Hydrogen Evolution Reaction on Ultra-Smooth Sputtered Nanocrystalline Ni Thin Films in Alkaline Media—From Intrinsic Activity to the Effects of Surface Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Ni Thin Layers
2.2. Materials Characterization
2.3. Electrochemical Measurements
2.4. Computational Modeling
3. Results
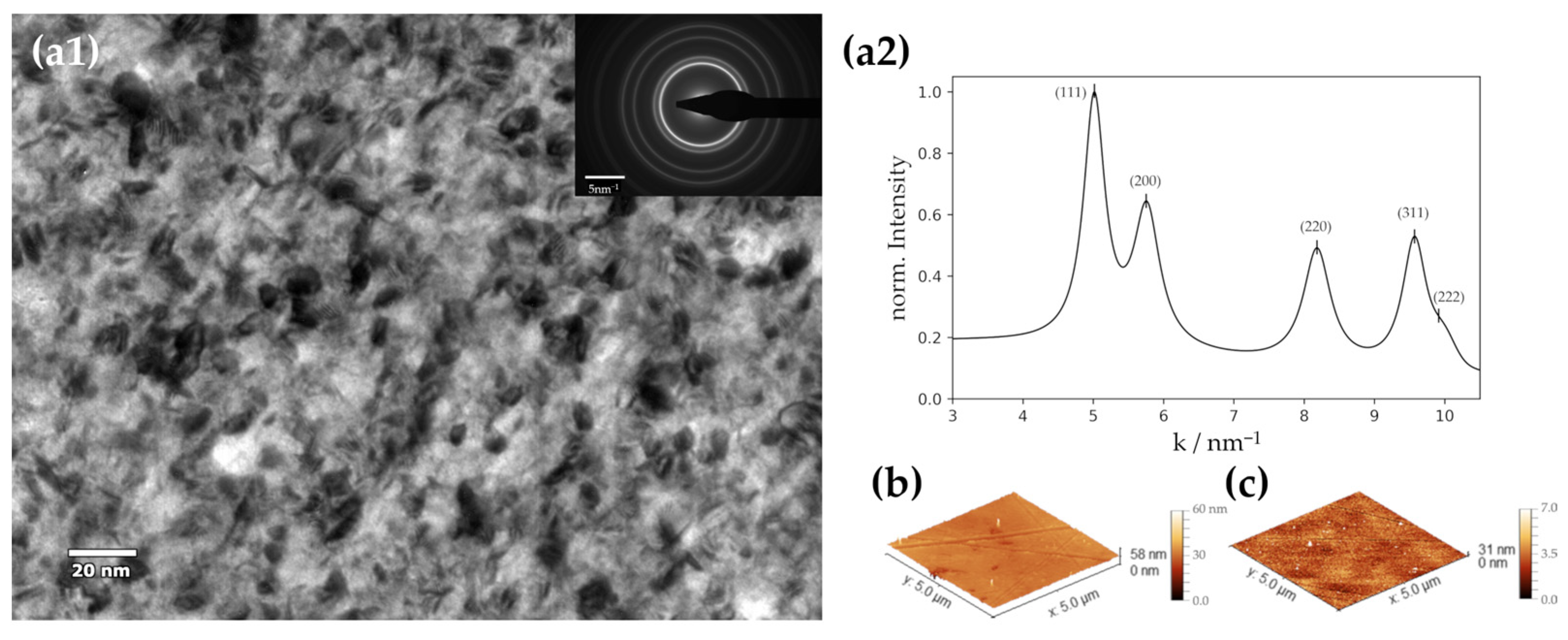
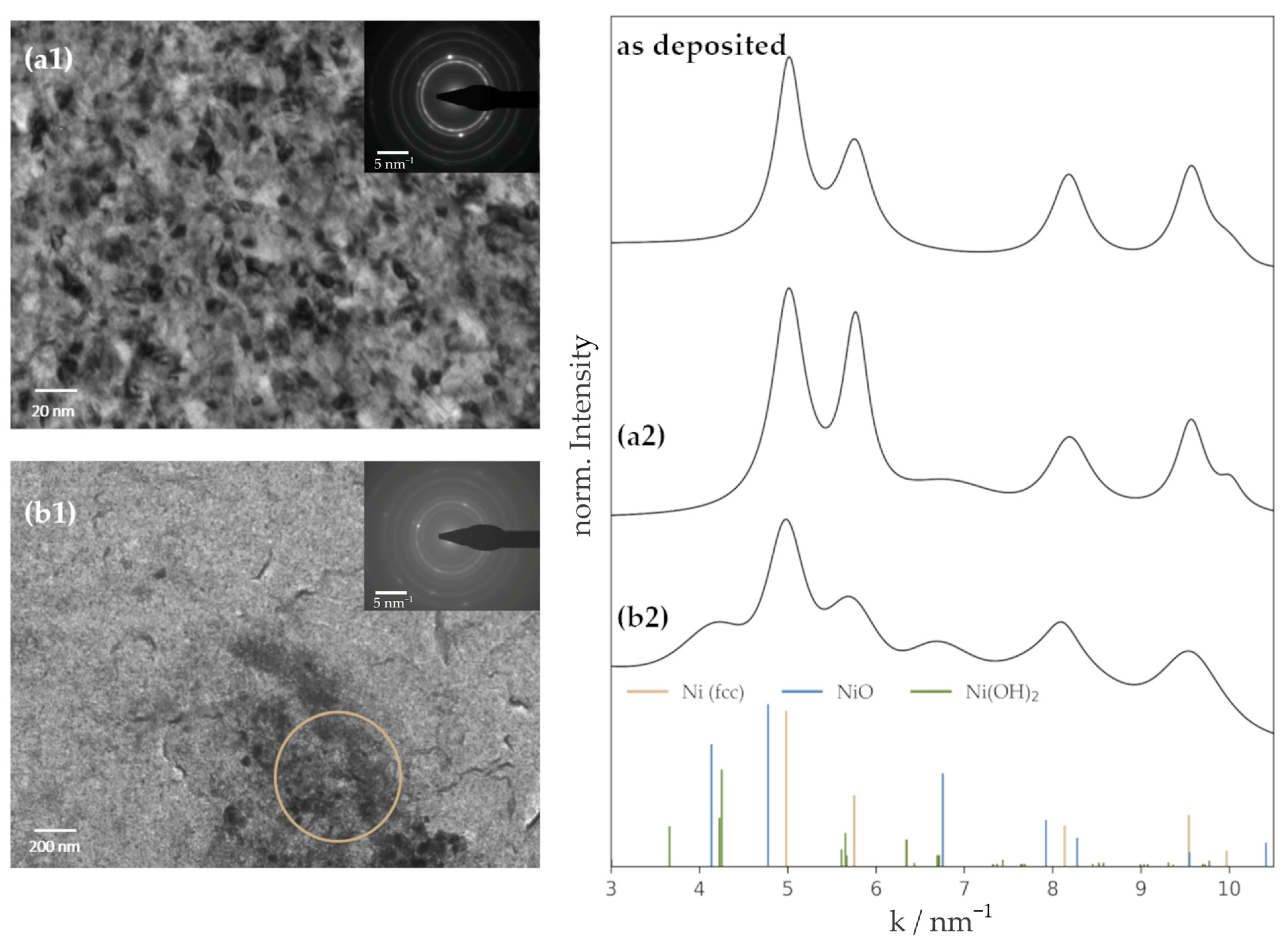
3.1. Properties of the Deposited Ni Films
3.2. HER Catalysis by the Deposited Ni Films
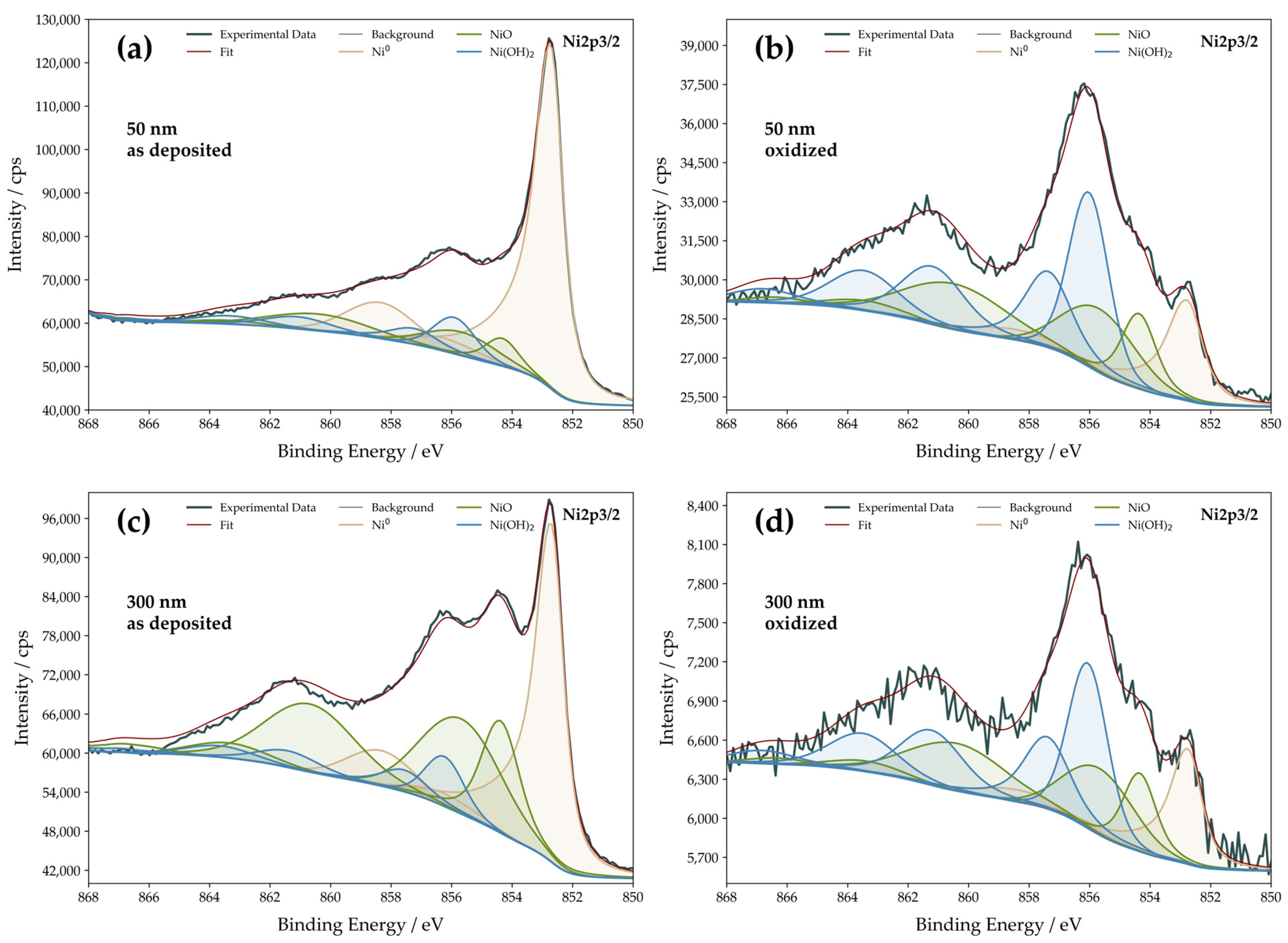
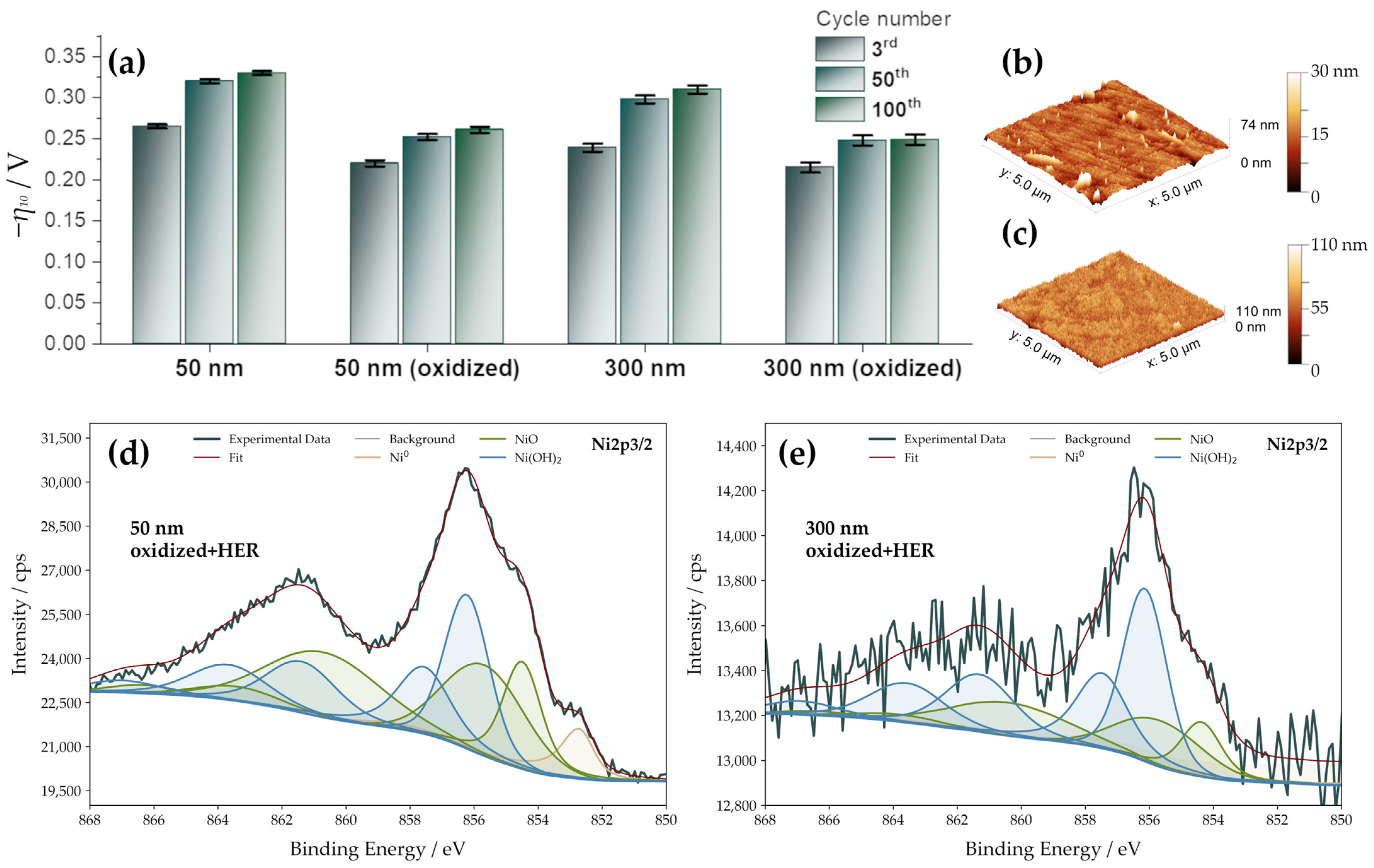
3.3. Surface Chemical Composition
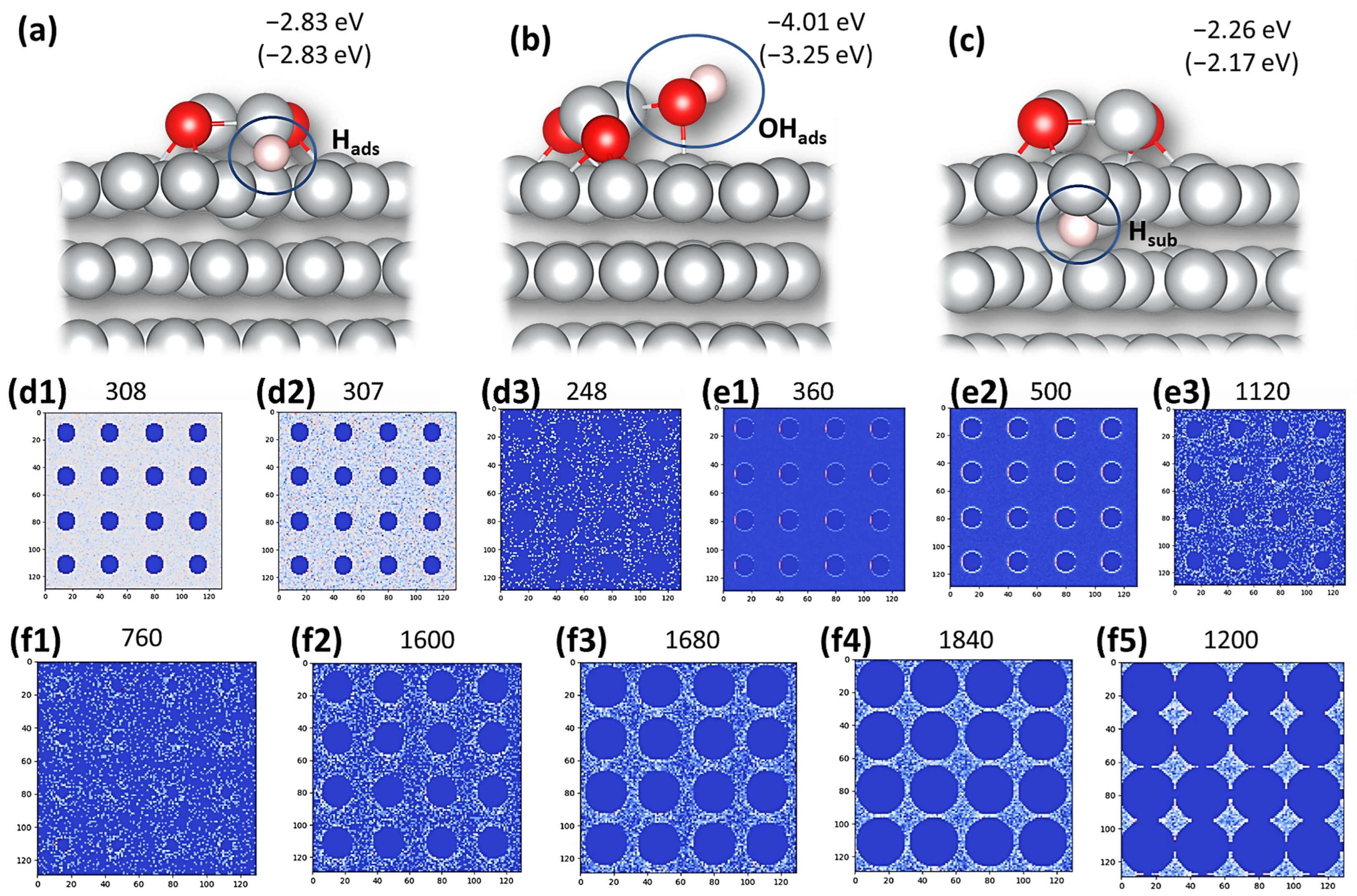
3.4. DFT Modeling and KMC Simulations
3.5. HER Activity upon Prolonged Cycling—The Crucial Role of Surface Oxidation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen Production for Energy: An Overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- IEA. Global Hydrogen Review 2021; IEA: Paris, France, 2021. [Google Scholar]
- Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, A.; Zhong, C.-J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Greeley, J.; Jaramillo, T.F.; Bonde, J.; Chorkendorff, I.; Nørskov, J.K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 2006, 5, 909–913. [Google Scholar] [CrossRef]
- Sabatier, P. Hydrogénations et déshydrogénations par catalyse. Eur. J. Inorg. Chem. 1911, 44, 1984–2001. [Google Scholar] [CrossRef]
- Gebremariam, G.K.; Jovanović, A.Z.; Dobrota, A.S.; Skorodumova, N.V.; Pašti, I.A. Hydrogen Evolution Volcano(es)—From Acidic to Neutral and Alkaline Solutions. Catalysts 2022, 12, 1541. [Google Scholar] [CrossRef]
- Lasia, A. Hydrogen Evolution Reaction. In Handbook of Fuel Cells, Fundamentals, Technology and Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2003; Volume 2, pp. 414–440. ISBN 0-471-49926-9. [Google Scholar]
