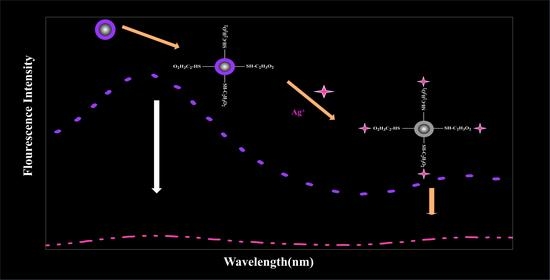
An Ethyl-Thioglycolate-Functionalized Fe3O4@ZnS Magnetic Fluorescent Nanoprobe for the Detection of Ag+ and Its Applications in Real Water Solutions
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Physical Characterizations
2.3. Synthesis of Fe3O4 Magnetic Nanoparticles
2.4. Synthesis of Fe3O4@ZnS Magnetic Nanoparticles
2.5. Synthesis of Fe3O4@ZnS@Ethyl Thioglycolate Magnetic Nanoparticles
2.6. Determination of Ag+ by Magnetic Fluorescence Nanoprobe Fe3O4@ZnS-SH
3. Results and Discussion
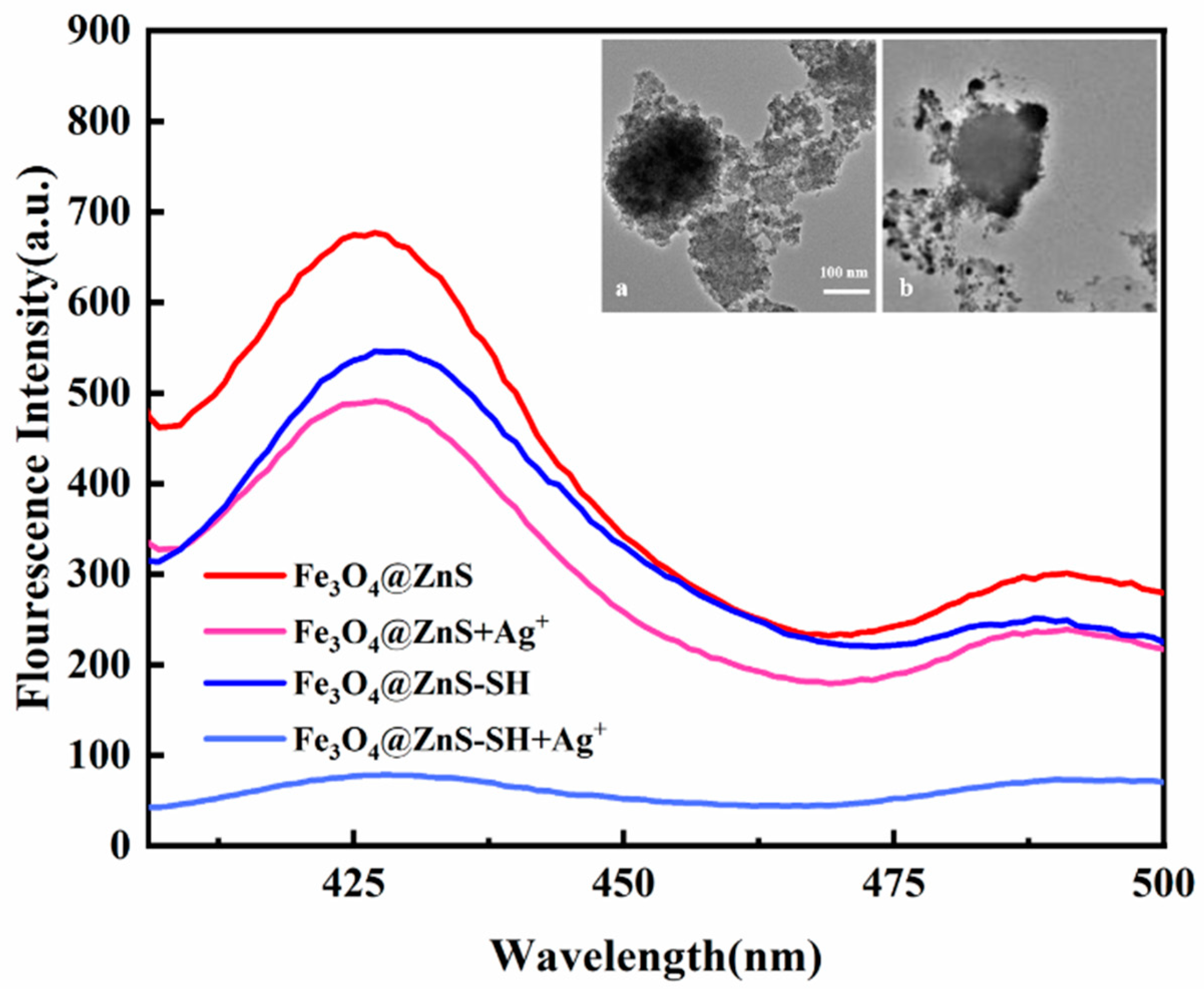
3.1. SEM and TEM Analysis
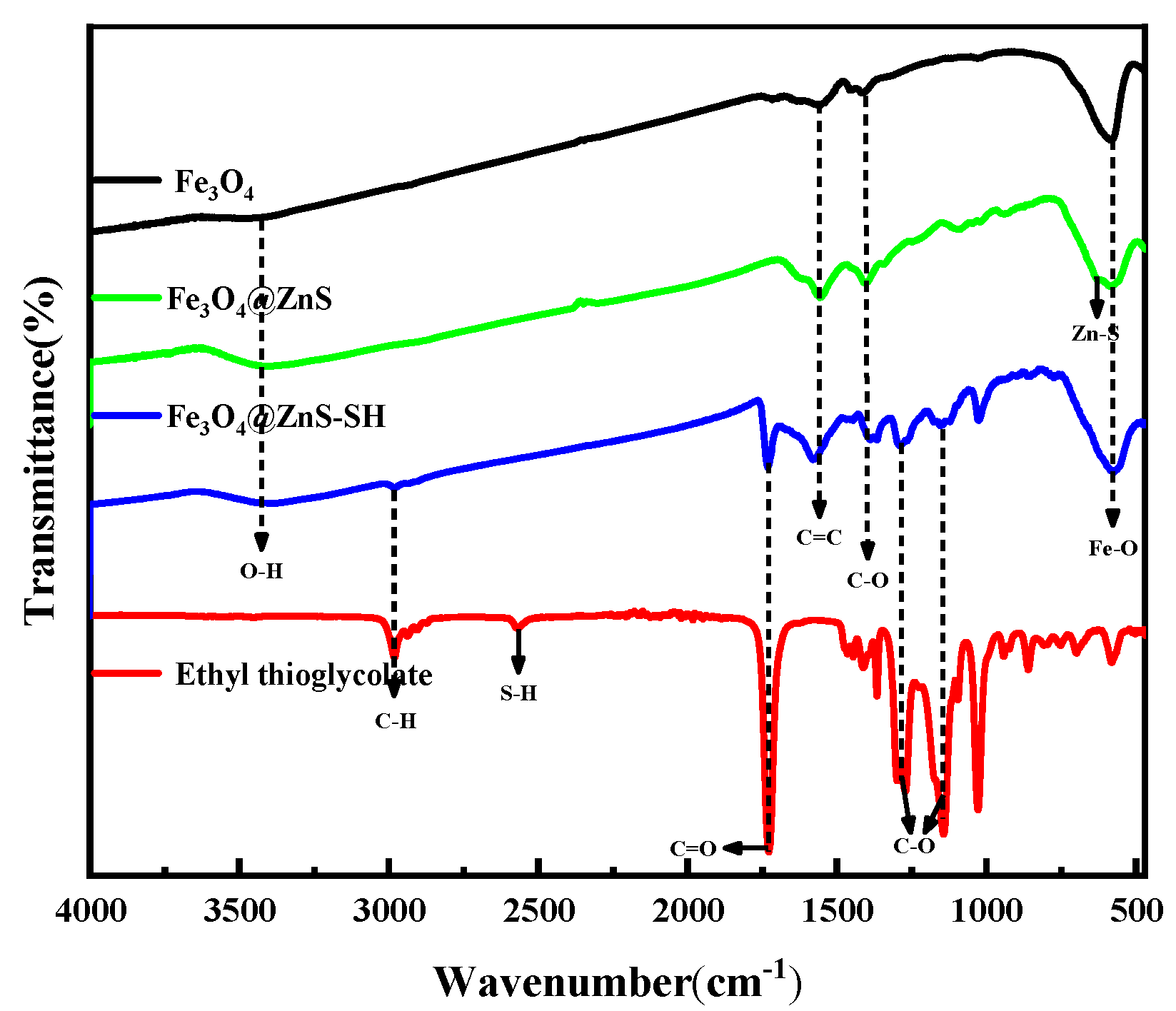
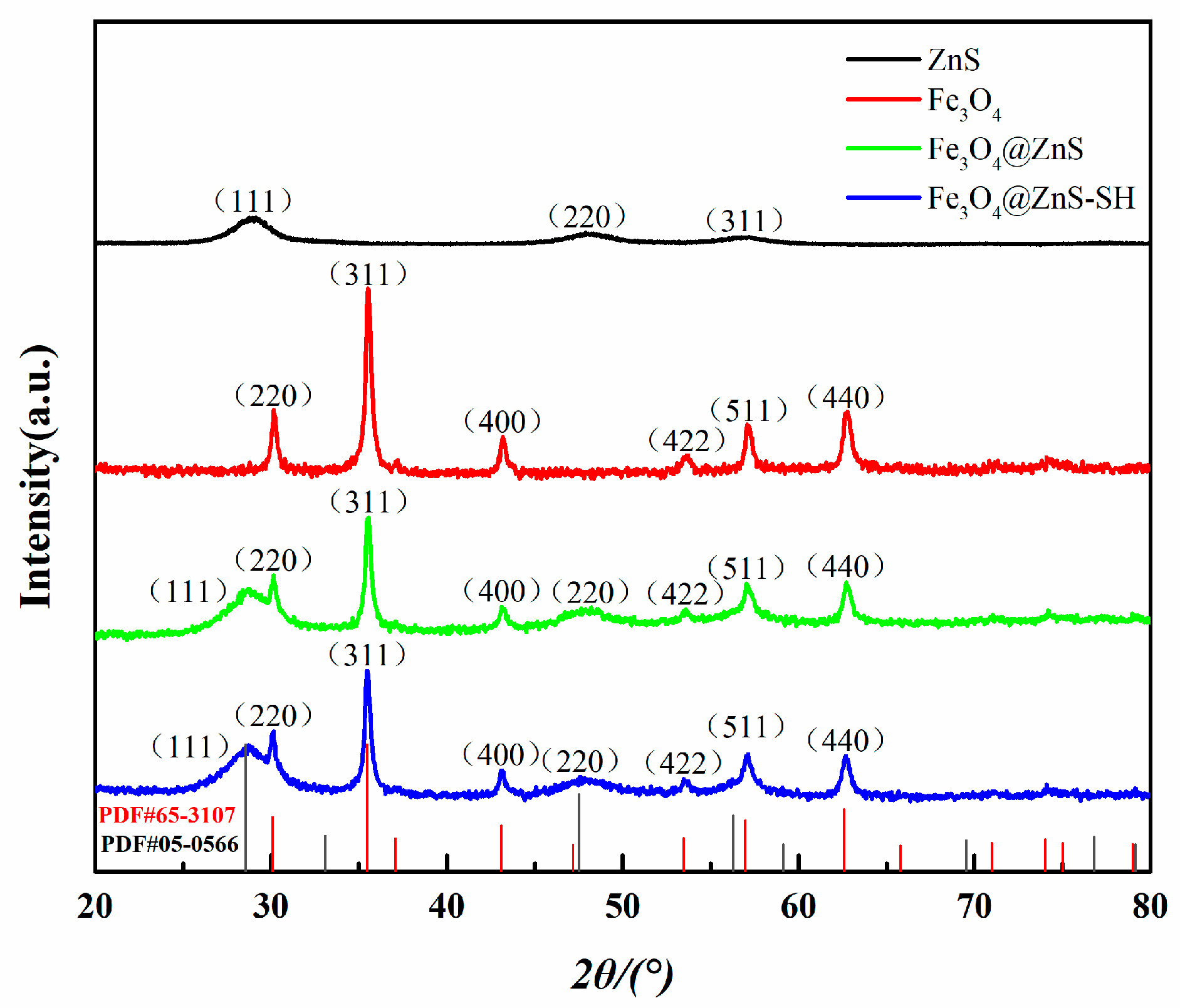
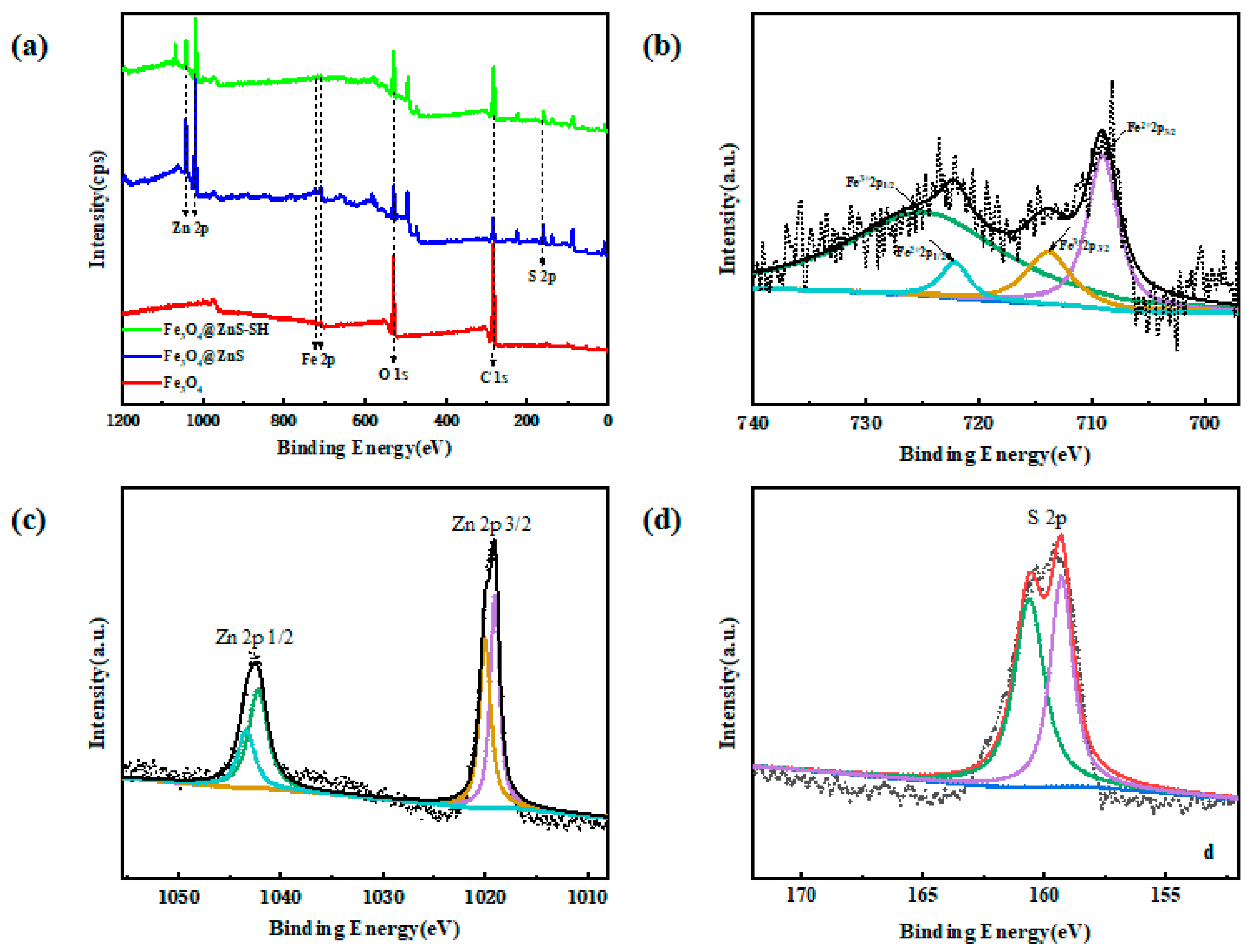
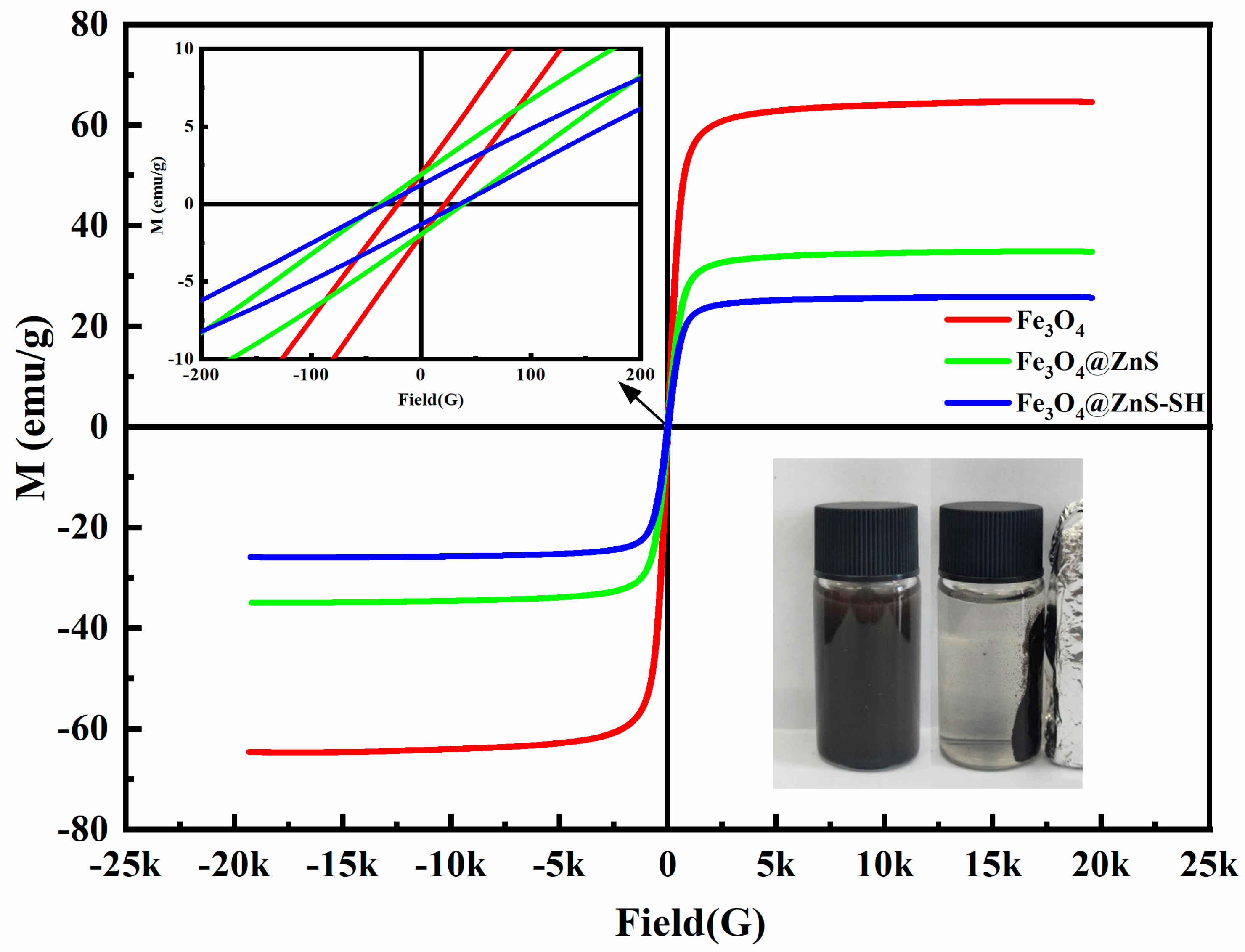
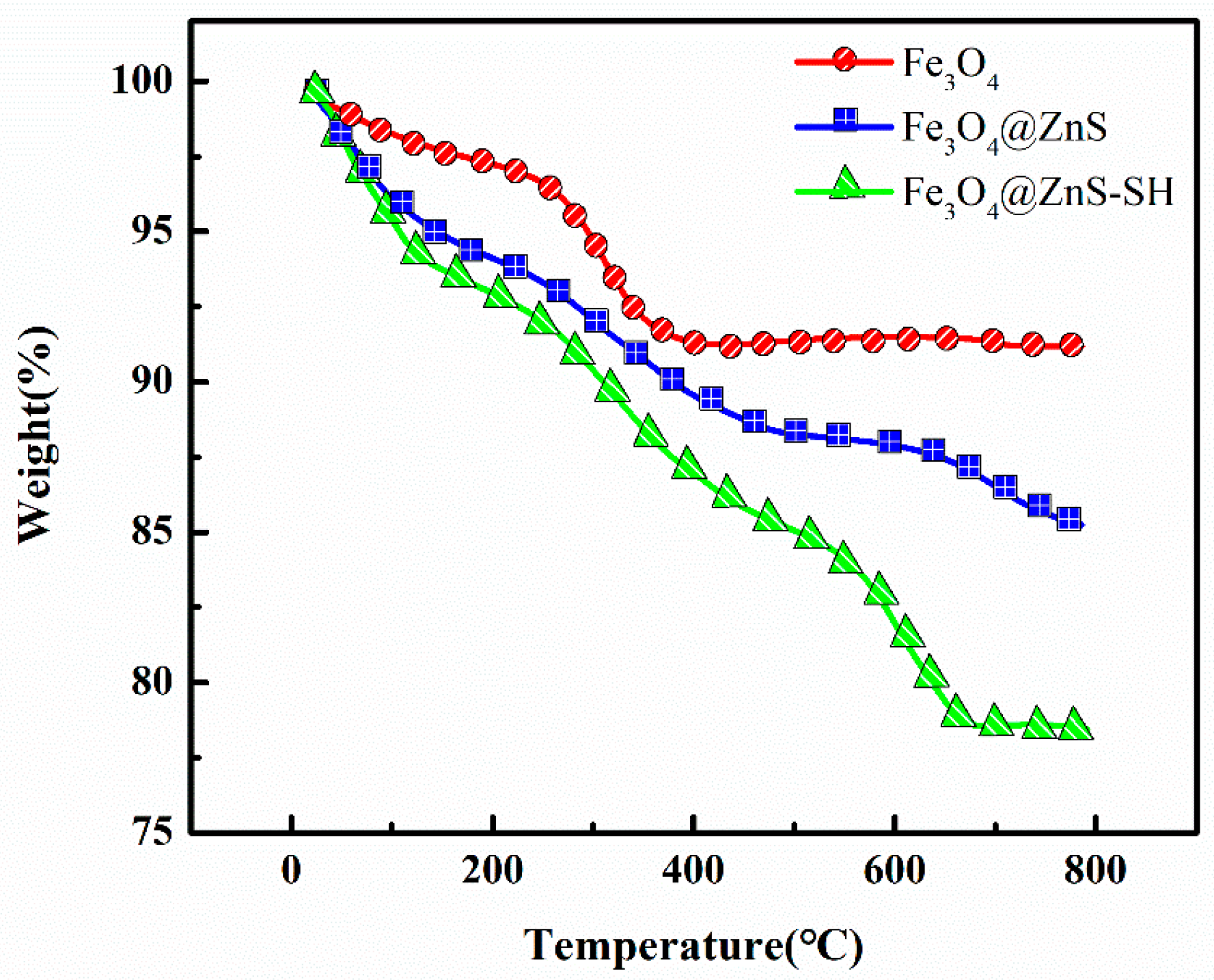
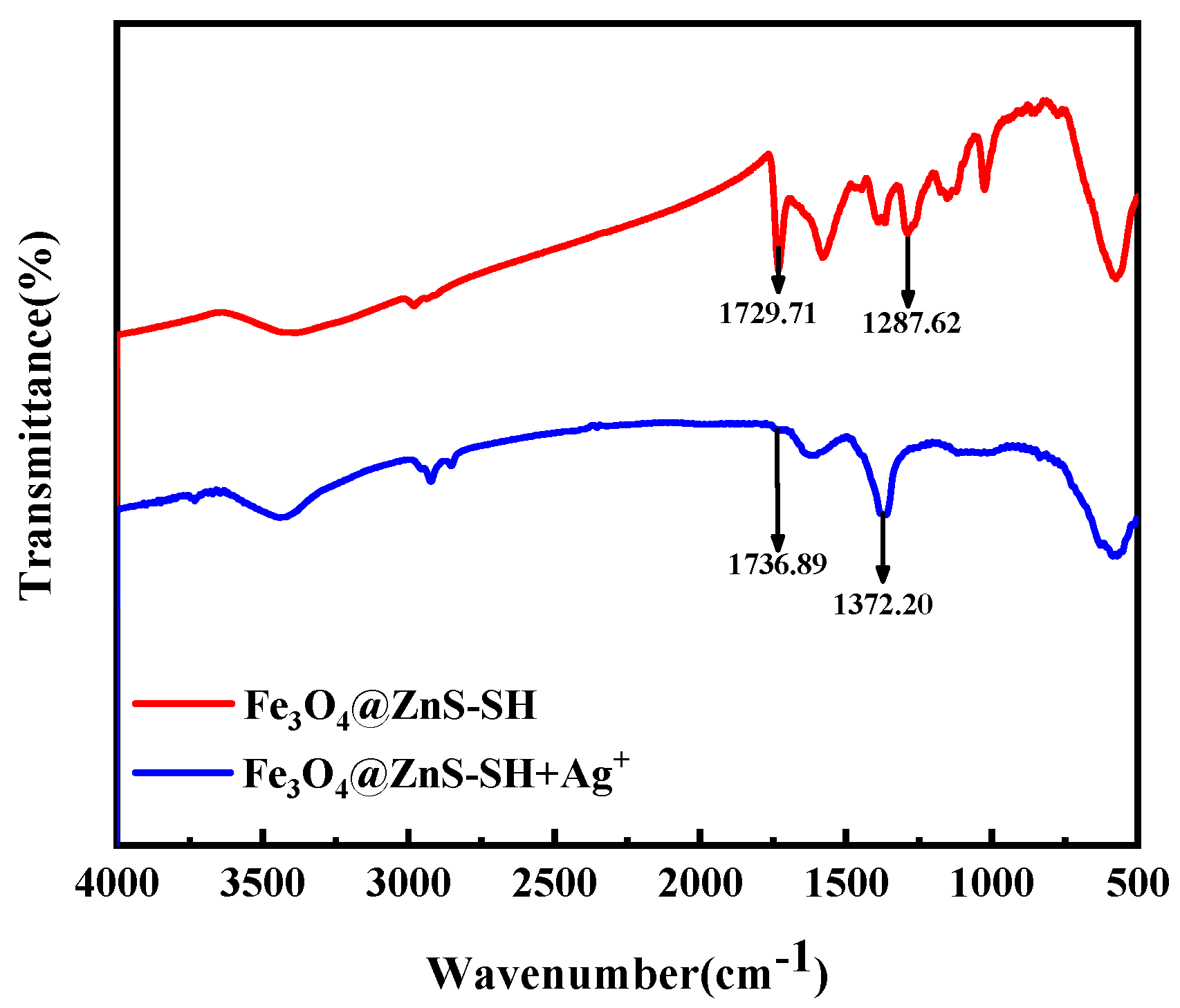
3.2. Characterization of Fe3O4@ZnS@Ethyl Thioglycolate Magnetic Nanoparticles
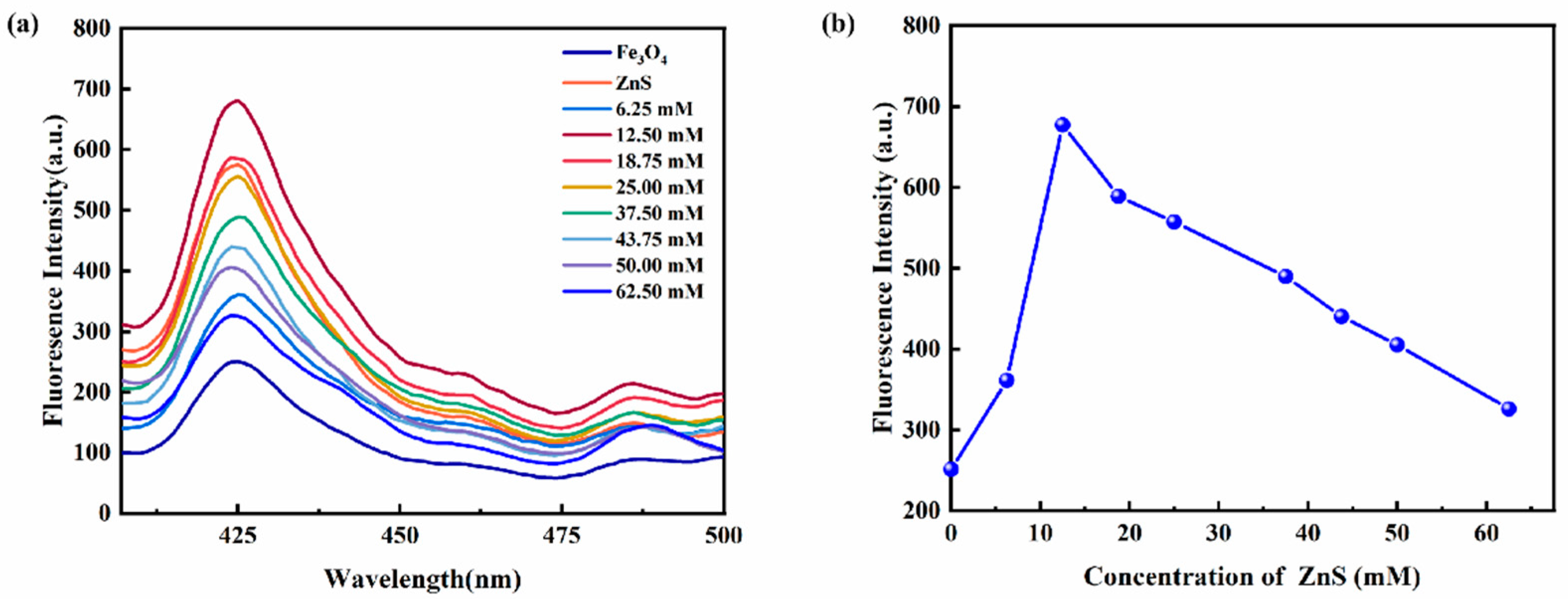
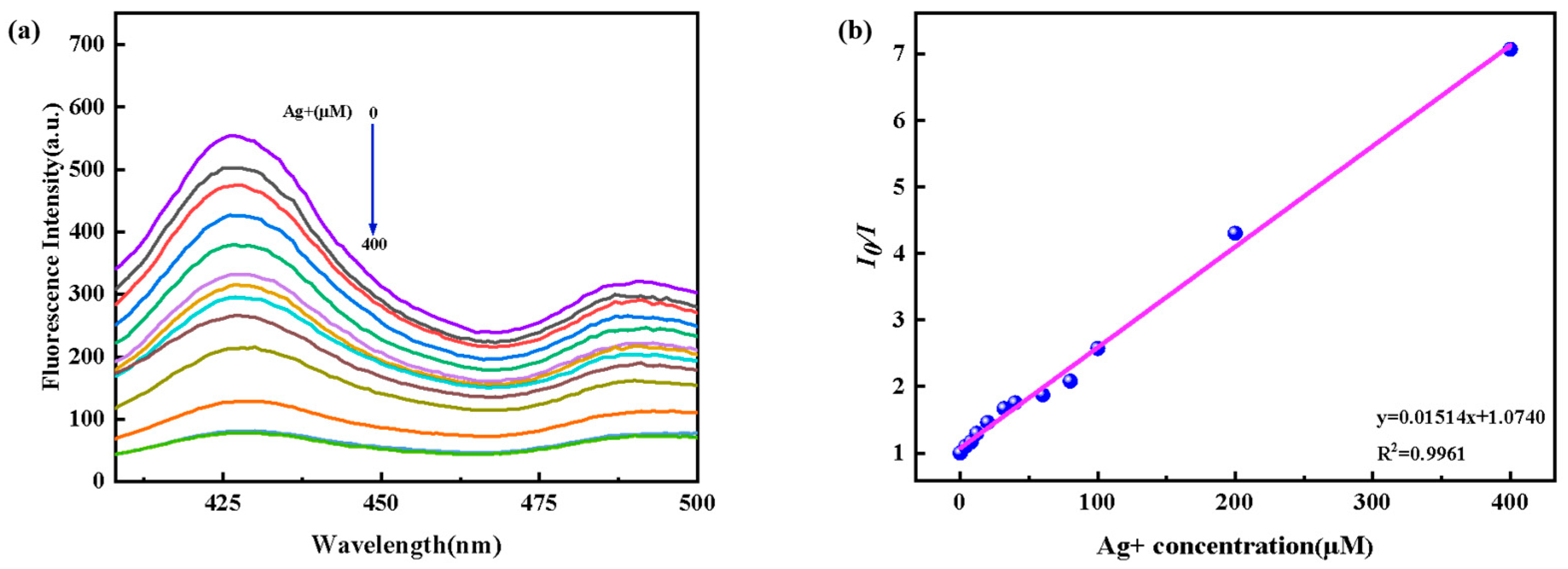
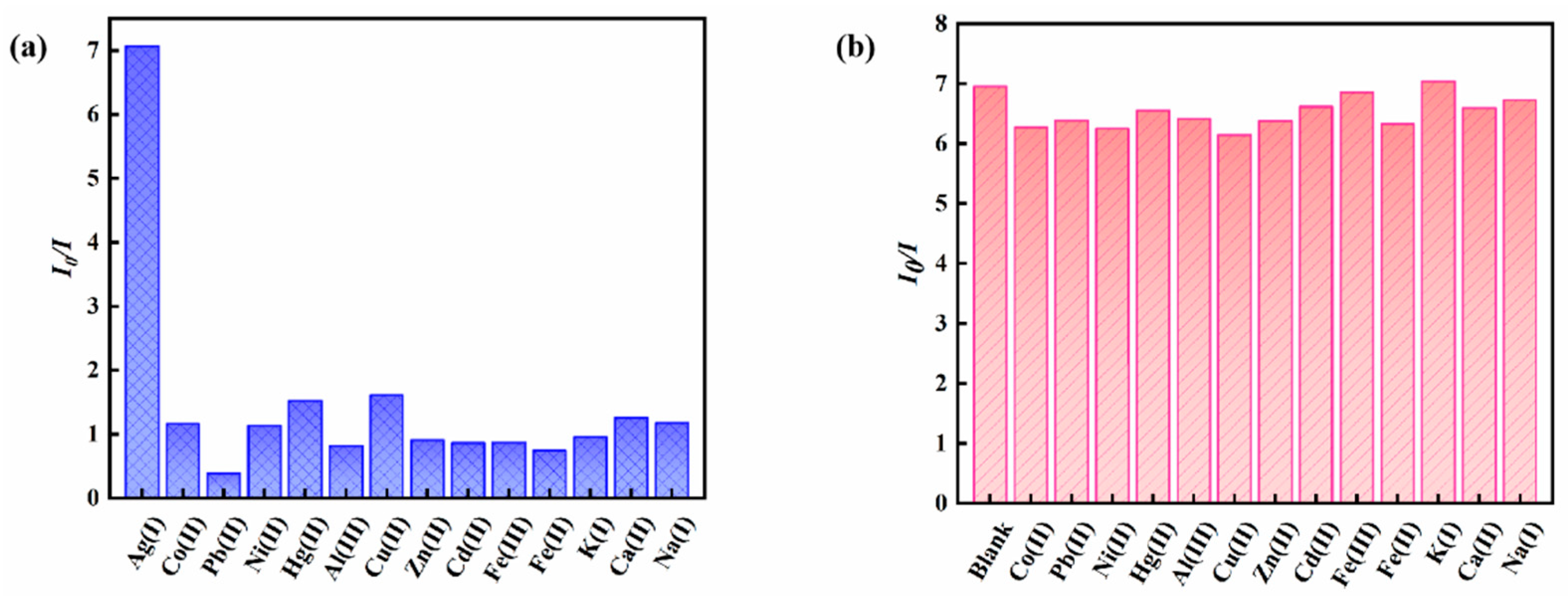
3.3. Performance Analysis of Magnetic Fluorescence Nanoprobe Fe3O4@ZnS-SH
3.4. Removal of Ag+
3.5. Detection of Ag+ in Real Samples
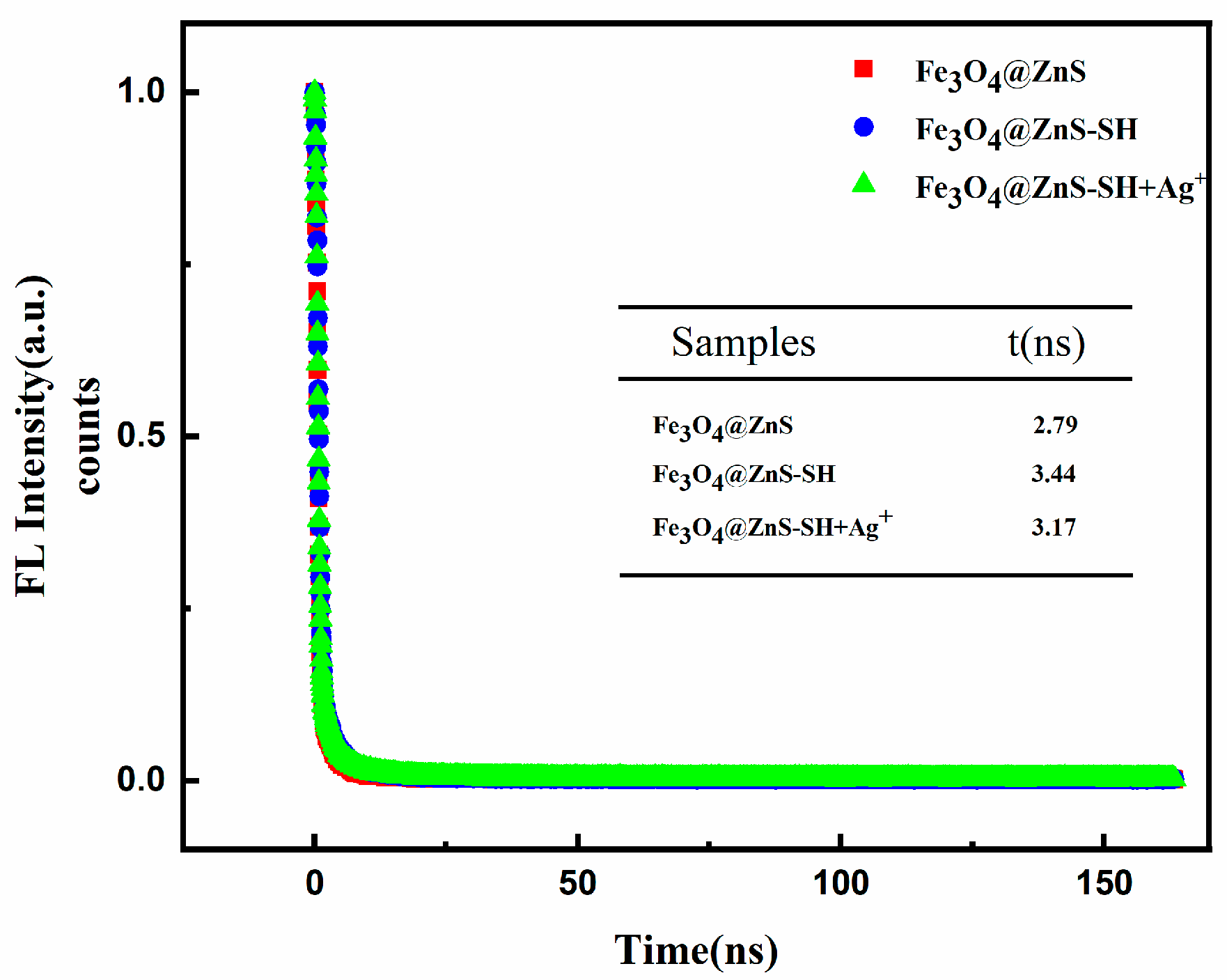
3.6. Detection Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carter, S.; Clough, R.; Fisher, A.; Gibson, B.; Russell, B.; Waack, J. Atomic spectrometry update: Review of advances in the analysis of metals, chemicals and materials. J. Anal. Atom. Spectrom. 2020, 35, 2410–2474. [Google Scholar] [CrossRef]
- Pan, X.-H.; Zu, J.-H.; Diao, J.-J.; Xue, Y.; Liu, S.-Y. Rapid and selective recovery of Ag(I) from simulative electroplating effluents by sulfydryl-rich covalent organic framework (COF-SH) with high adsorption capacity. Colloid. Surface. A 2022, 648, 129156. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.-D.; Cao, H.-Y.; Huang, Y.-M. An inorganic base stripping approach to synthesize N-doped Ti3C2 quantum dots as fluorescence nanoprobe for the simultaneous detection of Co2+ and Ag+ ions. Microchem. J. 2022, 180, 107629. [Google Scholar] [CrossRef]
