Electronic Characteristics, Stability and Water Oxidation Selectivity of High-Index BiVO4 Facets for Photocatalytic Application: A First Principle Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Geometric Optimization
3.2. Electronic Structures
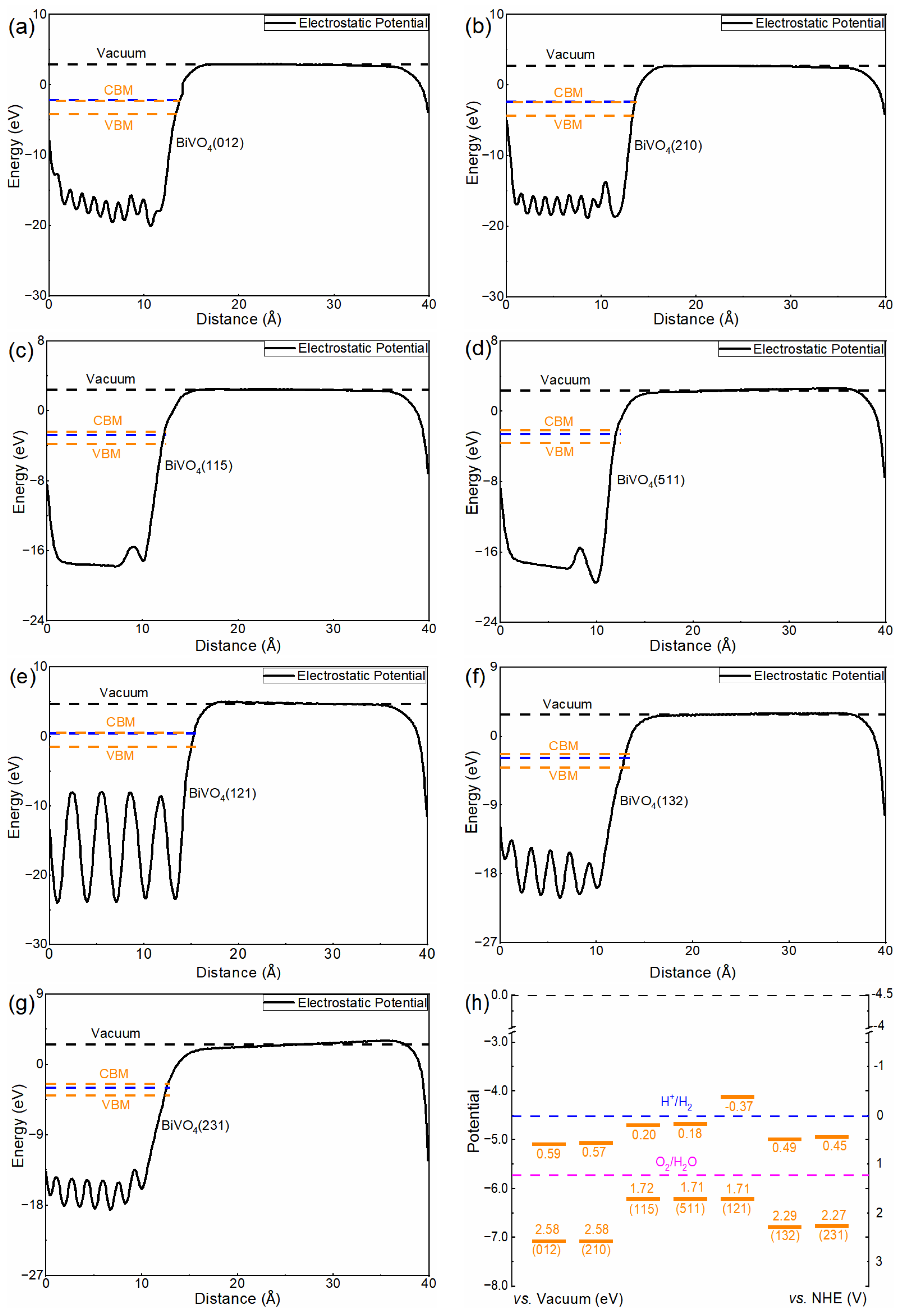
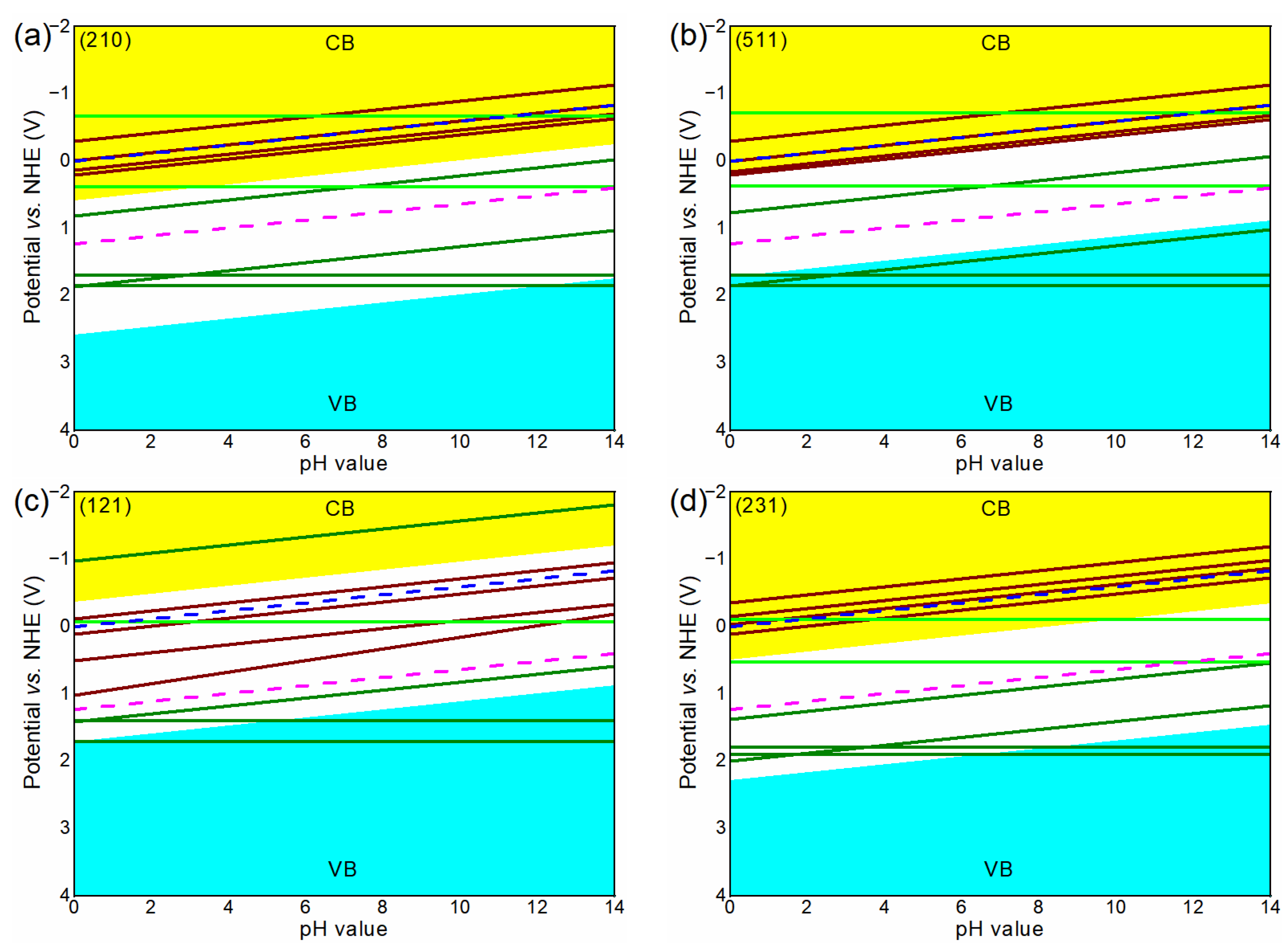
3.3. Band Alignments
3.4. Stability
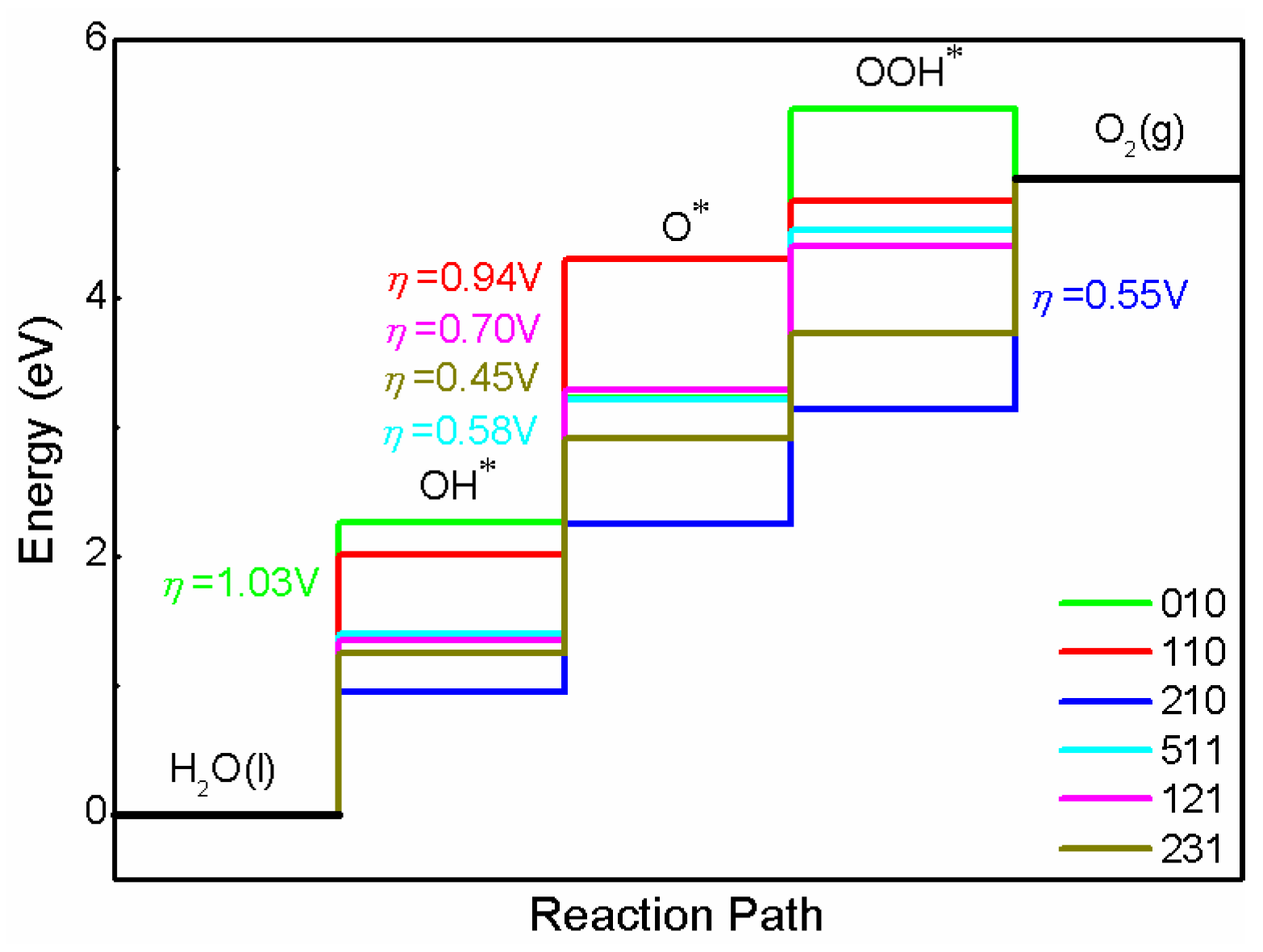
3.5. Overpotential
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Anantharaj, S.; Ede, S.R.; Sakthikumar, K.; Karthick, K.; Mishra, S.; Kundu, S. Recent Trends and Perspectives in Electrochemical Water Splitting with an Emphasis on Sulfide, Selenide, and Phosphide Catalysts of Fe, Co, and Ni: A Review. ACS Catal. 2016, 6, 8069–8097. [Google Scholar] [CrossRef]
- Kment, S.; Riboni, F.; Pausova, S.; Wang, L.; Wang, L.Y.; Han, H.; Hubicka, Z.; Krysa, J.; Schmuki, P.; Zboril, R. Photoanodes based on TiO2 and α-Fe2O3 for solar water splitting-superior role of 1D nanoarchitectures and of combined heterostructures. Chem. Soc. Rev. 2017, 46, 3716–3769. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Use of Highly-Ordered TiO2 Nanotube Arrays in Dye-Sensitized Solar Cells. Nano Lett. 2006, 6, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, L.; Li, W.; Gou, X. α-Fe2O3 Nanotubes in Gas Sensor and Lithium-Ion Battery Applications. Adv. Mater. 2005, 17, 582–586. [Google Scholar] [CrossRef]
- Mondschein, J.S.; Callejas, J.F.; Read, C.G.; Chen, J.Y.C.; Holder, C.F.; Badding, C.K.; Schaak, R.E. Crystalline Cobalt Oxide Films for Sustained Electrocatalytic OxygenEvolution under Strongly Acidic Conditions. Chem. Mater. 2017, 29, 950–957. [Google Scholar] [CrossRef]
- Eichhorn, J.; Kastl, C.; Cooper, J.K.; Ziegler, D.; Schwartzberg, A.M.; Sharp, I.D.; Toma, F.M. Nanoscale imaging of charge carrier transport in water splitting photoanodes. Nat. Commun. 2018, 9, 2597. [Google Scholar] [CrossRef] [Green Version]