- Ferriday, T.B.; Middleton, P.H.; Kolhe, M.L. Review of the Hydrogen Evolution Reaction—A Basic Approach. Energies 2021, 14, 8535. [Google Scholar] [CrossRef]
- Shen, L.-F.; Lu, B.-A.; Qu, X.-M.; Ye, J.-Y.; Zhang, J.-M.; Yin, S.-H.; Wu, Q.-H.; Wang, R.-X.; Shen, S.-Y.; Sheng, T.; et al. Does the oxophilic effect serve the same role for hydrogen evolution/oxidation reaction in alkaline media? Nano Energy 2019, 62, 601–609. [Google Scholar] [CrossRef]
- Strmcnik, D.; Uchimura, M.; Wang, C.; Subbaraman, R.; Danilovic, N.; van der Vliet, D.; Paulikas, A.P.; Stamenkovic, V.R.; Markovic, N.M. Improving the hydrogen oxidation reaction rate by promotion of hydroxyl adsorption. Nat. Chem. 2013, 5, 300–306. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef]
- Mahmood, N.; Yao, Y.; Zhang, J.-W.; Pan, L.; Zhang, X.; Zou, J.-J. Electrocatalysts for Hydrogen Evolution in Alkaline Electrolytes: Mechanisms, Challenges, and Prospective Solutions. Adv. Sci. 2018, 5, 1700464. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zheng, Y.; Sheng, W.; Xu, Z.J.; Jaroniec, M.; Qiao, S.-Z. Strategies for design of electrocatalysts for hydrogen evolution under alkaline conditions. Mater. Today 2020, 36, 125–138. [Google Scholar] [CrossRef]
- Gutić, S.J.; Metarapi, D.; Jovanović, A.Z.; Gebremariam, G.K.; Dobrota, A.S.; Vasiljević, B.N.; Pašti, I.A. Redrawing HER Volcano with Interfacial Processes—The Role of Hydrogen Spillover in Boosting H2 Evolution in Alkaline Media. Catalysts 2023, 13, 89. [Google Scholar] [CrossRef]
- Coutanceau, C.; Baranton, S.; Audichon, T. Hydrogen Production from Water Electrolysis. In Hydrogen Energy and Fuel Cells Primers, Hydrogen Electrochemical Production; Academic Press: Cambridge, MA, USA, 2018; pp. 17–62. ISBN 978-0-12-811250-2. [Google Scholar]