- Carter, S.; Clough, R.; Fisher, A.; Gibson, B.; Russell, B.; Waack, J. Atomic spectrometry update: Review of advances in the analysis of metals, chemicals and materials. J. Anal. Atom. Spectrom. 2018, 33, 1802–1848. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Zhang, L.; Hao, Y.-M.; Dong, W.-J.; Liu, Y.; Song, S.-M.; Shuang, S.-M.; Dong, C.; Gong, X.-J. Ratiometric fluorescent sensors for sequential on-off-on determination of riboflavin, Ag+ and l-cysteine based on NPCl-doped carbon quantum dots. Anal. Chim. Acta 2021, 1144, 1–13. [Google Scholar] [CrossRef]
- Greda, K.; Welna, M.; Pohl, P. Determination of Ag, Bi, Cd, Hg, Pb, Tl, and Zn by inductively coupled plasma mass spectrometry combined with vapor generation assisted by solution anode glow discharge—A preliminary study. Talanta 2022, 246, 123500. [Google Scholar] [CrossRef]
- Ji, Z.-P.; Shu, Y.; Xu, Q.; Hu, X.-Y. Ultrasensitive electrochemiluminescence determination of trace Ag ions based on the signal amplification caused by its catalytic effect on Mn(II) oxidation using graphite catheter as electrode. Talanta 2018, 187, 188–192. [Google Scholar] [CrossRef]
- Çetin, T.; Tokalıoğlu, Ş.; Ülgen, A.; Şahan, S.; Özentürk, İ.; Soykan, C. Synthesis/characterization of a new chelating resin and on-line solid phase extraction for the determination of Ag(I) and Pd(II) from water, cream, anode slime and converter samples by flow injection flame atomic absorption spectrometry. Talanta 2013, 105, 340–346. [Google Scholar] [CrossRef]
- Junk, T.; Rehkämper, M.; Laycock, A. High-sensitivity tracing of stable isotope labeled Ag nanoparticles in environmental samples using MC-ICP-MS. J. Anal. Atom. Spectrom. 2019, 34, 1173–1183. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, Y.-J.; Tuersun, T.; Yu, Y.; Zhi, J.-F. Simple preparation and highly selective detection of silver ions using an electrochemical sensor based on sulfur-doped graphene and a 3,3′,5,5′-tetramethylbenzidine composite modified electrode. Analyst 2018, 143, 2076–2082. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.-W.; Wu, M.-F.; Chen, S.; Zheng, R.-Q.; Xing, Y.-R.; He, L.; Duan, Y.-X.; Wang, X. Direct and sensitive determination of Cu, Pb, Cr and Ag in soil by laser ablation microwave plasma torch optical emission spectrometry. Talanta 2022, 246, 123516. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-J.; Zuo, G.-C.; Qi, X.-L.; Wei, W.; Pan, X.-H.; Su, T.; Zhang, J.