- Takata, T.; Jiang, J.; Sakata, Y. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 2020, 581, 411–414. [Google Scholar] [CrossRef]
- Irani, R.; Ahmet, I.Y.; Jang, J.W.; Berglund, P.; Plate, P.; Hohn, C.; Bottger, R.; Schmitt, S.W.; Dubourdieu, C.; Lardhi, S. Nature of Nitrogen Incorporation in BiVO4 Photoanodes through Chemical and Physical Methods. Sol. RRL 2020, 4, 1900290. [Google Scholar] [CrossRef] [Green Version]
- Malathi, A.; Madhavan, J.; Ashokkumar, M.; Arunachalam, P. A Review on BiVO4 Photocatalyst: Activity Enhancement Methods for Solar Photocatalytic Applications. Appl. Catal. A 2018, 555, 47–74. [Google Scholar]
- Sharp, I.D.; Cooper, J.K.; Toma, F.M.; Buonsanti, R. Bismuth Vanadate as a Platform for Accelerating Discovery and Development of Complex Transition-Metal Oxide Photoanodes. ACS Energy Lett. 2017, 2, 139–150. [Google Scholar] [CrossRef]
- Rossell, M.D.; Agrawal, P.; Borgschulte, A.; Hebert, C.; Passerone, D.; Erni, R. Direct Evidence of Surface Reduction in Monoclinic BiVO4. Chem. Mater. 2015, 27, 3593–3600. [Google Scholar] [CrossRef]
- Hong, S.J.; Lee, S.; Jang, J.S.; Lee, J.S. Heterojunction BiVO4/WO3 Electrodes for Enhanced Photoactivity of Water Oxidation. Energy Environ. Sci. 2011, 4, 1781–1787. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Guo, L.; Bao, N.; Grimes, C.A. Nanostructured WO3/BiVO4 Heterojunction Films for Efficient Photoelectrochemical Water Splitting. Nano Lett. 2011, 11, 1928–1933. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, Y.; Fan, X.; Li, Y.; Zhou, D.; Cai, B.; Wang, L.; Fan, K.; Zhang, K. Boosting Charge Transport in BiVO4 Photoanode for Solar Water Oxidation. Adv. Mater. 2022, 34, 2108178. [Google Scholar] [CrossRef]
- Wang, S.C.; Chen, P.; Bai, Y.; Yun, J.H.; Liu, G.; Wang, L.Z. New BiVO4 Dual Photoanodes with Enriched Oxygen Vacancies for Efficient Solar-Driven Water Splitting. Adv. Mater. 2018, 30, 1800486. [Google Scholar] [CrossRef]
