Direct Optical Patterning of Quantum Dots: One Strategy, Different Chemical Processes
Abstract
:1. Introduction
1.1. The Semiconductor Quantum Dots
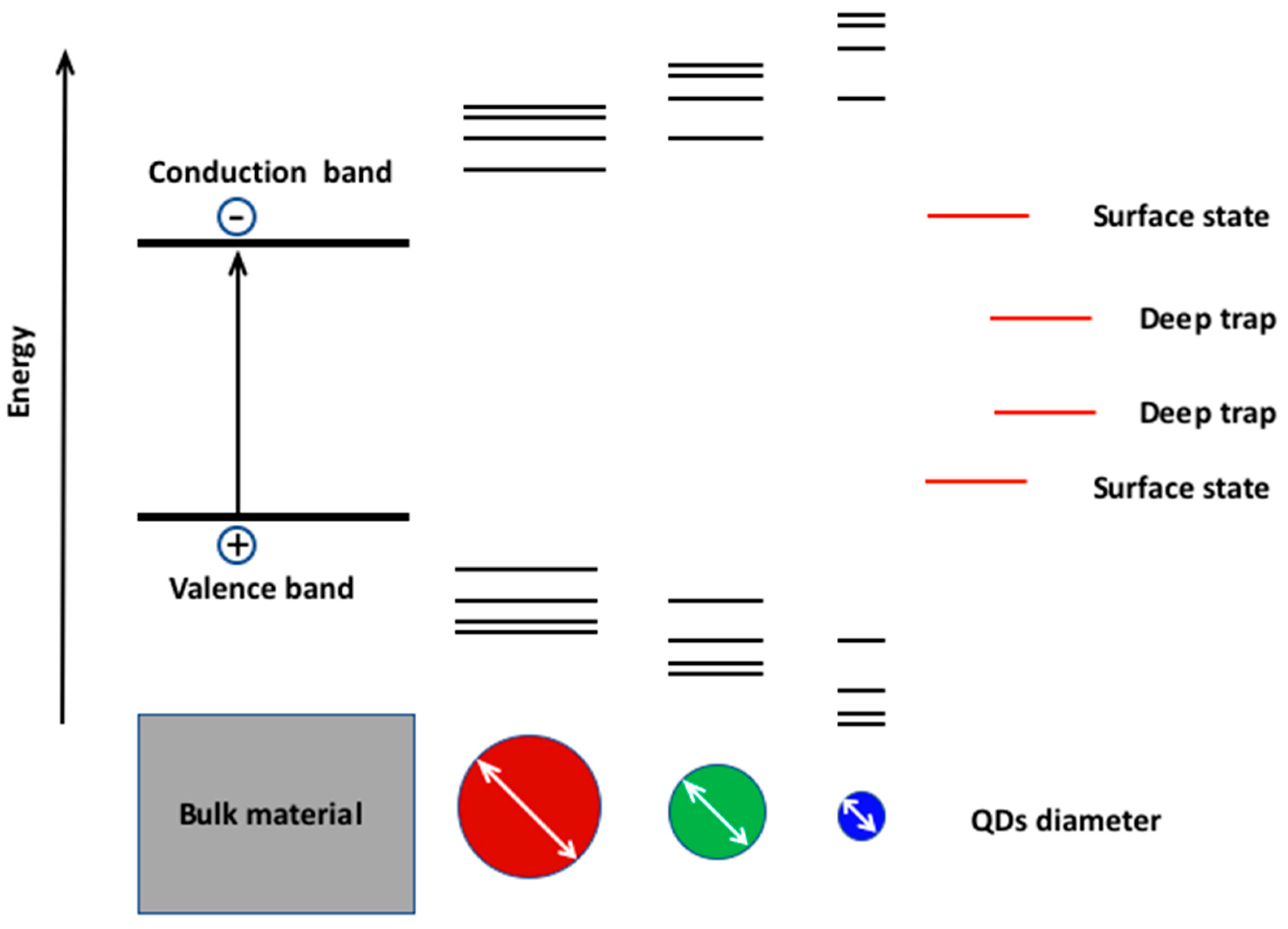
1.2. The Quantum Size Effect and Its Role in the Modulation of the Electro-Optical Properties of the scQDs
1.3. The Core@shell Systems
2. scQD Dispersion
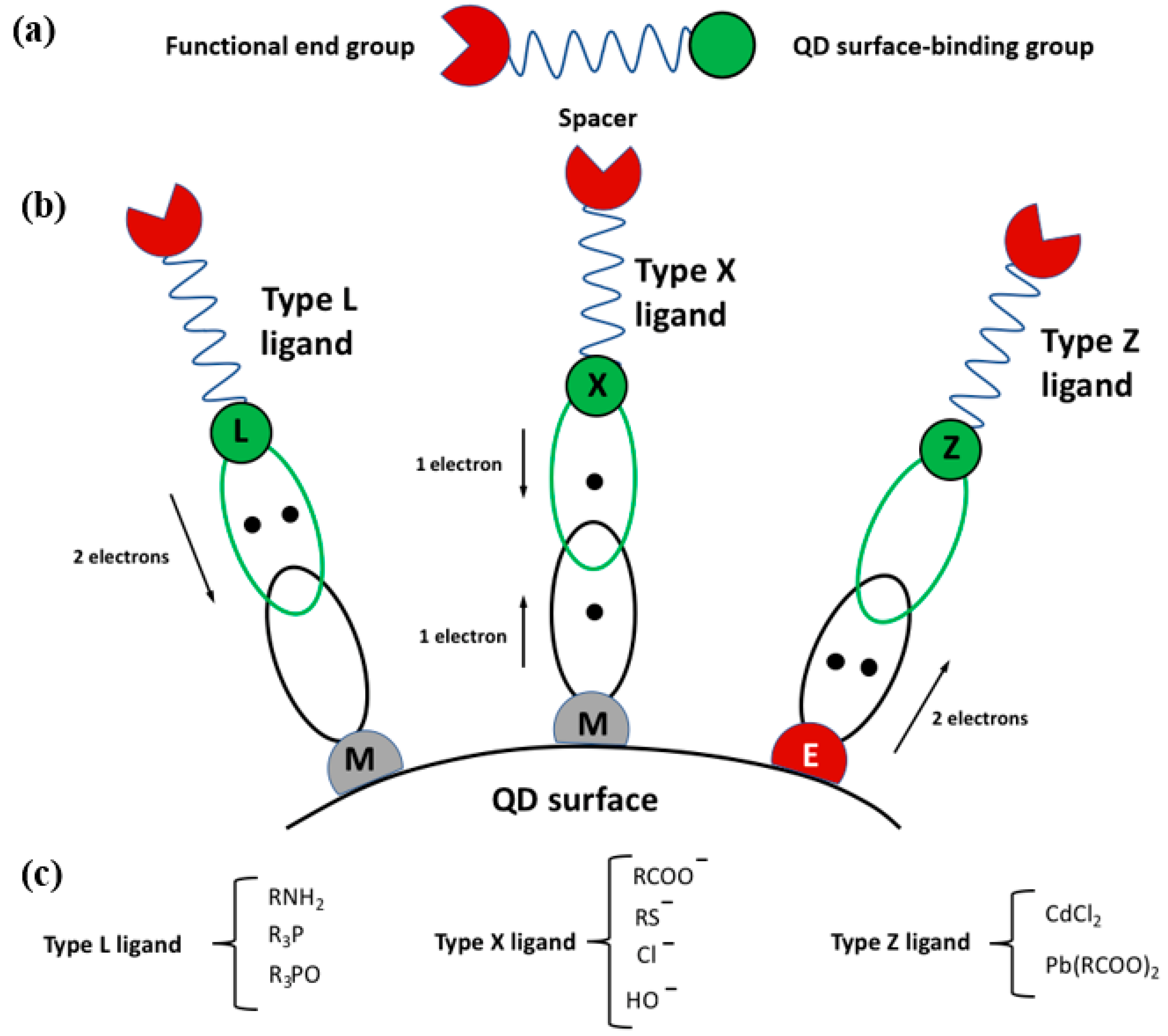
2.1. The Surface Ligands
2.2. The Ligands and the Surrounding Environment of the scQDs