-F.; Dong, W. Selective determination of Ag+ using Salecan derived nitrogen doped carbon dots as a fluorescent probe. Mat. Sci. Eng. C 2017, 77, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-Y.; Wang, X.-H.; Huang, S.-L.; Chen, J.-R.; Luo, H.-Q.; Li, N.-B. Evolving a high-performance bio-imaging tool derived from a compact fluorophore as well as creating a reaction-based fluorescent probe for precise determination of Ag+. Chem. Eng. J. 2022, 457, 140756. [Google Scholar] [CrossRef]
- Zeng, X.-D.; Chen, X.; Chen, J.; Ma, M.-S.; Jin, H.; Yu, S.-H.; Liu, Z.-G. A simple highly selective ratiometric fluorescent probe for detection of peroxynitrite and its bioimaging applications. Dye. Pigment. 2023, 210, 110993. [Google Scholar] [CrossRef]
- Zeng, X.-D.; Chen, J.; Yu, S.-H.; Liu, Z.-G.; Ma, M.-S. A highly selective and sensitive “turn-on” fluorescent probe for Fe2+ and its applications. J. Lumin. 2022, 250, 119069. [Google Scholar] [CrossRef]
- Zeng, X.-D.; Li, Z.-L.; Fu, J.; Jiang, C.; Ma, M.-S.; Zhu, L.; Jin, X. A novel ultrasensitive peroxynitrite-specific fluorescent probe and its bioimaging applications in living systems. Dye. Pigment. 2021, 186, 108982. [Google Scholar] [CrossRef]
- Zeng, X.-D.; Jiang, C.; Zhang, Q.; Chai, D.-K.; Ma, M.-S.; Chen, J.-Z.; Liu, G. A novel simple fluorescent probe for the detection of hydrogen peroxide in vivo with high selectivity. J. Lumin. 2021, 240, 118422. [Google Scholar] [CrossRef]
- Mastracco, P.; Gonzàlez-Rosell, A.; Evans, J.; Bogdanov, P.; Copp, S.M. Chemistry-Informed Machine Learning Enables Discovery of DNA-Stabilized Silver Nanoclusters with Near-Infrared Fluorescence. ACS Nano 2022, 16, 16322–16331. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Tang, C.-M.; Huang, M.-M.; Rong, X.; Lin, H.-Q.; Su, R.-X.; Wang, Y.-F.; Qi, W. One-Step Synthesis of Peptide- Gold Nanoclusters with Tunable Fluorescence and Enhanced Gene Delivery Efficiency. Langmuir 2022, 38, 14799–14807. [Google Scholar] [CrossRef]
- Xia, L.P.; Zhang, X.N.; Chen, Y.; Peng, J.; Liu, M.-R.; Huo, D.-Z.; Li, G.-Y.; He, H. A fluorescence turn-on strategy to achieve detection of captopril based on Ag nanoclusters. Chem. Phys. Lett. 2022, 807, 140085. [Google Scholar] [CrossRef]
