Highly Efficient Photo-Fenton Ag/Fe2O3/BiOI Z-Scheme Heterojunction for the Promoted Degradation of Tetracycline
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fe2O3 Nanoparticles, Ag/Fe2O3 Composite and Ag/Fe2O3/BiOI Heterojunction
2.3. Characterization
2.4. Photo-Fenton Activity Measurement
3. Results and Discussion
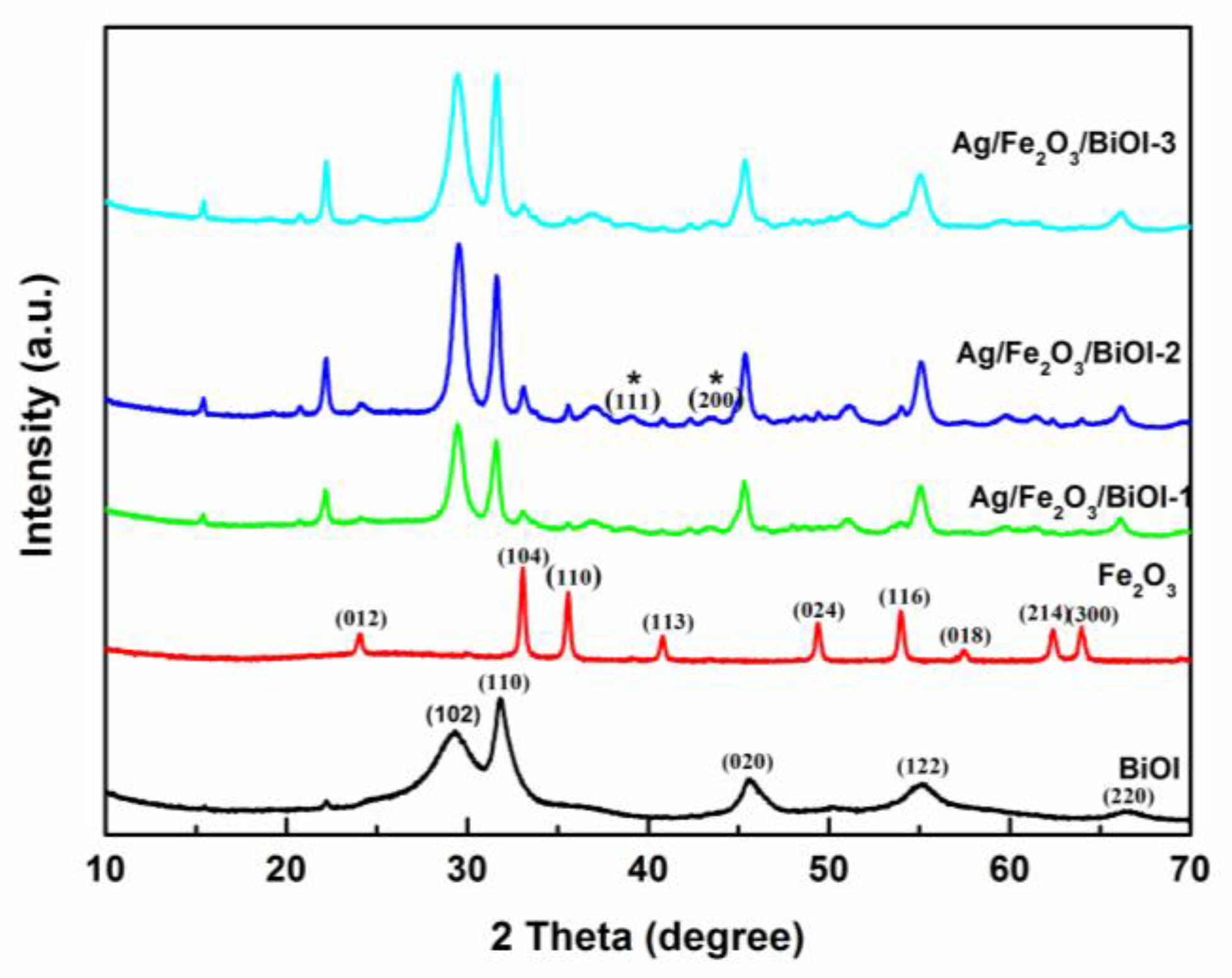
3.1. Structural Characterization of Prepared Samples
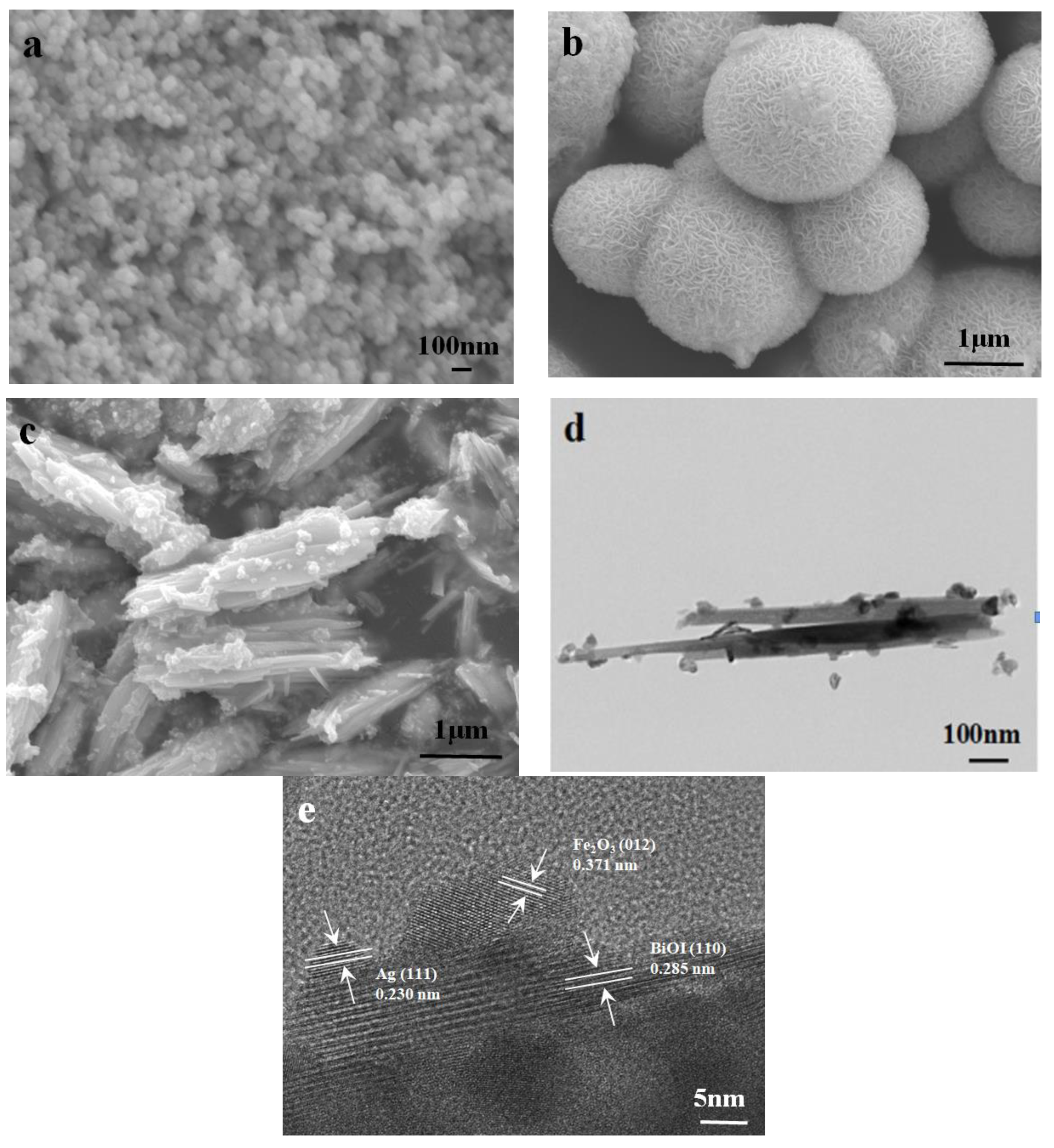
3.2. Morphological Analysis
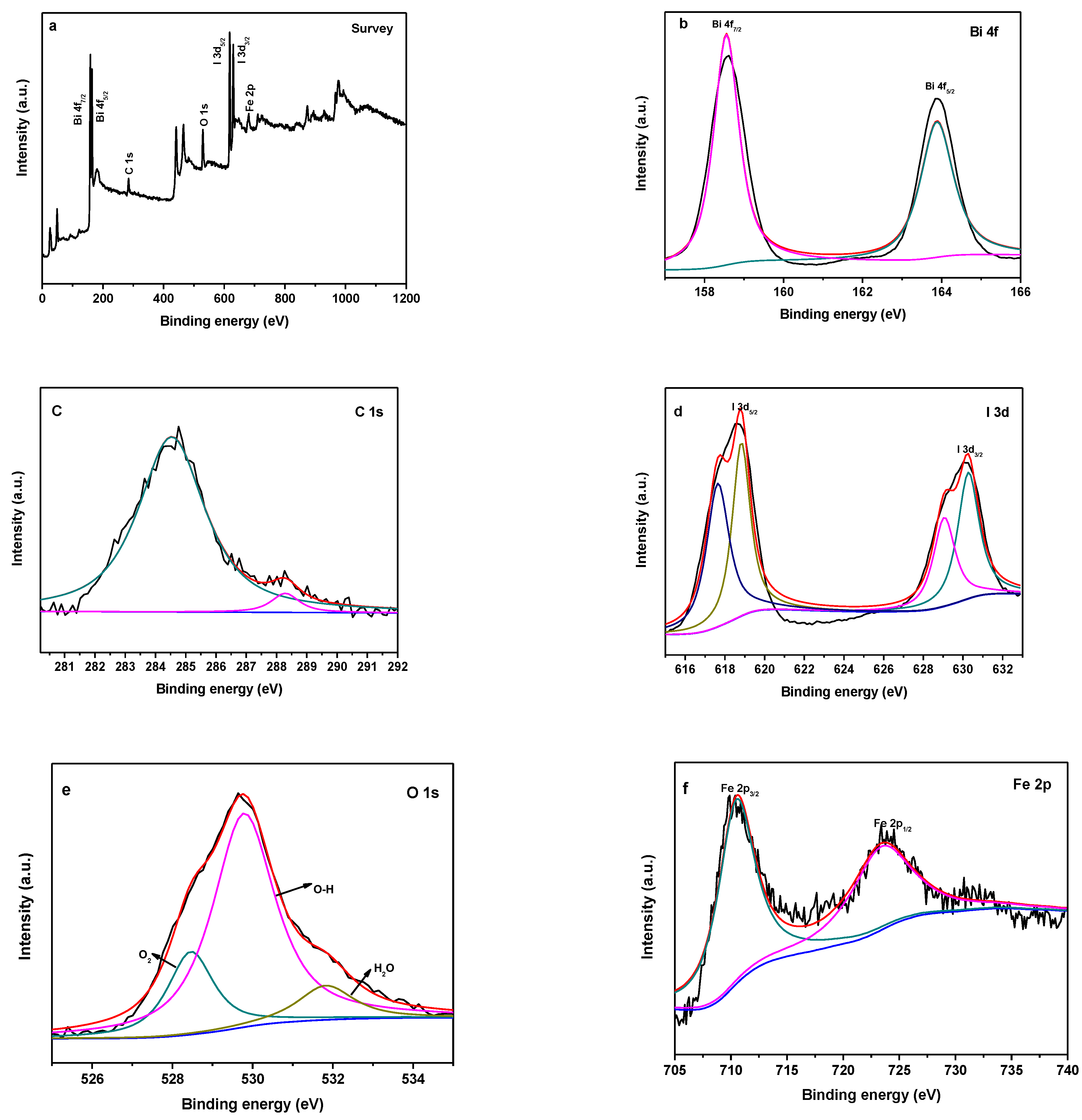
3.3. XPS Analysis
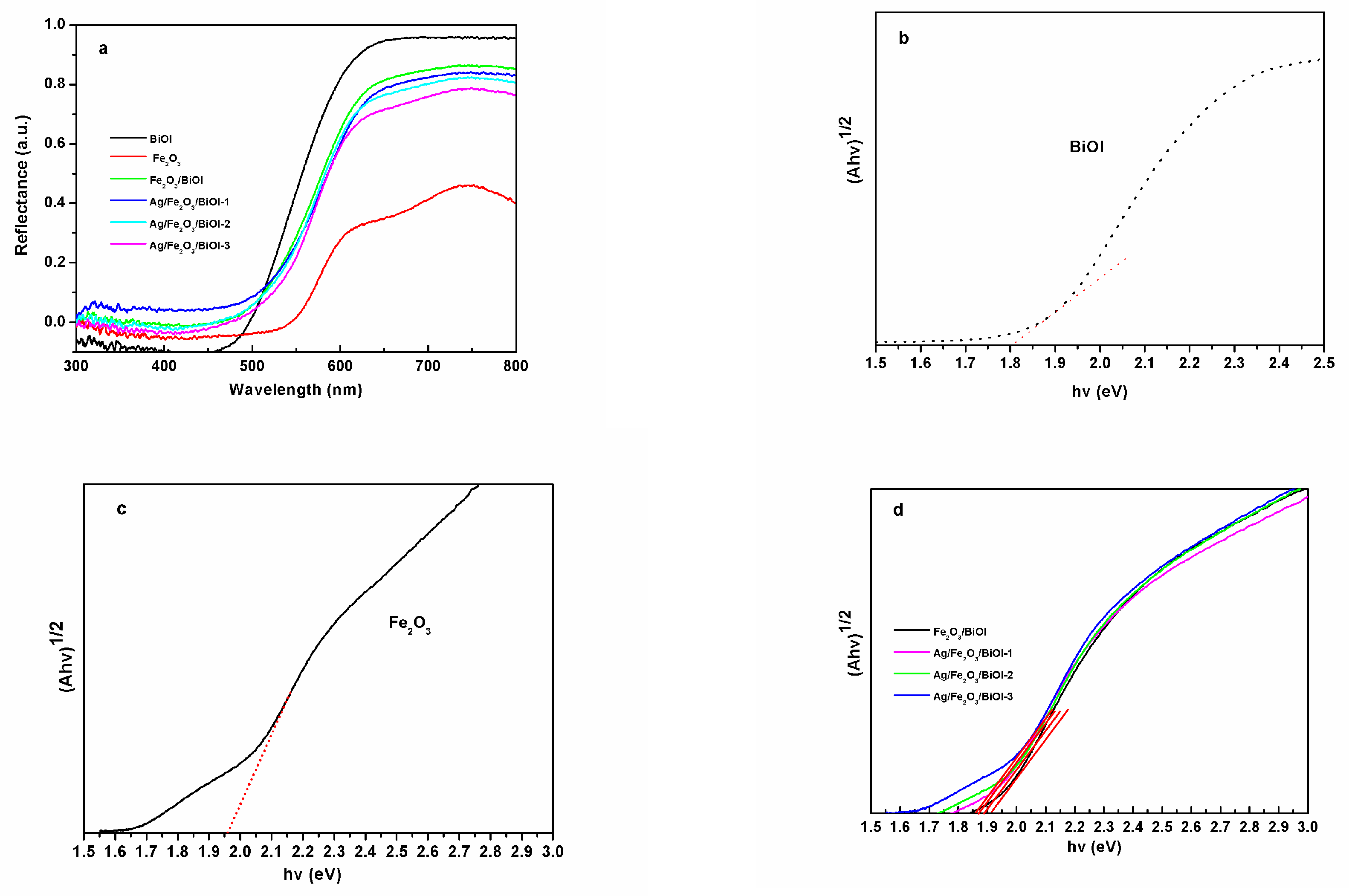
3.4. Ultraviolet-Visible Reflectance Spectra
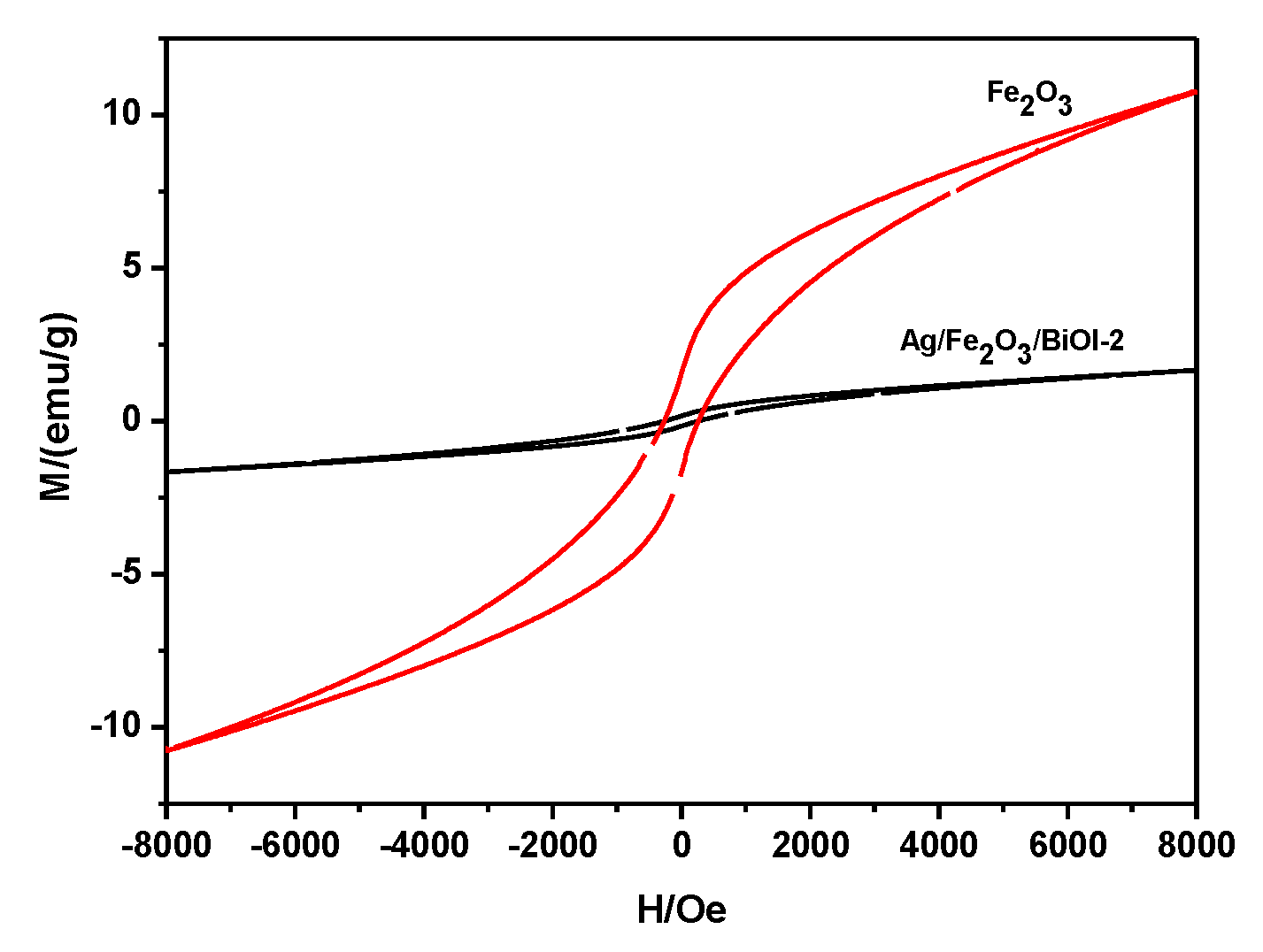
3.5. VSM Analysis
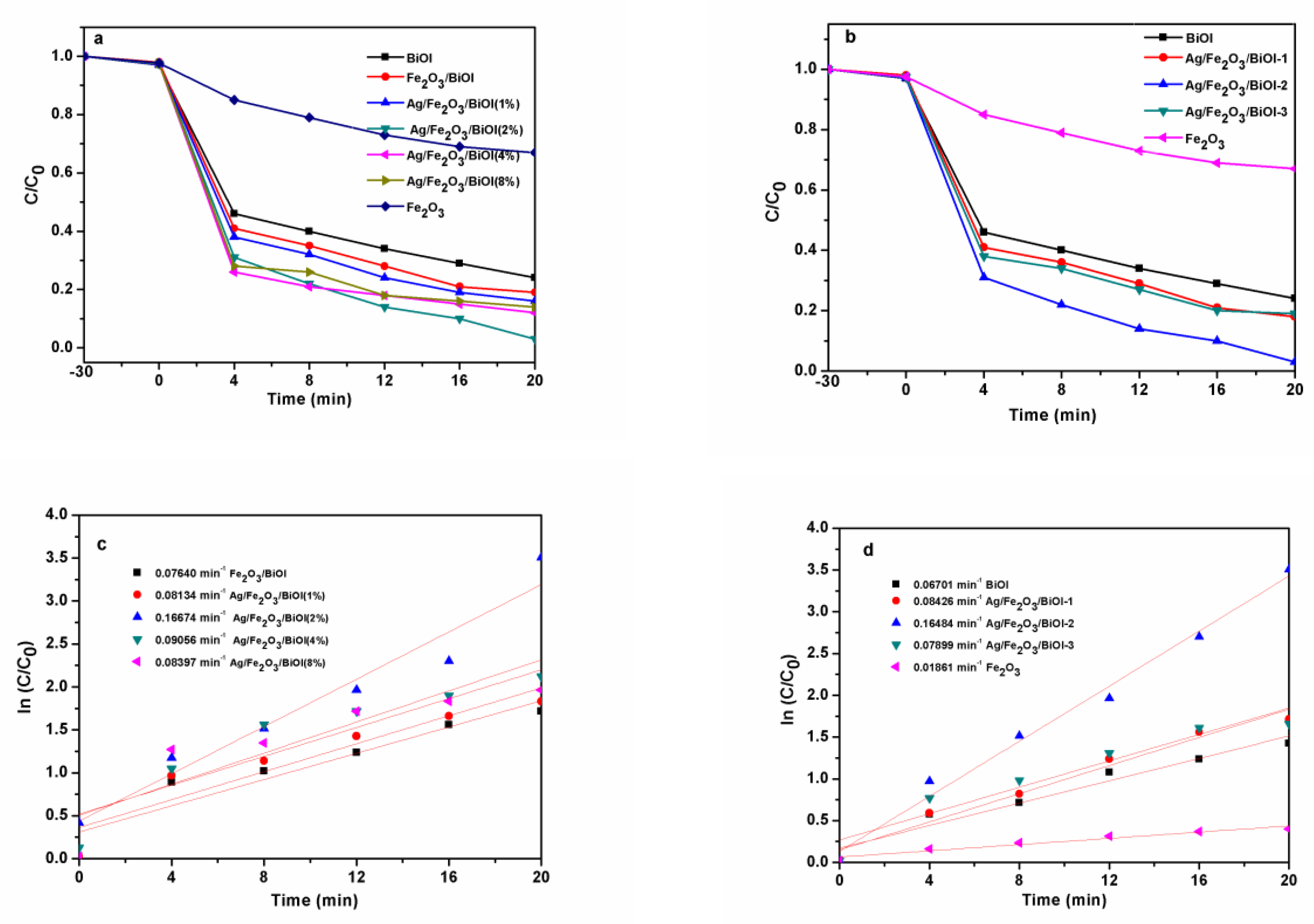
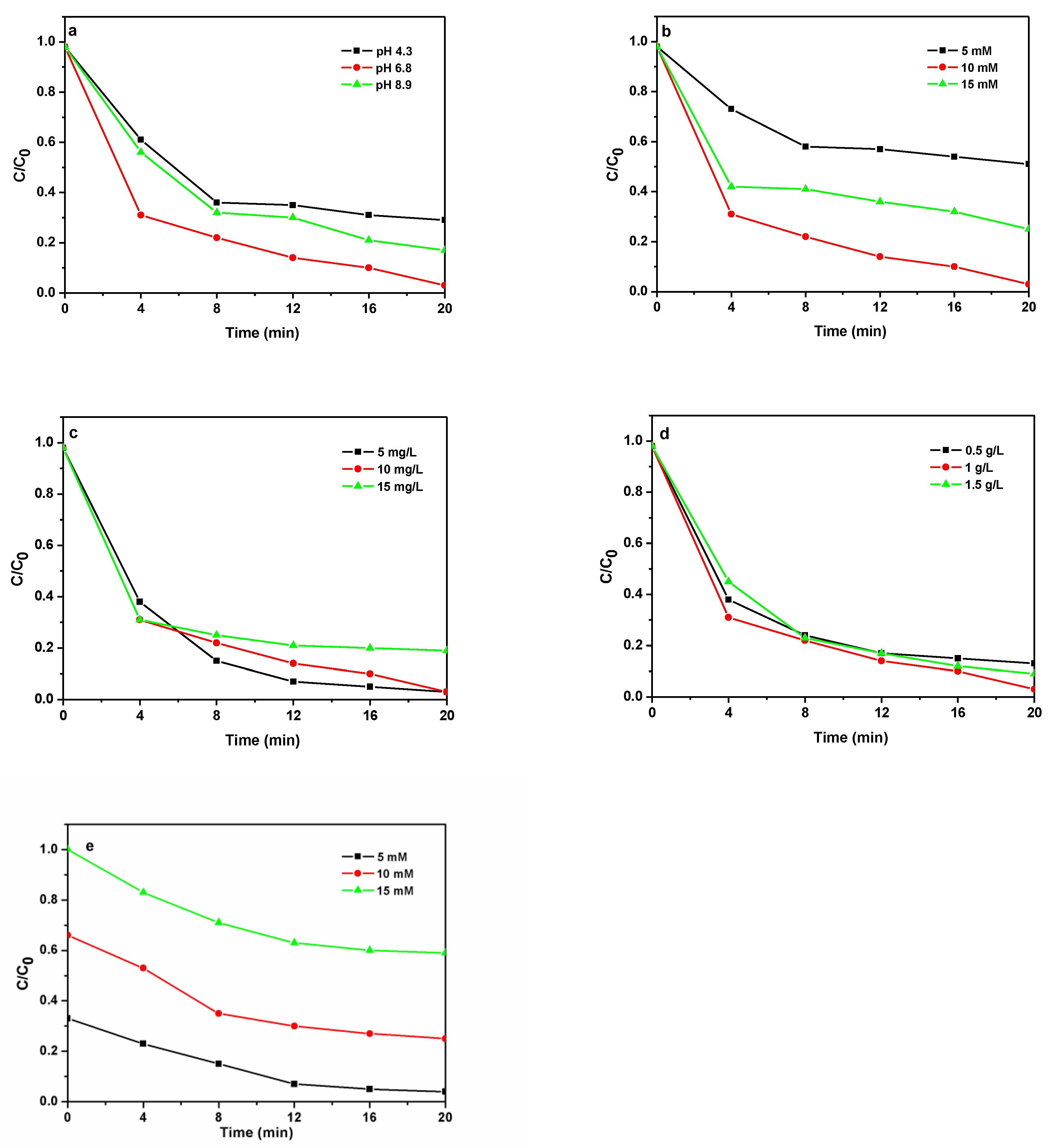
3.6. Evaluation of the Photo-Fenton Performance
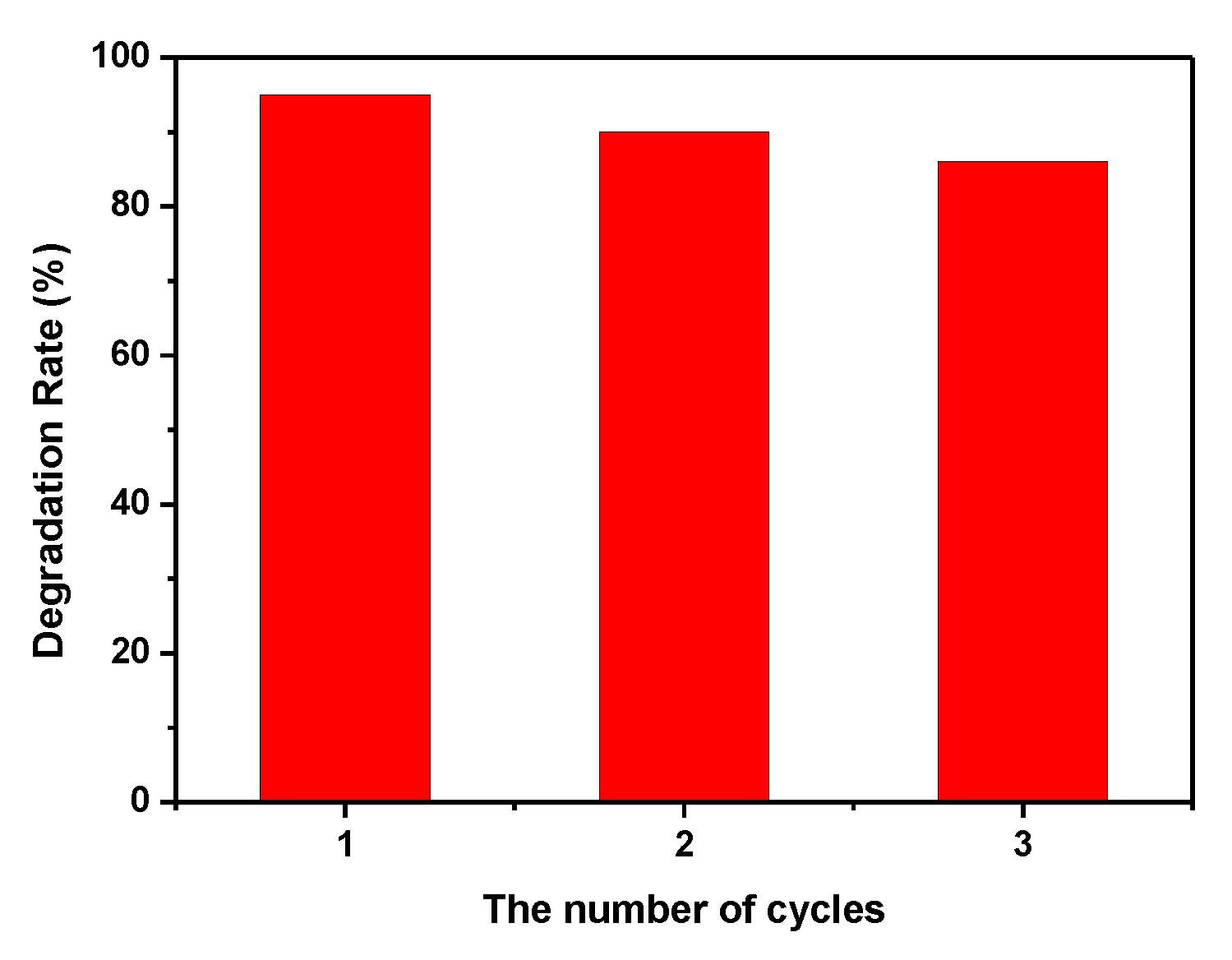
3.7. Catalyst Stability
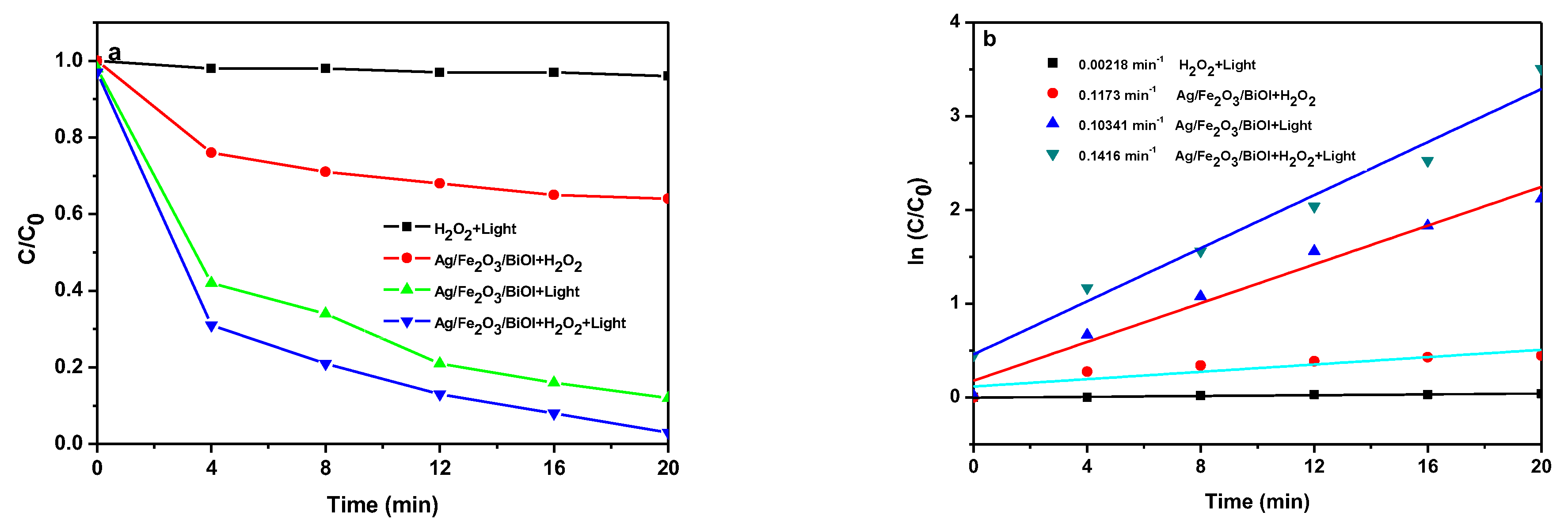
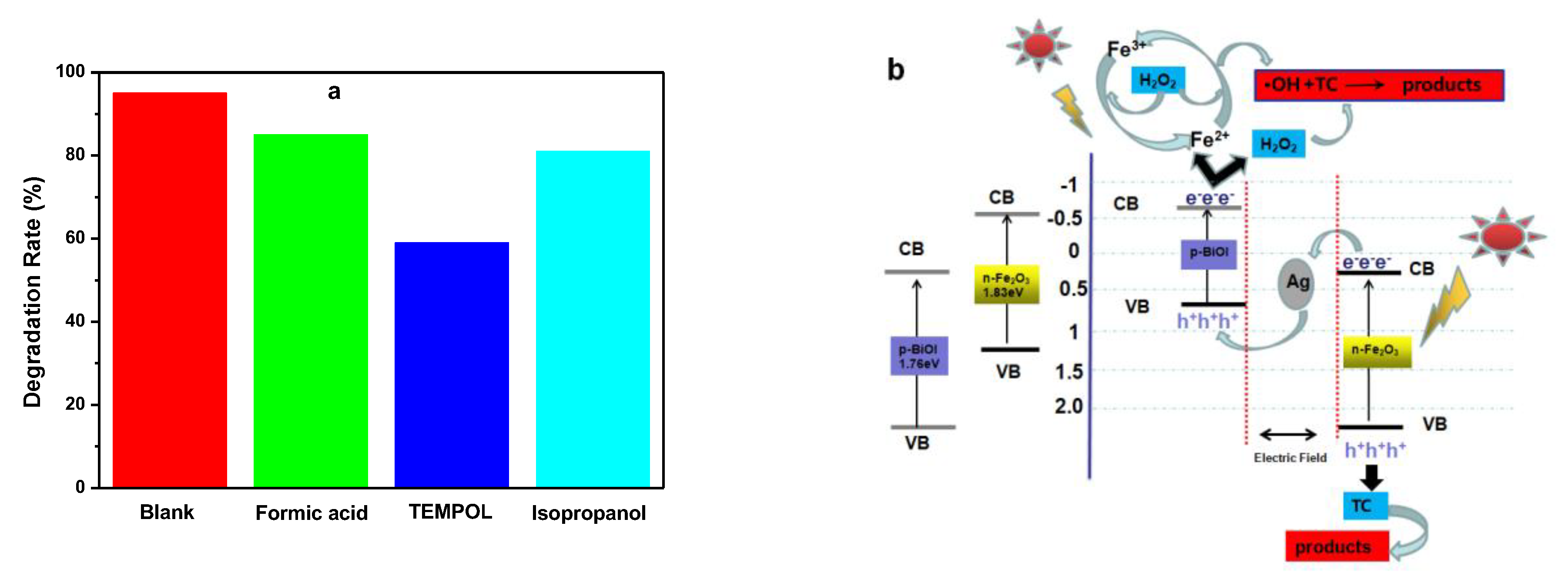
3.8. Photo-Fenton Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mo, S.; Zhang, Q.; Li, J.; Sun, Y.; Ren, Q.; Zou, S.; Zhang, Q.; Lu, J.; Fu, M.; Mo, D.; et al. Highly efficient mesoporous MnO2 catalysts for the total toluene oxidation: Oxygen-Vacancy defect engineering and involved intermediates using in situ DRIFTS. Appl. Catal. B 2020, 264, 118464. [Google Scholar] [CrossRef]
- Song, D.; Li, M.; Liao, L.; Guo, L.; Liu, H.; Wang, B.; Li, Z. High-crystallinity BiOCl nanosheets as efficient photocatalysts for norfloxacin antibiotic degradation. Nanomaterials 2023, 13, 1841. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Niu, C.; Guo, H.; Huang, D.W.; Liang, C.; Yang, Y.Y.; Tang, N.; Zhang, X.G. Integrating the Z-scheme heterojunction and hot electrons injection into a plasmonic-based Zn2In2S5/W18O49 composite induced improved molecular oxygen activation for photocatalytic degradation and antibacterial performance. J. Colloid Interface Sci. 2022, 610, 953–969. