Fabrication of Z-Type TiN@(A,R)TiO2 Plasmonic Photocatalyst with Enhanced Photocatalytic Activity
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Preparation of the Catalyst
2.3. Characterization Methods
2.4. Photocatalytic Performance
3. Results and Discussion
3.1. Structural Characterization of the Photocatalysts
3.2. Photodegradation Performance
| Photocatalyst | C0 (mg/L) | Dosage (mg) | Light Source | Degradation Rate | Time (min) | Kinetic Rate (min−1) | Ref. |
|---|---|---|---|---|---|---|---|
| Ag@TiO2 | 10 | 100 | 150 W Xe lamp | 98.2% | 120 | 0.0188 | [46] |
| TiO2 hollow boxes | 100 | 50 | Visible light | 96.5% | 240 | 0.0025 | [47] |
| Ag2O/TiO2 | 4.79 | 40 | UV light | 87.7% | 80 | 0.0277 | [48] |
| Ag/ZnO/AgO/TiO2 | 10 | 30 | 350 W Xe lamp | 99.3% | 100 | 0.0230 | [49] |
| Pt/A/R-TiO2 | - | - | UV light | 92.4% | 90 | 0.0280 | [50] |
| Bi2WO6/TiO2/Pt | 20 | 100 | UV light | 60.0% | 40 | 0.0210 | [51] |
| g-C3N4/TiO2 | 50 | 5 | Visible light | 87.0% | 300 | 0.0115 | [52] |
| A/R-TiO2 | 10 | 25 | UV light | About 100% | 50 | - | [53] |
| Au/A/R-TiO2 | - | - | UV light | 97% | 60 | 0.0470 | [54] |
| TiN@(A,R)TiO2 | 10 | 30 | Visible light | 97.0% | 90 | 0.0227 | This work |
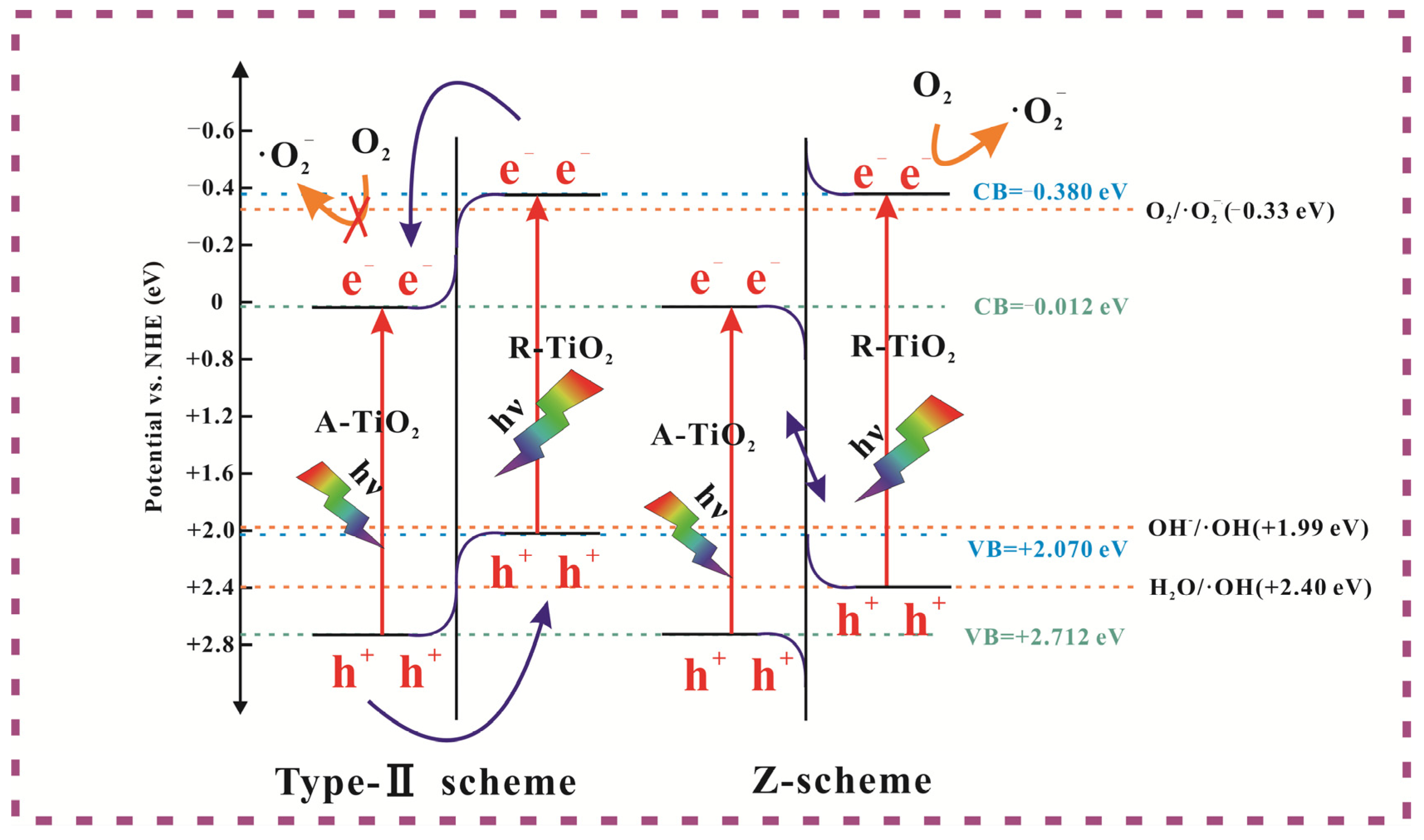
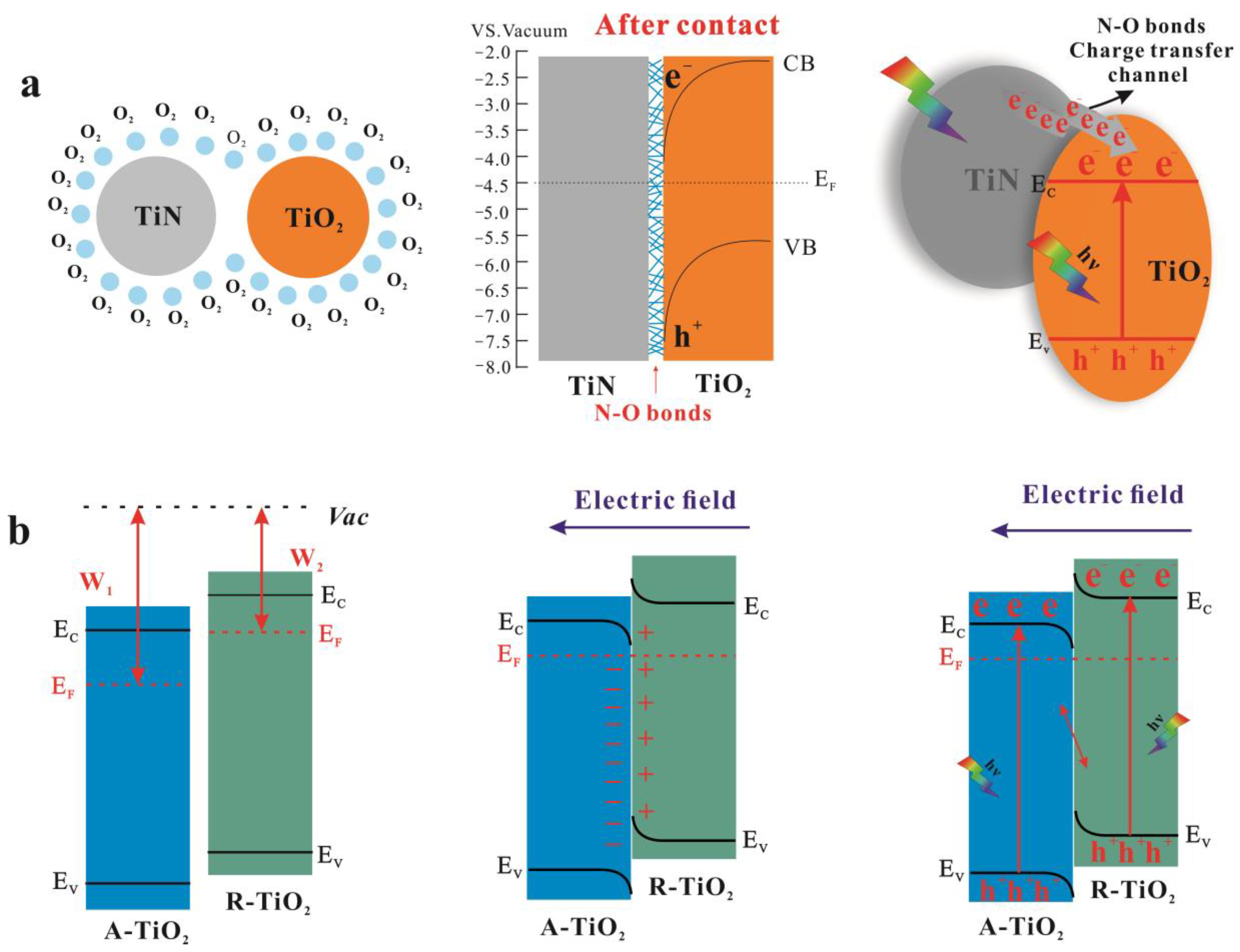
3.3. Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Masudy-Panah, S.; Siavash Moakhar, R.; Chua, C.S.; Kushwaha, A.; Dalapati, G.K. Stable and Efficient CuO Based Photocathode through Oxygen-Rich Composition and Au–Pd Nanostructure Incorporation for Solar-Hydrogen Production. ACS Appl. Mater. Interfaces 2017, 9, 27596–27606. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent Developments in Photocatalytic Water Treatment Technology: A Review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Domen, K. Particulate Photocatalysts for Water Splitting: Recent Advances and Future Prospects. ACS Energy Lett. 2019, 4, 542–549. [Google Scholar] [CrossRef]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-Photocatalytic Materials: Possibilities and Challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.-W.; Mohite, B.M. Role of Nanotechnology in Photocatalysis Application. Recent Pat. Nanotechnol. 2023, 17, 5–7. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kang, S.-W.; Fujishima, A.; Terashima, C. Enhanced Photocatalytic Degradation Activity Using the V2O5/RGO Composite. Nanomaterials 2023, 13, 338. [Google Scholar] [CrossRef]