- Yuan, C.; Liu, B.-H.; Liu, F.; Han, M.-Y.; Zhang, Z.-P. Fluorescence “Turn On” Detection of Mercuric Ion Based on Bis(dithiocarbamato)copper(II) Complex Functionalized Carbon Nanodots. Anal. Chem. 2014, 86, 1123–1130. [Google Scholar] [CrossRef]
- Maestro, L.M.; Rodríguez, E.M.; Rodríguez, F.S.; Iglesias-de la Cruz, M.C.; Juarranz, A.; Naccache, R.; Vetrone, F.; Jaque, D.; Capobianco, J.A.; Solé, J.G. CdSe Quantum Dots for Two-Photon Fluorescence Thermal Imaging. Nano Lett. 2010, 10, 5109–5115. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.S.; Harteveld, C.A.M.; Vancso, G.J.; Huskens, J.; Cloetens, P.; Vos, W.L. Targeted Positioning of Quantum Dots Inside 3D Silicon Photonic Crystals Revealed by Synchrotron X-ray Fluorescence Tomography. ACS Nano 2022, 16, 3674–3683. [Google Scholar] [CrossRef]
- Das, P.; Biswas, S.; Bhattacharya, S.S.; Nath, P. Carbon Nanodot-Neutral Red-Based Photometric and Fluorescence Sensing for Trace Detection of Nitrite in Water and Soil Using Smartphone. ACS Appl. Nano Mater. 2022, 5, 3265–3274. [Google Scholar] [CrossRef]
- Guo, X.-R.; Yang, J.; Wei, Y.-B.; Wang, L.-S.; Pi, J. Graphitic Carbon Nitride Quantum Dots in Dual-Mode Fluorescence Switching Platforms for Trace Analysis of Ag(I) and l-Cysteine. ACS Appl. Nano Mater. 2022, 5, 4230–4240. [Google Scholar] [CrossRef]
- Yang, B.; He, X.-Y.; Xu, H.-F.; Yao, J.-X.; Li, G. Ultrasensitive visualization detection of sodium borohydride based on surface treated transparent molybdenum oxide quantum dots. Mater. Lett. 2023, 331, 133431. [Google Scholar] [CrossRef]
- Li, Y.-C.; Jia, W.-X.; Yang, Z.-P.; Yang, J.; Chen, Y.-Y.; Yang, J.-Q.; Zhang, W.; Lu, C.-F.; Wang, X.-X. A fluorescent and colorimetric probe for MoO42-based on sulfur quantum dots nanozyme. J. Alloys Compd. 2023, 934, 167926. [Google Scholar] [CrossRef]
- Dhandapani, E.; Maadeswaran, P.; Raj, R.M.; Raj, V.; Kandiah, K.; Duraisamy, N. A potential forecast of carbon quantum dots (CQDs) as an ultrasensitive and selective fluorescence probe for Hg (II) ions sensing. Mat. Sci. Eng. 2023, 287, 116098. [Google Scholar] [CrossRef]
- Wang, H.-L.; Zhu, W.-J.; Fang, M.; Xu, Y.; Li, C. Turn-on fluorescence probe for high sensitive and selective detection of Ag+ by L-glutathione capped CdTe quantum dots in aqueous medium. J. Lumin. 2016, 180, 14–19. [Google Scholar] [CrossRef]
