Fabrication of UV-Curable Polysiloxane Coating with Tunable Refractive Index Based on Controllable Hydrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrolysis of MPS
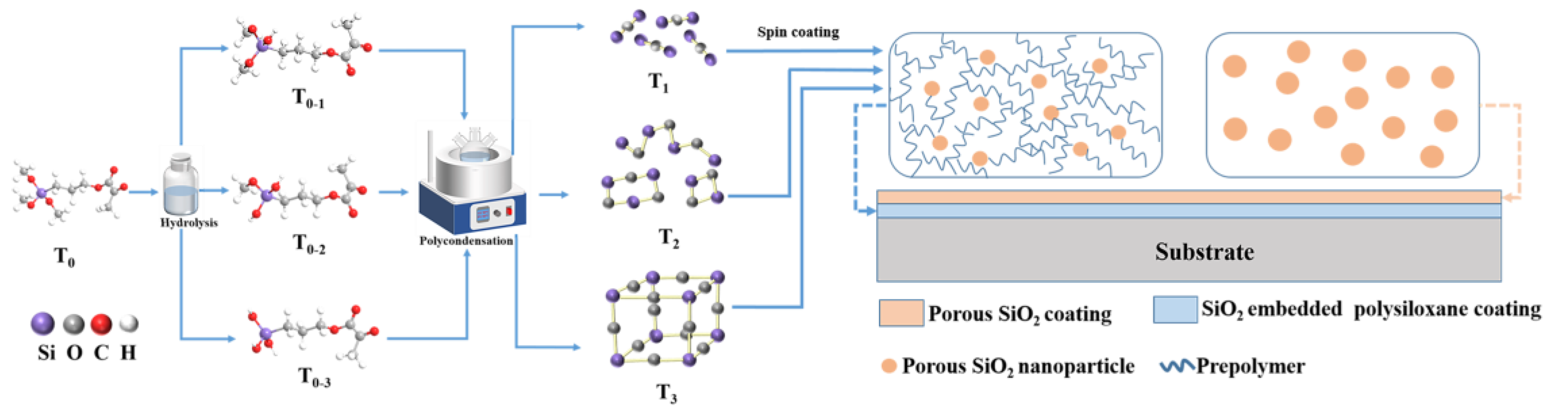
2.3. Preparation of Polysiloxane Prepolymer
2.4. Preparation of Porous SiO2 Nanoparticles