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, X.; Huang, S.; Li, Y.; Zhang, X.; Zeng, G.; Niu, L.; Ling, Y.; Zhang, Y. Facile construction of 2D g-C3N4 supported nanoflower-like NaBiO3 with direct Z-scheme heterojunctions and insight into its photocatalytic degradation of tetracycline. J. Hazard. Mater. 2021, 414, 125547. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Liu, X.M.; Zhao, Y.P.; Dionysiou, D.D. Aligned-FeOOH nanorods anchored on a graphene oxide-carbon nanotubes aerogel can serve as an effective Fenton-like oxidation catalyst. Appl. Catal. B-Environ. 2017, 213, 74–86. [Google Scholar] [CrossRef]
- Azha, S.F.; Sellaoui, L.; Yunus, E.H.E.; Yee, C.J.; Petriciolet, A.B.; Lamineb, A.B.; Ismail, S. Iron-modified composite adsorbent coating for azo dye removal and its regeneration by photo-Fenton process: Synthesis, characterization and adsorption mechanism interpretation. Chem. Eng. J. 2019, 361, 31–40. [Google Scholar] [CrossRef]
- Yan, X.J.; Tang, Y.; Ma, C.; Liu, Y.; Xu, J. Deactivation and regeneration of photocatalysts: A review. Desalin. Water Treat. 2018, 124, 160–176. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Zhu, R.L.; Yan, L.X.; Fu, H.Y.; Xi, Y.F.; Zhou, H.J.; Zhu, G.Q.; Zhu, J.X.; He, H.P. Visible-light Ag/AgBr/ferrihydrite catalyst with enhanced heterogeneous photo-Fenton reactivity via electron transfer from Ag/AgBr to ferrihydrite. Appl. Catal. B-Environ. 2018, 239, 280–289. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Zhu, R.L.; Xi, Y.F.; Xu, T.Y.; Yan, L.X.; Zhu, J.X.; Zhu, G.Q.; He, H.P. Heterogeneous photo-Fenton degradation of bisphenol A over Ag/AgCl/ferrihydrite catalysts under visible light. Chem. Eng. J. 2018, 346, 567–577. [Google Scholar] [CrossRef] [Green Version]