- Lou, Y.; Sun, W.; Jiang, L.; Fan, X.-G.; Zhang, K.-Y.; Chen, S.-P.; Li, Z.-Y.; Ji, J.-Y.; Ou, J.; Liao, L.; et al. Double-enhanced fluorescence of graphene quantum dots from cane molasses via metal and PEG modification for detecting metal ions and pigments. Opt. Mater. 2022, 133, 113037. [Google Scholar] [CrossRef]
- Dong, W.-Y.; Yan, J.-Q.; Wu, Q.-S.; Hu, X.-J. A fluorescent probe constructed of water-soluble dual-element-doped carbon quantum dots for rapid and highly sensitive detection of Ag+. Inorg. Chem. Commun. 2021, 134, 109052. [Google Scholar] [CrossRef]
- Shalali, F.; Cheraghi, S.; AliTaher, M. A sensitive electrochemical sensor amplified with ionic liquid and N-CQD/Fe3O4 nanoparticles for detection of raloxifene in the presence of tamoxifen as two essentials anticancer drugs. Mater. Chem. Phys. 2022, 278, 125658. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.-Y.; Wang, C.-Q.; Tian, X.-Y.; Chang, X.-H.; Ren, Y.; Yu, S.S. Applications of surface functionalized Fe3O4 NPs-based detection methods in food safety. Food Chem. 2021, 342, 128343. [Google Scholar] [CrossRef]
- Hatami, M.; Mohammadi-Rezaei, S.; Tahari, M.; Jing, D. Recent developments in magneto-hydrodynamic Fe3O4 nanofluids for different molecular applications: A review study. J. Molecul. Liq. 2018, 250, 244–258. [Google Scholar] [CrossRef]
- Rakhtshah, J. A comprehensive review on the synthesis, characterization, and catalytic application of transition-metal Schiff-base complexes immobilized on magnetic Fe3O4 nanoparticles. Coordin. Chem. Rev. 2022, 467, 214614. [Google Scholar] [CrossRef]
- Nafchi, R.F.; Ahmadi, R.; Heydari, M.; Rahimipour, M.R.; Molaei, M.J.; Unsworth, L. In Vitro Study: Synthesis and Evaluation of Fe3O4/CQD Magnetic/Fluorescent Nanocomposites for Targeted Drug Delivery, MRI, and Cancer Cell Labeling Applications. Langmuir 2022, 38, 3804–3816. [Google Scholar] [CrossRef] [PubMed]
- Koc, K.; Karakus, B.; Rajar, K.; Alveroglu, E. Synthesis and Characterization of ZnS@Fe3O4 Fluorescent-Magnetic Bifunctional Nanospheres. Superlattices Microstruct. 2017, 110, 198–204. [Google Scholar] [CrossRef]
- Song, X.-J.; Yang, F.; Fang, Q.-L.; Hu, H.-M.; Xu, F. Synthesis of Magnetic MWNTs/ZnS/Fe3O4 Nanocomposites and Their Enhanced Photocatalytic Activity. Synth. React. Inorg. M. 2016, 46, 638–641. [Google Scholar] [CrossRef]