- Carey, J.H.; Lawrence, J.; Tosine, H.M. Photodechlorination of PCB’s in the Presence of Titanium Dioxide in Aqueous Suspensions. Bull. Environ. Contam. Toxicol. 1976, 16, 697–701. [Google Scholar] [CrossRef]
- Poudel, M.B.; Kim, A.A. Silver Nanoparticles Decorated TiO2 Nanoflakes for Antibacterial Properties. Inorg. Chem. Commun. 2023, 152, 110675. [Google Scholar] [CrossRef]
- Hou, J.; Yang, C.; Wang, Z.; Jiao, S.; Zhu, H. Bi2O3 Quantum Dots Decorated Anatase TiO2 Nanocrystals with Exposed {001} Facets on Graphene Sheets for Enhanced Visible-Light Photocatalytic Performance. Appl. Catal. B Environ. 2013, 129, 333–341. [Google Scholar] [CrossRef]
- Kment, S.; Riboni, F.; Pausova, S.; Wang, L.; Wang, L.; Han, H.; Hubicka, Z.; Krysa, J.; Schmuki, P.; Zboril, R. Photoanodes Based on TiO2 and α-Fe2O3 for Solar Water Splitting—Superior Role of 1D Nanoarchitectures and of Combined Heterostructures. Chem. Soc. Rev. 2017, 46, 3716–3769. [Google Scholar] [CrossRef]
- Reddy, N.L.; Kumar, S.; Krishnan, V.; Sathish, M.; Shankar, M.V. Multifunctional Cu/Ag Quantum Dots on TiO2 Nanotubes as Highly Efficient Photocatalysts for Enhanced Solar Hydrogen Evolution. J. Catal. 2017, 350, 226–239. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, N.; Lou, L.; Iwasaki, K.; Wu, H.; Luo, Y.; Li, D.; Nakata, K.; Fujishima, A.; Meng, Q. Localized Surface Plasmon Resonance Enhanced Photocatalytic Hydrogen Evolution via Pt@Au NRs/C3N4 Nanotubes under Visible-Light Irradiation. Adv. Funct. Mater. 2019, 29, 1806774. [Google Scholar] [CrossRef]
- Boerigter, C.; Campana, R.; Morabito, M.; Linic, S. Evidence and Implications of Direct Charge Excitation as the Dominant Mechanism in Plasmon-Mediated Photocatalysis. Nat. Commun. 2016, 7, 10545. [Google Scholar] [CrossRef] [Green Version]
- Guler, U.; Kildishev, A.V.; Boltasseva, A.; Shalaev, V.M. Plasmonics on the Slope of Enlightenment: The Role of Transition Metal Nitrides. Faraday Discuss. 2015, 178, 71–86. [Google Scholar] [CrossRef]
- Lima, L.P.B.; Diniz, J.A.; Doi, I.; Godoy Fo, J. Titanium Nitride as Electrode for MOS Technology and Schottky Diode: Alternative Extraction Method of Titanium Nitride Work Function. Microelectron. Eng. 2012, 92, 86–90. [Google Scholar] [CrossRef]
