 2009, 131, 146–154. [Google Scholar] [CrossRef]
- Parlett, C.M.; Wilson, K.; Lee, A.F. Hierarchical Porous Materials: Catalytic Applications. Chem. Soc. Rev. 2013, 42, 3876–3893. [Google Scholar] [CrossRef]
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, S.; Deng, J.; Deng, S.; Wang, H.; Yan, H.; Dai, H. Morphologically Controlled Synthesis of Porous Spherical and Cubic LaMnO3 with High Activity for the Catalytic Removal of Toluene. ACS Appl. Mater. Interfaces 2014, 6, 17394–17401. [Google Scholar] [CrossRef]
- Lu, L.; Eychmüller, A. Ordered Macroporous Bimetallic Nanostructures: Design, Characterization, and Applications. Acc. Chem. Res. 2008, 41, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramirez, J.; Christensen, C.H.; Egeblad, K.; Christensen, C.H.; Groen, J.C. Hierarchical Zeolites: Enhanced Utilisation of Microporous Crystals in Catalysis by Advances in Materials Design. Chem. Soc. Rev. 2008, 37, 2530–2542. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Amini, M.; Tarassoli, A.; Eftekhari-Sis, B.; Ghasemian, N.; Jabbari, E. Transition metal oxide nanoparticles as efficient catalysts in oxidation reactions. Nano-Struct. Nano-Objects 2018, 14, 19–48. [Google Scholar] [CrossRef]
- De, S.; Dutta, S.; Patra, A.K.; Bhaumik, A.; Saha, B. Self-assembly of mesoporous TiO2 nanospheres via aspartic acid templating pathway and its catalytic application for 5-hydroxymethyl-furfural synthesis. J. Mater. Chem. 2011, 21, 17505–17510. [Google Scholar] [CrossRef]
- Pal, N.; Chakraborty, D.; Bhaumik, A.; Ali, M. Mesoporous Zn-Ti mixed oxide nanostructure: A new bifunctional catalyst for partial oxidation and bezylation reactions. J. Inorg. Organomet. Polym. Mater. 2022, 32, 3141–3152. [Google Scholar] [CrossRef]
- Pramanik, M.; Bhaumik, A. Organic–inorganic hybrid supermicroporous Iron(III) phosphonate nanoparticles as an efficient catalyst for the synthesis of biofuels. Chem. Eur. J. 2013, 19, 8507–8514. [Google Scholar] [CrossRef]
- Abdelrahman, A.E.; Al-Farraj, E.-S. Facile synthesis and characterizations of mixed metal oxide nanoparticles for the efficient photocatalytic degradation of rhodamine B and congo red dyes. Nanomaterials 2022, 12, 3992. [Google Scholar] [CrossRef]
- Paul, M.; Pal, N.; Bhaumik, A. Mesoporous Nickel-Aluminum mixed oxide: A promising catalyst in hydride-transfer reactions. Eur. J. Inorg. Chem. 2010, 5, 5129–5134. [Google Scholar] [CrossRef]
- Paul, M.; Pal, N.; Mondal, J.; Sasidharan, M.; Bhaumik, A. New mesoporous magnesium-aluminum mixed oxide and its catalytic activity in liquid phase Baeyer-Villiger oxidation reaction. Chem. Eng. Sci. 2012, 71, 564–572. [Google Scholar] [CrossRef]
- Abdelrahman, A.E.; Hegazey, R.M.; Ismail, H.S.; El-Feky, H.H.; Khedr, M.A.; Khairy, M.; Ammar, A.M. Facile synthesis and characterization of β-cobalt hydroxide/hydrohausmannite/ramsdellitee/spertiniite and tenorite/cobalt manganese oxide/manganese oxide as novel nanocomposites for efficient photocatalytic degradation of methylene blue dye. Arab. J. Chem. 2022, 15, 104372. [Google Scholar] [CrossRef]
- Cote, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- El-Kaderi, H.M.; Hunt, J.R.; Mendoza-Cortés, J.L.; Côté, A.P.; Taylor, R.E.; O’Keeffe, M.; Yaghi, O.M. Designed Synthesis of 3D Covalent Organic Frameworks. Science 2007, 316, 268–272. [Google Scholar] [CrossRef]
- Wu, D.; Xu, F.; Sun, B.; Fu, R.; He, H.; Matyjaszewski, K. Design and preparation of porous polymers. Chem. Rev. 2012, 112, 3959. [Google Scholar] [CrossRef]
- Kramer, S.; Bennedsen, N.R.; Kegnæs, S. Porous organic polymers containing active metal centers as catalysts for synthetic organic chemistry. ACS Catal. 2018, 8, 6961–6982. [Google Scholar] [CrossRef]