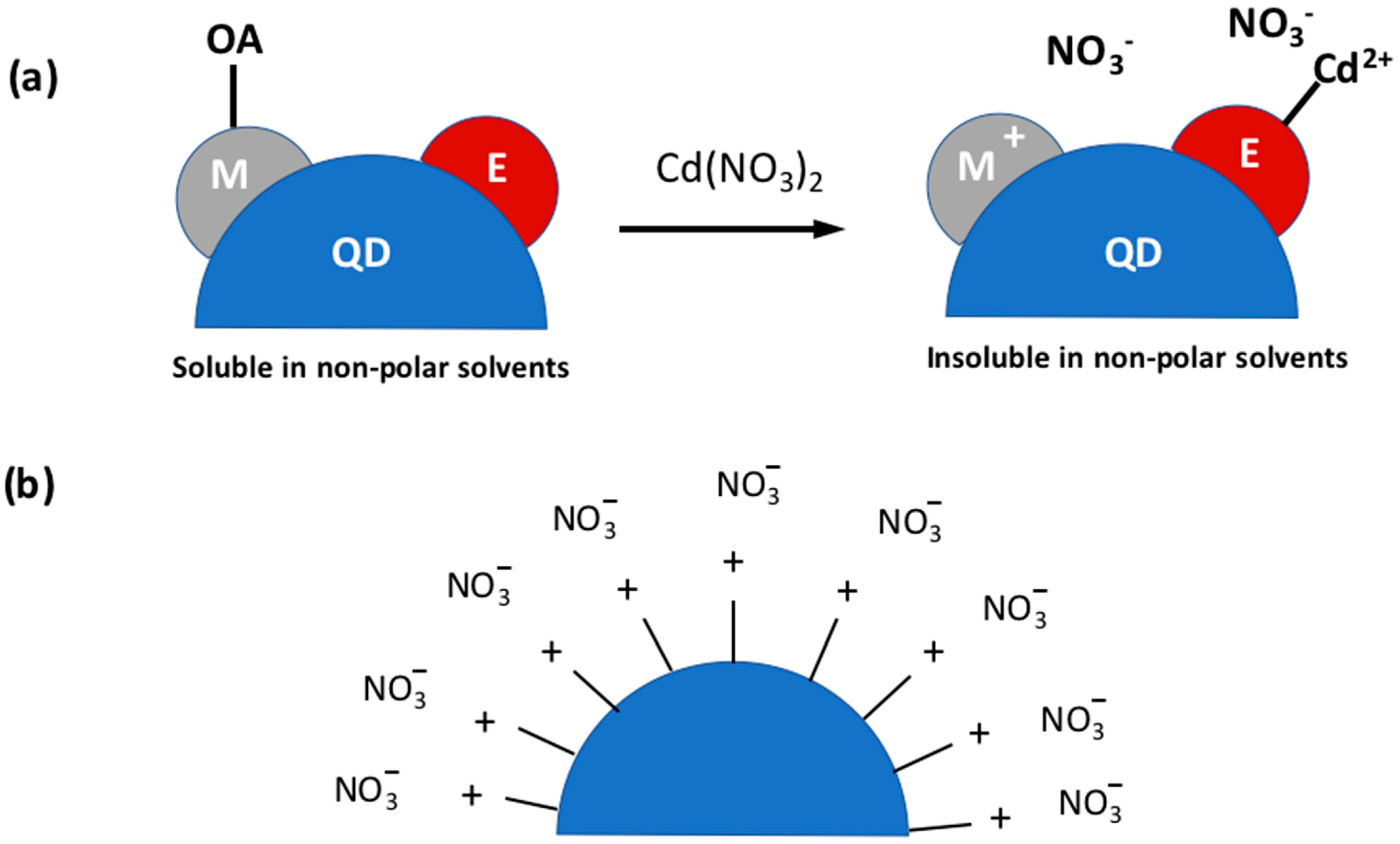
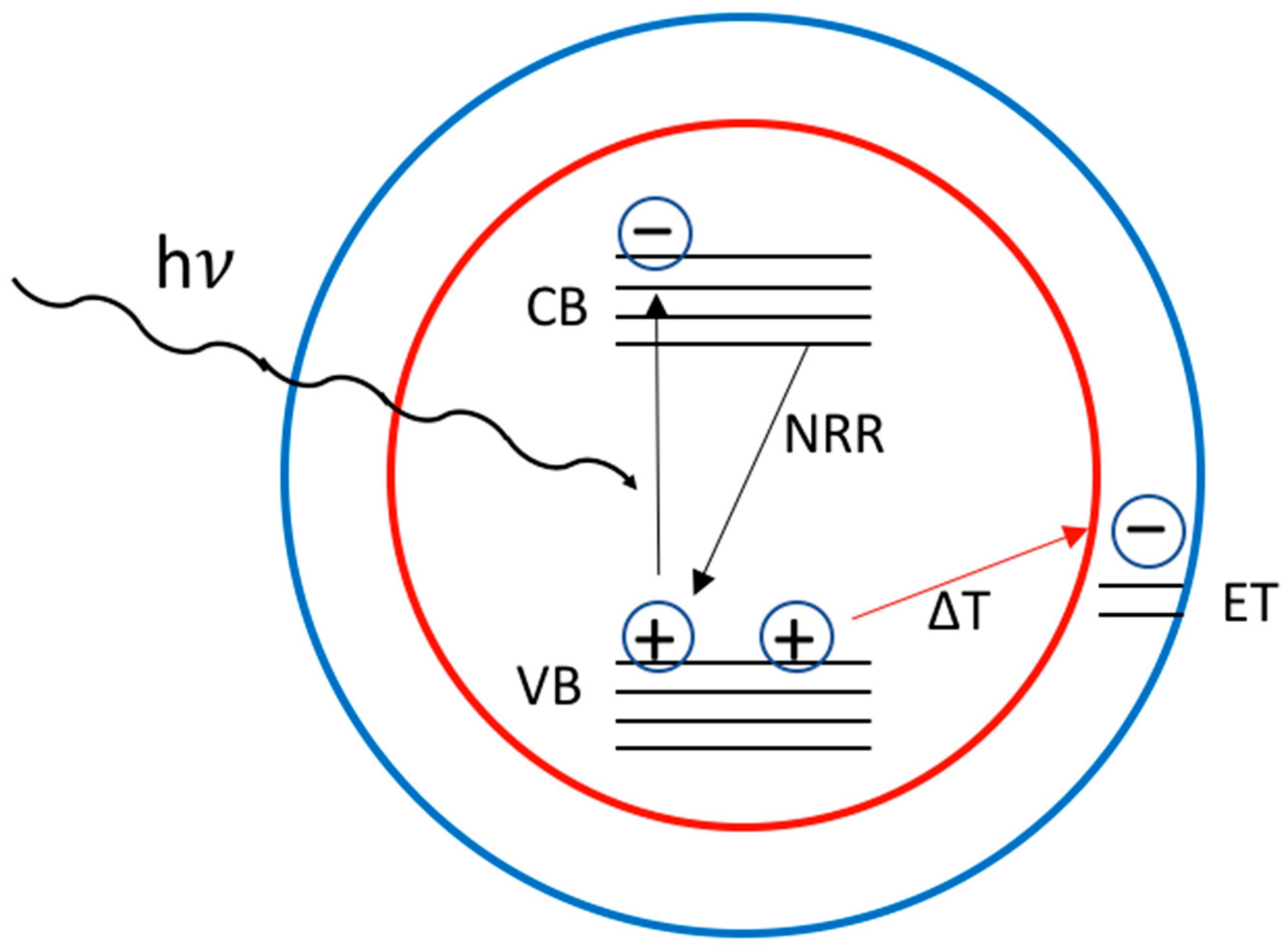
3. QD Stability
3.1. The Effect of Oxygen and Moisture
3.2. The Effect of the Temperature
4. Stabilization of the QDs at a Single and Collective Level
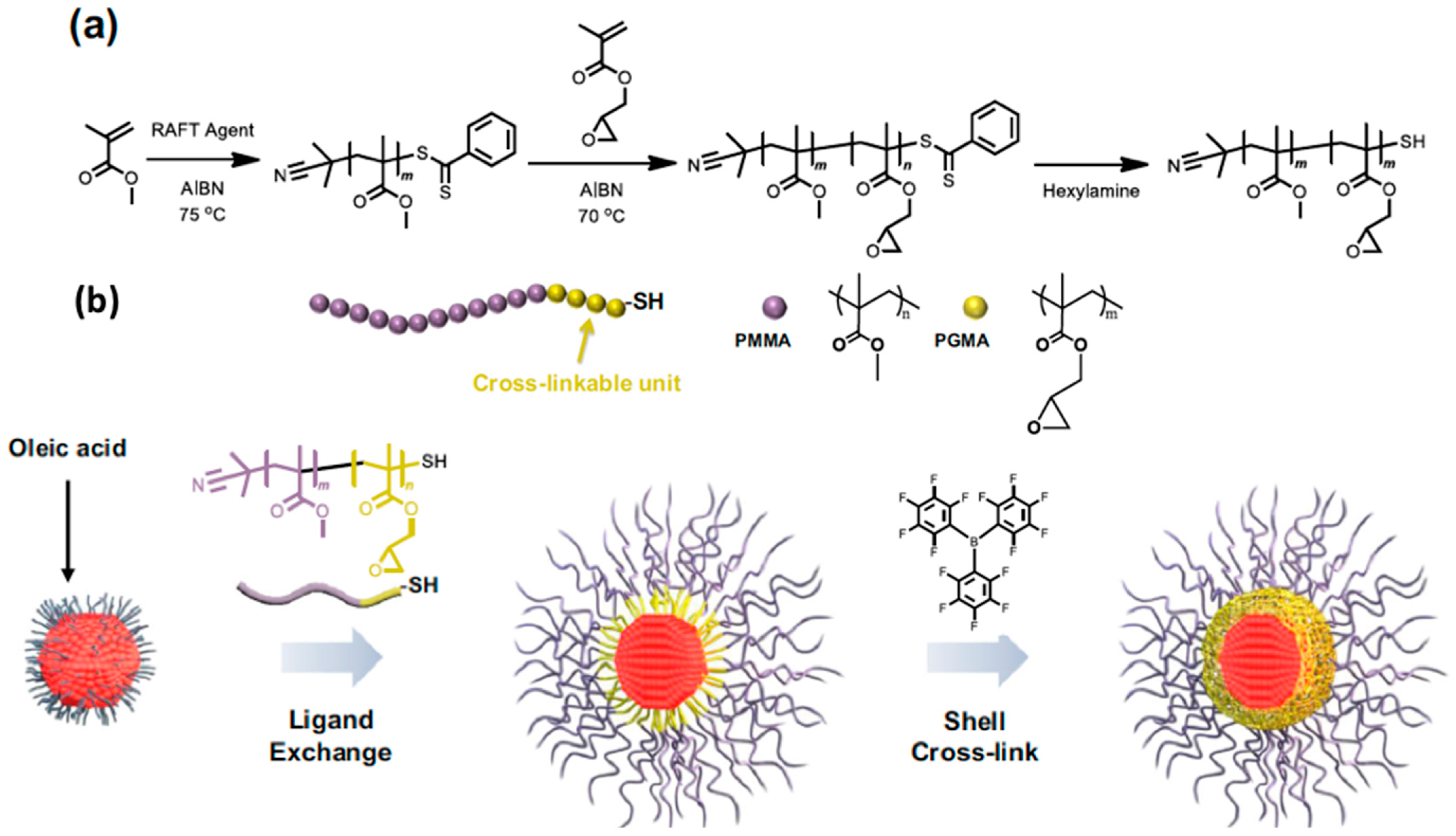
4.1. Single QD Encapsulation
4.2. Collective QD Encapsulation
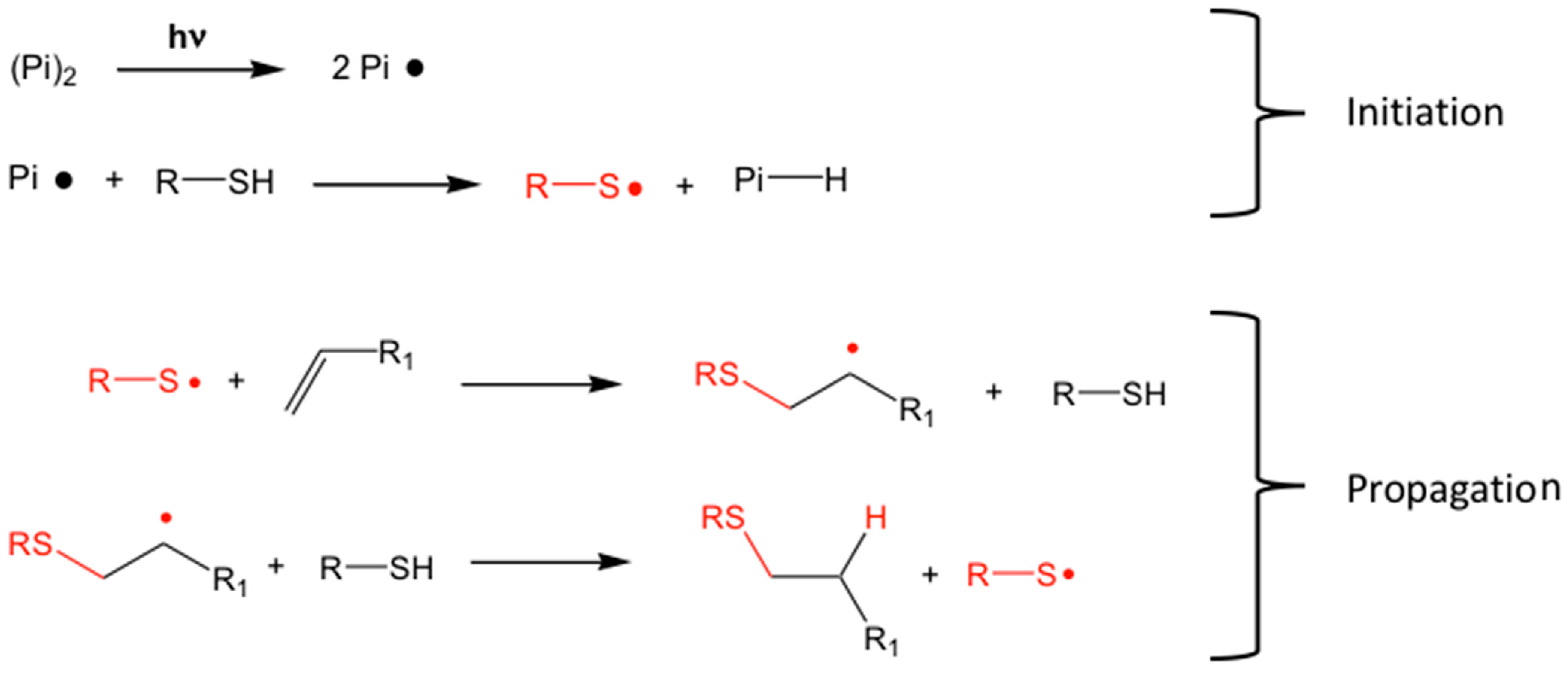
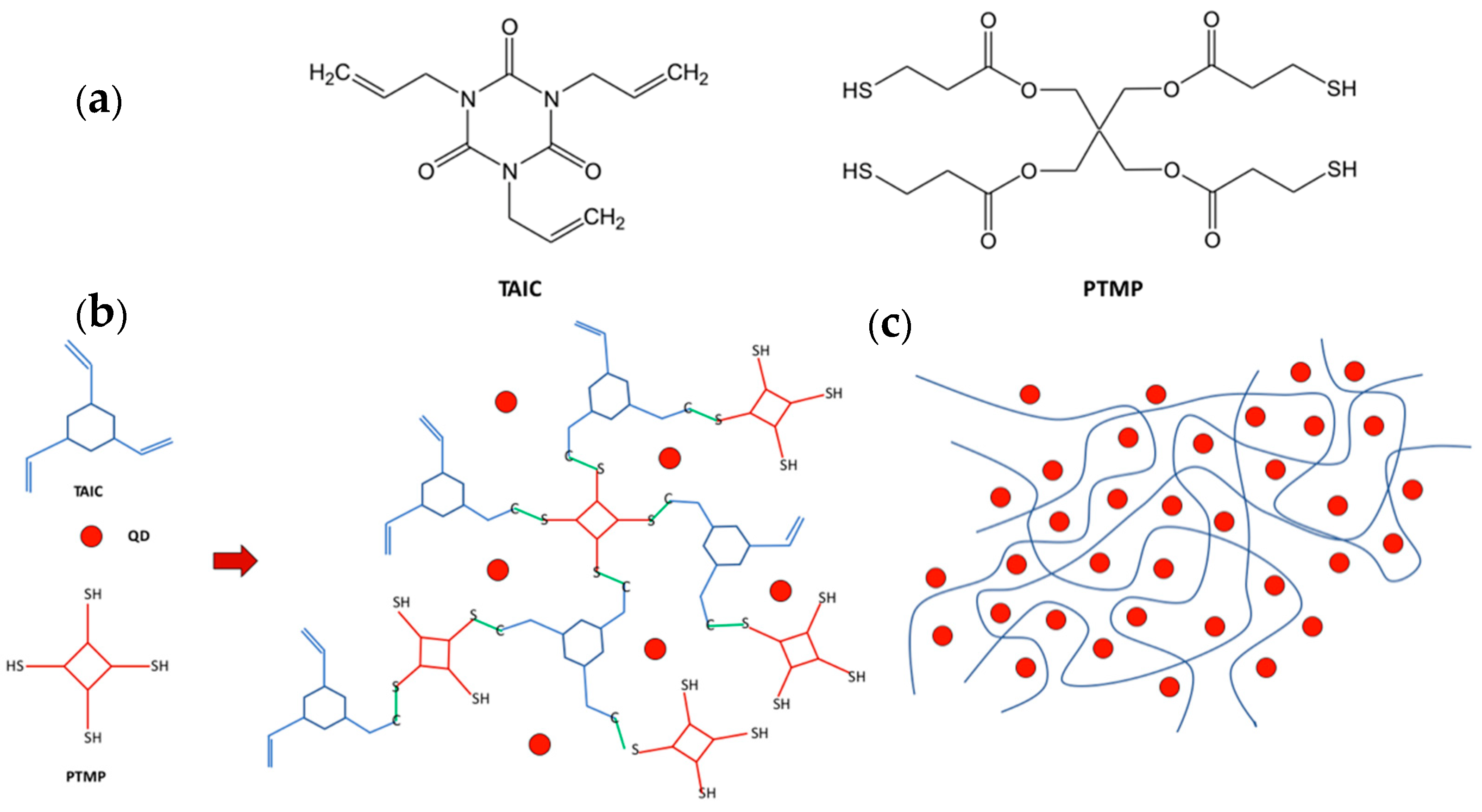
