Activating Hydrogen Evolution Reaction on Carbon Nanotube via Aryl Functionalisation: The Role of Hybrid sp2–sp3 Interface and Curvature
Abstract
1. Introduction
2. Computational Details
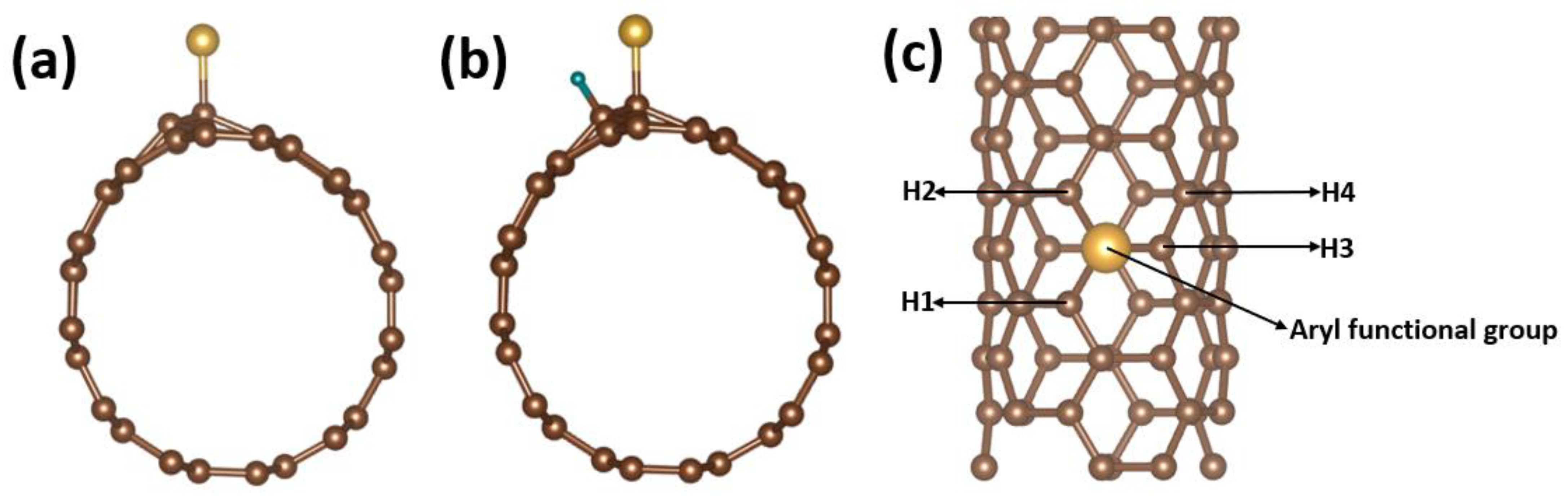
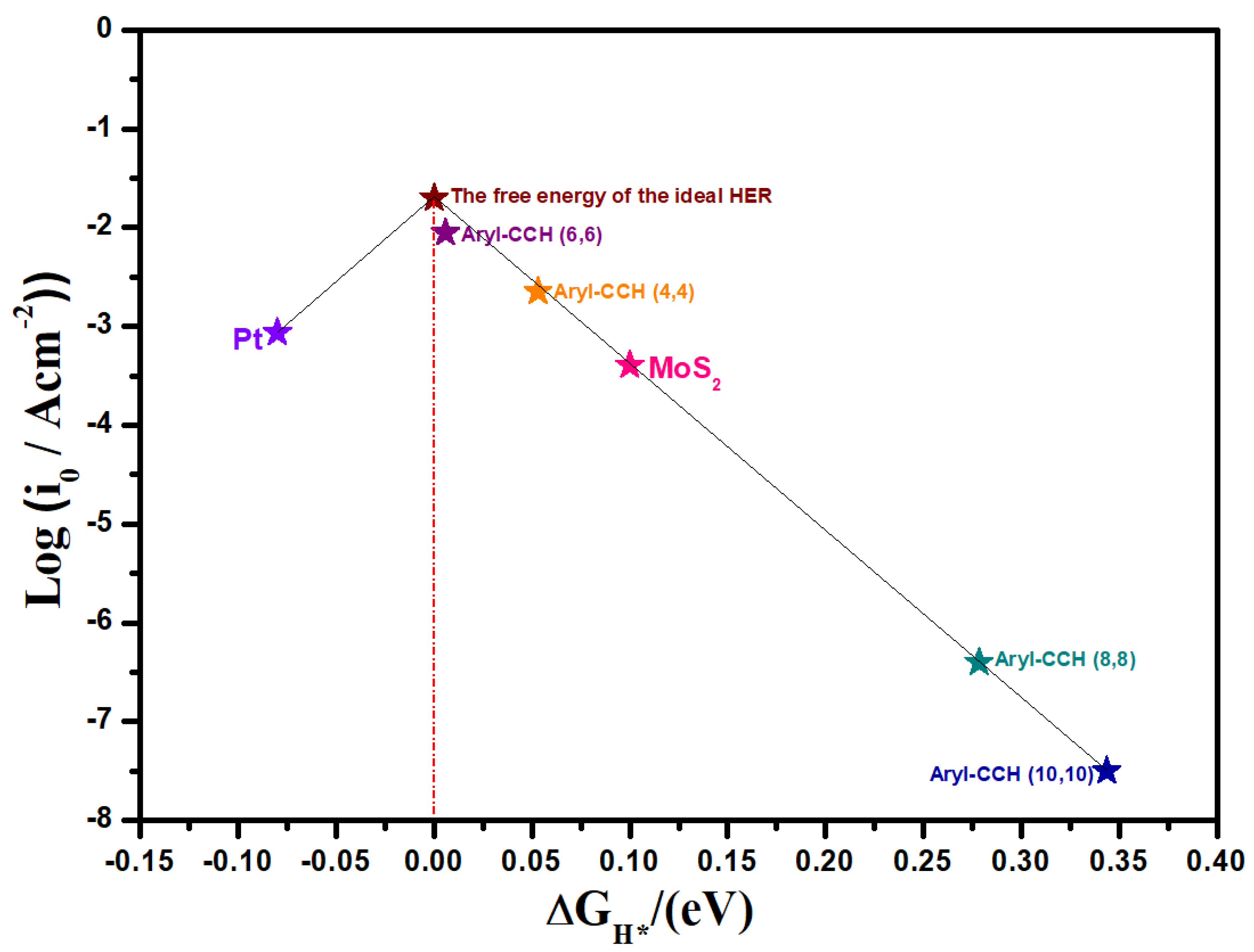
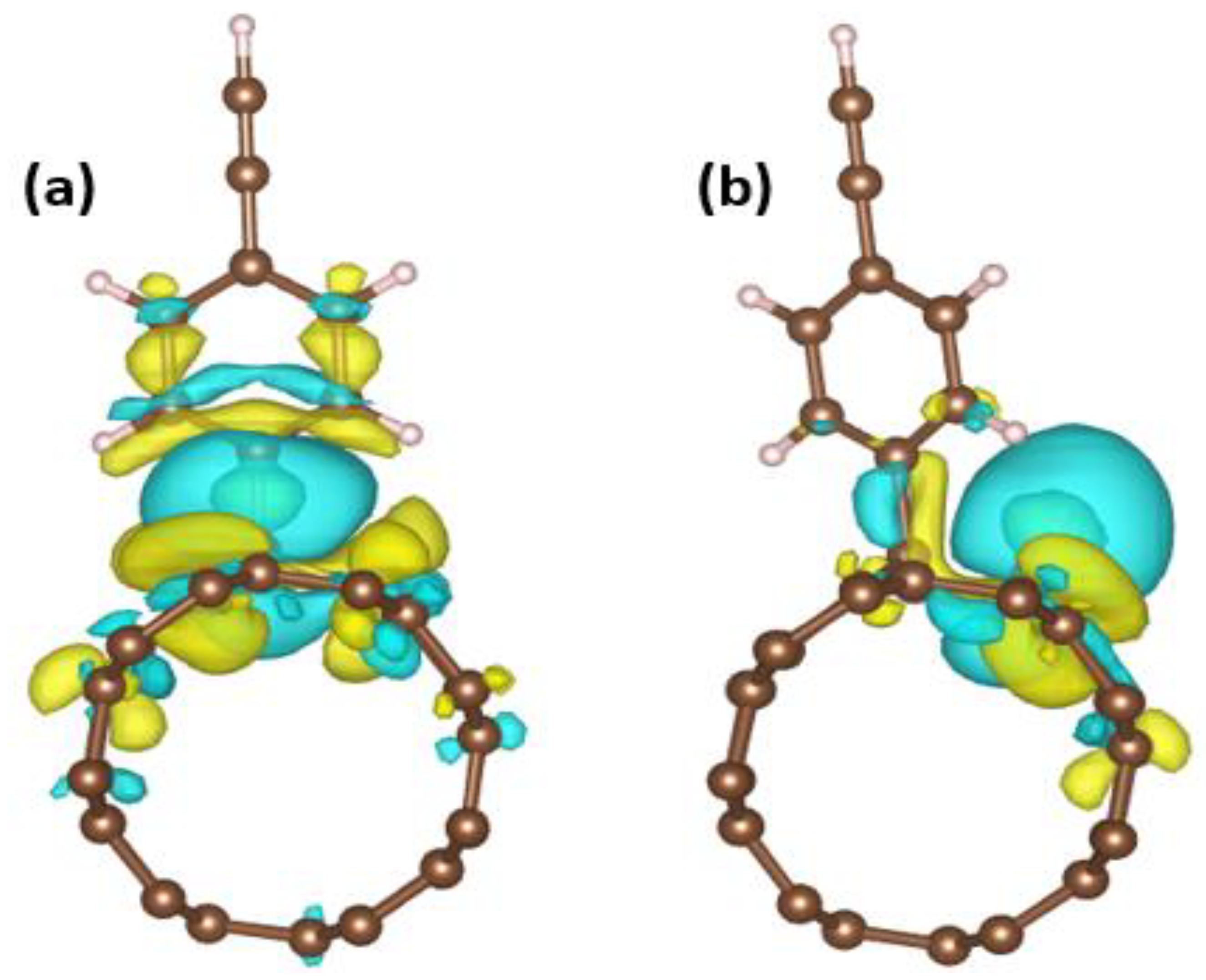
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, C.-H.; Huang, C.-W.; Wu, J.C. Hydrogen production from semiconductor-based photocatalysis via water splitting. Catalysts 2012, 2, 490–516. [Google Scholar] [CrossRef]
- Sharma, S.; Agarwal, S.; Jain, A. Significance of Hydrogen as Economic and Environmentally Friendly Fuel. Energies 2021, 14, 7389. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Teramura, K.; Lu, D.; Takata, T.; Saito, N.; Inoue, Y.; Domen, K. Photocatalyst releasing hydrogen from water. Nature 2006, 440, 295. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Yu, Y.; Ma, Q.; Chen, B.; Zhang, H. 2D transition-metal-dichalcogenide-nanosheet-based composites for photocatalytic and electrocatalytic hydrogen evolution reactions. Adv. Mater. 2016, 28, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Hasani, A.; Tekalgne, M.; Van Le, Q.; Jang, H.W.; Kim, S.Y. Two-dimensional materials as catalysts for solar fuels: Hydrogen evolution reaction and CO2 reduction. J. Mater. Chem. A 2019, 7, 430–454. [Google Scholar] [CrossRef]
- Murthya, A.; Madhavana, J.; Muruganb, K. Recent advances in hydrogen evolution reaction catalysts on carbon/carbon-based supports in acid media. J. Power Sources 2018, 398, 9–26. [Google Scholar] [CrossRef]
- Safizadeh, F.; Ghali, E.; Houlachi, G. Electrocatalysis developments for hydrogen evolution reaction in alkaline solutions–a review. Int. J. Hydrogren Energy 2015, 40, 256–274. [Google Scholar] [CrossRef]
- Tahir, M.B.; Batool, A. Chapter 3—Recent development in sustainable technologies for clean hydrogen evolution: Current scenario and future perspectives. In Sustainable Materials and Green Processing for Energy Conversion; Elsevier: Amsterdam, The Netherlands, 2022; pp. 97–130. [Google Scholar]