- David, M.; Ocampo-Martínez, C.; Sanchez-Pena, R. Advances in alkaline water electrolyzers: A review. J. Energy Storage 2019, 23, 392–403. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Oshchepkov, A.G.; Bonnefont, A.; Savinova, E.R. Metal–metal (hydr)oxide heterostructures for electrocatalysis of hydrogen electrode reactions. Curr. Opin. Electrochem. 2021, 26, 100667. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, Q.; Zhong, Y.; Tahini, H.A.; Shao, Z.; Wang, H. Metal oxide-based materials as an emerging family of hydrogen evolution electrocatalysts. Energy Environ. Sci. 2020, 13, 3361–3392. [Google Scholar] [CrossRef]
- Subbaraman, R.; Tripkovic, D.; Chang, K.-C.; Strmcnik, D.; Paulikas, A.P.; Hirunsit, P.; Chan, M.; Greeley, J.; Stamenkovic, V.; Markovic, N.M. Trends in activity for the water electrolyser reactions on 3d M(Ni,Co,Fe,Mn) hydr(oxy)oxide catalysts. Nat. Mater. 2012, 11, 550–557. [Google Scholar] [CrossRef]
- Xu, H.; Li, F.; Li, Z.; Liu, Q.; Li, B.; Xu, L.; He, G. Research Progress of Metal Oxide as Cathode Materials for Hydrogen Evolution. Int. J. Electrochem. Sci. 2022, 17, 221249. [Google Scholar] [CrossRef]
- Gong, M.; Wang, D.-Y.; Chen, C.-C.; Hwang, B.-J.; Dai, H. A mini review on nickel-based electrocatalysts for alkaline hydrogen evolution reaction. Nano Res. 2016, 9, 28–46. [Google Scholar] [CrossRef]
- Gong, M.; Zhou, W.; Tsai, M.-C.; Zhou, J.; Guan, M.; Lin, M.-C.; Zhang, B.; Hu, Y.; Wang, D.-Y.; Yang, J.; et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nat. Commun. 2014, 5, 4695. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Guo, P.; Xiong, Z.; Sun, S.; Wang, H.; Gao, Y. Ni/NiO hybrid nanostructure supported on biomass carbon for visible-light photocatalytic hydrogen evolution. J. Mater. Sci. 2021, 56, 12775–12788. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking Hydrogen Evolving Reaction and Oxygen Evolving Reaction Electrocatalysts for Solar Water Splitting Devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Leetmaa, M.; Skorodumova, N.V. KMCLib 1.1: Extended random number support and technical updates to the KMCLib general framework for kinetic Monte-Carlo simulations. Comput. Phys. Commun. 2015, 196, 611–613. [Google Scholar] [CrossRef]
- Pašti, I.A.; Leetmaa, M.; Skorodumova, N.V. General principles for designing supported catalysts for hydrogen evolution reaction based on conceptual Kinetic Monte Carlo modeling. Int. J. Hydrogen Energy 2016, 41, 2526–2538. [Google Scholar] [CrossRef]
- Landau, M.V.; Vidruk, R.; Vingurt, D.; Fuks, D.; Herskowitz, M. Grain boundaries in nanocrystalline catalytic materials as a source of surface chemical functionality. Rev. Chem. Eng. 2014, 30, 379–401. [Google Scholar] [CrossRef]
- Jiang, R.; Fu, J.; Wang, Z.; Dong, C. Grain Boundary—A Route to Enhance Electrocatalytic Activity for Hydrogen Evolution Reaction. Appl. Sci. 2022, 12, 4290. [Google Scholar] [CrossRef]
- Gammer, C.; Mangler, C.; Rentenberger, C.; Karnthaler, H. Quantitative local profile analysis of nanomaterials by electron diffraction. Scr. Mater. 2010, 63, 312–315. [Google Scholar] [CrossRef]
- Guo, W.; Kim, J.; Kim, H.; Hong, S.; Kim, S.; Ahn, S. Electrochemically activated Ni@Ni(OH)2 heterostructure as efficient hydrogen evolution reaction electrocatalyst for anion exchange membrane water electrolysis. Mater. Today Chem. 2022, 24, 100994. [Google Scholar] [CrossRef]
- Danilovic, N.; Subbaraman, R.; Strmcnik, D.; Chang, K.-C.; Paulikas, A.P.; Stamenkovic, V.R.; Markovic, N.M. Enhancing the Alkaline Hydrogen Evolution Reaction Activity through the Bifunctionality of Ni(OH)2/Metal Catalysts. Angew. Chem. Int. Ed. 2012, 51, 12495–12498. [Google Scholar] [CrossRef]