4.2.1. The Thiol-Ene Network
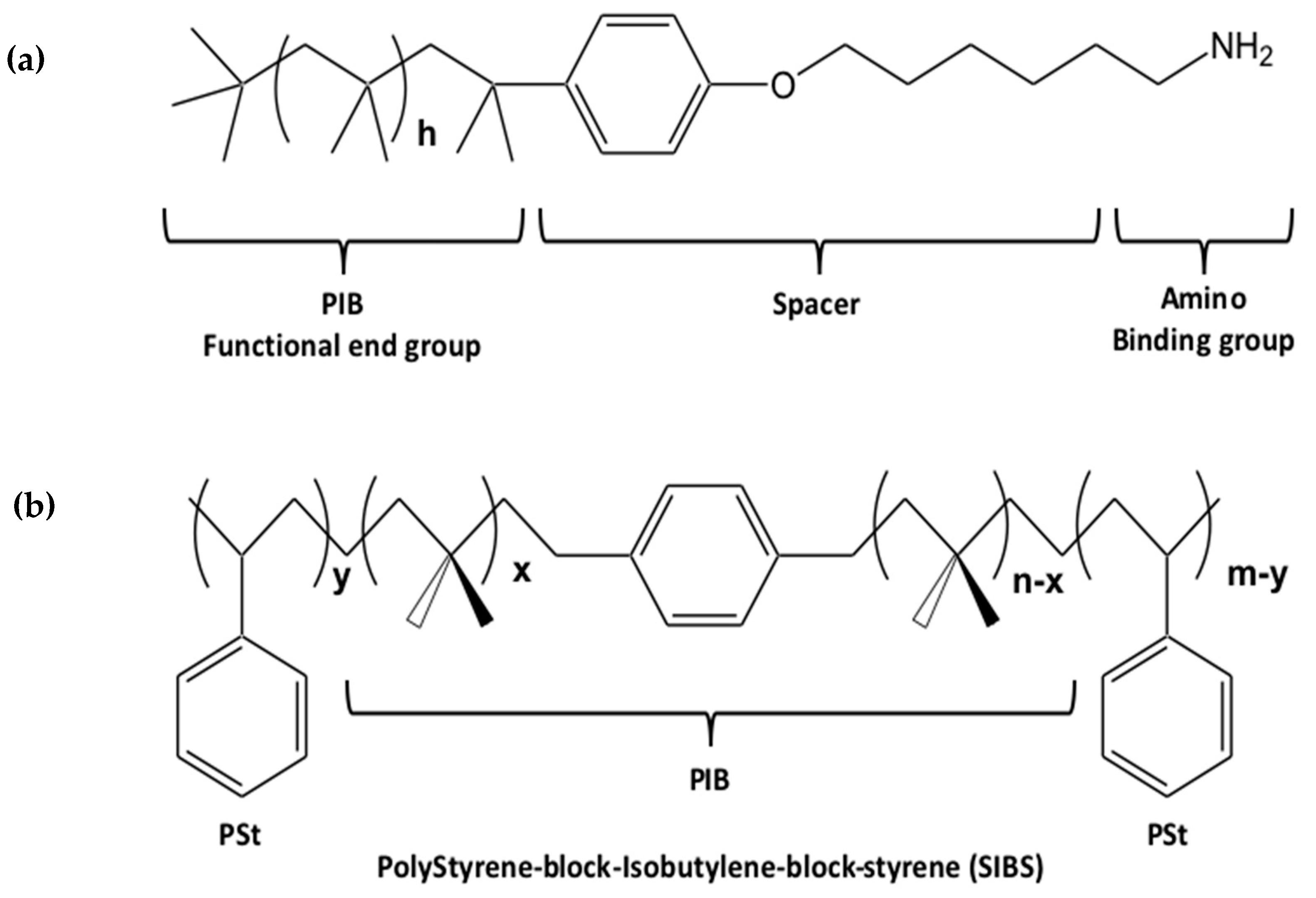
4.2.2. The Polymers
4.2.3. The Siloxanes
5. Quantum Dots Direct Optical Patterning (DOP)
5.1. Direct Optical Patterning of scQDs with Thiol-Ene Cross-Linkers
5.2. Direct Optical Patterning of scQDs with Siloxanes
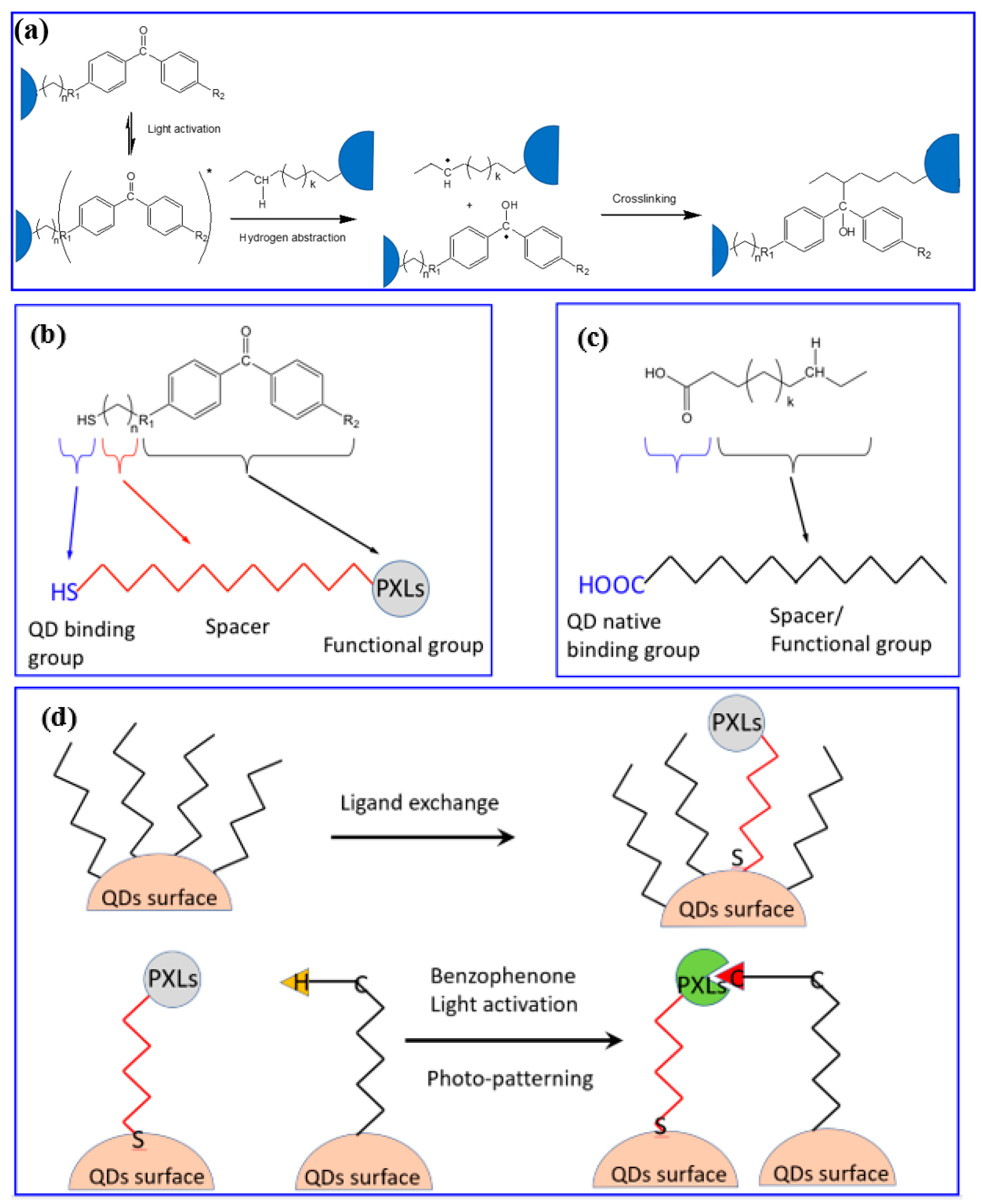
5.3. Direct Optical Patterning via In Situ Ligand Exchange (DOLFIN)
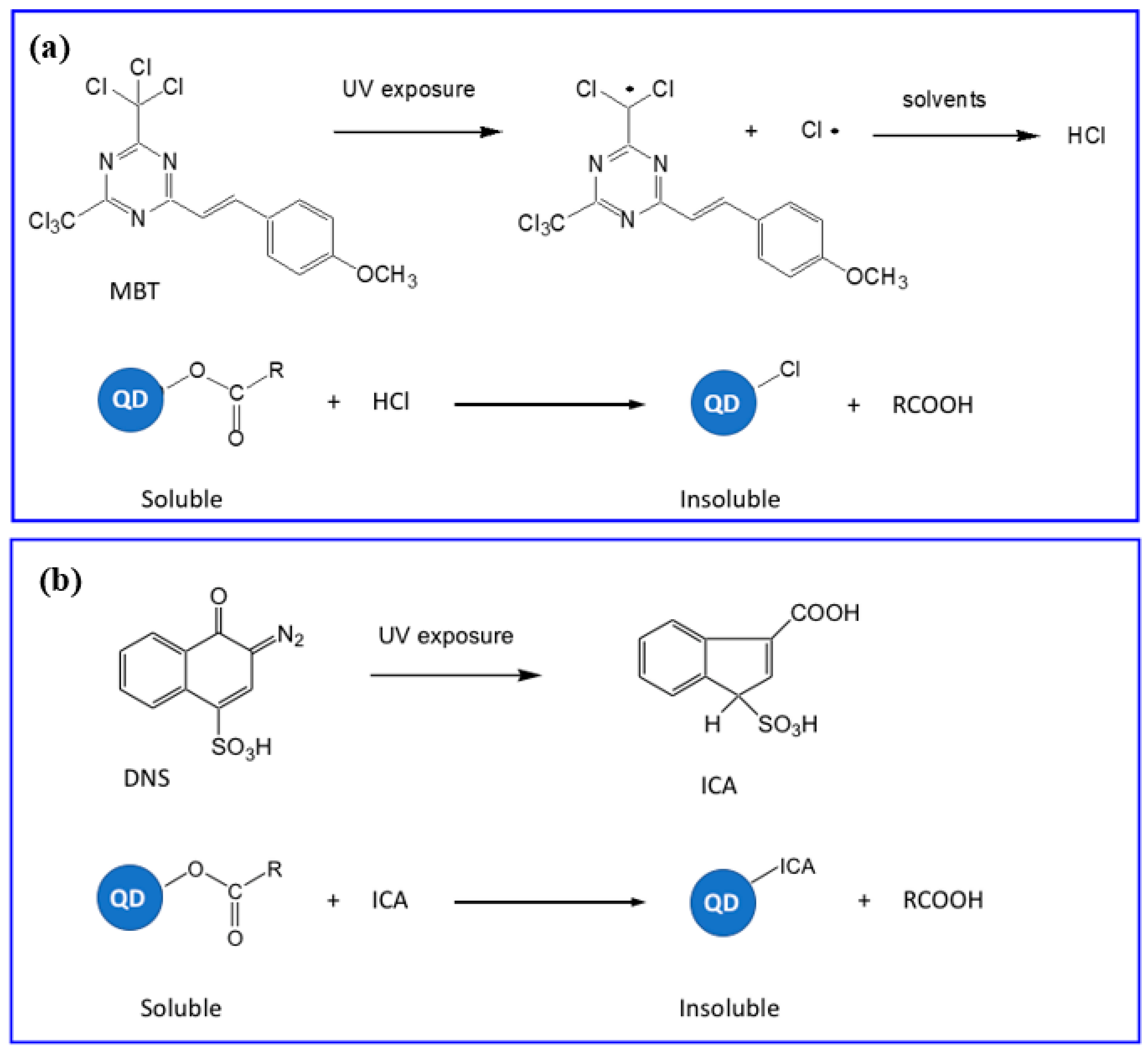