- Al-Hamdi, A.M.; Rinner, U.; Sillanpää, M. Tin Dioxide as a Photocatalyst for Water Treatment: A Review. Process Saf. Environ. Prot. 2017, 107, 190–205. [Google Scholar] [CrossRef]
- Mascaretti, L.; Barman, T.; Bricchi, B.R.; Münz, F.; Li Bassi, A.; Kment, Š.; Naldoni, A. Controlling the Plasmonic Properties of Titanium Nitride Thin Films by Radiofrequency Substrate Biasing in Magnetron Sputtering. Appl. Surf. Sci. 2021, 554, 149543. [Google Scholar] [CrossRef]
- Fakhouri, H.; Arefi-Khonsari, F.; Jaiswal, A.K.; Pulpytel, J. Enhanced Visible Light Photoactivity and Charge Separation in TiO2/TiN Bilayer Thin Films. Appl. Catal. Gen. 2015, 492, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Clatworthy, E.B.; Yick, S.; Murdock, A.T.; Allison, M.C.; Bendavid, A.; Masters, A.F.; Maschmeyer, T. Enhanced Photocatalytic Hydrogen Evolution with TiO2 –TiN Nanoparticle Composites. J. Phys. Chem. C 2019, 123, 3740–3749. [Google Scholar] [CrossRef]
- Cherevan, A.S.; Gebhardt, P.; Shearer, C.J.; Matsukawa, M.; Domen, K.; Eder, D. Interface Engineering in Nanocarbon–Ta2O5 Hybrid Photocatalysts. Energy Env. Sci 2014, 7, 791–796. [Google Scholar] [CrossRef]
- Bai, S.; Ge, J.; Wang, L.; Gong, M.; Deng, M.; Kong, Q.; Song, L.; Jiang, J.; Zhang, Q.; Luo, Y.; et al. A Unique Semiconductor-Metal-Graphene Stack Design to Harness Charge Flow for Photocatalysis. Adv. Mater. 2014, 26, 5689–5695. [Google Scholar] [CrossRef]
- Maarisetty, D.; Baral, S.S. Defect Engineering in Photocatalysis: Formation, Chemistry, Optoelectronics, and Interface Studies. J. Mater. Chem. A 2020, 8, 18560–18604. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, Y.; Kang, Y.; Niu, P.; Kang, X.; Yang, Z.; Ye, H.; Liu, G. Strong Interface Contact between NaYF4:Yb,Er and CdS Promoting Photocatalytic Hydrogen Evolution of NaYF4:Yb,Er/CdS Composites. J. Mater. Sci. Technol. 2022, 102, 1–7. [Google Scholar] [CrossRef]
- Li, J.; Gao, L.; Sun, J.; Zhang, Q.; Guo, J.; Yan, D. Synthesis of Nanocrystalline Titanium Nitride Powders by Direct Nitridation of Titanium Oxide. J. Am. Ceram. Soc. 2001, 84, 3045–3047. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Zhu, S.; Liu, Y.; Zhang, X.; Wang, J.; Braun, A. Covalent S-O Bonding Enables Enhanced Photoelectrochemical Performance of Cu2S/Fe2O3 Heterojunction for Water Splitting. Small 2021, 17, 2100320. [Google Scholar] [CrossRef] [PubMed]