- Lim, D.; Kim, S.; Kim, N.; Oh, E.; Shim, S.E.; Baeck, S.-H. Strongly Coupled Ni/Ni(OH)2 Hybrid Nanocomposites as Highly Active Bifunctional Electrocatalysts for Overall Water Splitting. ACS Sustain. Chem. Eng. 2020, 8, 4431–4439. [Google Scholar] [CrossRef]
- Faid, A.Y.; Barnett, A.O.; Seland, F.; Sunde, S. Ni/NiO nanosheets for alkaline hydrogen evolution reaction: In situ electrochemical-Raman study. Electrochim. Acta 2020, 361, 137040. [Google Scholar] [CrossRef]
- Alsabet, M.; Grden, M.; Jerkiewicz, G. Electrochemical Growth of Surface Oxides on Nickel. Part 1: Formation of α-Ni(OH)2 in Relation to the Polarization Potential, Polarization Time, and Temperature. Electrocatalysis 2011, 2, 317–330. [Google Scholar] [CrossRef]
- Alsabet, M.; Grden, M.; Jerkiewicz, G. Electrochemical Growth of Surface Oxides on Nickel. Part 2: Formation of β-Ni(OH)2 and NiO in Relation to the Polarization Potential, Polarization Time, and Temperature. Electrocatalysis 2014, 5, 136–147. [Google Scholar] [CrossRef]
- Hall, D.S.; Bock, C.; MacDougall, B.R. The Electrochemistry of Metallic Nickel: Oxides, Hydroxides, Hydrides and Alkaline Hydrogen Evolution. J. Electrochem. Soc. 2013, 160, F235–F243. [Google Scholar] [CrossRef]
- Grden, M.; Klimek, K.; Czerwinski, A. A quartz crystal microbalance study on a metallic nickel electrode. J. Solid State Electrochem. 2004, 8, 390–397. [Google Scholar] [CrossRef]
- Alsabet, M.; Grdeń, M.; Jerkiewicz, G. Electrochemical Growth of Surface Oxides on Nickel. Part 3: Formation of β-NiOOH in Relation to the Polarization Potential, Polarization Time, and Temperature. Electrocatalysis 2015, 6, 60–71. [Google Scholar] [CrossRef]