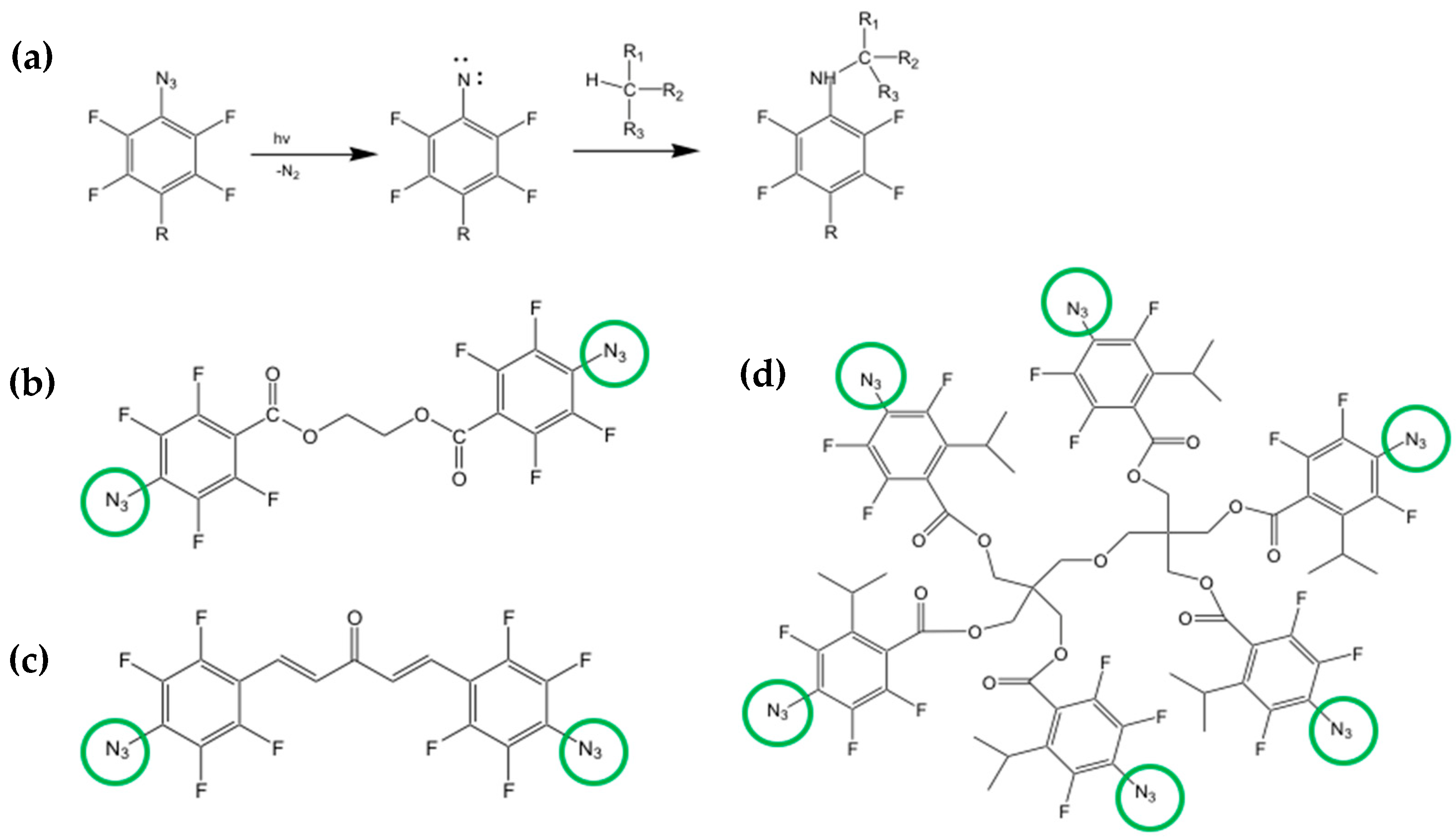
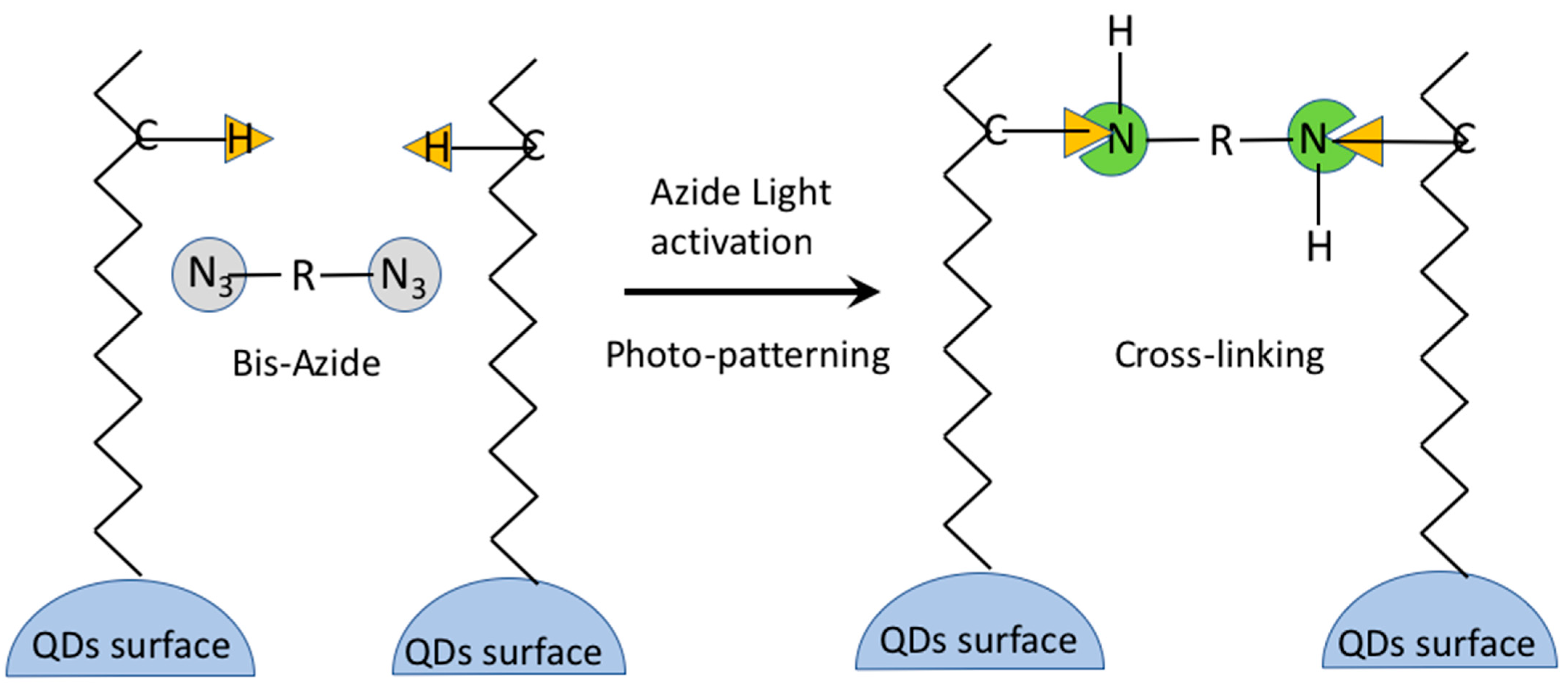
5.4. Direct Photolithography of scQDs via Photo-Active Cross-Linkers
5.5. Direct Optical Patterning of scQDs via Their Direct Synthesis
6. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Introduction to Semiconductor Lithography. In Fundamental Principles of Optical Lithography; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 1–28. ISBN 978-0-470-72387-6.