- Guo, R.; Yan, A.; Xu, J.; Xu, B.; Li, T.; Liu, X.; Yi, T.; Luo, S. Effects of morphology on the visible-light-driven photocatalytic and bactericidal properties of BiVO4/CdS heterojunctions: A discussion on photocatalysis mechanism. J. Alloys Compd. 2020, 817, 153246–153257. [Google Scholar] [CrossRef]
- Wang, S.; He, T.; Chen, P.; Du, A.; Ostrikov, K.; Huang, W.; Wang, L. In Situ Formation of Oxygen Vacancies Achieving Near-Complete Charge Separation in Planar BiVO4 Photoanodes. Adv. Mater. 2020, 32, 2001385. [Google Scholar] [CrossRef]
- Gaikwad, M.A.; Suryawanshi, U.P.; Ghorpade, U.V.; Jang, J.S.; Suryawanshi, M.P.; Kim, J.H. Emerging Surface, Bulk, and Interface Engineering Strategies on BiVO4 for Photoelectrochemical Water Splitting. Small 2022, 18, 2105084. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, D.; Jiao, X. Monoclinic Structured BiVO4 Nanosheets: Hydrothermal Preparation, Formation Mechanism, and Coloristic and Photocatalytic Properties. J. Phys. Chem. B 2006, 110, 2668–2673. [Google Scholar] [CrossRef]
- Xi, G.; Ye, J. Synthesis of Bismuth Vanadate Nanoplates with Exposed {001} Facets and Enhanced Visible-light Photocatalytic Properties. Chem. Commun. 2010, 46, 1893–1895. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jiang, H.; Zong, X.; Xu, Q.; Ma, Y.; Li, G.; Li, C. Crystal Facet Dependence of Water Oxidation on BiVO4 sheets under Visible Light Irradiation. Chem. Eur. J. 2011, 17, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, F.; Wang, D.; Yang, J.; Li, M.; Zhu, J.; Zhou, X.; Han, H.; Li, C. Spatial Separation of Photogenerated Electrons and Holes Among {010} and {110} Crystal Facets of BiVO4. Nat. Commun. 2013, 4, 1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.; Feng, J.; Pan, W. Enhanced Photocatalytic Activity in Electrospun Bismuth Vanadate Nanofibers with Phase Junction. ACS Appl. Mater. Interfaces 2015, 7, 9638–9644. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Z.; Zou, Z. Structure and Energetics of Low-Index Stoichiometric Monoclinic Clinobisvanite BiVO4 Surfaces. RSC Adv. 2011, 1, 874–883. [Google Scholar] [CrossRef]