- Grdeń, M.; Jerkiewicz, G. Influence of Surface Treatment on the Kinetics of the Hydrogen Evolution Reaction on Bulk and Porous Nickel Materials. Electrocatalysis 2019, 10, 173–183. [Google Scholar] [CrossRef]
- Krstajić, N.; Popović, M.; Grgur, B.; Vojnović, M.; Šepa, D. On the kinetics of the hydrogen evolution reaction on nickel in alkaline solution: Part I. The mechanism. J. Electroanal. Chem. 2001, 512, 16–26. [Google Scholar] [CrossRef]
- Liang, Z.; Ahn, H.S.; Bard, A.J. A Study of the Mechanism of the Hydrogen Evolution Reaction on Nickel by Surface Interrogation Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2017, 139, 4854–4858. [Google Scholar] [CrossRef]
- Jovanović, A.Z.; Bijelić, L.; Dobrota, A.S.; Skorodumova, N.V.; Mentus, S.V.; Pašti, I.A. Enhancement of hydrogen evolution reaction kinetics in alkaline media by fast galvanic displacement of nickel with rhodium—From smooth surfaces to electrodeposited nickel foams. Electrochim. Acta 2022, 414, 140214. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; McIntyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Legrand, D.; Bancroft, G.M. Interpretation of Ni2p XPS spectra of Ni conductors and Ni insulators. Phys. Chem. Miner. 2000, 27, 357–366. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Yuan, X.; Dong, W.; Huang, F. Rational composition and structural design of in situ grown nickel-based electrocatalysts for efficient water electrolysis. J. Mater. Chem. A 2016, 4, 167–172. [Google Scholar] [CrossRef]
- Subbaraman, R.; Tripkovic, D.; Strmcnik, D.; Chang, K.-C.; Uchimura, M.; Paulikas, A.P.; Stamenkovic, V.; Markovic, N.M. Enhancing Hydrogen Evolution Activity in Water Splitting by Tailoring Li+-Ni(OH)2-Pt Interfaces. Science 2011, 334, 1256–1260. [Google Scholar] [CrossRef]