- Sung, S.H.; Yoon, H.; Lim, J.; Char, K. Reusable Stamps for Printing Sub-100 nm Patterns of Functional Nanoparticles. Small 2012, 8, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Onses, M.S.; Lim, J.B.; Nam, S.; Oh, N.; Kim, H.; Yu, K.J.; Lee, J.W.; Kim, J.-H.; Kang, S.-K.; et al. High-Resolution Patterns of Quantum Dots Formed by Electrohydrodynamic Jet Printing for Light-Emitting Diodes. Nano Lett. 2015, 15, 969–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García de Arquer, F.P.; Talapin, D.V.; Klimov, V.I.; Arakawa, Y.; Bayer, M.; Sargent, E.H. Semiconductor quantum dots: Technological progress and future challenges. Science 2021, 373, eaaz8541. [Google Scholar] [CrossRef]
- Bayer, M. Bridging Two Worlds: Colloidal versus Epitaxial Quantum Dots. Ann. Phys. 2019, 531, 1900039. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Jia, R.; Xu, J.; Song, L.; Liu, Y.; Zhang, Y.; Ullah, S.; Dai, J. Strategies of Improving CsPbX3 Perovskite Quantum Dots Optical Performance. Front. Mater. 2022, 9, 845977. [Google Scholar] [CrossRef]
- Song, Z.; Zhao, J.; Liu, Q. Luminescent perovskites: Recent advances in theory and experiments. Inorg. Chem. Front. 2019, 6, 2969–3011. [Google Scholar] [CrossRef]
- Haydous, F.; Gardner, J.M.; Cappel, U.B. The impact of ligands on the synthesis and application of metal halide perovskite nanocrystals. J. Mater. Chem. A 2021, 9, 23419–23443. [Google Scholar] [CrossRef]
- Efros, A.L.; Brus, L.E. Nanocrystal Quantum Dots: From Discovery to Modern Development. ACS Nano 2021, 15, 6192–6210. [Google Scholar] [CrossRef]
- Weidman, M.C.; Beck, M.E.; Hoffman, R.S.; Prins, F.; Tisdale, W.A. Monodisperse, Air-Stable PbS Nanocrystals via Precursor Stoichiometry Control. ACS Nano 2014, 8, 6363–6371. [Google Scholar] [CrossRef] [Green Version]
- Mocatta, D.; Cohen, G.; Schattner, J.; Millo, O.; Rabani, E.; Banin, U. Heavily Doped Semiconductor Nanocrystal Quantum Dots. Science 2011, 332, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Xiao, P.; Zhang, Z.; Ge, J.; Deng, Y.; Chen, X.; Zhang, J.-R.; Deng, Z.; Kambe, Y.; Talapin, D.V.; Wang, Y. Surface passivation of intensely luminescent all-inorganic nanocrystals and their direct optical patterning. Nat. Commun. 2023, 14, 49. [Google Scholar] [CrossRef]
- Todescato, F.; Fortunati, I.; Minotto, A.; Signorini, R.; Jasieniak, J.J.; Bozio, R. Engineering of Semiconductor Nanocrystals for Light Emitting Applications. Materials 2016, 9, 672. [Google Scholar] [CrossRef] [Green Version]
- Reiss, P.; Protière, M.; Li, L. Core/Shell Semiconductor Nanocrystals. Small 2009, 5, 154–168. [Google Scholar] [CrossRef]
- Eagle, F.W.; Park, N.; Cash, M.; Cossairt, B.M. Surface Chemistry and Quantum Dot Luminescence: Shell Growth, Atomistic Modification, and Beyond. ACS Energy Lett. 2021, 6, 977–984. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Cao, Z.; Shu, Y.; Qin, H.; Su, B.; Peng, X. Quantum Dots with Highly Efficient, Stable, and Multicolor Electrochemiluminescence. ACS Cent. Sci. 2020, 6, 1129–1137. [Google Scholar] [CrossRef]
- Li, J.J.; Wang, Y.A.; Guo, W.; Keay, J.C.; Mishima, T.D.; Johnson, M.B.; Peng, X. Large-Scale Synthesis of Nearly Monodisperse CdSe/CdS Core/Shell Nanocrystals Using Air-Stable Reagents via Successive Ion Layer Adsorption and Reaction. J. Am. Chem. Soc. 2003, 125, 12567–12575. [Google Scholar] [CrossRef]
- Bae, W.K.; Char, K.; Hur, H.; Lee, S. Single-Step Synthesis of Quantum Dots with Chemical Composition Gradients. Chem. Mater. 2008, 20, 531–539. [Google Scholar] [CrossRef]
- Hanifi, D.A.; Bronstein, N.D.; Koscher, B.A.; Nett, Z.; Swabeck, J.K.; Takano, K.; Schwartzberg, A.M.; Maserati, L.; Vandewal, K.; van de Burgt, Y.; et al. Redefining near-unity luminescence in quantum dots with photothermal threshold quantum yield. Science 2019, 363, 1199–1202. [Google Scholar] [CrossRef] [Green Version]
- Meinardi, F.; Colombo, A.; Velizhanin, K.A.; Simonutti, R.; Lorenzon, M.; Beverina, L.; Viswanatha, R.; Klimov, V.I.; Brovelli, S. Large-area luminescent solar concentrators based on ‘Stokes-shift-engineered’ nanocrystals in a mass-polymerized PMMA matrix. Nat. Photonics 2014, 8, 392–399. [Google Scholar] [CrossRef]
- Selopal, G.S.; Abdelkarim, O.; Kumar, P.; Jin, L.; Liu, J.; Zhao, H.; Yurtsever, A.; Vidal, F.; Wang, Z.M.; Rosei, F. Role of Interfacial Engineering of “Giant” Core–Shell Quantum Dots. ACS Appl. Energy Mater. 2022, 5, 1447–1459. [Google Scholar] [CrossRef]
- Carbone, L.; Nobile, C.; De Giorgi, M.; Sala, F.D.; Morello, G.; Pompa, P.; Hytch, M.; Snoeck, E.; Fiore, A.; Franchini, I.R.; et al. Synthesis and Micrometer-Scale Assembly of Colloidal CdSe/CdS Nanorods Prepared by a Seeded Growth Approach. Nano Lett. 2007, 7, 2942–2950. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Zhang, W.; Schneider, J.; Halpert, J.E.; Rogach, A.L. Luminescent Down-Conversion Semiconductor Quantum Dots and Aligned Quantum Rods for Liquid Crystal Displays. Adv. Sci. 2019, 6, 1901345. [Google Scholar] [CrossRef]
- Diroll, B.T.; Guzelturk, B.; Po, H.; Dabard, C.; Fu, N.; Makke, L.; Lhuillier, E.; Ithurria, S. 2D II–VI Semiconductor Nanoplatelets: From Material Synthesis to Optoelectronic Integration. Chem. Rev. 2023, 123, 3543–3624. [Google Scholar] [CrossRef]
- Onal, A.; Sadeghi, S.; Melikov, R.; Karatum, O.; Eren, G.O.; Nizamoglu, S. Quantum Dot to Nanorod Transition for Efficient White-Light-Emitting Diodes with Suppressed Absorption Losses. ACS Photonics 2022, 9, 3268–3278. [Google Scholar] [CrossRef]
- Giansante, C. Library Design of Ligands at the Surface of Colloidal Nanocrystals. Acc. Chem. Res. 2020, 53, 1458–1467. [Google Scholar] [CrossRef]
- Boles, M.A.; Ling, D.; Hyeon, T.; Talapin, D.V. The surface science of nanocrystals. Nat. Mater. 2016, 15, 141–153. [Google Scholar] [CrossRef]
- Hines, D.A.; Forrest, R.P.; Corcelli, S.A.; Kamat, P.V. Predicting the Rate Constant of Electron Tunneling Reactions at the CdSe–TiO2 Interface. J. Phys. Chem. B 2015, 119, 7439–7446. [Google Scholar] [CrossRef]
- Grisorio, R.; Quarta, D.; Fiore, A.; Carbone, L.; Paolo Suranna, G.; Giansante, C. The dynamic surface chemistry of colloidal metal chalcogenide quantum dots. Nanoscale Adv. 2019, 1, 3639–3646. [Google Scholar] [CrossRef]
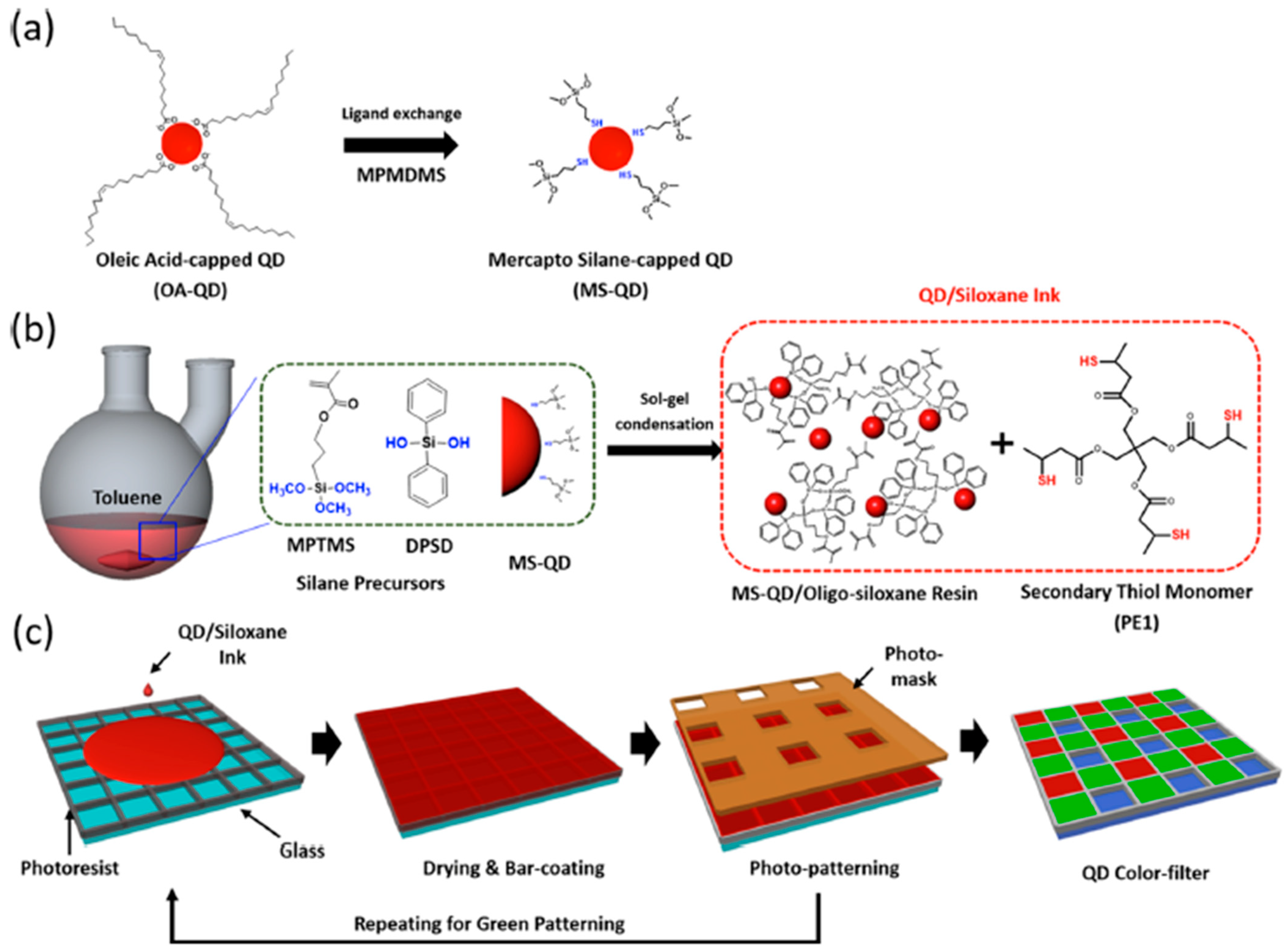
- Kim, Y.H.; Koh, S.; Lee, H.; Kang, S.-M.; Lee, D.C.; Bae, B.-S. Photo-Patternable Quantum Dots/Siloxane Composite with Long-Term Stability for Quantum Dot Color Filters. ACS Appl. Mater. Interfaces 2020, 12, 3961–3968. [Google Scholar] [CrossRef]