- Bockris, J.M.; Potter, E. The mechanism of the cathodic hydrogen evolution reaction. J. Electrochem. Soc. 1952, 99, 169. [Google Scholar] [CrossRef]
- Zhou, W.; Jia, J.; Lu, J.; Yang, L.; Hou, D.; Li, G.; Chen, S. Recent developments of carbon-based electrocatalysts for hydrogen evolution reaction. Nano Energy 2016, 28, 29–43. [Google Scholar] [CrossRef]
- Eftekhari, A. Electrocatalysts for hydrogen evolution reaction. Int. J. Hydrogren Energy 2017, 42, 11053–11077. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Guo, X.; Chen, C.; Dong, C.-L.; Liu, R.-S.; Han, C.-P.; Li, Y.; Gogotsi, Y.; Wang, G. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nat. Catal. 2018, 1, 985–992. [Google Scholar] [CrossRef]
- Chen, R.; Yang, C.; Cai, W.; Wang, H.-Y.; Miao, J.; Zhang, L.; Chen, S.; Liu, B. Use of platinum as the counter electrode to study the activity of nonprecious metal catalysts for the hydrogen evolution reaction. ACS Energy Lett. 2017, 2, 1070–1075. [Google Scholar] [CrossRef]
- Davodi, F.; Mühlhausen, E.; Tavakkoli, M.; Sainio, J.; Jiang, H.; Gökce, B.; Marzun, G.; Kallio, T. Catalyst Support Effect on the Activity and Durability of Magnetic Nanoparticles: Toward Design of Advanced Electrocatalyst for Full Water Splitting. ACS Appl. Mater. Intefaces 2018, 10, 31300–31311. [Google Scholar] [CrossRef]
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.-K.; Liu, L.-M. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Fang, S.; Zhu, X.; Liu, X.; Gu, J.; Liu, W.; Wang, D.; Zhang, W.; Lin, Y.; Lu, J.; Wei, S. Uncovering near-free platinum single-atom dynamics during electrochemical hydrogen evolution reaction. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Cui, W.; Liu, Q.; Cheng, N.; Asiri, A.M.; Sun, X. Activated carbon nanotubes: A highly-active metal-free electrocatalyst for hydrogen evolution reaction. Chem. Commun. 2014, 50, 9340–9342. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Z.; Zhang, M.; Tan, Y.; Jia, S.; Liu, A. Green electroless plating of cuprous oxide nanoparticles onto carbon nanotubes as efficient electrocatalysts for hydrogen evolution reaction. Appl. Surf. Sci. 2021, 548, 149218. [Google Scholar] [CrossRef]