- Kirchon, A.; Zhang, P.; Li, J.; Joseph, E.; Chen, W.; Zhou, H. Effect of isomorphic metal substitution on the fenton and photo-Fenton degradation of methylene blue using Fe-based metal-organic frameworks. ACS Appl. Mater. Interfaces 2020, 12, 9292–9299. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, H.; Li, K.; Zhao, G.; Ren, F. Fe-based metal-organic frameworks as Fenton-like catalysts for highly efficient degradation of tetracycline hydro-chloride over a wide pH range: Acceleration of Fe(II)/ Fe(III) cycle under visible light irradiation. Appl. Catal. B Environ. 2020, 263, 118–282. [Google Scholar] [CrossRef]
- Qian, X.F.; Wu, Y.W.; Kan, M.; Fang, M.Y.; Yue, D.T.; Zeng, J.; Zhao, Y.X. FeOOH quantum dots coupled g-C3N4 for visible light driving photo- Fenton degradation of organic pollutants. Appl. Catal. B-Environ. 2018, 237, 513–520. [Google Scholar] [CrossRef]
- Zhang, S.W.; Gao, H.H.; Huang, Y.S.; Wang, X.X.; Hayat, T.; Li, J.X.; Xu, X.J.; Wang, X.K. Ultrathin g-C3N4 nanosheets coupled with amorphous Cu-doped FeOOH nanoclusters as 2D/0D heterogeneous catalysts for water remediation. Environ. Sci. Nano 2018, 5, 1179–1190. [Google Scholar] [CrossRef]
- Wen, X.J.; Zhang, C.; Niu, C.G.; Zhang, L.; Huang, D.W.; Wang, X.Y.; Zhang, X.G.; Zeng, G.M. Facile synthesis of a visible light a-Fe2O3/BiOBr composite with high photocatalytic performance. RSC Adv. 2016, 6, 4035–4042. [Google Scholar] [CrossRef]
- Huang, H.W.; Xiao, K.; He, Y.; Zhang, T.R.; Dong, F.; Du, X.; Zhang, Y.H. In situ assembly of BiOI@Bi12O17Cl2 p-n junction: Charge induced unique front-lateral surfaces couplingheterostructure with high exposure of BiOI {001} active facets for robust and nonselective photocatalysis. Appl. Catal. B-Environ. 2016, 199, 75–86. [Google Scholar] [CrossRef]