- Sleight, A.; Chen, H.; Ferretti, A.; Cox, D. Crystal growth and structure of BiVO4. Mater. Res. Bull. 1979, 14, 1571–1581. [Google Scholar] [CrossRef]
- Hu, J.; He, H.; Zhou, X.; Li, Z.; Shen, Q.; Luo, W.; Alsaedi, A.; Hayat, T.; Zhou, Y.; Zou, Z. BiVO4 Tubular Structures: Oxygen Defect-Rich and Largely Exposed Reactive {010} Facets Synergistically Boost Photocatalytic Water Oxidation and Selective N-N Coupling Reaction of 5-Amino-1H-Tetrazole. Chem. Commun. 2019, 55, 5635–5638. [Google Scholar] [CrossRef]
- Lardhi, S.; Cavallo, L.; Harb, M. Significant Impact of Exposed Facets on the BiVO4 Material Performance for Photocatalytic Water Splitting Reactions. J. Phys. Chem. Lett. 2020, 11, 5497–5503. [Google Scholar] [CrossRef]
- Li, P.; Chen, X.; He, H.; Zhou, X.; Zhou, Y.; Zou, Z. Polyhedral 30-Faceted BiVO4 Microcrystals Predominantly Enclosed by High-Index Planes Promoting Photocatalytic Water-Splitting Activity. Adv. Mater. 2018, 30, 1703119. [Google Scholar] [CrossRef]
- Hu, J.; He, H.; Li, L.; Zhou, X.; Li, Z.; Shen, Q.; Wu, C.; Asiri, A.M.; Zhou, Y.; Zou, Z. Highly Symmetrical, 24-faceted, Concave BiVO4 Polyhedron Bounded by Multiple High-Index Facets for Prominent Photocatalytic O2 Evolution under Visible Light. Chem. Commun. 2019, 55, 4777–4780. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Geerlings, P.; De Proft, F.; Langenaeker, W. Conceptual density functional theory. Chem Rev. 2003, 103, 1793–1873. [Google Scholar] [CrossRef]
- Perdew, P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.W.; Ping, Y.; Galli, G.A.; Choi, K.S. Simultaneous enhancements in photon absorption and charge transport of bismuth vanadate photoanodes for solar water splitting. Nat. Commun. 