- Bashir, A.; Mehvish, A.; Khalil, M. Advanced Carbon Materials for Sustainable and Emerging Applications. In 21st Century Advanced Carbon Materials for Engineering Applications—A Comprehensive Handbook; Books on Demand: Copenhagen, Denmark, 2021. [Google Scholar]
- Melchionna, M.; Marchesan, S.; Pratoa, M.; Fornasiero, P. Carbon nanotubes and catalysis: The many facets of a successful marriage. Catal. Sci. Technol. 2015, 5, 3859–3875. [Google Scholar] [CrossRef]
- Holmberg, N.; Laasonen, K. Ab initio electrochemistry: Exploring the hydrogen evolution reaction on carbon nanotubes. J. Phys. Chem. C 2015, 119, 16166–16178. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, D.; Guo, F.; Guo, H.; Liu, Y.; Yin, Y.; Hu, H.; Wang, X. Facile one-pot supercritical synthesis of MoS2/pristine graphene nanohybrid as a highly active advanced electrocatalyst for hydrogen evolution reaction. Appl. Surf. Sci. 2020, 531, 147282. [Google Scholar] [CrossRef]
- Kong, D.; Cha, J.J.; Wang, H.; Lee, H.R.; Cui, Y. First-row transition metal dichalcogenide catalysts for hydrogen evolution reaction. Energy Environ. Sci. 2013, 6, 3553–3558. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, R.; Zhang, J.; Shi, Y.; Zhang, B. Anion-exchange synthesis of nanoporous FeP nanosheets as electrocatalysts for hydrogen evolution reaction. Chem. Commun. 2013, 49, 6656–6658. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Gong, M.; Chou, H.-L.; Pan, C.-J.; Chen, H.-A.; Wu, Y.; Lin, M.-C.; Guan, M.; Yang, J.; Chen, C.-W. Highly active and stable hybrid catalyst of cobalt-doped FeS2 nanosheets–carbon nanotubes for hydrogen evolution reaction. J. Am. Chem. Soc. 2015, 137, 1587–1592. [Google Scholar] [CrossRef]
- Nasir, S.; Hussein, M.; Zainal, Z.; Yusof, N. Carbon-Based Nanomaterials/Allotropes: A Glimpse of Their Synthesis, Properties and Some Applications. Materials 2018, 11, 295. [Google Scholar] [CrossRef]
- Liu, C.; Dai, Z.; Zhang, J.; Jin, Y.; Li, D.; Sun, C. Two-dimensional boron sheets as metal-free catalysts for hydrogen evolution reaction. J. Phys. Chem. C 2018, 122, 19051–19055. [Google Scholar] [CrossRef]