- Li, D.; Xu, K.; Zhang, C. Improvement of photocatalytic performance by building multiple heterojunction structures of anatase-rutile/BiOI composite fibers. Nanomaterials 2022, 12, 3906. [Google Scholar] [CrossRef]
- Ye, K.H.; Chai, Z.; Gu, J.; Yu, X.; Zhao, C.; Zhang, Y.; Mai, W. BiOI-BiVO4 photoanodes with significantly improvedsolar water splitting capability: P-n junction to expand solaradsorption range and facilitate charge carrier dynamics. Nanos Energy 2015, 18, 222–231. [Google Scholar] [CrossRef]
- Mousavi, M.; Habibi, Y.A. Magnetically separable ternary g-C3N4/Fe3O4/BiOI nanocomposites: Novel visible-light-driven photocatalysts based on graphitic carbon nitride. J. Colloid Interface Sci. 2016, 465, 83–92. [Google Scholar] [CrossRef]
- Hu, X.; Han, S.; Zhu, Y. Facet-controlled synthesis of polyhedral hematite/carbon composites with enhanced photoactivity. Appl. Surf. Sci. 2018, 443, 227–235. [Google Scholar] [CrossRef]
- Yan, X.; Wu, Y.; Li, D.; Luo, C.; Wang, Y.; Hu, J.; Li, G.; Li, P.; Jiang, H.; Zhang, W. Facile synthesis of ring-like α-Fe2O3 assembly composed of small hematite particles for highly efficient photocatalysis. J. Mater. Sci. Mater. Electron. 2018, 29(4), 2610–2617. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Wang, X.; Nie, G.D.; Zhang, J.; Zhang, S.X.; Cao, N.; Yan, S.Y.; Long, Y.Z. Highly flexible Fe2O3/TiO2 composite nanofibers for photocatalysis and utraviolet detection. J. Phys. Chem. Solid. 2018, 121, 236–246. [Google Scholar] [CrossRef]