- Michaelides, A.; Liu, Z.-P.; Zhang, C.J.; Alavi, A.; King, D.A.; Hu, P. Identification of General Linear Relationships between Activation Energies and Enthalpy Changes for Dissociation Reactions at Surfaces. J. Am. Chem. Soc. 2003, 125, 3704–3705. [Google Scholar] [CrossRef] [PubMed]
- Abouatallah, R.; Kirk, D.; Graydon, J. Long-term electrolytic hydrogen permeation in nickel and the effect of vanadium species addition. Electrochim. Acta 2002, 47, 2483–2494. [Google Scholar] [CrossRef]
- Rommal, H.E.G.; Morgan, P.J. The Role of Absorbed Hydrogen on the Voltage-Time Behavior of Nickel Cathodes in Hydrogen Evolution. J. Electrochem. Soc. 1988, 135, 343–346. [Google Scholar] [CrossRef]
- Soares, D.M.; Teschke, O.; Torriani, I. Hydride Effect on the Kinetics of the Hydrogen Evolution Reaction on Nickel Cathodes in Alkaline Media. J. Electrochem. Soc. 1992, 139, 98–105. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
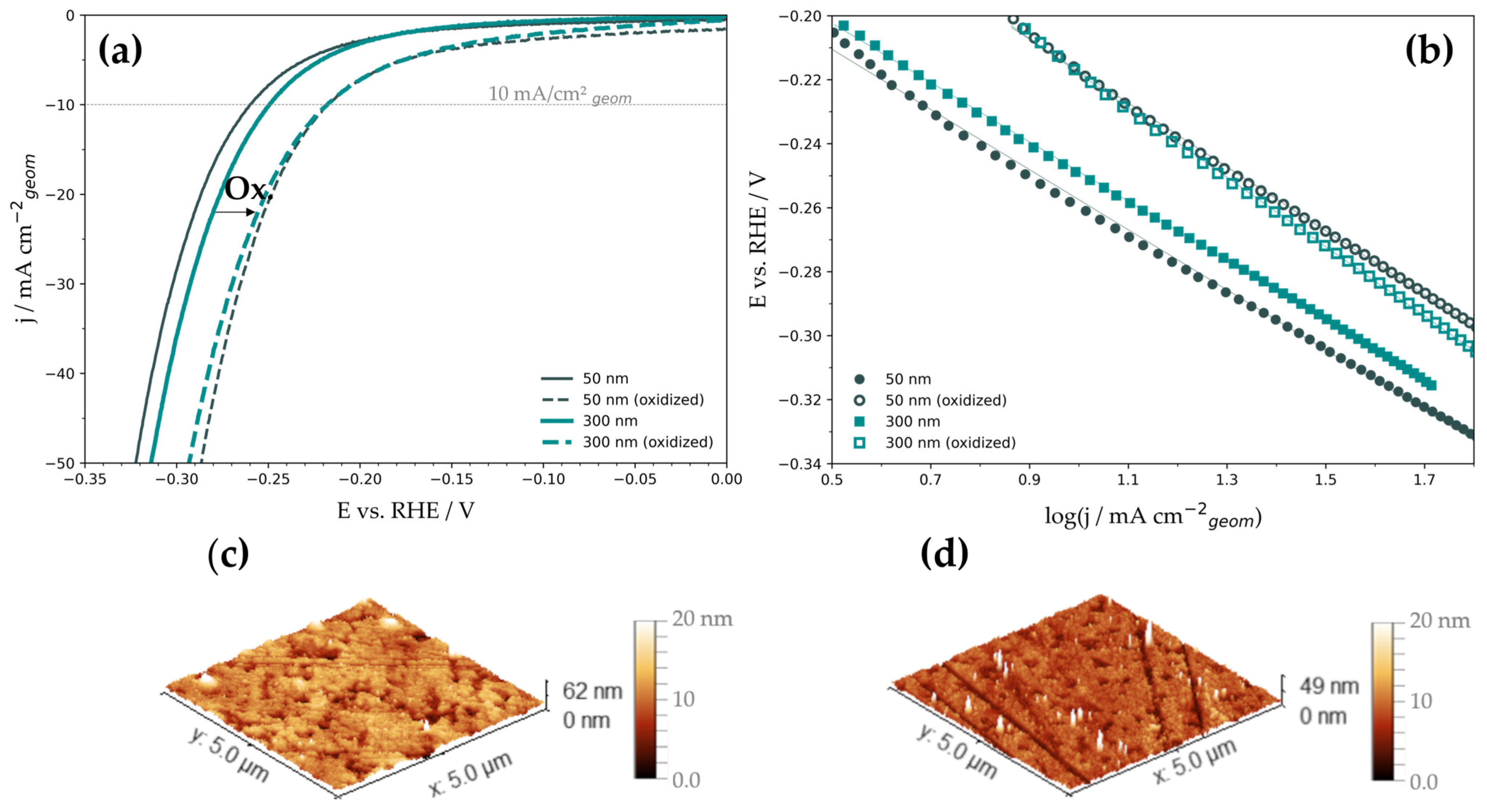
| Film Thickness | Film State | η10 (V) | b (mV dec−1) | log(j0/A cm−2geom) | RF |
|---|---|---|---|---|---|
| 50 nm | as-deposited | −(0.263 ± 0.003) | −(89 ± 4) | −(4.92 ± 0.15) | 1.004 ± 0.071 |
| oxidized | −(0.220 ± 0.004) | −(92 ± 2) | −(4.42 ± 0.13) | 1.006 ± 0.070 | |
| 300 nm | as-deposited | −(0.256 ± 0.005) | −(92 ± 3) | −(4.71 ± 0.14) | 1.004 ± 0.070 |
| oxidized | −(0.215 ± 0.006) | −(108 ± 5) | −(4.03 ± 0.12) | 1.011 ± 0.071 | |
| Benchmarks [27] | |||||
| Ni (electrodeposited) | −(0.26 ± 0.04) | / | / | 16 ± 2 | |
| Pt (electrodeposited) | −(0.10 ± 0.02) | / | / | 10 ± 2 | |
| Film Thickness | Film State | Ni0 | Ni2+ [NiO] | Ni2+ [Ni(OH)2] |
|---|---|---|---|---|
| 50 nm | as-deposited | 65.6 | 18.2 | 16.2 |
| oxidized | 15.4 | 34.5 | 50.1 | |
| 300 nm | as-deposited | 39.2 | 47.9 | 12.9 |
| oxidized | 16.4 | 35.3 | 48.3 |