- Ning, Q.; Zhang, L.; Liu, C.; Li, X.; Xu, C.; Hou, X. Boosting Photogenerated Carriers for Organic Pollutant Degradation via In-Situ Constructing Atom-to-Atom TiO2/ZrTiO4 Heterointerface. Ceram. Int. 2021, 47, 33298–33308. [Google Scholar] [CrossRef]
- Guan, Y.; Hu, S.; Li, P.; Zhao, Y.; Wang, F.; Kang, X. In-Situ Synthesis of Highly Efficient Direct Z-Scheme Cu3P/g-C3N4 Heterojunction Photocatalyst for N2 Photofixation. Nano 2019, 14, 1950083. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Ma, D. Advances in 2D/2D Z-Scheme Heterojunctions for Photocatalytic Applications. Sol. RRL 2021, 5, 2000397. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Jaroniec, M. Direct Z-Scheme Photocatalysts: Principles, Synthesis, and Applications. Mater. Today 2018, 21, 1042–1063. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Xu, C.; Wu, Y.; Hu, X.; Hou, X. Boron-Doped Rutile TiO2/Anatase TiO2/ZrTiO4 Ternary Heterojunction Photocatalyst with Optimized Phase Interface and Band Structure. Ceram. Int. 2020, 46, 20943–20953. [Google Scholar] [CrossRef]
- Kaur, M.; Shinde, S.L.; Ishii, S.; Jevasuwan, W.; Fukata, N.; Yu, M.-W.; Li, Y.; Ye, J.; Nagao, T. Marimo-Bead-Supported Core–Shell Nanocomposites of Titanium Nitride and Chromium-Doped Titanium Dioxide as a Highly Efficient Water-Floatable Green Photocatalyst. ACS Appl. Mater. Interfaces 2020, 12, 31327–31339. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Peng, F.; Wang, H.; Yu, H.; Yang, J. Preparation of Nitrogen Doped TiO2 Photocatalyst by Oxidation of Titanium Nitride with H2O2. Mater. Res. Bull. 2011, 46, 840–844. [Google Scholar] [CrossRef]
- Huang, D.G.; Liao, S.J.; Zhou, W.B.; Quan, S.Q.; Liu, L.; He, Z.J.; Wan, J.B. Synthesis of Samarium- and Nitrogen-Co-Doped TiO2 by Modified Hydrothermal Method and Its Photocatalytic Performance for the Degradation of 4-Chlorophenol. J. Phys. Chem. Solids 2009, 70, 853–859. [Google Scholar] [CrossRef]