- Basak, A.; Karak, S.; Banerjee, R. Covalent Organic Frameworks as Porous Pigments for Photocatalytic Metal-Free C–H Borylation. J. Am. Chem. Soc. 2023, 145, 7592–7599. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Bhattacharjee, S.; Chatterjee, R.; Bhaumik, A. A new nitrogen rich porous organic polymer for ultra-high CO2 uptake and as an excellent organocatalyst for CO2 fixation reactions. J. CO2 Util. 2022, 65, 102236. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, G.; Li, H. Selective Binding and Removal of Guests in a Microporous Metal-Organic Framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H. Hydrothermal Synthesis of a Metal-Organic Framework Containing Large Rectangular Channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Gascon, J.; Hernandez-Alonso, M.D.; Almeida, A.R.; van Klink, G.P.M.; Kapteijn, F.; Mul, G. Isoreticular MOFs as Efficient Photocatalysts with Tunable Band Gap: An Operando FTIR Study of the Photoinduced Oxidation of Propylene. ChemSusChem 2008, 1, 981–983. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Gates, B.C. Catalysis by Metal Organic Frameworks: Perspective and Suggestions for Future Research. ACS Catal. 2019, 9, 1779–1798. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.-Q.; Jiang, H.-L.; Sun, L.-B. Metal–Organic Frameworks for Heterogeneous Basic Catalysis. Chem. Rev. 2017, 117, 8129–8176. [Google Scholar] [CrossRef]
- Jones, C.W. Metal-organic frameworks and covalent organic frameworks: Emerging advances and applications. JACS Au 2022, 2, 1504–1505. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, K.; Han, X.; He, Y.; Chen, B. Recent Progress on Porous Mofs for Process-Efficient Hydrocarbon Separation, Luminescent Sensing, And Information Encryption. Chem. Commun. 2022, 58, 747–770. [Google Scholar] [CrossRef]
- Gouda, S.P.; Dhakshinamoorthy, A.; Rokhum, S.L. Metal-organic framework as a heterogeneous catalyst for biodiesel production: A review. Chem. Eng. J. Adv. 2022, 12, 100415. [Google Scholar] [CrossRef]
- Bavykina, A.; Kolobov, N.; Khan, I.S.; Bau, J.A.; Ramirez, A.; Gascon, J. Metal-organic frameworks in heterogeneous catalysis: Recent progress, new trends, and future perspectives. Chem. Rev. 2020, 120, 8468–8535. [Google Scholar] [CrossRef] [PubMed]
- Nikseresht, A.; Ghasemi, S.; Parak, S. [Cu3(BTC)2]: A metal-organic framework as an environment-friendly and economically catalyst for the synthesis of tacrine analogues by Friedländer ceaction under conventional and ultrasound irradiation. Polyhedron 2018, 151, 112–117. [Google Scholar] [CrossRef]
- Kurisingal, J.-F.; Rachuri, Y.; Gu, Y.; Chitumalla, R.-K.; Vuppala, S.; Jang, J.; Bisht, K.-K.; Suresh, E.; Park, D.-W. Facile green synthesis of new Copper-based metal–organic frameworks: Experimental and theoretical study of the CO2 fixation reaction. ACS Sustain. Chem. Eng. 2020, 8, 10822–10832. [Google Scholar] [CrossRef]
- Caratelli, C.; Hajek, J.; Cirujano, F.G.; Waroquier, M.; Llabrés i Xamena, F.X.; Van Speybroeck, V. Nature of active sites on UiO-66 and beneficial influence of water in the catalysis of Fischer esterification. J. Catal. 2017, 352, 401–414. [Google Scholar] [CrossRef]
- Cao, C.C.; Chen, C.X.; Wei, Z.W.; Qiu, Q.F.; Zhu, N.X.; Xiong, Y.Y.; Jiang, J.J.; Wang, D.; Su, C.Y. Catalysis through Dynamic Spacer Installation of Multivariate Functionalities in Metal-Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 2589–2593. [Google Scholar] [CrossRef]
- Chakraborty, D.; Shyamal, S.; Bhaumik, A. A new porous Ni-W mixed metal phosphonate open framework material for efficient photoelectrochemical OER. ChemCatChem 2020, 12, 1504–1511. [Google Scholar] [CrossRef]