- Hahm, D.; Lim, J.; Kim, H.; Shin, J.-W.; Hwang, S.; Rhee, S.; Chang, J.H.; Yang, J.; Lim, C.H.; Jo, H.; et al. Direct patterning of colloidal quantum dots with adaptable dual-ligand surface. Nat. Nanotechnol. 2022, 17, 952–958. [Google Scholar] [CrossRef]
- Yang, Y.; Qin, H.; Jiang, M.; Lin, L.; Fu, T.; Dai, X.; Zhang, Z.; Niu, Y.; Cao, H.; Jin, Y.; et al. Entropic Ligands for Nanocrystals: From Unexpected Solution Properties to Outstanding Processability. Nano Lett. 2016, 16, 2133–2138. [Google Scholar] [CrossRef]
- Anderson, N.C.; Hendricks, M.P.; Choi, J.J.; Owen, J.S. Ligand Exchange and the Stoichiometry of Metal Chalcogenide Nanocrystals: Spectroscopic Observation of Facile Metal-Carboxylate Displacement and Binding. J. Am. Chem. Soc. 2013, 135, 18536–18548. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Alivisatos, A.P. Colloidal nanocrystal synthesis and the organic–inorganic interface. Nature 2005, 437, 664–670. [Google Scholar] [CrossRef] [Green Version]
- Nasilowski, M.; Mahler, B.; Lhuillier, E.; Ithurria, S.; Dubertret, B. Two-Dimensional Colloidal Nanocrystals. Chem. Rev. 2016, 116, 10934–10982. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Chen, D.; Li, Z.; Peng, X. Symmetry-Breaking for Formation of Rectangular CdSe Two-Dimensional Nanocrystals in Zinc-Blende Structure. J. Am. Chem. Soc. 2017, 139, 10009–10019. [Google Scholar] [CrossRef]
- Li, Z.; Peng, X. Size/Shape-Controlled Synthesis of Colloidal CdSe Quantum Disks: Ligand and Temperature Effects. J. Am. Chem. Soc. 2011, 133, 6578–6586. [Google Scholar] [CrossRef]
- Giansante, C. Surface Chemistry Control of Colloidal Quantum Dot Band Gap. J. Phys. Chem. C 2018, 122, 18110–18116. [Google Scholar] [CrossRef]
- Ehlert, S.; Stegelmeier, C.; Pirner, D.; Förster, S. A General Route to Optically Transparent Highly Filled Polymer Nanocomposites. Macromolecules 2015, 48, 5323–5327. [Google Scholar] [CrossRef]
- Noh, M.; Kim, T.; Lee, H.; Kim, C.-K.; Joo, S.-W.; Lee, K. Fluorescence quenching caused by aggregation of water-soluble CdSe quantum dots. Colloids Surf. Physicochem. Eng. Asp. 2010, 359, 39–44. [Google Scholar] [CrossRef]
- Kang, T.; Um, K.; Park, J.; Chang, H.; Lee, D.C.; Kim, C.-K.; Lee, K. Minimizing the fluorescence quenching caused by uncontrolled aggregation of CdSe/CdS core/shell quantum dots for biosensor applications. Sens. Actuators B Chem. 2016, 222, 871–878. [Google Scholar] [CrossRef]
- Reitinger, N.; Hohenau, A.; Köstler, S.; Krenn, J.R.; Leitner, A. Radiationless energy transfer in CdSe/ZnS quantum dot aggregates embedded in PMMA. Phys. Status Solidi A 2011, 208, 710–714. [Google Scholar] [CrossRef]
- Shiman, D.I.; Sayevich, V.; Meerbach, C.; Nikishau, P.A.; Vasilenko, I.V.; Gaponik, N.; Kostjuk, S.V.; Lesnyak, V. Robust Polymer Matrix Based on Isobutylene (Co)polymers for Efficient Encapsulation of Colloidal Semiconductor Nanocrystals. ACS Appl. Nano Mater. 2019, 2, 956–963. [Google Scholar] [CrossRef]
- Kovalenko, M.V.; Scheele, M.; Talapin, D.V. Colloidal Nanocrystals with Molecular Metal Chalcogenide Surface Ligands. Science 2009, 324, 1417–1420. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, S.; Zhang, X.; Xiang, L.; Xie, B.; Luo, X. Enhancing oxygen/moisture resistance of quantum dots by short-chain, densely cross-linked silica glass network. Nanotechnology 2022, 33, 465202. [Google Scholar] [CrossRef]
- Nirmal, M.; Dabbousi, B.O.; Bawendi, M.G.; Macklin, J.J.; Trautman, J.K.; Harris, T.D.; Brus, L.E. Fluorescence intermittency in single cadmium selenide nanocrystals. Nature 1996, 383, 802–804. [Google Scholar] [CrossRef]
- Qin, H.; Meng, R.; Wang, N.; Peng, X. Photoluminescence Intermittency and Photo-Bleaching of Single Colloidal Quantum Dot. Adv. Mater. 2017, 29, 1606923. [Google Scholar] [CrossRef]
- Moon, H.; Lee, C.; Lee, W.; Kim, J.; Chae, H. Stability of Quantum Dots, Quantum Dot Films, and Quantum Dot Light-Emitting Diodes for Display Applications. Adv. Mater. 2019, 31, 1804294. [Google Scholar] [CrossRef]
- Lee, W.; Son, H.J.; Lee, D.-K.; Kim, B.; Kim, H.; Kim, K.; Ko, M.J. Suppression of photocorrosion in CdS/CdSe quantum dot-sensitized solar cells: Formation of a thin polymer layer on the photoelectrode surface. Synth. Met. 2013, 165, 60–63. [Google Scholar] [CrossRef]
- Vokhmintcev, K.V.; Guhrenz, C.; Gaponik, N.; Nabiev, I.; Samokhvalov, P.S. Quenching of quantum dots luminescence under light irradiation and its influence on the biological application. J. Phys. Conf. Ser. 2017, 784, 012014. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, S.; Qin, H.; Zhou, J.; Peng, X. Oxygen Stabilizes Photoluminescence of CdSe/CdS Core/Shell Quantum Dots via Deionization. J. Am. Chem. Soc. 2020, 142, 4254–4264. [Google Scholar] [CrossRef]
- Hu, Z.; Shu, Y.; Qin, H.; Hu, X.; Peng, X. Water Effects on Colloidal Semiconductor Nanocrystals: Correlation of Photophysics and Photochemistry. J. Am. Chem. Soc. 2021, 143, 18721–18732. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Carrión, C.; Cárdenas, S.; Simonet, B.M.; Valcárcel, M. Quantum dots luminescence enhancement due to illumination with UV/Vis light. Chem. Commun. 2009, 35, 5214–5226. [Google Scholar] [CrossRef]
- Hines, D.A.; Becker, M.A.; Kamat, P.V. Photoinduced Surface Oxidation and Its Effect on the Exciton Dynamics of CdSe Quantum Dots. J. Phys. Chem. C 2012, 116, 13452–13457. [Google Scholar] [CrossRef]
- Jo, J.H.; Heo, H.S.; Lee, K. Assessing Stability of Nanocomposites Containing Quantum Dot/Silica Hybrid Particles with Different Morphologies at High Temperature and Humidity. Chem. Mater. 2020, 32, 10538–10544. [Google Scholar] [CrossRef]
- Cai, X.; Martin, J.E.; Shea-Rohwer, L.E.; Gong, K.; Kelley, D.F. Thermal Quenching Mechanisms in II–VI Semiconductor Nanocrystals. J. Phys. Chem. C 2013, 117, 7902–7913. [Google Scholar] [CrossRef]
- Woo, J.Y.; Kim, K.N.; Jeong, S.; Han, C.-S. Thermal behavior of a quantum dot nanocomposite as a color converting material and its application to white LED. Nanotechnology 2010, 21, 495704. [Google Scholar] [CrossRef]
- Javaux, C.; Mahler, B.; Dubertret, B.; Shabaev, A.; Rodina, A.V.; Efros, A.L.; Yakovlev, D.R.; Liu, F.; Bayer, M.; Camps, G.; et al. Thermal activation of non-radiative Auger recombination in charged colloidal nanocrystals. Nat. Nanotechnol. 2013, 8, 206–212. [Google Scholar] [CrossRef]
- Orfield, N.J.; Majumder, S.; McBride, J.R.; Yik-Ching Koh, F.; Singh, A.; Bouquin, S.J.; Casson, J.L.; Johnson, A.D.; Sun, L.; Li, X.; et al. Photophysics of Thermally-Assisted Photobleaching in “Giant” Quantum Dots Revealed in Single Nanocrystals. ACS Nano 2018, 12, 4206–4217. [Google Scholar] [CrossRef]