- Divyasri, Y.V.; Lakshmana Reddy, N.; Lee, K.; Sakar, M.; Navakoteswara Rao, V.; Venkatramu, V.; Shankar, M.V.; Gangi Reddy, N.C. Optimization of N Doping in TiO2 Nanotubes for the Enhanced Solar Light Mediated Photocatalytic H2 Production and Dye Degradation. Environ. Pollut. 2021, 269, 116170. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, C.; Wang, W.; Chen, L.; Li, X.; Wu, Y. Oxygen Vacancy Mediated Band-Gap Engineering via B-Doping for Enhancing Z-Scheme A-TiO2/R-TiO2 Heterojunction Photocatalytic Performance. Nanomaterials 2023, 13, 794. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhaifallah, M.; Abdelkareem, M.A.; Rezk, H.; Alhumade, H.; Nassef, A.M.; Olabi, A.G. Co-decorated Reduced Graphene/Titanium Nitride Composite as an Active Oxygen Reduction Reaction Catalyst with Superior Stability. Int. J. Energy Res. 2021, 45, 1587–1598. [Google Scholar] [CrossRef]
- Wang, W.-K.; Chen, J.-J.; Gao, M.; Huang, Y.-X.; Zhang, X.; Yu, H.-Q. Photocatalytic Degradation of Atrazine by Boron-Doped TiO2 with a Tunable Rutile/Anatase Ratio. Appl. Catal. B Environ. 2016, 195, 69–76. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Yu, Z.; Wei, X.; Wu, W.D.; Wang, X.; Wu, Z. Facile Synthesis of Mesoporous Anatase/Rutile/Hematite Triple Heterojunctions for Superior Heterogeneous Photo-Fenton Catalysis. Appl. Catal. B Environ. 2020, 263, 118335. [Google Scholar] [CrossRef]
- Hashemizadeh, I.; Golovko, V.B.; Choi, J.; Tsang, D.C.W.; Yip, A.C.K. Photocatalytic Reduction of CO2 to Hydrocarbons Using Bio-Templated Porous TiO2 Architectures under UV and Visible Light. Chem. Eng. J. 2018, 347, 64–73. [Google Scholar] [CrossRef]
- Hao, H.; Shi, J.-L.; Xu, H.; Li, X.; Lang, X. N-Hydroxyphthalimide-TiO2 Complex Visible Light Photocatalysis. Appl. Catal. B Environ. 2019, 246, 149–155. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, X.; Xing, Z.; Yang, L. Synthesis and Characterization of N-Doped TiO2 and Its Enhanced Visible-Light Photocatalytic Activity. Arab. J. Chem. 2016, 9, S1706–S1711. [Google Scholar] [CrossRef]
- Sarkar, A.; Khan, G.G. The Formation and Detection Techniques of Oxygen Vacancies in Titanium Oxide-Based Nanostructures. Nanoscale 2019, 11, 3414–3444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, J.; Hua, L.; Li, S.; Zhang, X.; Sheng, W.; Cao, S. High Photocatalytic Activity of Hierarchical SiO2@C-Doped TiO2 Hollow Spheres in UV and Visible Light towards Degradation of Rhodamine B. J. Hazard. Mater. 2017, 340, 309–318. [Google Scholar] [CrossRef]