- Chakraborty, D.; Chowdhury, A.; Chandra, M.; Jana, R.; Shyamal, S.; Bhunia, M.K.; Chandra, D.; Hara, M.; Pradhan, D.; Datta, A.; et al. Novel tetradentate phosphonate ligand based bioinspired Co metal–organic frameworks: Robust electrocatalyst for the Hydrogen evolution reaction in different mediums. Cryst. Growth Des. 2021, 21, 2614–2623. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Sun, C.; Liu, P.; Li, L.; Yang, Z.; Feng, X.; Huo, F.; Lu, X. CuO/Cu2O porous composites: Shape and composition controllable fabrication inherited from metal organic frameworks and further application in CO oxidation. J. Mater. Chem. A 2015, 3, 5294–5298. [Google Scholar] [CrossRef]
- Shen, W.; Dong, X.; Zhu, Y.; Chen, H.; Shi, J. Mesoporous CeO2 and CuO-loaded mesoporous CeO2: Synthesis, characterization, and CO catalytic oxidation property. Microporous Mesoporous Mater. 2005, 85, 157–162. [Google Scholar] [CrossRef]
- Xie, Q.; Zhao, Y.; Guo, H.; Lu, A.; Zhang, X.; Wang, L.; Chen, M.-S.; Peng, D.-L. Facile preparation of well-dispersed CeO2-ZnO composite hollow microspheres with enhanced catalytic activity for CO oxidation. ACS Appl. Mater. Interfaces 2014, 6, 421–428. [Google Scholar] [CrossRef]
- Xu, L.; Song, H.; Chou, L. One-pot synthesis of ordered mesoporous NiO-CaO-Al2O3 composite oxides for catalyzing CO2 reforming of CH4. ACS Catal. 2012, 2, 1331–1342. [Google Scholar] [CrossRef]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal–Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-N.; Song, X.-Z.; Song, S.-Y.; Zhang, H.-J. Highly Efficient Heterogeneous Catalytic Materials Derived from Metal-Organic Framework Supports/Precursors. Coord. Chem. Rev. 2017, 337, 80–96. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Zhang, R.; Jiao, L.; Jiang, H.L. Metal-Organic Frameworks-Derived Porous Materials for Catalysis. Coord. Chem. Rev. 2018, 362, 1–23. [Google Scholar] [CrossRef]
- Shen, K.; Chen, X.D.; Chen, J.Y.; Li, Y.W. Development of MOF-Derived Carbon-Based Nanomaterials for Efficient Catalysis. ACS Catal. 2016, 6, 5887–5903. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, B.; Shi, W.; Chen, X.; Xu, Z.; Li, S.; Chi, Y.R.; Yang, Y.; Lu, J.; Huang, W.; et al. Site-selective catalysis of a multifunctional linear molecule: The steric hindrance of metal–organic framework channels. Adv. Mater. 2018, 30, 1800643. [Google Scholar] [CrossRef]
- Lippi, R.; Howard, S.C.; Barron, H.; Easton, C.D.; Madsen, I.C.; Waddington, L.J.; Vogt, C.; Hill, M.R.; Sumby, C.J.; Doonan, C.J.; et al. Highly active catalyst for CO2 methanation derived from a metal organic framework template. J. Mater. Chem. A 2017, 5, 12990–12997. [Google Scholar] [CrossRef]
- Zhang, S.; Yin, H.; Wang, J.; Zhu, S.; Xiong, Y. Catalytic cracking of biomass tar using Ni nanoparticles embedded carbon nanofiber/porous carbon catalysts. Energy 2021, 216, 119285. [Google Scholar] [CrossRef]
- Mehdipour-Ataei, S.; Aram, E. Mesoporous carbon-based materials: A review of synthesis, modification, and applications. Catalysts 2023, 13, 2. [Google Scholar] [CrossRef]
- Gogoi, D.; Karmur, R.S.; Das, M.R.; Ghosh, N.N. Cu and CoFe2O4 nanoparticles decorated hierarchical porous carbon: An excellent catalyst for reduction of nitroaromatics and microwave-assisted antibiotic degradation. Appl. Catal. B Environ. 2022, 312, 121407. [Google Scholar] [CrossRef]
- Huang, A.; He, Y.; Zhou, Y.; Zhou, Y.; Yang, Y.; Zhang, J.; Luo, L.; Mao, Q.; Hou, D.; Yang, J. A review of recent applications of porous metals and metal oxide in energy storage, sensing and catalysis. J. Mater. Sci. 2019, 54, 949–973. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.M. Nanoporous Metals: Fabrication Strategies and Advanced Electrochemical Applications in Catalysis, Sensing and Energy Systems. Chem. Soc. Rev. 2012, 41, 7016–7031. [Google Scholar] [PubMed]