- Wu, R.; Zhang, J.; Shi, Y.; Liu, D.; Zhang, B. Metallic WO2–carbon mesoporous nanowires as highly efficient electrocatalysts for hydrogen evolution reaction. J. Am. Chem. Soc. 2015, 137, 6983–6986. [Google Scholar] [CrossRef]
- Shahraei, A.; Moradabadi, A.; Martinaiou, I.; Lauterbach, S.; Klemenz, S.; Dolique, S.; Kleebe, H.-J.; Kaghazchi, P.; Kramm, U.I. Elucidating the origin of hydrogen evolution reaction activity in mono-and bimetallic metal-and nitrogen-doped carbon catalysts (Me–N–C). ACS Appl. Mater. Interfaces 2017, 9, 25184–25193. [Google Scholar] [CrossRef]
- Xiao, P.; Sk, M.A.; Thia, L.; Ge, X.; Lim, R.J.; Wang, J.-Y.; Lim, K.H.; Wang, X. Molybdenum phosphide as an efficient electrocatalyst for the hydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 2624–2629. [Google Scholar] [CrossRef]
- Rahman, U.; Humayun, M.; Ghani, U.; Usman, M.; Ullah, H.; Khan, A.; El-Metwaly, N.; Khan, A. MXenes as Emerging Materials: Synthesis, Properties, and Applications. Molecules 2022, 27, 4909. [Google Scholar] [CrossRef]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef]
- Xiao, P.; Buijnsters, J.G.; Zhao, Y.; Yu, H.; Xu, X.; Zhu, Y.; Tang, D.; Zhu, J.; Zhao, Z. Fullerene-like WS2 supported Pd catalyst for hydrogen evolution reaction. J. Catal. 2019, 380, 215–223. [Google Scholar] [CrossRef]
- Gao, G.; O’Mullane, A.P.; Du, A. 2D MXenes: A new family of promising catalysts for the hydrogen evolution reaction. Acs Catal. 2017, 7, 494–500. [Google Scholar] [CrossRef]
- Guo, Z.; Zhou, J.; Zhu, L.; Sun, Z. MXene: A promising photocatalyst for water splitting. J. Mater. Chem. A 2016, 4, 11446–11452. [Google Scholar] [CrossRef]
- Fang, W.; Wang, J.; Hu, Y.; Cui, X.; Zhu, R.; Zhang, Y.; Yue, C.; Dang, J.; Cui, W.; Zhao, H. Metal-organic framework derived Fe-Co-CN/reduced graphene oxide for efficient HER and OER. Electrochim. Acta 2021, 365, 137384. [Google Scholar] [CrossRef]
- Jo, W.-K.; Moru, S.; Lee, D.-E.; Tonda, S. Cobalt-and iron-coordinated graphitic carbon nitride on reduced graphene oxide: A nonprecious bimetallic M–Nx–C analogue electrocatalyst for efficient oxygen reduction reaction in acidic media. Appl. Surf. Sci. 2020, 531, 147367. [Google Scholar] [CrossRef]