- Zhao, Y.; Riemersma, C.; Pietra, F.; Koole, R.; de Mello Donegá, C.; Meijerink, A. High-Temperature Luminescence Quenching of Colloidal Quantum Dots. ACS Nano 2012, 6, 9058–9067. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Saeed, M.H.; Zhang, S.; Wei, H.; Gao, Y.; Zou, C.; Zhang, L.; Yang, H. Luminescence Enhancement, Encapsulation, and Patterning of Quantum Dots Toward Display Applications. Adv. Funct. Mater. 2022, 32, 2109472. [Google Scholar] [CrossRef]
- Lv, W.; Li, L.; Xu, M.; Hong, J.; Tang, X.; Xu, L.; Wu, Y.; Zhu, R.; Chen, R.; Huang, W. Improving the Stability of Metal Halide Perovskite Quantum Dots by Encapsulation. Adv. Mater. 2019, 31, 1900682. [Google Scholar] [CrossRef]
- Zhou, S.; Xie, B.; Yang, X.; Zhang, X.; Luo, X. Superior hydrophobic silica-coated quantum dot for stable optical performance in humid environments. Nanotechnology 2022, 33, 195202. [Google Scholar] [CrossRef]
- Wang, N.; Koh, S.; Jeong, B.G.; Lee, D.; Kim, W.D.; Park, K.; Nam, M.K.; Lee, K.; Kim, Y.; Lee, B.-H.; et al. Highly luminescent silica-coated CdS/CdSe/CdS nanoparticles with strong chemical robustness and excellent thermal stability. Nanotechnology 2017, 28, 185603. [Google Scholar] [CrossRef]
- Ko, J.; Jeong, B.G.; Chang, J.H.; Joung, J.F.; Yoon, S.-Y.; Lee, D.C.; Park, S.; Huh, J.; Yang, H.; Bae, W.K.; et al. Chemically resistant and thermally stable quantum dots prepared by shell encapsulation with cross-linkable block copolymer ligands. NPG Asia Mater. 2020, 12, 19. [Google Scholar] [CrossRef]
- Dankert, F.; von Hänisch, C. Siloxane Coordination Revisited: Si−O Bond Character, Reactivity and Magnificent Molecular Shapes. Eur. J. Inorg. Chem. 2021, 2021, 2907–2927. [Google Scholar] [CrossRef]
- Koole, R.; van Schooneveld, M.M.; Hilhorst, J.; de Mello Donegá, C.; Hart, D.C.; van Blaaderen, A.; Vanmaekelbergh, D.; Meijerink, A. On the Incorporation Mechanism of Hydrophobic Quantum Dots in Silica Spheres by a Reverse Microemulsion Method. Chem. Mater. 2008, 20, 2503–2512. [Google Scholar] [CrossRef] [Green Version]
- Goryacheva, O.A.; Guhrenz, C.; Schneider, K.; Beloglazova, N.V.; Goryacheva, I.Y.; De Saeger, S.; Gaponik, N. Silanized Luminescent Quantum Dots for the Simultaneous Multicolor Lateral Flow Immunoassay of Two Mycotoxins. ACS Appl. Mater. Interfaces 2020, 12, 24575–24584. [Google Scholar] [CrossRef] [PubMed]
- Chemtob, A.; Versace, D.-L.; Belon, C.; Croutxé-Barghorn, C.; Rigolet, S. Concomitant Organic−Inorganic UV-Curing Catalyzed by Photoacids. Macromolecules 2008, 41, 7390–7398. [Google Scholar] [CrossRef]
- Lowe, A.B. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis. Polym. Chem. 2010, 1, 17–36. [Google Scholar] [CrossRef]
- Kade, M.J.; Burke, D.J.; Hawker, C.J. The power of thiol-ene chemistry. J. Polym. Sci. Part Polym. Chem. 2010, 48, 743–750. [Google Scholar] [CrossRef]
- Dondoni, A. The Emergence of Thiol–Ene Coupling as a Click Process for Materials and Bioorganic Chemistry. Angew. Chem. Int. Ed. 2008, 47, 8995–8997. [Google Scholar] [CrossRef]
- Chan, J.W.; Hoyle, C.E.; Lowe, A.B. Sequential Phosphine-Catalyzed, Nucleophilic Thiol−Ene/Radical-Mediated Thiol−Yne Reactions and the Facile Orthogonal Synthesis of Polyfunctional Materials. J. Am. Chem. Soc. 2009, 131, 5751–5753. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Malak, S.T.; Jung, J.; Yoon, Y.J.; Lin, C.H.; Kim, S.; Lee, K.M.; Ma, R.; White, T.J.; Bunning, T.J.; et al. Robust, Uniform, and Highly Emissive Quantum Dot–Polymer Films and Patterns Using Thiol–Ene Chemistry. ACS Appl. Mater. Interfaces 2017, 9, 17435–17448. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.P.; Mackey, N.M.; Confait, B.S.; Heaps, D.T.; Deng, X.; Todd, M.L.; Stevenson, S.; Zhou, H.; Hoyle, C.E. Dispersion of Gold Nanoparticles in UV-Cured, Thiol−Ene Films by Precomplexation of Gold−Thiol. Chem. Mater. 2008, 20, 5240–5245. [Google Scholar] [CrossRef]
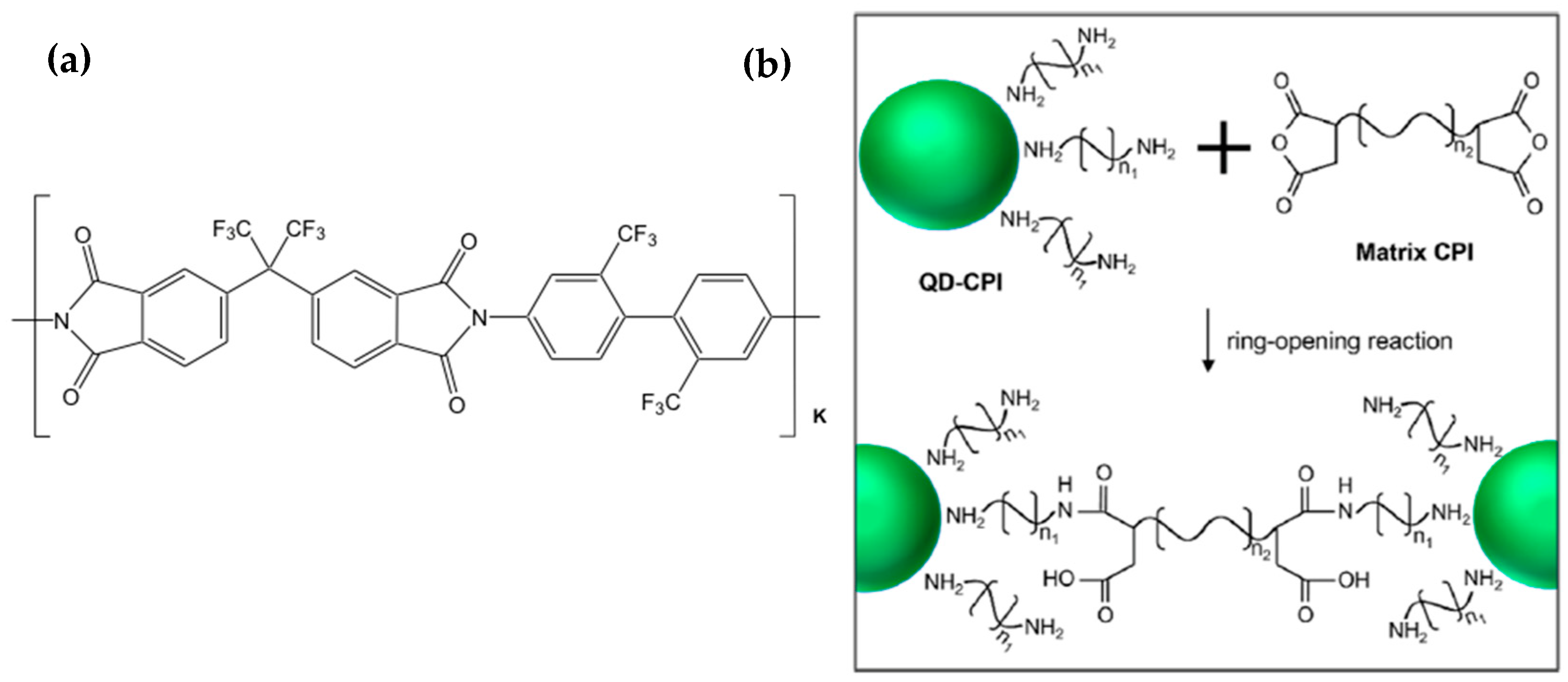
- Heo, H.S.; Jo, J.H.; Lee, S.J.; Yun, C.; Choi, S.-H.; Lee, J.H.; Lee, K. Fabrication of Cross-Linked Quantum Dot/Colorless-Polyimide Nanocomposites for Highly Efficient and Long-Term Stable Light-Emitting Diodes. Chem. Mater. 2022, 34, 6958–6967. [Google Scholar] [CrossRef]
- Zhang, E.; Chen, H.; Dai, X.; Liu, X.; Yang, W.; Liu, W.; Dong, Z.; Qiu, X.; Ji, X. Influence of molecular weight on scaling exponents and critical concentrations of one soluble 6FDA-TFDB polyimide in solution. J. Polym. Res. 2017, 24, 47. [Google Scholar] [CrossRef]
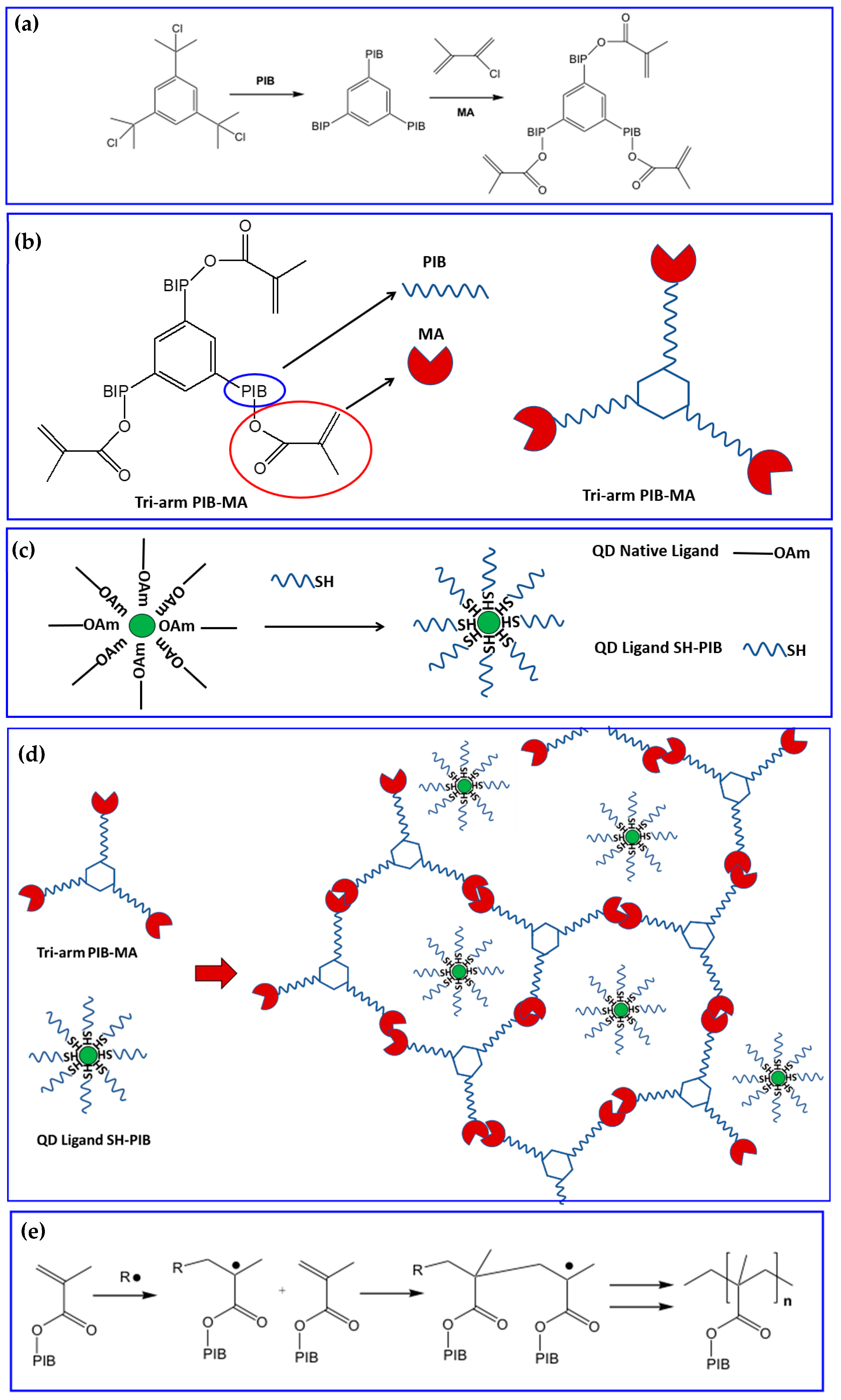
- Prudnikau, A.; Shiman, D.I.; Ksendzov, E.; Harwell, J.; Bolotina, E.A.; Nikishau, P.A.; Kostjuk, S.V.; Samuel, I.D.W.; Lesnyak, V. Design of cross-linked polyisobutylene matrix for efficient encapsulation of quantum dots. Nanoscale Adv. 2021, 3, 1443–1454. [Google Scholar] [CrossRef]
- Yang, B.; Parada, C.M.; Storey, R.F. Synthesis, Characterization, and Photopolymerization of Polyisobutylene Phenol (Meth)acrylate Macromers. Macromolecules 2016, 49, 6173–6185. [Google Scholar] [CrossRef]
- Lim, Y.-W.; Jin, J.; Bae, B.-S. Optically Transparent Multiscale Composite Films for Flexible and Wearable Electronics. Adv. Mater. 2020, 32, 1907143. [Google Scholar] [CrossRef]
- Ro, H.W.; Soles, C.L. Silsesquioxanes in nanoscale patterning applications. Mater. Today 2011, 14, 20–33. [Google Scholar] [CrossRef]
- Lee, H.E.; Lee, D.; Lee, T.-I.; Jang, J.; Jang, J.; Lim, Y.-W.; Shin, J.H.; Kang, S.-M.; Choi, G.-M.; Joe, D.J.; et al. Siloxane Hybrid Material-Encapsulated Highly Robust Flexible μLEDs for Biocompatible Lighting Applications. ACS Appl. Mater. Interfaces 2022, 14, 28258–28269. [Google Scholar] [CrossRef]
- Kuk, S.K.; Jang, J.; Han, H.J.; Lee, E.; Oh, H.; Kim, H.Y.; Jang, J.; Lee, K.T.; Lee, H.; Jung, Y.S.; et al. Siloxane-Encapsulated Upconversion Nanoparticle Hybrid Composite with Highly Stable Photoluminescence against Heat and Moisture. ACS Appl. Mater. Interfaces 2019, 11, 15952–15959. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Yoon, D.-E.; Kang, S.-M.; Kim, Y.H.; Lee, I.; Lee, H.; Kim, Y.H.; Lee, D.C.; Bae, B.-S. Exceptionally stable quantum dot/siloxane hybrid encapsulation material for white light-emitting diodes with a wide color gamut. Nanoscale 2019, 11, 14887–14895. [Google Scholar] [CrossRef] [PubMed]
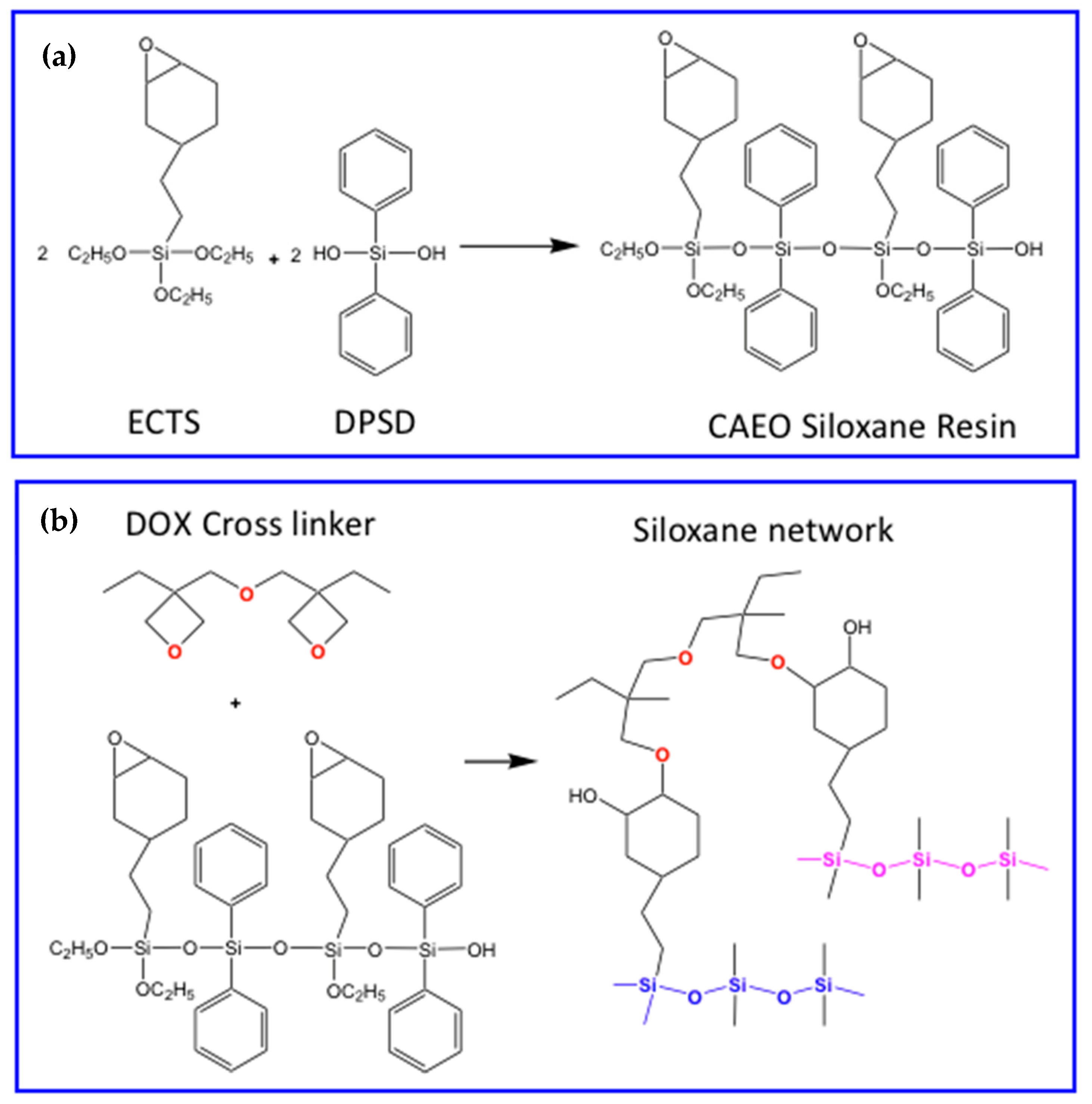
- Yang, S.; Jin, J.; Kwak, S.-Y.; Bae, B.-S. Photocurable transparent cycloaliphatic epoxy hybrid materials crosslinked by oxetane. J. Appl. Polym. Sci. 2012, 126, E380–E386. [Google Scholar] [CrossRef]