2015, 6, 8769. [Google Scholar] [CrossRef] [Green Version]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jonsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Mathew, K.; Sundararaman, R.; Letchworth-Weaver, K.; Arias, T.A.; Hennig, R.G. Implicit Solvation Model for Density-Functional Study of Nanocrystal Surfaces and Reaction Pathways. J. Chem. Phys. 2014, 140, 084106. [Google Scholar] [CrossRef] [Green Version]
- Laraib, I.; Carneiro, M.A.; Janotti, A. Effects of Doping on the Crystal Structure of BiVO4. J. Phys. Chem. C 2019, 123, 26752–26757. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, W.; Gu, Q. Ab Initio Calculation of Surface-Controlled Photocatalysis in Multiple-Phase BiVO4. J. Phys. Chem. C 2022, 126, 9541–9550. [Google Scholar] [CrossRef]
- Hu, J.; Chen, W.; Zhao, X.; Su, H.; Chen, Z. Anisotropic Electronic Characteristics, Adsorption, and Stability of Low-Index BiVO4 Surfaces for Photoelectrochemical Applications. ACS Appl. Mater. Interfaces 2018, 10, 5475–5484. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, Y.; Xiang, Y.; Zhu, Z. Vacancy defect engineered BiVO4 with low-index surfaces for photocatalytic application: A first principles study. RSC Adv. 2022, 12, 31317. [Google Scholar] [CrossRef]
- Van de Walle, C.G.; Martin, R.M. Theoretical calculations of heterojunction discontinuities in the Si/Ge system. Phys. Rev. B 1986, 34, 5621. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, L.W. Thermodynamic Oxidation and Reduction Potentials of Photocatalytic Semiconductors in Aqueous Solution. Chem. Mater. 2012, 24, 3659–3666. [Google Scholar] [CrossRef] [Green Version]
- Ding, C.; Shi, J.; Wang, D.; Wang, Z.; Wang, N.; Liu, G.; Xiong, F.; Li, C. Visible Light Driven overall Water Splitting Using Cocatalyst/BiVO4 Photoanode with Minimized Bias. Phys. Chem. Chem. Phys. 2013, 15, 4589–4595. [Google Scholar] [CrossRef] [PubMed]