- Qiu, H.-J.; Xu, H.-T.; Liu, L.; Wang, Y. Correlation of the Structure and Applications of Dealloyed Nanoporous Metals in Catalysis and Energy Conversion/Storage. Nanoscale 2015, 7, 386–400. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Li, C.M. Nanostructured catalysts in fuel cells. J. Mater. Chem. 2011, 21, 4027. [Google Scholar] [CrossRef]
- Shui, J.; Chen, C.; Li, J.C.M. Evolution of Nanoporous Pt-Fe Alloy Nanowires by Dealloying and Their Catalytic Property for Oxygen Reduction Reaction. Adv. Funct. Mater. 2011, 21, 3357–3362. [Google Scholar] [CrossRef]
- Li, Y.; Ding, Y. Porous AgCl/Ag nanocomposites with enhanced visible light photocatalytic properties. J. Phys. Chem. C 2010, 114, 3175–3179. [Google Scholar] [CrossRef]
- Koya, A.N.; Zhu, X.; Ohannesian, N.; Yanik, A.A.; Alabastri, A.; Proietti Zaccaria, R.; Krahne, R.; Shih, W.-C.; Garoli, D. Nanoporous Metals: From Plasmonic Properties to Applications in Enhanced Spectroscopy and Photocatalysis. ACS Nano 2021, 15, 6038–6060. [Google Scholar] [CrossRef]
- Wang, W.; Kang, S.; Zhou, W.; Vikesland, P.J. Environmental Routes of Virus Transmission and the Application of Nanomaterial-Based Sensors for Virus Detection. Environ. Sci. Nano 2023, 10, 393–423. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and Nanoparticles: Sources and Toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- De, S.; Balu, D.M.; van der Waal, J.C.; Luque, R. Biomass-derived porous carbon materials: Synthesis and catalytic applications. ChemCatChem 2015, 7, 1608–1629. [Google Scholar] [CrossRef]
- Yaraki, M.T.; Tan, Y.N. Metal nanoparticles-enhanced biosensors: Synthesis, design and applications in fluorescence enhancement and surface-enhanced Raman scattering. Chem. Asian J. 2020, 15, 3180–3208. [Google Scholar] [CrossRef]
- Kim, M.; Shim, J.; Li, T.; Lee, J.; Park, H.G. Fabrication of nanoporous nanocomposites entrapping Fe3O4 magnetic nanoparticles and oxidases for colorimetric biosensing. Chem. Eur. J. 2011, 17, 10700–10707. [Google Scholar]
- Wang, J.; Zhao, C.; Zhou, F.; Lu, H.; Huang, Z.; Yao, C.; Song, C. Dual mimic enzyme properties of Fe nanoparticles embedded in two-dimensional carbon nanosheets for colorimetric detection of biomolecules. Analyst 2023, 148, 146–152. [Google Scholar] [CrossRef]
- Swaidan, A.; Barras, A.; Addad, A.; Tahon, J.-F.; Toufaily, J.; Hamieh, T.; Szunerits, S.; Boukherroub, R. Colorimetric sensing of dopamine in beef meat using copper sulfide encapsulated within bovine serum albumin functionalized with copper phosphate (CuS-BSA-Cu3(PO4)2) nanoparticles. J. Colloid Interface Sci. 2021, 582, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Jin, S.; Wang, L. Metal nanoparticles-based nanoplatforms for colorimetric sensing: A review. Rev. Anal. Chem. 2021, 40, 11. [Google Scholar] [CrossRef]
- Cao, X.; Yang, H.; Wei, Q.; Yang, Y.; Liu, M.; Liu, Q.; Zhang, X. Fast colorimetric sensing of H2O2 and glutathione based on Pt deposited on NiCo layered double hydroxide with double peroxidase-/oxidase-like activity. Inorg. Chem. Commun. 2021, 123, 108331. [Google Scholar] [CrossRef]
- Gaviria-Arroyave, M.I.; Cano, J.B.; Gustavo, A.P. Nanomaterial-based fluorescent biosensors for monitoring environmental pollutants: A critical review. Talanta Open 2020, 2, 100006. [Google Scholar] [CrossRef]
- Willner, M.; Vikesland, P.J. Nanomaterial enabled sensors for environmental contaminants. J. Nanobiotechnol. 2018, 16, 95. [Google Scholar]