- Poudel, M.B.; Awasthi, G.P.; Kim, H.J. Novel Insight into the Adsorption of Cr(VI) and Pb(II) Ions by MOF Derived Co-Al Layered Double Hydroxide @hematite Nanorods on 3D Porous Carbon Nanofiber Network. Chem. Eng. J. 2021, 417, 129312. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cheng, Z.; Cui, L.; Duan, T.; Anan, A.; Zhang, C.; Kang, L. Controllable Synthesis of Ag@TiO2 Heterostructures with Enhanced Photocatalytic Activities under UV and Visible Excitation. RSC Adv. 2016, 6, 1844–1850. [Google Scholar] [CrossRef]
- Zhao, X.; Du, Y.; Zhang, C.; Tian, L.; Li, X.; Deng, K.; Chen, L.; Duan, Y.; Lv, K. Enhanced Visible Photocatalytic Activity of TiO2 Hollow Boxes Modified by Methionine for RhB Degradation and NO Oxidation. Chin. J. Catal. 2018, 39, 736–746. [Google Scholar] [CrossRef]
- Liu, G.; Wang, G.; Hu, Z.; Su, Y.; Zhao, L. Ag2O Nanoparticles Decorated TiO2 Nanofibers as a p-n Heterojunction for Enhanced Photocatalytic Decomposition of RhB under Visible Light Irradiation. Appl. Surf. Sci. 2019, 465, 902–910. [Google Scholar] [CrossRef]
- Bian, H.; Zhang, Z.; Xu, X.; Gao, Y.; Wang, T. Photocatalytic Activity of Ag/ZnO/AgO/TiO2 Composite. Phys. E Low-Dimens. Syst. Nanostructures 2020, 124, 114236. [Google Scholar] [CrossRef]
- Wang, W.-K.; Chen, J.-J.; Zhang, X.; Huang, Y.-X.; Li, W.-W.; Yu, H.-Q. Self-Induced Synthesis of Phase-Junction TiO2 with a Tailored Rutile to Anatase Ratio below Phase Transition Temperature. Sci. Rep. 2016, 6, 20491. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Zhao, K.; Zhao, Y.; Zhu, S.; Yuan, X.; Huo, M.; Zhang, Y.; Qiu, Y. Bi2WO6/TiO2/Pt Nanojunction System: A UV–Vis Light Responsive Photocatalyst with High Photocatalytic Performance. Colloids Surf. Physicochem. Eng. Asp. 2015, 481, 252–260. [Google Scholar] [CrossRef]
- Li, Y.; Lv, K.; Ho, W.; Dong, F.; Wu, X.; Xia, Y. Hybridization of Rutile TiO2 (R-TiO2) with g-C3N4 Quantum Dots (CN QDs): An Efficient Visible-Light-Driven Z-Scheme Hybridized Photocatalyst. Appl. Catal. B Environ. 2017, 202, 611–619. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Y.; He, D.; Zhang, J.; Fan, Z.; Xie, T. Interface Junction at Anatase/Rutile in Mixed-Phase TiO2: Formation and Photo-Generated Charge Carriers Properties. Chem. Phys. Lett. 2011, 504, 71–75. [Google Scholar] [CrossRef]