- Yu, P.; Wang, F.; Shifa, T.A.; Zhan, X.; Lou, X.; Xia, F.; He, J. Earth abundant materials beyond transition metal dichalcogenides: A focus on electrocatalyzing hydrogen evolution reaction. Nano Energy 2019, 58, 244–276. [Google Scholar] [CrossRef]
- Zahra, R.; Pervaiz, E.; Yang, M.; Rabi, O.; Saleem, Z.; Ali, M.; Farrukh, S. A review on nickel cobalt sulphide and their hybrids: Earth abundant, pH stable electro-catalyst for hydrogen evolution reaction. Int. J. Hydrogren Energy 2020, 45, 24518–24543. [Google Scholar] [CrossRef]
- Mohammed-Ibrahim, J.; Sun, X. Recent progress on earth abundant electrocatalysts for hydrogen evolution reaction (HER) in alkaline medium to achieve efficient water splitting–A review. J. Energy Chem. 2019, 34, 111–160. [Google Scholar] [CrossRef]
- McKone, J.R.; Marinescu, S.C.; Brunschwig, B.S.; Winkler, J.R.; Gray, H.B. Earth-abundant hydrogen evolution electrocatalysts. Chem. Sci. 2014, 5, 865–878. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Hao, X.-F.; Jiang, Z.; Sun, X.-P.; Xu, D.; Wang, J.; Zhong, H.-X.; Meng, F.-L.; Zhang, X.-B. C and N hybrid coordination derived Co–C–N complex as a highly efficient electrocatalyst for hydrogen evolution reaction. J. Am. Chem. Soc. 2015, 137, 15070–15073. [Google Scholar] [CrossRef] [PubMed]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, M.S.; Atarod, M.; Sajjadi, M.; Isaabadi, Z. An Introduction to Green Nanotechnology; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Sulaiman, M.R.; Gupta, R.K. Advanced carbon-based nanostructured materials for fuel cells. In Nanotechnology in Fuel Cells; Elsevier: Amsterdam, The Netherlands, 2022; pp. 201–227. [Google Scholar]
- Yang, L.; Lv, Y.; Cao, D. Co, N-codoped nanotube/graphene 1D/2D heterostructure for efficient oxygen reduction and hydrogen evolution reactions. J. Mater. Chem. A 2018, 6, 3926–3932. [Google Scholar] [CrossRef]
- Katsnelson, M. Graphene: Carbon in two dimensions. Mater. Today 2007, 10, 20–27. [Google Scholar] [CrossRef]