- Cao, Q.; Teng, Y.; Yang, X.; Wang, J.; Wang, E.A. Label-free fluorescent molecular beacon based on DNA-Ag nanoclusters for the construction of versatile biosensors. Biosens. Bioelectron. 2015, 74, 318–321. [Google Scholar] [CrossRef]
- Nampi, P.P.; Kartha, C.C.; Jose, G.; Kumar, A.; Anilkumar, T.; Varma, H. Sol-gel nanoporous silica as substrate for immobilization of conjugated biomolecules for application as fluorescence resonance energy transfer (FRET) based biosensor. Sens. Actuators B Chem. 2013, 185, 252–257. [Google Scholar] [CrossRef]
- Chakraborty, D.; Bej, S.; Chatterjee, R.; Banerjee, P.; Bhaumik, A. A new phosphonate-based Mn-MOF in recognising arginine over lysine in aqueous medium and other bio-fluids with “Sepsis” disease remediation. Chem. Eng. J. 2022, 446, 136916. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, Z.; Ma, W.; Wang, R.; Zhang, C.; Liu, J. A porous organic polymer nanosphere-based fluorescent biosensing platform for simultaneous detection of multiplexed DNA via electrostatic attraction and π–π stacking interactions. RSC Adv. 2021, 11, 38820–38828. [Google Scholar] [CrossRef] [PubMed]
- Abuzeid, H.R.; EL-Mahdy, A.F.M.; Kuo, S.W. Covalent Organic Frameworks: Design Principles, Synthetic Strategies, and Diverse Applications. Giant 2021, 6, 100054. [Google Scholar] [CrossRef]
- Dong, J.; Li, X.; Peh, S.B.; Yuan, Y.D.; Wang, Y.; Ji, D.; Peng, S.; Liu, G.; Ying, S.; Yuan, D.; et al. Restriction of Molecular Rotors in Ultrathin Two-Dimensional Covalent Organic Framework Nanosheets for Sensing Signal Amplification. Chem. Mater. 2019, 31, 146–160. [Google Scholar] [CrossRef]
- Ai, R.; He, Y. Covalent Organic Framework-Inspired Chromogenic System for Visual Colorimetric Detection of Carcinogenic 3, 3′-Diaminobenzidine. Sens. Actuators B Chem. 2020, 304, 127372. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, J.; Gao, F.; Zhang, Q. Covalent Organic Frameworks: Recent Progress in Biomedical Applications. ACS Nano 2023, 17, 1879–1905. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, H.; Xue, W.; Chang, Y.; Li, Y.; Guo, X.; Zhong, C. Rigidifying induced fluorescence enhancement in 2D porous covalent triazine framework nanosheets for the simultaneously luminous detection and adsorption removal of antibiotics. Chem. Eng. J. 2020, 384, 123382. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, Y.; Zheng, A.; Cai, Z.; Huang, A.; Zeng, J.; Liu, X.; Liu, J. A fluorescence-based immunoassay for galectin-4 using gold nanoclusters and a composite consisting of glucose oxidase and a metal-organic framework. Microchim. Acta 2017, 184, 1933. [Google Scholar] [CrossRef]
- Pal, N.; Banerjee, S.; Bhaumik, A. A facile route for the syntheses of Ni(OH)2 and NiO nanostructures as potential candidates for non-enzymatic glucose sensor. J. Colloid Interface Sci. 2018, 516, 121–127. [Google Scholar] [CrossRef]
- Feng, K.-J.; Yang, Y.-H.; Wang, Z.-L.; Jiang, J.-H.; Shen, G.-L.; Yu, R.-Q. A nano-porous CeO2/Chitosan composite film as the immobilization matrix for colorectal cancer DNA sequence-selective electrochemical biosensor. Talanta 2006, 70, 561–565. [Google Scholar] [CrossRef]
- Yun, D.; Song, M.J.; Hwang, S.; Hong, S.I. Fabrication and electrochemical characterization of nanoporous silicon electrode for Amperometric urea biosensor. Jpn. J. Appl. Phys. 2012, 51, 06FG02. [Google Scholar] [CrossRef]