- Yu, Y.; Wen, W.; Qian, X.-Y.; Liu, J.-B.; Wu, J.-M. UV and Visible Light Photocatalytic Activity of Au/TiO2 Nanoforests with Anatase/Rutile Phase Junctions and Controlled Au Locations. Sci. Rep. 2017, 7, 41253. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Ding, X.; Yang, X.; Wang, P.; Li, S.; Lu, Z.; Chen, H. Oxygen Vacancy Boosted Photocatalytic Decomposition of Ciprofloxacin over Bi2MoO6: Oxygen Vacancy Engineering, Biotoxicity Evaluation and Mechanism Study. J. Hazard. Mater. 2019, 364, 691–699. [Google Scholar] [CrossRef]
- Baran, T.; Wojtyła, S.; Minguzzi, A.; Rondinini, S.; Vertova, A. Achieving Efficient H2O2 Production by a Visible-Light Absorbing, Highly Stable Photosensitized TiO2. Appl. Catal. B Environ. 2019, 244, 303–312. [Google Scholar] [CrossRef]
- Lakshmanareddy, N.; Navakoteswara Rao, V.; Cheralathan, K.K.; Subramaniam, E.P.; Shankar, M.V. Pt/TiO2 Nanotube Photocatalyst—Effect of Synthesis Methods on Valance State of Pt and Its Influence on Hydrogen Production and Dye Degradation. J. Colloid Interface Sci. 2019, 538, 83–98. [Google Scholar] [CrossRef]
- Poudel, M.B.; Kim, A.A.; Lohani, P.C.; Yoo, D.J.; Kim, H.J. Assembling Zinc Cobalt Hydroxide/Ternary Sulfides Heterostructure and Iron Oxide Nanorods on Three-Dimensional Hollow Porous Carbon Nanofiber as High Energy Density Hybrid Supercapacitor. J. Energy Storage 2023, 60, 106713. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, S.; Zhang, J.; Zhong, W.; Huang, X. Cu2−xSe/CdS Composite Photocatalyst with Enhanced Visible Light Photocatalysis Activity. Appl. Surf. Sci. 2019, 478, 762–769. [Google Scholar] [CrossRef]
- Yin, W.; Bai, L.; Zhu, Y.; Zhong, S.; Zhao, L.; Li, Z.; Bai, S. Embedding Metal in the Interface of a p-n Heterojunction with a Stack Design for Superior Z-Scheme Photocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2016, 8, 23133–23142. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Ma, R.; Jia, D.; Wen, T.; Ai, Y.; Zhao, G.; Fang, F.; Hu, B.; Wang, X. Molybdenum (VI)-oxo Clusters Incorporation Activates g-C3N4 with Simultaneously Regulating Charge Transfer and Reaction Centers for Boosting Photocatalytic Performance. Adv. Funct. Mater. 2022, 32, 2204175. [Google Scholar] [CrossRef]
- Guan, Y.; Liu, W.; Zuo, S.; Yuan, K.; Wu, F.; Ji, J.; Yao, C. Double Z-Scheme TiO2 (R)/C-TiO2 (A) Heterojunction Greatly Enhanced Efficiency of Photocatalytic Desulfurization under Sunlight. J. Mater. Sci. Mater. Electron. 2020, 31, 22297–22311. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, Z.; Zhu, C.; Hu, X.; Zhang, X.; Zhu, H.; Fan, J.; Lin, S.H. C/B Codoping Effect on Band Gap Narrowing and Optical Performance of TiO2 Photocatalyst: A Spin-Polarized DFT Study. J. Mater. Chem. A 2013, 1, 4516. [Google Scholar] [CrossRef]