- McDowell, M.T.; Lichterman, M.F.; Spurgeon, J.M.; Hu, S.; Sharp, I.D.; Brunschwig, B.S.; Lewis, N.S. Improved Stability of Polycrystalline Bismuth Vanadate Photoanodes by use of Dual-layer Thin TiO2/Ni Coatings. J. Phys. Chem. C 2014, 118, 19618–19624. [Google Scholar] [CrossRef] [Green Version]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Stevanovic, V.; Lany, S.; Zhang, X.; Zunger, A. Correcting density functional theory for accurate predictions of compound enthalpies of formation: Fitted elemental-phase reference energies. Phys. Rev. B 2012, 85, 115104. [Google Scholar] [CrossRef] [Green Version]
- Genscher, H. Electrochemical Behavior of Semiconductors under Illumination. J. Electrochem. Soc. 1966, 113, 1174–1182. [Google Scholar] [CrossRef]
- Park, H.S.; Leonard, K.C.; Bard, A.J. Surface Interrogation Scanning Electrochemical Microscopy (SI-SECM) of Photoelectrochemistry at a W/Mo-BiVO4 Semiconductor Electrode: Quantification of Hydroxyl Radicals during Water Oxidation. J. Phys. Chem. C 2013, 117, 12093–12102. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Xiang, Y.; Zhu, Z. Electronic Characteristics, Stability and Water Oxidation Selectivity of High-Index BiVO4 Facets for Photocatalytic Application: A First Principle Study. Nanomaterials 2023, 13, 2023. https://doi.org/10.3390/nano13132023
Zhang Z, Xiang Y, Zhu Z. Electronic Characteristics, Stability and Water Oxidation Selectivity of High-Index BiVO4 Facets for Photocatalytic Application: A First Principle Study. Nanomaterials. 2023; 13(13):2023. https://doi.org/10.3390/nano13132023
Chicago/Turabian StyleZhang, Zhiyuan, Yuqi Xiang, and Zhihong Zhu. 2023. "Electronic Characteristics, Stability and Water Oxidation Selectivity of High-Index BiVO4 Facets for Photocatalytic Application: A First Principle Study" Nanomaterials 13, no. 13: 2023. https://doi.org/10.3390/nano13132023
APA StyleZhang, Z., Xiang, Y., & Zhu, Z. (2023). Electronic Characteristics, Stability and Water Oxidation Selectivity of High-Index BiVO4 Facets for Photocatalytic Application: A First Principle Study. Nanomaterials, 13(13), 2023. https://doi.org/10.3390/nano13132023