- Mehraj, O.; Pirzada, B.M.; Mir, N.A.; Khan, M.Z.; Sabir, S. A highly efficient visible-light-driven novel p-n junction Fe2O3/BiOI photocatalyst: Surface decoration of BiOI nanosheets with Fe2O3 nanoparticles. Appl. Surf. Sci. 2016, 387, 642–651. [Google Scholar] [CrossRef]
- Niu, J.; Dai, P.; Wang, K.; Zhang, Z.; Zhang, Q.; Yao, B.; Yu, X. Microwave-assisted synthesis of high efficient α-Fe2O3/BiOI composites and its performance in photocatalytic degardation of organic pollutants. Adv. Powder Technol. 2020, 31, 2327–2336. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, Z.; Zhang, C.; Huang, D.; Zeng, G.; Xiao, R.; Lai, C.; Zhou, C.; Guo, H.; Xue, W.; et al. Construction of iodine vacancy-rich BiOI/Ag@AgI Z-scheme heterojunction photocatalysts for visible-light-driven tetracycline degradation: Transformation pathways and mechanism insight. Chem. Eng. J. 2018, 349, 808–821. [Google Scholar] [CrossRef]
- Li, C.; Zhang, A.; Zhang, L.; Song, J.; Su, S.; Sun, Z.; Xiang, J. Enhanced photocatalyticactivity and characterization of magnetic Ag/BiOI/ZnFe2O4 composites for Hg0 removal under fluorescent light irradiation. Appl. Surf. Sci. 2018, 433, 914–926. [Google Scholar] [CrossRef]
- Zhong, S.; Wang, B.; Zhou, H.; Li, C.; Peng, X.; Zhang, S. Fabrication and characterization of Ag/BiOI/GO composites with enhanced photocatalytic activity. J. Alloys Compd. 2019, 806, 401–409. [Google Scholar] [CrossRef]
- Fayeza, S.U.; Khan, A.A.; Jan, A.; Aain, S.Q.; Neto, E.P.F.; Serge-Correales, Y.E.; Parveen, R.; Wender, H.; Rodrigues-Filho, U.P.; Ribeiro, S.J.L. Enhanced photoactivity of BiVO4/Ag/Ag2O Z-scheme photocatalyst for efficient environmental remediation under natural sunlight and low-cost LED illumination. Colloids Surf. A 2020, 600, 124946. [Google Scholar]