- Ding, Y.-D.; Sun, Y.-W.; Hong, X.; Cong, T.; Wen, X.-K.; Zhao, H.-Y. Fabrication of Fe3O4/ZnS nanocomposites towards ultrasensitive resonant Raman scattering-based immunoassays. Mater. Lett. 2019, 253, 354–357. [Google Scholar] [CrossRef]
- Mondal, D.K.; Phukan, G.; Paul, N.; Borah, J.P. Improved self heating and optical properties of bifunctional Fe3O4/ZnS nanocomposites for magnetic hyperthermia application. J. Magn. Magn. Mater. 2021, 528, 167809. [Google Scholar] [CrossRef]
- Dafeh, S.R.; Iranmanesh, P.; Salarizadeh, P. Fabrication, optimization, and characterization of ultra-small superparamagnetic Fe3O4 and biocompatible Fe3O4@ZnS core/shell magnetic nanoparticles: Ready for biomedicine applications. Mat. Sci. Eng. C 2019, 98, 205–212. [Google Scholar] [CrossRef]
- Stefan, M.; Leostean, C.; Pana, O.; Soran, M.L.; Suciu, R.C.; Gautron, E.; Chauvet, O. Synthesis and characterization of Fe3O4@ZnS and Fe3O4@Au@ZnScore-shell nanoparticles. Appl. Surf. Sci. 2014, 288, 180–192. [Google Scholar] [CrossRef]
- Feng, L.-P.; Zhang, L.-X.; Chen, X.; Zhang, C.-X.; Mao, G.-J.; Wang, H. A visible light-driven photoelectrochemical sensor for mercury (II) with “turn-on” signal output through in-situ formation of double type-II heterostructure using CdS nanowires and ZnS quantum dots. Chem. Eng. J. 2022, 441, 136073. [Google Scholar] [CrossRef]
- Gerulová, K.; Kucmanová, A.; Sanny, Z.; Garaiová, Z.; Seiler, E.; Čaplovičová, M.; Čaplovič, Ľ.; Palcut, M. Fe3O4-PEI Nanocomposites for Magnetic Harvesting of Chlorella vulgaris, Chlorella ellipsoidea, Microcystis aeruginosa, and Auxenochlorella protothecoides. Nanomaterials 2022, 12, 1786. [Google Scholar] [CrossRef] [PubMed]
- Savvidou, M.G.; Dardavila, M.M.; Georgiopoulou, I.; Louli, V.; Stamatis, H.; Kekos, D.; Voutsas, E. Optimization of Microalga Chlorella vulgaris Magnetic Harvesting. Nanomaterials 2021, 11, 1614. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.-H.; Chen, H.; Jiang, F.; Hu, Z.-X.; Wang, X. Construction of Acetaldehyde-Modified g-C3N4 Ultrathin Nanosheets via Ethylene Glycol-Assisted Liquid Exfoliation for Selective Fluorescence Sensing of Ag+. ACS Appl. Mater. Interfaces 2018, 10, 44624–44633. [Google Scholar] [CrossRef]