- Gumilar, G.; Kaneti, Y.V.; Henzie, J.; Chatterjee, S.; Na, J.; Yuliarto, B.; Nugraha, N.; Patah, A.; Bhaumik, A.; Yamauchi, Y. General synthesis of hierarchical sheet/plate-like M-BDC (M = Cu, Mn, Ni, and Zr) metal–organic frameworks for electrochemical nonenzymatic glucose sensing. Chem. Sci. 2020, 11, 3644–3655. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Garcia-Gancedo, L.; Flewitt, A.J.; Xie, H.; Moussy, F.; Milne, W.I. A critical review of glucose biosensors based on carbon nanomaterials: Carbon nanotubes and graphene. Sensors 2012, 12, 5996–6022. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Gupta, V.K.; Huseinov, A.; Rahm, C.E.; Gazica, K.; Ivarez, N.T. Highly sensitive non-enzymatic glucose sensor based on carbon nanotube microelectrode set. Sens. Actuators B Chem. 2021, 348, 130688. [Google Scholar] [CrossRef]
- Kang, X.; Wang, J.; Wu, H.; Aksay, I.A.; Liu, J.; Lin, Y. Glucose oxidase-graphene-chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens. Bioelectron. 2009, 25, 901–905. [Google Scholar] [CrossRef]
- Shin, J.-H.; Lee, M.-J.; Choi, J.-H.; Song, J.-A.; Kim, T.-H.; Oh, B.-K. Electrochemical H2O2 biosensor based on horseradish peroxidase encapsulated protein nanoparticles with reduced graphene oxide-modified gold electrode. Nano Converg. 2020, 7, 39. [Google Scholar] [CrossRef]
- Baig, N.; Kawde, A.N. A cost-effective disposable graphene-modified electrode decorated with alternating layers of Au NPs for the simultaneous detection of dopamine and uric acid in human urine. RSC Adv. 2016, 6, 80756–80765. [Google Scholar] [CrossRef]
- Ma, X.; Pang, C.; Li, S.; Xiong, Y.; Li, J.; Luo, J.; Yang, Y. Synthesis of Zr-Coordinated Amide Porphyrin-Based Two-Dimensional Covalent Organic Framework at Liquid-Liquid Interface for Electrochemical Sensing of Tetracycline. Biosens. Bioelectron. 2019, 146, 111734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, Y.; Geng, W.; Liu, H. Rapid Detection of Tryptamine by Optosensor with Molecularly Imprinted Polymers Based on Carbon Dots-Embedded Covalent-Organic Frameworks. Sens. Actuators B Chem. 2019, 285, 546–552. [Google Scholar] [CrossRef]
- Zhang, X.; Chi, K.N.; Li, D.L.; Deng, Y.; Ma, Y.C.; Xu, Q.Q.; Hu, R.; Yang, Y.H. 2D-Porphrinic Covalent Organic Framework-Based Aptasensor with Enhanced Photoelectrochemical Response for the Detection of C-Reactive Protein. Biosens. Bioelectron. 2019, 129, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Duan, F.; Huang, X.; Liu, Y.; Zhou, N.; Xia, L.; Zhang, Z.; Du, M. A Multiple Aptasensor for Ultrasensitive Detection of MiRNAs by Using Covalent-Organic Framework Nanowire as Platform and Shell-Encoded Gold Nanoparticles as Signal Labels. Anal. Chim. Acta 2019, 1082, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, N.; Xie, Y.; Chu, H.; Wang, Y.; Wang, Y. In-situ anchoring bimetallic nanoparticles on covalent organic framework as an ultrasensitive electrochemical sensor for levodopa detection. Talanta 2021, 225, 122072. [Google Scholar] [CrossRef]
- Cui, J.; Kan, L.; Cheng, F.; Liu, J.; He, L.; Xue, Y.; Fang, S.; Zhang, Z. Construction of bifunctional electrochemical biosensors for the sensitive detection of the SARS-CoV-2 N-gene based on porphyrin porous organic polymers. Dalton Trans. 2022, 51, 2094–2104. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Modica, J.A.; Howarth, A.J.; Vargas, E.L.; Moghadam, P.Z.; Snurr, R.Q.; Mrksich, M.; Hupp, J.T.; Farha, O.K. Toward design rules for enzyme immobilization in hierarchical mesoporous metal-organic frameworks. Chem 2016, 1, 154–169. [Google Scholar] [CrossRef]
- Mehta, J.; Bhardwaj, N.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Recent advances in enzyme immobilization techniques: Metal-organic frameworks as novel substrates. Coord. Chem. Rev. 2016, 322, 30–40. [Google Scholar] [CrossRef]
- Qiu, Q.; Chen, H.; Wang, Y.; Ying, Y. Recent Advances in the Rational Synthesis and Sensing Applications of Metal-Organic Framework Biocomposites. Coord. Chem. Rev. 2019, 387, 60–78. [Google Scholar] [CrossRef]