- Kashiwaya, S.; Morasch, J.; Streibel, V.; Toupance, T.; Jaegermann, W.; Klein, A. The Work Function of TiO2. Surfaces 2018, 1, 73–89. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-Scheme Heterojunction Photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Li, C.; Yang, W.; Liu, L.; Sun, W.; Li, Q. In Situ Growth of TiO2 on TiN Nanoparticles for Non-Noble-Metal Plasmonic Photocatalysis. RSC Adv. 2016, 6, 72659–72669. [Google Scholar] [CrossRef]
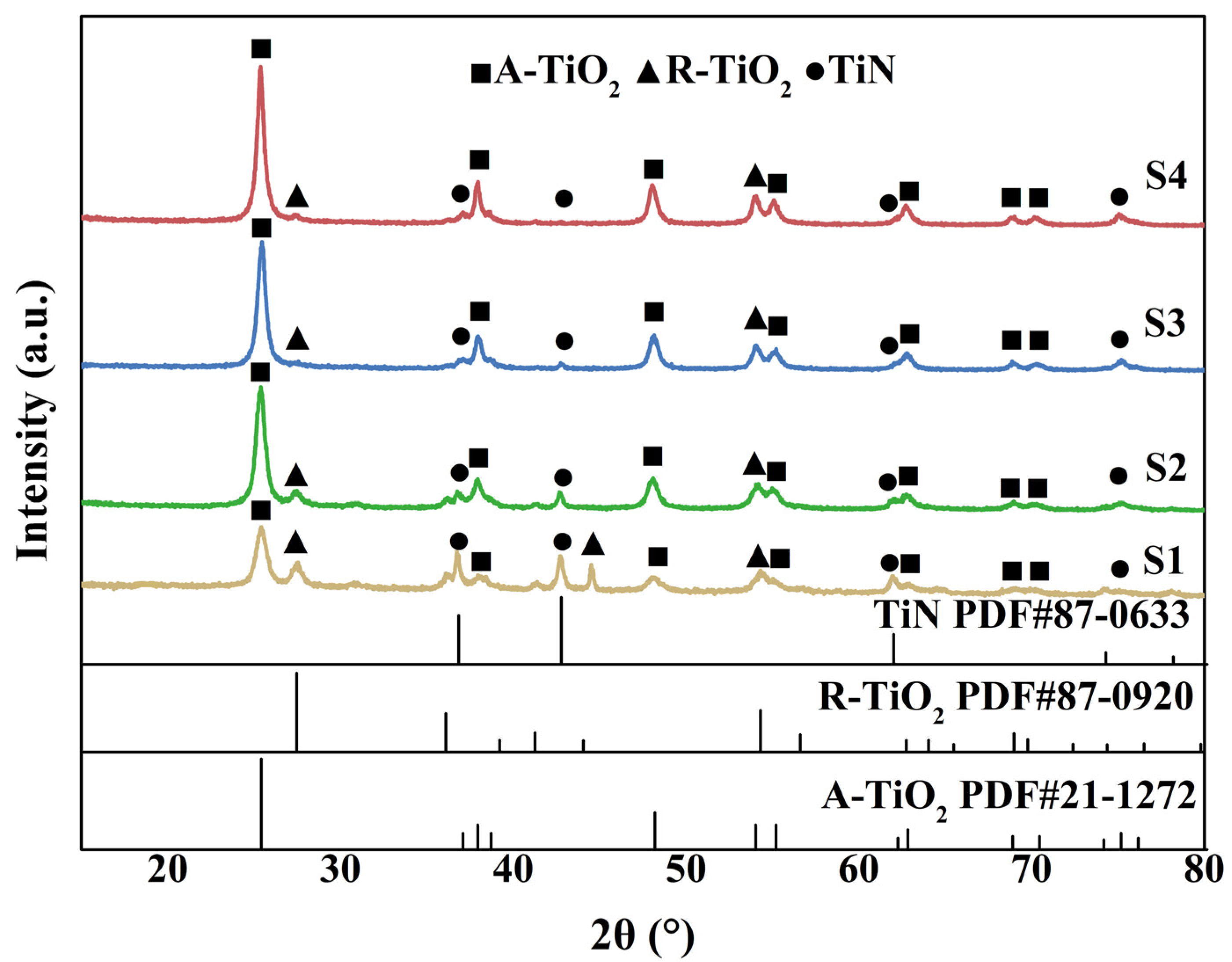
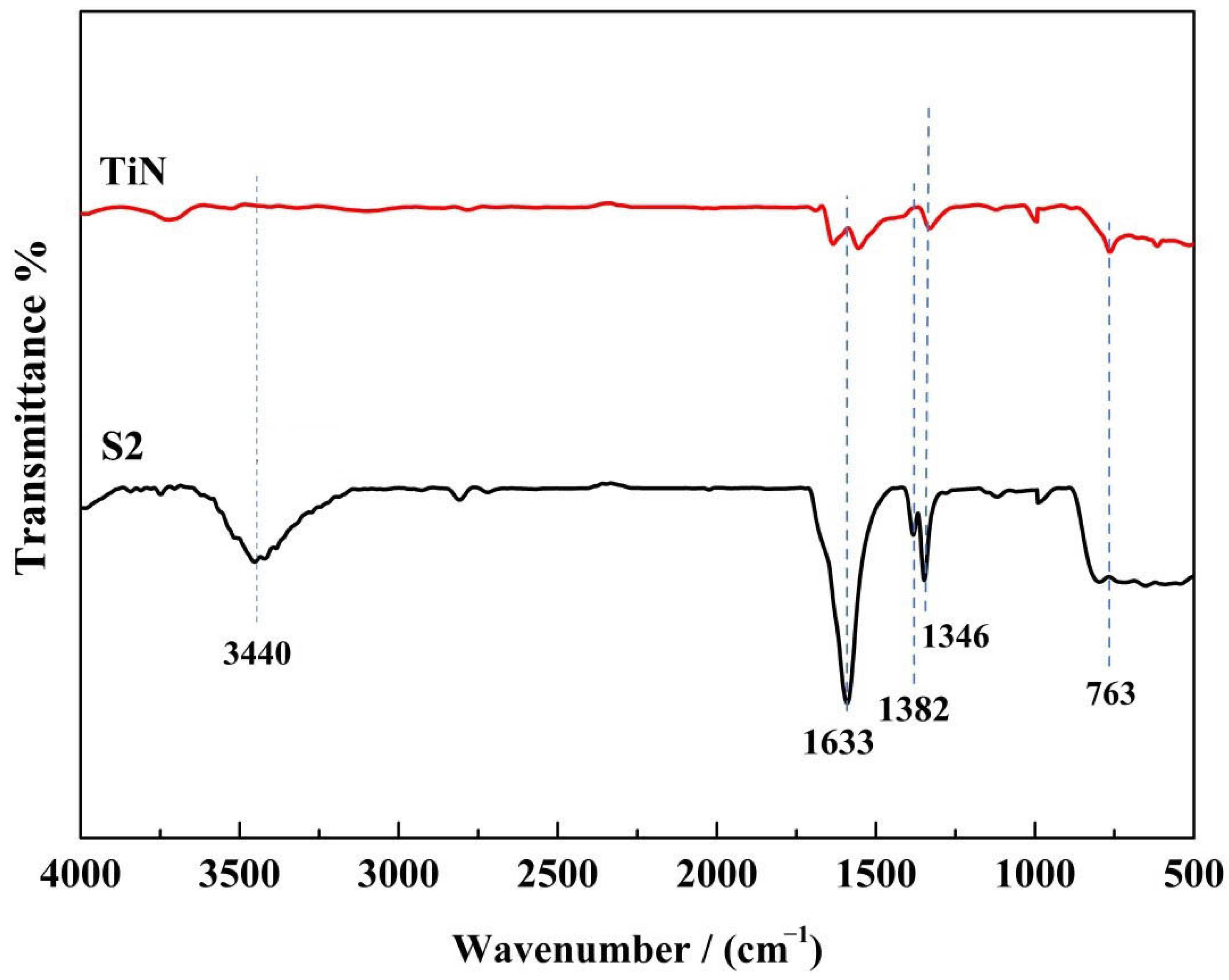
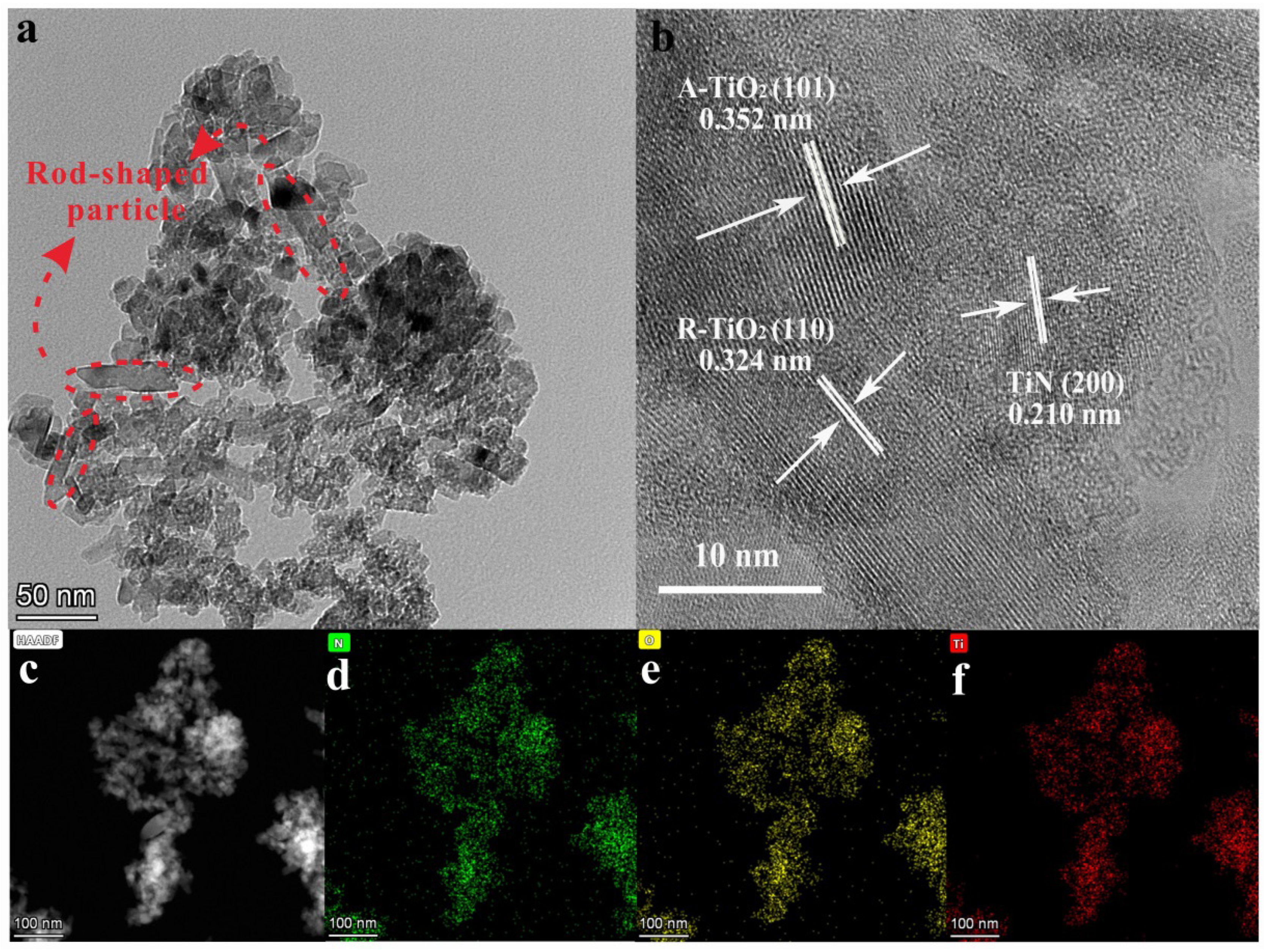
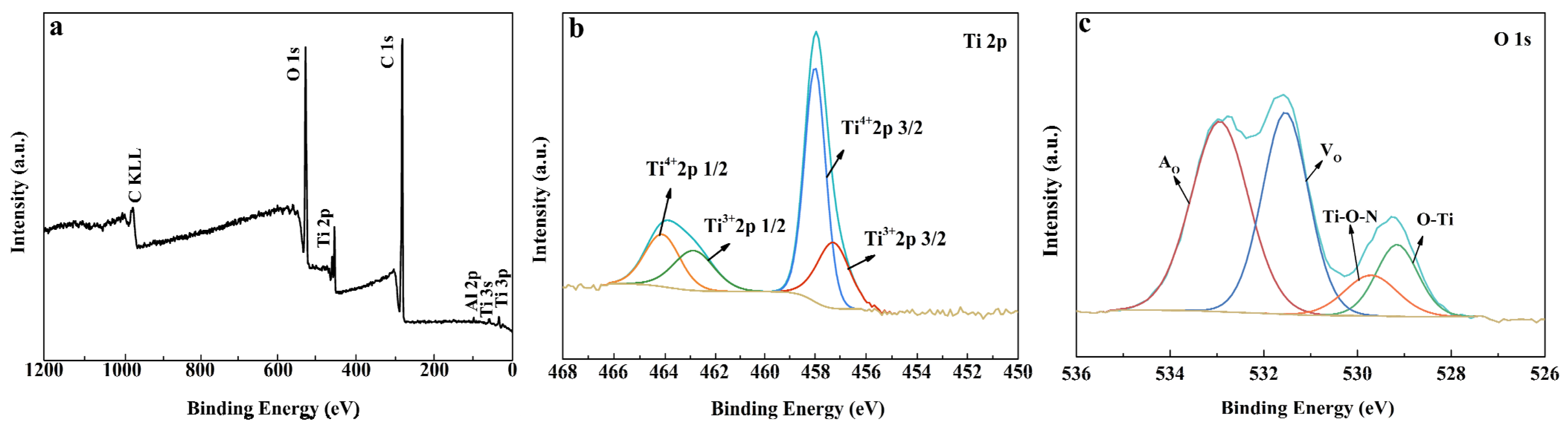
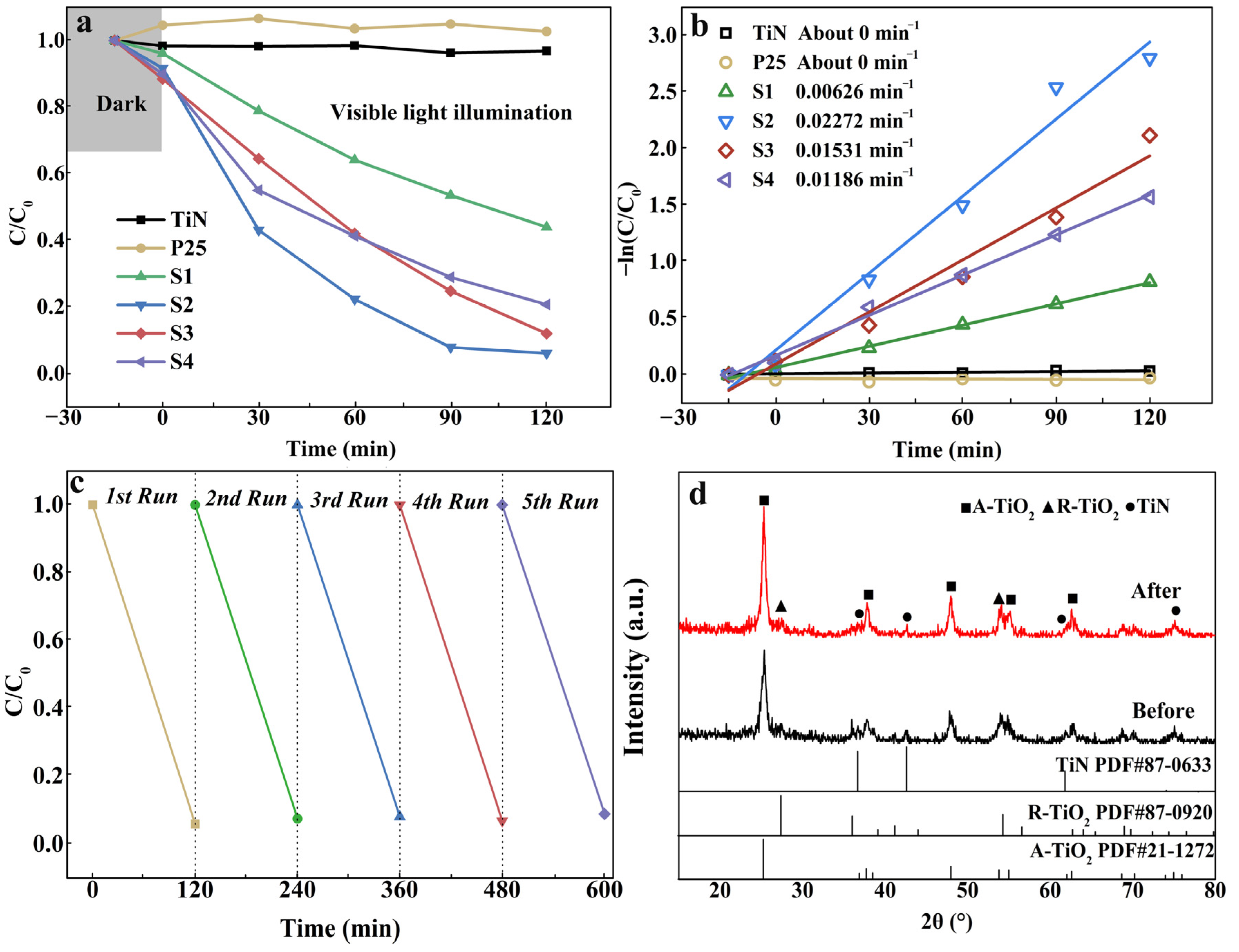

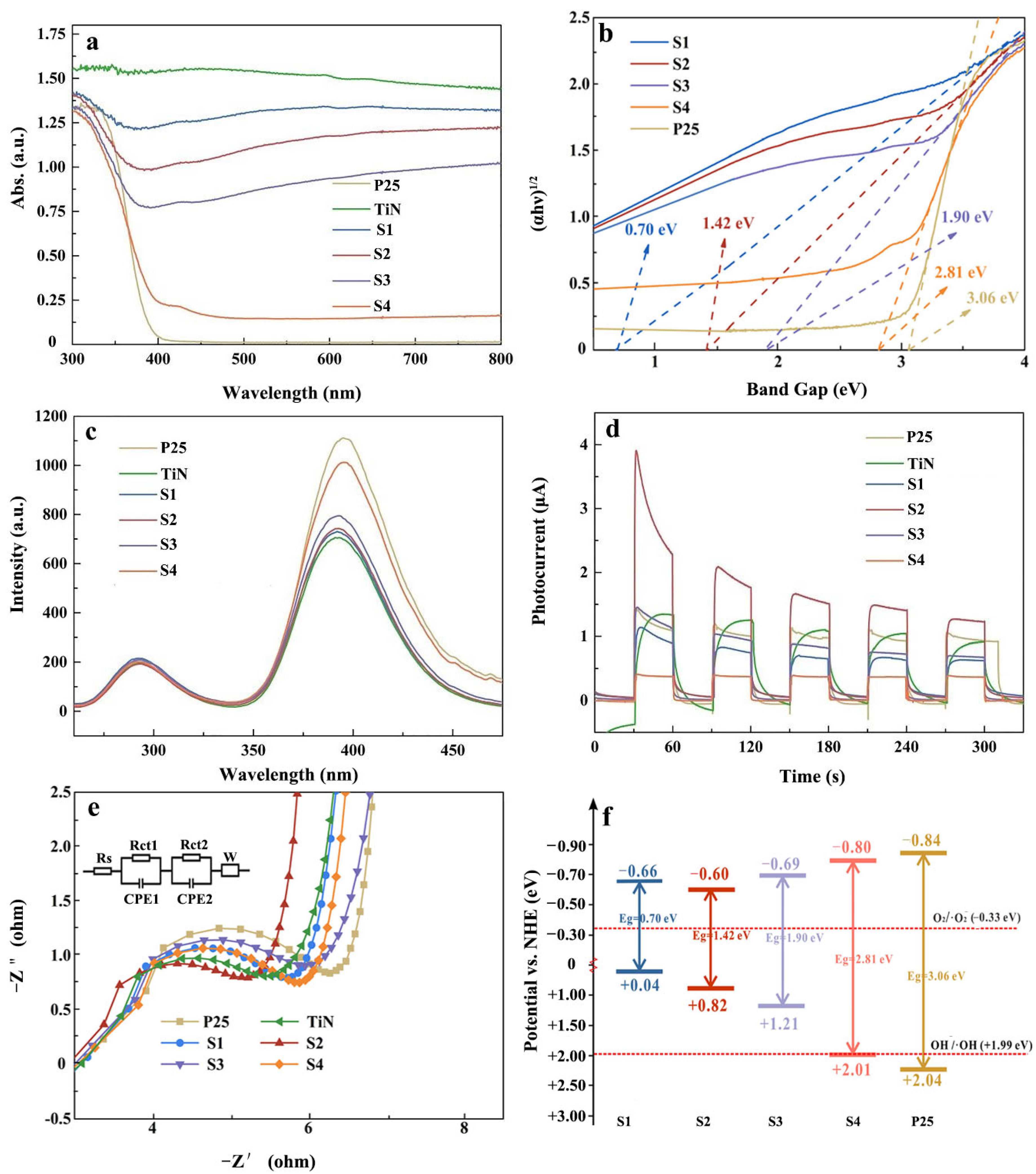
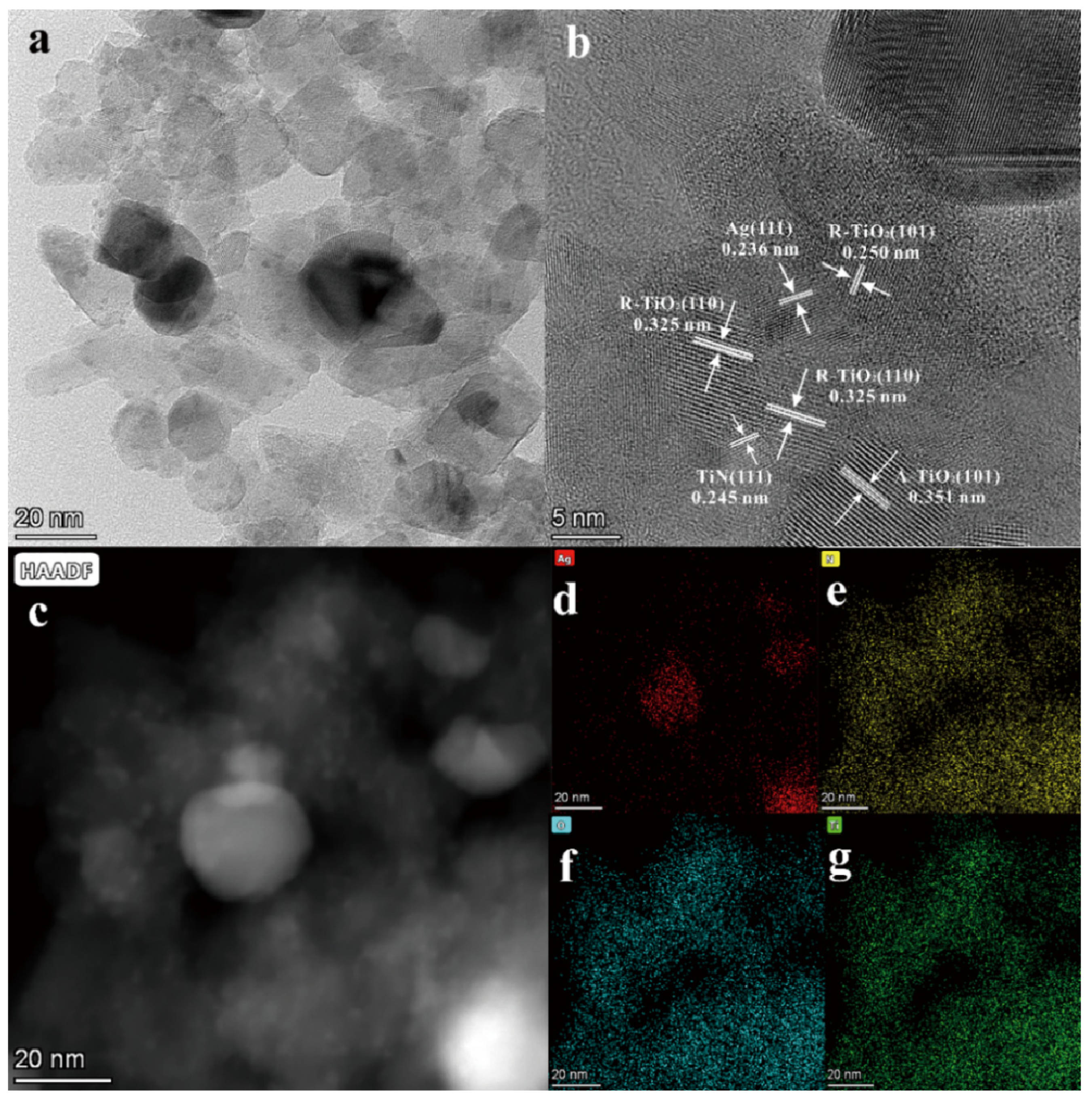
| Samples | Content of H2O2 (wt%) | TiO2/wt% | TiN/wt% | ||
|---|---|---|---|---|---|
| A-TiO2 | R-TiO2 | A-TiO2:R-TiO2 | |||
| TiN | 0 | - | - | - | 100% |
| S1 | 0.5 | 80.9% | 19.1% | 4.25 | 21.1% |
| S2 | 1.0 | 93.8% | 6.2% | 15.13 | 7.2% |
| S3 | 2.5 | 97.3% | 2.7% | 36.40 | 1.7% |
| S4 | 5.0 | 98.3% | 1.7% | 57.82 | 0.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Wu, Y.; Chen, L.; Xu, C.; Liu, C.; Li, C. Fabrication of Z-Type TiN@(A,R)TiO2 Plasmonic Photocatalyst with Enhanced Photocatalytic Activity. Nanomaterials 2023, 13, 1984. https://doi.org/10.3390/nano13131984
Wang W, Wu Y, Chen L, Xu C, Liu C, Li C. Fabrication of Z-Type TiN@(A,R)TiO2 Plasmonic Photocatalyst with Enhanced Photocatalytic Activity. Nanomaterials. 2023; 13(13):1984. https://doi.org/10.3390/nano13131984
Chicago/Turabian StyleWang, Wanting, Yuanting Wu, Long Chen, Chenggang Xu, Changqing Liu, and Chengxin Li. 2023. "Fabrication of Z-Type TiN@(A,R)TiO2 Plasmonic Photocatalyst with Enhanced Photocatalytic Activity" Nanomaterials 13, no. 13: 1984. https://doi.org/10.3390/nano13131984
APA StyleWang, W., Wu, Y., Chen, L., Xu, C., Liu, C., & Li, C. (2023). Fabrication of Z-Type TiN@(A,R)TiO2 Plasmonic Photocatalyst with Enhanced Photocatalytic Activity. Nanomaterials, 13(13), 1984. https://doi.org/10.3390/nano13131984