| Film Thickness | Film State | Ni0 [at.%] | Ni2+ (NiO) [at.%] | Ni2+ (Ni(OH)2) [at.%] |
|---|---|---|---|---|
| 50 nm | oxidized + HER | 6.6 | 48.9 | 44.5 |
| 300 nm | oxidized + HER | 4.9 | 33.4 | 61.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neumüller, D.; Rafailović, L.D.; Jovanović, A.Z.; Skorodumova, N.V.; Pašti, I.A.; Lassnig, A.; Griesser, T.; Gammer, C.; Eckert, J. Hydrogen Evolution Reaction on Ultra-Smooth Sputtered Nanocrystalline Ni Thin Films in Alkaline Media—From Intrinsic Activity to the Effects of Surface Oxidation. Nanomaterials 2023, 13, 2085. https://doi.org/10.3390/nano13142085
Neumüller D, Rafailović LD, Jovanović AZ, Skorodumova NV, Pašti IA, Lassnig A, Griesser T, Gammer C, Eckert J. Hydrogen Evolution Reaction on Ultra-Smooth Sputtered Nanocrystalline Ni Thin Films in Alkaline Media—From Intrinsic Activity to the Effects of Surface Oxidation. Nanomaterials. 2023; 13(14):2085. https://doi.org/10.3390/nano13142085
Chicago/Turabian StyleNeumüller, Daniela, Lidija D. Rafailović, Aleksandar Z. Jovanović, Natalia V. Skorodumova, Igor A. Pašti, Alice Lassnig, Thomas Griesser, Christoph Gammer, and Jürgen Eckert. 2023. "Hydrogen Evolution Reaction on Ultra-Smooth Sputtered Nanocrystalline Ni Thin Films in Alkaline Media—From Intrinsic Activity to the Effects of Surface Oxidation" Nanomaterials 13, no. 14: 2085. https://doi.org/10.3390/nano13142085
APA StyleNeumüller, D., Rafailović, L. D., Jovanović, A. Z., Skorodumova, N. V., Pašti, I. A., Lassnig, A., Griesser, T., Gammer, C., & Eckert, J. (2023). Hydrogen Evolution Reaction on Ultra-Smooth Sputtered Nanocrystalline Ni Thin Films in Alkaline Media—From Intrinsic Activity to the Effects of Surface Oxidation. Nanomaterials, 13(14), 2085. https://doi.org/10.3390/nano13142085