- Sun, Q.; Pan, Y.; Wang, X.; Li, H.; Farmakes, J.; Aguila, B.; Yang, Z.; Ma, S. Mapping out the Degree of Freedom of Hosted Enzymes in Confined Spatial Environments. Chem 2019, 5, 3184–3195. [Google Scholar] [CrossRef]
- Ma, W.; Jiang, Q.; Yu, P.; Yang, L.; Mao, L. Zeolitic Imidazolate Framework-Based Electrochemical Biosensor for in Vivo Electrochemical Measurements. Anal. Chem. 2013, 85, 7550. [Google Scholar] [CrossRef]
- Liu, X.; Chen, W.; Lian, M.; Chen, X.; Lu, Y.; Yang, W. Enzyme immobilization on ZIF-67/MWCNT composite engenders high sensitivity electrochemical sensing. J. Electroanal. Chem. 2019, 833, 505–511. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Shan, D.; Dong, H.F.; Cosnier, S.; Al-Ghanim, K.A.; Ahmad, Z.; Mahboob, S.; Zhang, X.J. DNA-Mediated Nanoscale Metal–Organic Frameworks for Ultrasensitive Photoelectrochemical Enzyme-Free Immunoassay. Anal. Chem. 2018, 90, 12284–12291. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Y.; Zhang, X.; Liu, P.; He, M.; Li, C.; Wang, Y. Near-infrared Photoactive Yb-MOF Functionalized with a Large Conjugate Ionic Liquid: Synthesis and Application for Photoelectrochemical Immunosensing of Carcinoma Embryonic Antigen. Nanoscale 2021, 13, 9757–9765. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, S.; Bari, M.R.; Pirsa, S.; Amiri, S. Use of bacterial cellulose film modified by polypyrrole/TiO2-Ag nanocomposite for detecting and measuring the growth of pathogenic bacteria. Carbohydr. Polym. 2019, 232, 115801. [Google Scholar] [CrossRef] [PubMed]
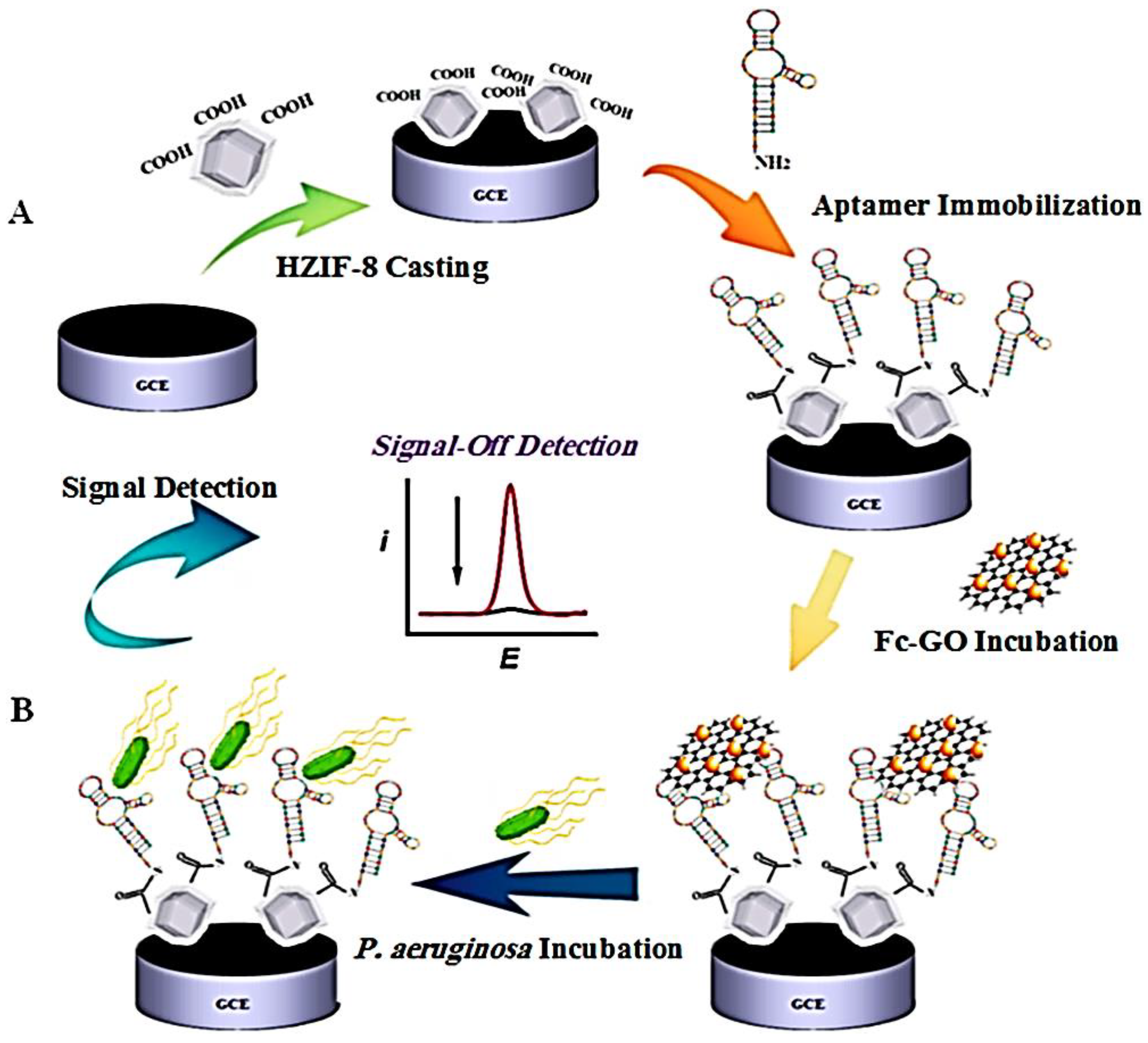
- Shahrokhian, S.; Ranjbar, S. Development of a Sensitive Diagnostic Device Based on Zeolitic Imidazolate Frameworks-8 Using Ferrocene–Graphene Oxide as Electroactive Indicator for Pseudomonas Aeruginosa Detection. ACS Sustain. Chem. Eng. 2019, 7, 12760–12769. [Google Scholar] [CrossRef]
- Eskandarinezhad, S.; Ahmad, I.; Nourollahileilan, W.M.; Khosla, A.; Ahmad, T. Review- metal and metal oxide nanoparticles/nanocomposites as electrochemical biosensors for cancer detection. J. Electrochem. Soc. 2022, 169, 047504. [Google Scholar] [CrossRef]
- Pal, N.; Saha, B.; Kundu, S.K.; Bhaumik, A.; Banerjee, S. A highly efficient non-enzymatic glucose biosensor based on a nanostructured NiTiO3/NiO material. New J. Chem. 2015, 39, 8035–8043. [Google Scholar] [CrossRef]
- Prathap, M.A.A.; Kaur, B.; Srivastava, R. Direct synthesis of metal oxide incorporated mesoporous SBA-15, and their applications in non-enzymatic sensing of glucose. J. Colloid Interface Sci. 2012, 381, 143–151. [Google Scholar] [CrossRef]
- Pal, N.; Banerjee, S.; Choi, E.; Cho, E.-B. Facile one-pot synthesis of yolk-shell structured Ni doped mesoporous silica and its application in enzyme-free glucose sensor. ChemistrySelect 2018, 3, 6029–6034. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, H.; Ni, Y.; Zhang, Q.; Chen, J. Porous nanosheet-based ZnO microspheres for the construction of direct electrochemical biosensors. Biosens. Bioelectron. 2008, 24, 93–98. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Gomes, A.R.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, A.P.T. Graphene based sensors and biosensors. Trends Anal. Chem. 2017, 91, 53–66. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Barrow, C.J.; Yang, W. Molecularly engineered graphene surfaces for sensing applications: A review. Anal. Chim. Acta 2015, 859, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kuma, S.; Sreejith, S.; Mandal, A.K.; Ma, X.; Zhao, Y. Immobilizing gold nanoparticles in mesoporous silica covered reduced graphene oxide: A hybrid material for cancer cell detection through hydrogen peroxide sensing. ACS Appl. Mater. Interfaces 2014, 16, 13648–13656. [Google Scholar]