- Jang, J.; Kim, Y.-H.; Park, S.; Yoo, D.; Cho, H.; Jang, J.; Jeong, H.B.; Lee, H.; Yuk, J.M.; Park, C.B.; et al. Extremely Stable Luminescent Crosslinked Perovskite Nanoparticles under Harsh Environments over 1.5 Years. Adv. Mater. 2021, 33, 2005255. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, X.; Yin, J. A water-soluble supramolecular-structured photoinitiator between methylated β-cyclodextrin and 2,2-dimethoxy-2-phenylacetophenone. J. Appl. Polym. Sci. 2007, 105, 3819–3823. [Google Scholar] [CrossRef]
- Kim, H.Y.; Yoon, D.-E.; Jang, J.; Lee, D.; Choi, G.-M.; Chang, J.H.; Lee, J.Y.; Lee, D.C.; Bae, B.-S. Quantum Dot/Siloxane Composite Film Exceptionally Stable against Oxidation under Heat and Moisture. J. Am. Chem. Soc. 2016, 138, 16478–16485. [Google Scholar] [CrossRef]
- Yang, J.; Choi, M.K.; Yang, U.J.; Kim, S.Y.; Kim, Y.S.; Kim, J.H.; Kim, D.-H.; Hyeon, T. Toward Full-Color Electroluminescent Quantum Dot Displays. Nano Lett. 2021, 21, 26–33. [Google Scholar] [CrossRef]
- Li, X.; Kundaliya, D.; Tan, Z.J.; Anc, M.; Fang, N.X. Projection lithography patterned high-resolution quantum dots/thiol-ene photo-polymer pixels for color down conversion. Opt. Express 2019, 27, 30864–30874. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, G.; Li, F.; Shi, J.; Zhong, H. Direct in situ photolithography of perovskite quantum dots based on photocatalysis of lead bromide complexes. Nat. Commun. 2022, 13, 6713. [Google Scholar] [CrossRef]
- Kim, J.-S.; Yang, S.; Park, H.; Bae, B.-S. Photo-curable siloxane hybrid material fabricated by a thiol–ene reaction of sol–gel synthesized oligosiloxanes. Chem. Commun. 2011, 47, 6051–6053. [Google Scholar] [CrossRef]
- Ozdemir, R.; Van Avermaet, H.; Erdem, O.; Schiettecatte, P.; Hens, Z.; Aubert, T. Quantum Dot Patterning and Encapsulation by Maskless Lithography for Display Technologies. ACS Appl. Mater. Interfaces 2023, 15, 9629–9637. [Google Scholar] [CrossRef]
- Wang, Y.; Fedin, I.; Zhang, H.; Talapin, D.V. Direct optical lithography of functional inorganic nanomaterials. Science 2017, 357, 385–388. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.; Pan, J.-A.; Wu, H.; Lan, X.; Coropceanu, I.; Wang, Y.; Cho, W.; Hill, E.A.; Anderson, J.S.; Talapin, D.V. Direct Optical Patterning of Quantum Dot Light-Emitting Diodes via In Situ Ligand Exchange. Adv. Mater. 2020, 32, 2003805. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hahm, D.; Kim, K.; Rhee, S.; Lee, M.; Kim, S.; Chang, J.H.; Park, H.W.; Lim, J.; Lee, M.; et al. High-resolution patterning of colloidal quantum dots via non-destructive, light-driven ligand crosslinking. Nat. Commun. 2020, 11, 2874. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Weng, K.; Lu, S.; Li, F.; Abudukeremu, H.; Zhang, L.; Yang, Y.; Hou, J.; Qiu, H.; Fu, Z.; et al. Direct optical patterning of perovskite nanocrystals with ligand cross-linkers. Sci. Adv. 2022, 8, eabm8433. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, M.; Park, S.Y.; Park, M.; Kim, J.; Sitapure, N.; Hahm, D.; Rhee, S.; Lee, D.; Jo, H.; et al. Nondestructive Photopatterning of Heavy-Metal-Free Quantum Dots. Adv. Mater. 2022, 34, 2205504. [Google Scholar] [CrossRef]
- Liu, L.-H.; Yan, M. Perfluorophenyl Azides: New Applications in Surface Functionalization and Nanomaterial Synthesis. Acc. Chem. Res. 2010, 43, 1434–1443. [Google Scholar] [CrossRef] [Green Version]
- Antolini, F.; Orazi, L. Quantum Dots Synthesis through Direct Laser Patterning: A Review. Front. Chem. 2019, 7, 252. [Google Scholar] [CrossRef] [Green Version]
- Antolini, F.; Limosani, F.; Carcione, R. Direct Laser Patterning of CdTe QDs and Their Optical Properties Control through Laser Parameters. Nanomaterials 2022, 12, 1551. [Google Scholar] [CrossRef] [PubMed]
- Carcione, R.; Limosani, F.; Antolini, F. Cadmium Telluride Nanocomposite Films Formation from Thermal Decomposition of Cadmium Carboxylate Precursor and Their Photoluminescence Shift from Green to Red. Crystals 2021, 11, 253. [Google Scholar] [CrossRef]
- Cotta, M.A. Quantum Dots and Their Applications: What Lies Ahead? ACS Appl. Nano Mater. 2020, 3, 4920–4924. [Google Scholar] [CrossRef]
- Mallick, S.; Kumar, P.; Koner, A.L. Freeze-Resistant Cadmium-Free Quantum Dots for Live-Cell Imaging. ACS Appl. Nano Mater. 2019, 2, 661–666. [Google Scholar] [CrossRef]
- Saran, R.; Curry, R.J. Lead sulphide nanocrystal photodetector technologies. Nat. Photonics 2016, 10, 81–92. [Google Scholar] [CrossRef]
- Osypiw, A.R.C.; Lee, S.; Jung, S.-M.; Leoni, S.; Smowton, P.M.; Hou, B.; Kim, J.M.; Amaratunga, G.A.J. Solution-processed colloidal quantum dots for light emission. Mater. Adv. 2022, 3, 6773–6790. [Google Scholar] [CrossRef]
- Wen, Z.; Zhou, Z.; Liu, H.; Wang, Z.; Li, X.; Fang, F.; Wang, K.; Teo, K.L.; Sun, X.W. Color revolution: Toward ultra-wide color gamut displays. J. Phys. Appl. Phys. 2021, 54, 213002. [Google Scholar] [CrossRef]
- Lu, S.; Fu, Z.; Li, F.; Weng, K.; Zhou, L.; Zhang, L.; Yang, Y.; Qiu, H.; Liu, D.; Qing, W.; et al. Beyond a Linker: The Role of Photochemistry of Crosslinkers in the Direct Optical Patterning of Colloidal Nanocrystals. Angew. Chem. Int. Ed. 2022, 61, e202202633. [Google Scholar] [CrossRef]
- Qiao, W.; Huang, W.; Liu, Y.; Li, X.; Chen, L.-S.; Tang, J.-X. Toward Scalable Flexible Nanomanufacturing for Photonic Structures and Devices. Adv. Mater. 2016, 28, 10353–10380. [Google Scholar] [CrossRef]
- Son, Y.; Yeo, J.; Moon, H.; Lim, T.W.; Hong, S.; Nam, K.H.; Yoo, S.; Grigoropoulos, C.P.; Yang, D.-Y.; Ko, S.H. Nanoscale Electronics: Digital Fabrication by Direct Femtosecond Laser Processing of Metal Nanoparticles. Adv. Mater. 2011, 23, 3176–3181. [Google Scholar] [CrossRef]









| DOP Technology | Patterning Resolution (µm) | Device Realized | Device Stability |
|---|---|---|---|
| Thiol-ene | 5 [92] | n.d. | n.d. |
| Siloxanes | 10 [84] | [31] | 30 days 85% Humidity Harsh conditions |
| DOLFIN | 1.5 [96] | [96] | Comparison with standard device |
| Cross-linkers | 2.5 [99] | [99] | Comparison with standard device |
| Laser | 30 [102] | n.d. | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antolini, F. Direct Optical Patterning of Quantum Dots: One Strategy, Different Chemical Processes. Nanomaterials 2023, 13, 2008. https://doi.org/10.3390/nano13132008
Antolini F. Direct Optical Patterning of Quantum Dots: One Strategy, Different Chemical Processes. Nanomaterials. 2023; 13(13):2008. https://doi.org/10.3390/nano13132008
Chicago/Turabian StyleAntolini, Francesco. 2023. "Direct Optical Patterning of Quantum Dots: One Strategy, Different Chemical Processes" Nanomaterials 13, no. 13: 2008. https://doi.org/10.3390/nano13132008
APA StyleAntolini, F. (2023). Direct Optical Patterning of Quantum Dots: One Strategy, Different Chemical Processes. Nanomaterials, 13(13), 2008. https://doi.org/10.3390/nano13132008