- Li, T.; Luo, G.; Liu, K.; Li, X.; Sun, D.; Xu, L.; Li, Y.; Tang, Y. Encapsulation of Ni3Fe nanoparticles in N-doped carbon nanotube–grafted carbon nanofibers as high-efficiency hydrogen evolution electrocatalysts. Adv. Funct. Mater. 2018, 28, 1805828. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, R.; Liu, Y.; Ha, Y.; Guo, Y.; Sun, D.; Liu, M.; Fang, F. Ultrafine Co nanoparticles encapsulated in carbon-nanotubes-grafted graphene sheets as advanced electrocatalysts for the hydrogen evolution reaction. Adv. Mater. 2018, 30, 1802011. [Google Scholar] [CrossRef]
- Jin, D.; Johnson, L.R.; Raman, A.S.; Ming, X.; Gao, Y.; Du, F.; Wei, Y.; Chen, G.; Vojvodic, A.; Gogotsi, Y. Computational screening of 2D ordered double transition-metal carbides (MXenes) as electrocatalysts for hydrogen evolution reaction. J. Phys. Chem. C 2020, 124, 10584–10592. [Google Scholar] [CrossRef]
- Chen, X.; Liu, G.; Zheng, W.; Feng, W.; Cao, W.; Hu, W.; Hu, P. Vertical 2D MoO2/MoSe2 Core–Shell Nanosheet Arrays as High-Performance Electrocatalysts for Hydrogen Evolution Reaction. Adv. Funct. Mater. 2016, 26, 8537–8544. [Google Scholar] [CrossRef]
- Liang, X.; Wu, C.-M.L. Metal-free two-dimensional phosphorus carbide as an efficient electrocatalyst for hydrogen evolution reaction comparable to platinum. Nano Energy 2020, 71, 104603. [Google Scholar] [CrossRef]
- Yao, Y.; Jin, Z.; Chen, Y.; Gao, Z.; Yan, J.; Liu, H.; Wang, J.; Li, Y.; Liu, S.F. Graphdiyne-WS2 2D-Nanohybrid electrocatalysts for high-performance hydrogen evolution reaction. Carbon 2018, 129, 228–235. [Google Scholar] [CrossRef]
- Zhang, S.; Chowdari, B.; Wen, Z.; Jin, J.; Yang, J. Constructing highly oriented configuration by few-layer MoS2: Toward high-performance lithium-ion batteries and hydrogen evolution reactions. ACS Nano 2015, 9, 12464–12472. [Google Scholar] [CrossRef]
- Stenina, I.A.; Yaroslavtsev, A.B. Nanomaterials for lithium-ion batteries and hydrogen energy. Pure Appl. Chem. 2017, 89, 1185–1194. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Sun, Y.; Zhang, P.; Gao, X.; Zhang, W.; Guo, J. MoS2-graphene hybrid nanosheets constructed 3D architectures with improved electrochemical performance for lithium-ion batteries and hydrogen evolution. Electrochim. Acta 2016, 189, 224–230. [Google Scholar] [CrossRef]