- Chen, L.Y.; Lang, X.Y.; Fujita, T.; Chen, M.W. Nanoporous Gold for Enzyme-Free Electrochemical Glucose Sensors. Scr. Mater. 2011, 65, 17–20. [Google Scholar] [CrossRef]
- Zhong, G.; Liu, A.; Chen, X.; Wang, K.; Lian, Z.; Liu, Q.; Chen, Y.; Du, M.; Lin, X. Electrochemical biosensor based on nanoporous gold electrode for detection of PML/RARα fusion gene. Biosens. Bioelectron. 2011, 26, 3812–3817. [Google Scholar] [CrossRef]
- Qiu, H.; Lu, L.; Huang, X.; Zhang, Z.; Qu, Y. Immobilization of horseradish peroxidase on nanoporous copper and its potential applications. Bioresour. Technol 2010, 101, 9415–9420. [Google Scholar] [CrossRef] [PubMed]
- Ennaert, T.; Van Aelst, J.; Dijkmans, J.; De Clercq, R.; Schutyser, W.; Dusselier, M.; Verboekend, D.; Sels, B.F. Potential and Challenges of Zeolite Chemistry in the Catalytic Conversion of Biomass. Chem. Soc. Rev. 2016, 45, 584–611. [Google Scholar] [CrossRef]
- Perot, G.; Guisnet, M. Advantages and disadvantages of zeolites as catalysts in organic chemistry. J. Mol. Catal. 1990, 61, 173–196. [Google Scholar] [CrossRef]
- Chen, L.-H.; Sun, M.-H.; Wang, Z.; Yang, W.; Xie, Z.; Su, B.-L. Hierarchically structured zeolites: From design to application. Chem. Rev. 2020, 120, 11194–11294. [Google Scholar] [CrossRef]














| Analytical Technique | Instrument | Properties |
|---|---|---|
| Powder X-ray diffraction (small and wide angle) | Powder X-ray diffractometer | Mesostructure, porosity, phase purity, and crystallinity |
| Brunauer–Emmett–Teller (BET) surface area analysis | BET surface area analyzer | Surface area, porosity, pore-diameter, pore volume, and shapes of pores |
| Transmission electron microscopy (TEM) | Transmission electron microscope | Internal nanostructure, particle size, pore crystallinity, and aggregation |
| Scanning electron microscopy (SEM) | Scanning electron microscope | Morphology, particle size and distribution, shape, and aggregation |
| Atomic force microscopy | Atomic force microscope | Particle size and distribution, shape, structure, and aggregation |
| X-ray photoelectron spectroscopy | X-ray photoelectron spectroscope | Oxidation state and chemical composition of surface |
| Fourier transform infrared spectroscopy | Fourier transform infrared spectroscope | Chemical bonding and bonding connectivity |
| UV-visible spectroscopy | UV-visible spectrophotometer | Chemical environment |
| Thermogravimetric analysis | Thermogravimetric analyzer | Thermal stability |
| Dynamic light scattering | Dynamic light scattering instruments | Size distribution |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pal, N.; Chakraborty, D.; Cho, E.-B.; Seo, J.G. Recent Developments on the Catalytic and Biosensing Applications of Porous Nanomaterials. Nanomaterials 2023, 13, 2184. https://doi.org/10.3390/nano13152184
Pal N, Chakraborty D, Cho E-B, Seo JG. Recent Developments on the Catalytic and Biosensing Applications of Porous Nanomaterials. Nanomaterials. 2023; 13(15):2184. https://doi.org/10.3390/nano13152184
Chicago/Turabian StylePal, Nabanita, Debabrata Chakraborty, Eun-Bum Cho, and Jeong Gil Seo. 2023. "Recent Developments on the Catalytic and Biosensing Applications of Porous Nanomaterials" Nanomaterials 13, no. 15: 2184. https://doi.org/10.3390/nano13152184
APA StylePal, N., Chakraborty, D., Cho, E.-B., & Seo, J. G. (2023). Recent Developments on the Catalytic and Biosensing Applications of Porous Nanomaterials. Nanomaterials, 13(15), 2184. https://doi.org/10.3390/nano13152184