- Zhu, B.-C.; Yang, P.-L.; Yu, H.-Q.; Yan, L.-G.; Wei, Q.; Du, B. Development of a novel water-soluble magnetic fluorescent nanoparticle for the selective detection and removal of Cu2+. Nanotechnology 2013, 24, 495502. [Google Scholar] [CrossRef]
- Peng, X.-Q.; Zhang, W.; Gai, L.-G.; Jiang, H.-H.; Wang, Y.; Zhao, L.-C. Dedoped Fe3O4/PPy nanocomposite with high anti-interfering ability for effective separation of Ag(I) from mixed metal-ion solution. Chem. Eng. J. 2015, 218, 197–205. [Google Scholar] [CrossRef]
- Ghanei-Motlagh, M.; Fayazi, M.; Taher, M.A.; Jalalinejad, A. Application of magnetic nanocomposite modified with a thiourea based ligand for the preconcentration and trace detection of silver(I) ions by electrothermal atomic absorption spectrometry. Chem. Eng. J. 2016, 290, 53–62. [Google Scholar] [CrossRef]
- Yin, X.-C.; Long, J.; Xi, Y.; Luo, X.-B. Recovery of silver from wastewater using a new magnetic photocatalytic ion-imprinted polymer. ACS Sustain. Chem. Eng. 2017, 5, 2090–2097. [Google Scholar] [CrossRef]
- Wei, W.; Li, A.; Pi, S.; Wang, Q.; Zhou, L.; Yang, J.; Ma, F.; Ni, B. Synthesis of Core-Shell magnetic nanocomposite Fe3O4@microbial extracellular polymeric substances for simultaneous redox sorption and recovery of silver ions as silver nanoparticles. ACS Sustain. Chem. Eng. 2018, 1, 749–756. [Google Scholar] [CrossRef]
- Zhao, J.-J.; Luan, L.-P.; Li, Z.-W.; Duan, Z.-F.; Li, Y.-F.; Zheng, S.-B.; Xue, Z.-X.; Xu, W.-L.; Niu, Y.-Z. The adsorption property and mechanism for Hg(II) and Ag(I) by Schiff base functionalized magnetic Fe3O4 from aqueous solution. J. Alloys Compd. 2020, 825, 154051. [Google Scholar] [CrossRef]
- Luan, L.-P.; Tang, B.-T.; Liu, Y.-F.; Wang, A.-L.; Zhang, B.-B.; Xu, W.-L.; Niu, Y.-Z. Selective capture of Hg(II) and Ag(I) from water by sulfur-functionalized polyamidoamine dendrimer/magnetic Fe3O4 hybrid materials. Sep. Purif. Technol. 2021, 257, 117902. [Google Scholar] [CrossRef]
- Luo, L.-X.; Ye, Y.; Wang, H.; Liu, Z.; Li, J.-Q. Rapid detection of Ag+ in food using cholesteric chiral artificial receptor L5. Microchem. J. 2023, 190, 108633. [Google Scholar]



| Type of Sensor | Detection Range | Detection Limit | Reference |
|---|---|---|---|
| N-doped Ti3C2 quantum | 0.5–150 μM | 0.1 μM | [3] |
| NPCl-doped carbon quantum dots | 15.89–27.66 μM | 26.38 μM | [5] |