- Lasia, A. Hydrogen evolution reaction. In Handbook of Fuel Cells; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; p. 815. [Google Scholar]
- Lasia, A. Mechanism and kinetics of the hydrogen evolution reaction. Int. J. Hydrogren Energy 2019, 44, 19484–19518. [Google Scholar] [CrossRef]
- Kamran, M.; Fazal, M. Chapter 1—Fundamentals of renewable energy systems. In Renewable Energy Conversion Systems; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–19. [Google Scholar]
- Dincer, I.; Rosen, M. Chapter 4—Exergy, environment, and sustainable development. In Environment and Sustainable Development; Springer: Warsaw, Poland, 2021; pp. 61–89. [Google Scholar]
- Zorn, N.; Berger, F.; Zaumseil, J. Charge Transport in and Electroluminescence from sp3-Functionalized Carbon Nanotube Networks. ACS Nano 2021, 15, 10451–10463. [Google Scholar] [CrossRef]
- Li, M.; Riaz, A.; Wederhake, M.; Fink, K.; Saha, A.; Dehm, S.; He, X.; Schöppler, F.; Kappes, M.; Htoon, H.; et al. Electroluminescence from Single-Walled Carbon Nanotubes with Quantum Defects. ACS Nano 2022, 16, 11742–11754. [Google Scholar] [CrossRef]
- Zaumseil, J. Luminescent Defects in Single-Walled Carbon Nanotubes for Applications. Adv. Opt. Mater. 2022, 10, 2101576. [Google Scholar] [CrossRef]
- Oskin, P.; Demkina, I.; Dmitrieva, E.; Alferov, S. Functionalization of Carbon Nanotubes Surface by Aryl Groups: A Review. Nanomaterials 2023, 13, 1630. [Google Scholar] [CrossRef]
- Vautrin-Ul, C. Modification of sp2 carbon Allotropes with Diazonium Salts-Focus on Carbon Nanotubes Functionalization. Aryl Diazonium Salts Relat. Compd. Surf. Chem. Appl. 2022, 31, 137–156. [Google Scholar]
- Zhou, F.; Zhou, Y.; Liu, G.-G.; Wang, C.-T.; Wang, J. Recent advances in nanostructured electrocatalysts for hydrogen evolution reaction. Rare Met. 2021, 40, 3375–3405. [Google Scholar] [CrossRef]