- Zhang, D.; Tan, G.; Wang, M.; Li, B.; Dang, M.; Ren, H.; Xia, A. The enhanced photocatalytic activity of Ag-OVs-(001) BiOCl by separating secondary excitons under double SPR effects. Appl. Surf. Sci. 2020, 526, 146689. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, G.; Siahrostami, S.; Chen, Z.; Liu, K.; Xie, J.; Liao, L.; Wu, T.; Lin, D.; Liu, Y.; et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal. 2018, 1, 156–162. [Google Scholar] [CrossRef]
- Jiang, J.; Gao, J.; Li, T.; Chen, Y.; Wu, Q.; Xie, T.; Lin, Y.; Dong, S. Visible-light-driven photo-Fenton reaction with α-Fe2O3/BiOI at near neutral pH: Boosted photogenerated charge separation, optimum operating parameters and mechanism insight. J. Colloid Interf. Sci. 2019, 554, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, K.; Sindhuja, E. Fabrication of cost effective g-C3N4+Ag activated ZnO photocatalyst in thin film form for enhanced visible light responsive dye degradation. Mater. Chem. Phys. 2019, 221, 203–215. [Google Scholar] [CrossRef]
- Chen, R.; Wang, H.; Wu, H.; Sheng, J.; Li, J.; Cui, W.; Dong, F. SrTiO3/BiOI heterostructure: Interfacial charge separation, enhanced photocatalytic activity, and reaction mechanism. Chin. J. Catal. 2020, 41, 710–718. [Google Scholar] [CrossRef]
- Ma, Q.; Hu, X.; Liu, N.; Sharma, A.; Zhang, C.; Kawazoe, N.; Chen, G.; Yang, Y. Polyethylene glycol (PEG)-modified Ag/Ag2O/Ag3PO4/Bi2WO6 photocatalyst film with enhanced efficiency and stability under solar light. J. Colloid Interface Sci. 2020, 569, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.J.; Reddy, D.A.; Ma, R.; Kim, Y.; Kim, T.K. Reduced-graphene-oxide-wrapped BiOI-AgI heterostructured nanocomposite as a high-performance photocatalyst for dye degradation under solar light irradiation. Solid State Sci. 2016, 61, 32–39. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Lin, C.; Lin, Q.; Jin, Y.B.; Wang, Y.H.; Zhang, Z.Z.; Lina, H.X.; Long, J.L.; Wang, X.X. CuI-BiOI/Cu film for enhanced photo-induced charge separation and visible light antibacterial activity. Appl. Catal. B-Environ. 2018, 235, 238–245. [Google Scholar] [CrossRef]