| Nitrogen0doped carbon dots | 0–40 μM | 0.11 μM | [12] |
| Dual-element-doped carbon quantum dots | 0.1–700 μM | 0.03 μM | [31] |
| Acetaldehyde-modified g-C3N4 nanosheets | 0–30 μM | 1.0 μM | [46] |
| Fe3O4@ZnS-SH | 0–400 μM | 0.20 μM | This work |
| Adsorbents | Maximum Adsorption Capacities (mg/g) | Ref. |
|---|---|---|
| Fe3O4/polypyrrole nanocomposite | 143.3 | [48] |
| Activated carbon/γ-Fe2O3- 4,4′-bis-(3-phenylthiourea)diphenyl methane | 32.6 | [49] |
| Fe3O4@SiO2@Tio2- Ion imprinting polymers | 35.48 | [50] |
| Fe3O4@SiO2-HO-S | 166.32 | [51] |
| Fe3O4-sulfur-functionalized polyamidoamine | 139.32 | [52] |
| Fe3O4@microbial extracellular polymeric substance | 47.6 | [53] |
| Fe3O4@ZnS-SH | 107.022 | This work |
| Sample | Added (μM) | Measured (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Tap water | 1 5.0 10.0 | 0.98 5.29 10.30 | 98.00 105.80 103.00 | 2.3 3.1 2.9 |
| Mineral water | 1 5.0 10.0 | 1.02 4.96 10.75 | 102.00 99.20 107.50 | 3.2 2.7 2.5 |
| Electrolysis wastewater | 1 5.0 10.0 | 0.87 4.43 8.75 | 87.00 88.60 87.50 | 4.5 3.9 4.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Chen, J.; Ma, M.; Yu, S.; Liu, Z.; Zeng, X. An Ethyl-Thioglycolate-Functionalized Fe3O4@ZnS Magnetic Fluorescent Nanoprobe for the Detection of Ag+ and Its Applications in Real Water Solutions. Nanomaterials 2023, 13, 1992. https://doi.org/10.3390/nano13131992
Chen X, Chen J, Ma M, Yu S, Liu Z, Zeng X. An Ethyl-Thioglycolate-Functionalized Fe3O4@ZnS Magnetic Fluorescent Nanoprobe for the Detection of Ag+ and Its Applications in Real Water Solutions. Nanomaterials. 2023; 13(13):1992. https://doi.org/10.3390/nano13131992
Chicago/Turabian StyleChen, Xin, Jie Chen, Mingshuo Ma, Shihua Yu, Zhigang Liu, and Xiaodan Zeng. 2023. "An Ethyl-Thioglycolate-Functionalized Fe3O4@ZnS Magnetic Fluorescent Nanoprobe for the Detection of Ag+ and Its Applications in Real Water Solutions" Nanomaterials 13, no. 13: 1992. https://doi.org/10.3390/nano13131992
APA StyleChen, X., Chen, J., Ma, M., Yu, S., Liu, Z., & Zeng, X. (2023). An Ethyl-Thioglycolate-Functionalized Fe3O4@ZnS Magnetic Fluorescent Nanoprobe for the Detection of Ag+ and Its Applications in Real Water Solutions. Nanomaterials, 13(13), 1992. https://doi.org/10.3390/nano13131992