- Greeley, J.; Nørskov, J.K.; Mavrikakis, M. Electronic structure and catalysis on metal surfaces. Annu. Rev. Phys. Chem. 2002, 53, 319–348. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Gross, E.; Kohn, W. Time-Dependent Density-Functional Theory. Adv. Quantum Chem. 1990, 21, 255–291. [Google Scholar]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ernzerhof, M.; Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 1996, 105, 9982–9985. [Google Scholar] [CrossRef]
- Tsai, C.; Abild-Pedersen, F.; Nørskov, J.K. Tuning the MoS2 edge-site activity for hydrogen evolution via support interactions. Nano Lett. 2014, 14, 1381–1387. [Google Scholar] [CrossRef]
- Atkins, P.; Atkins, P.W.; de Paula, J. Atkins’ Physical Chemistry; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 2005, 152, J23. [Google Scholar] [CrossRef]
- Greeley, J.; Jaramillo, T.F.; Bonde, J.; Chorkendorff, I.; Nørskov, J.K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 2006, 5, 909–913. [Google Scholar] [CrossRef]
- Lv, X.; Wei, W.; Wang, H.; Huang, B.; Dai, Y. Multifunctional electrocatalyst PtM with low Pt loading and high activity T towards hydrogen and oxygen electrode reactions: A computational study. Appl. Catal. B Environ. 2019, 255, 117743. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.; Kour, G.; Sun, Z.; Du, A.; Mao, X. Activating Hydrogen Evolution Reaction on Carbon Nanotube via Aryl Functionalisation: The Role of Hybrid sp2–sp3 Interface and Curvature. Nanomaterials 2023, 13, 2122. https://doi.org/10.3390/nano13142122
Ahmed M, Kour G, Sun Z, Du A, Mao X. Activating Hydrogen Evolution Reaction on Carbon Nanotube via Aryl Functionalisation: The Role of Hybrid sp2–sp3 Interface and Curvature. Nanomaterials. 2023; 13(14):2122. https://doi.org/10.3390/nano13142122
Chicago/Turabian StyleAhmed, Muhammad, Gurpreet Kour, Ziqi Sun, Aijun Du, and Xin Mao. 2023. "Activating Hydrogen Evolution Reaction on Carbon Nanotube via Aryl Functionalisation: The Role of Hybrid sp2–sp3 Interface and Curvature" Nanomaterials 13, no. 14: 2122. https://doi.org/10.3390/nano13142122
APA StyleAhmed, M., Kour, G., Sun, Z., Du, A., & Mao, X. (2023). Activating Hydrogen Evolution Reaction on Carbon Nanotube via Aryl Functionalisation: The Role of Hybrid sp2–sp3 Interface and Curvature. Nanomaterials, 13(14), 2122. https://doi.org/10.3390/nano13142122