- Tang, X.; Jia, R.Y.; Zhai, T.; Xia, H. Hierarchical Fe3O4@Fe2O3 core-shell nanorod arrays as high-performance anodes for asymmetric supercapacitors, ACS Appl. Mater. Interfaces 2015, 7, 27518–27525. [Google Scholar] [CrossRef]
- Bai, Y.J.; Bai, H.Y.; Qu, K.G.; Wang, F.G.; Guan, P.; Xu, D.B.; Fan, W.Q.; Shi, W.D. In-situ approach to fabricate BiOI photocathode with oxygen vacancies: Understanding the N2 reduced behavior in photoelectrochemical system. Chem. Eng. J. 2019, 362, 349–356. [Google Scholar] [CrossRef]
- Li, N.; Zhang, J.; Tian, Y.; Zhao, J.; Zhang, J.; Zuo, W. Precisely controlled fabrication of magnetic 3D γ-Fe2O3@ZnO core-shell photocatalyst with enhanced activity: Ciprofloxacin degradation and mechanism insight. Chem. Eng. J. 2017, 308, 377–385. [Google Scholar] [CrossRef]
- Yan, S.D.; Shi, Y.; Tao, Y.F.; Zhang, H. Enhanced persulfate-mediated photocatalytic oxidation of bisphenol A using bioelectricity and a g-C3N4 /Fe2O3 heterojunction. Chem. Eng. J. 2019, 359, 933–943. [Google Scholar] [CrossRef]
- Jing, H.; Gao, Y.; Wang, X.; He, H.; Li, Q.; Pei, W. Preparation of Z-scheme spherical BiOI/Fe2O3/BiOBr for water pollution treatment. Appl. Organomet. Chem. 2023, 37, 6953–6963. [Google Scholar] [CrossRef]
- Zheng, J.; Jiao, Z. Magnetic recyclable bismuth oxyiodide/polyacrylic anion exchange resin composites with enhanced photocatalytic activity under visible light. J. Colloid Interface Sci. 2017, 504, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.B.; Zhao, H.Y.; Zhao, G.H. Iron-copper bimetallic nanoparticles embedded within ordered mesoporous carbon as effective and stable heterogeneous Fenton catalyst for the degradation of organic contaminants. Appl. Catal. B-Environ. 2015, 164, 396–406. [Google Scholar] [CrossRef]
- Ma, Y.; Li, M.; Jiang, J.; Li, T.; Wang, X.; Song, Y. In-situ prepared MIL-53(Fe)/BiOI photocatalyst for efficient degradation of tetracycline under visible-light driven photo-Fenton system: Investigation of performance and mechanism. J. Alloys Compd. 2012, 870, 159524–159533. [Google Scholar] [CrossRef]

| Samples | Ag (at.%) | Fe (at.%) | O (at.%) | Bi (at.%) | I (at.%) |
|---|---|---|---|---|---|
| Ag/Fe2O3/BiOI-1 | 0.19 | 2.36 | 14.91 | 10.72 | 71.82 |
| Ag/Fe2O3/BiOI-2 | 0.20 | 3.47 | 21.89 | 9.67 | 64.77 |
| Ag/Fe2O3/BiOI-3 | 0.20 | 4.78 | 30.18 | 8.42 | 56.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Liu, G.; Jiao, Z. Highly Efficient Photo-Fenton Ag/Fe2O3/BiOI Z-Scheme Heterojunction for the Promoted Degradation of Tetracycline. Nanomaterials 2023, 13, 1991. https://doi.org/10.3390/nano13131991
Zheng J, Liu G, Jiao Z. Highly Efficient Photo-Fenton Ag/Fe2O3/BiOI Z-Scheme Heterojunction for the Promoted Degradation of Tetracycline. Nanomaterials. 2023; 13(13):1991. https://doi.org/10.3390/nano13131991
Chicago/Turabian StyleZheng, Jingjing, Guoxia Liu, and Zhengbo Jiao. 2023. "Highly Efficient Photo-Fenton Ag/Fe2O3/BiOI Z-Scheme Heterojunction for the Promoted Degradation of Tetracycline" Nanomaterials 13, no. 13: 1991. https://doi.org/10.3390/nano13131991
APA StyleZheng, J., Liu, G., & Jiao, Z. (2023). Highly Efficient Photo-Fenton Ag/Fe2O3/BiOI Z-Scheme Heterojunction for the Promoted Degradation of Tetracycline. Nanomaterials, 13(13), 1991. https://doi.org/10.3390/nano13131991