2.5. Preparation of Polysiloxane Coating
2.6. Preparation of bi-Layer Antireflection Coating System
2.7. Characterizations
3. Results
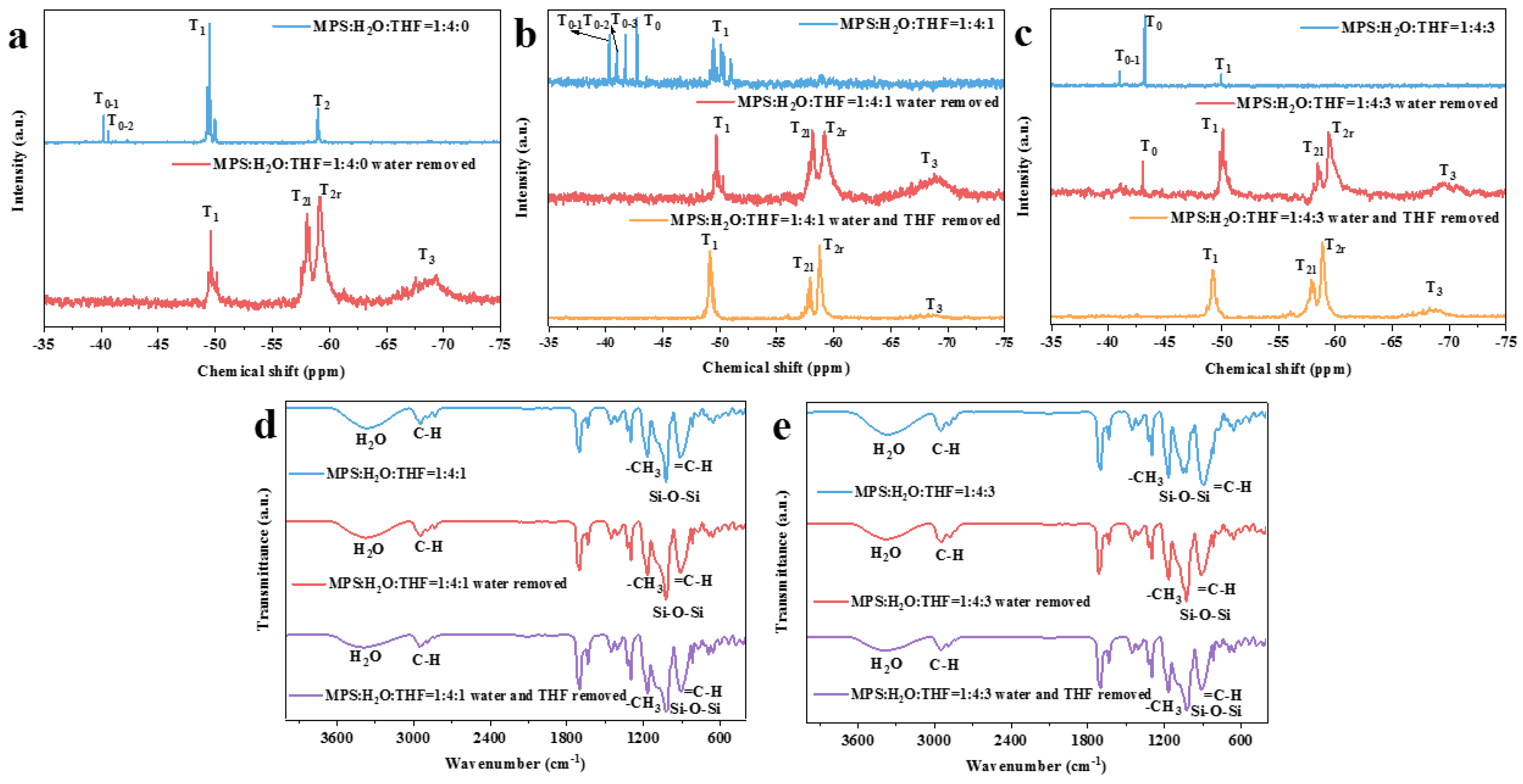
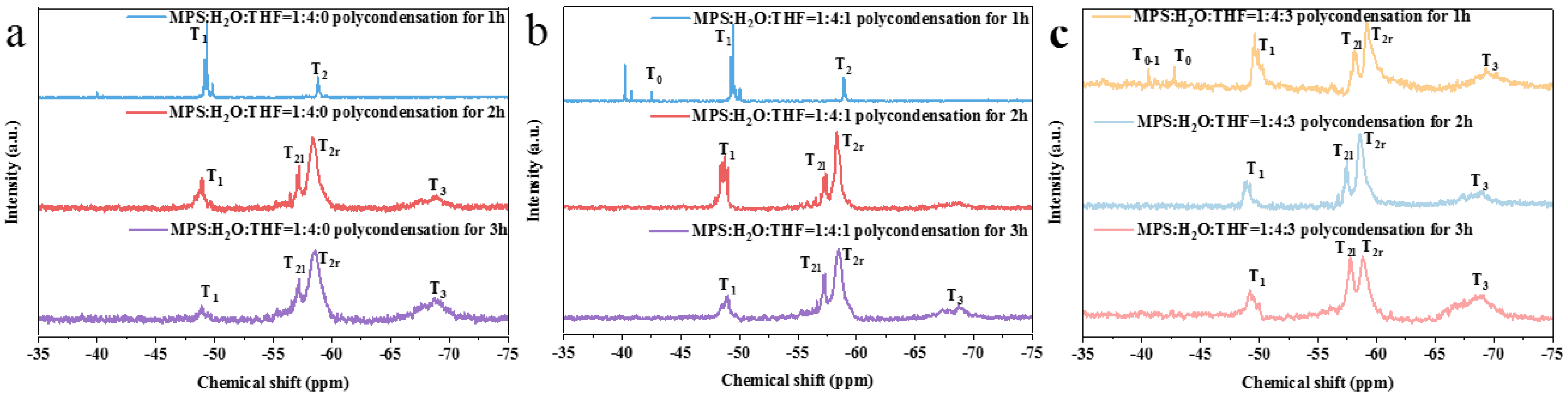
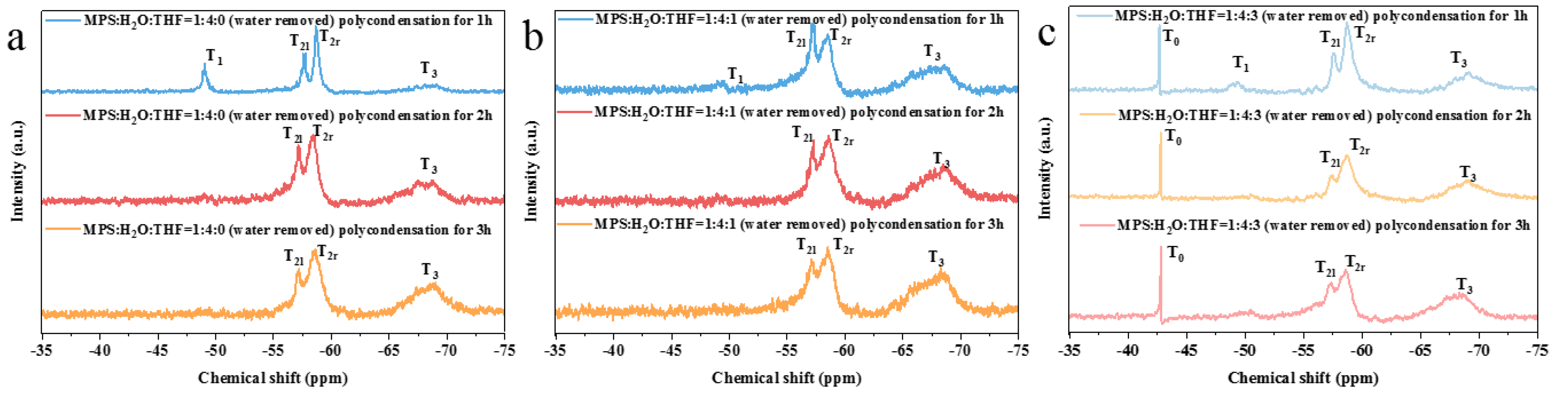
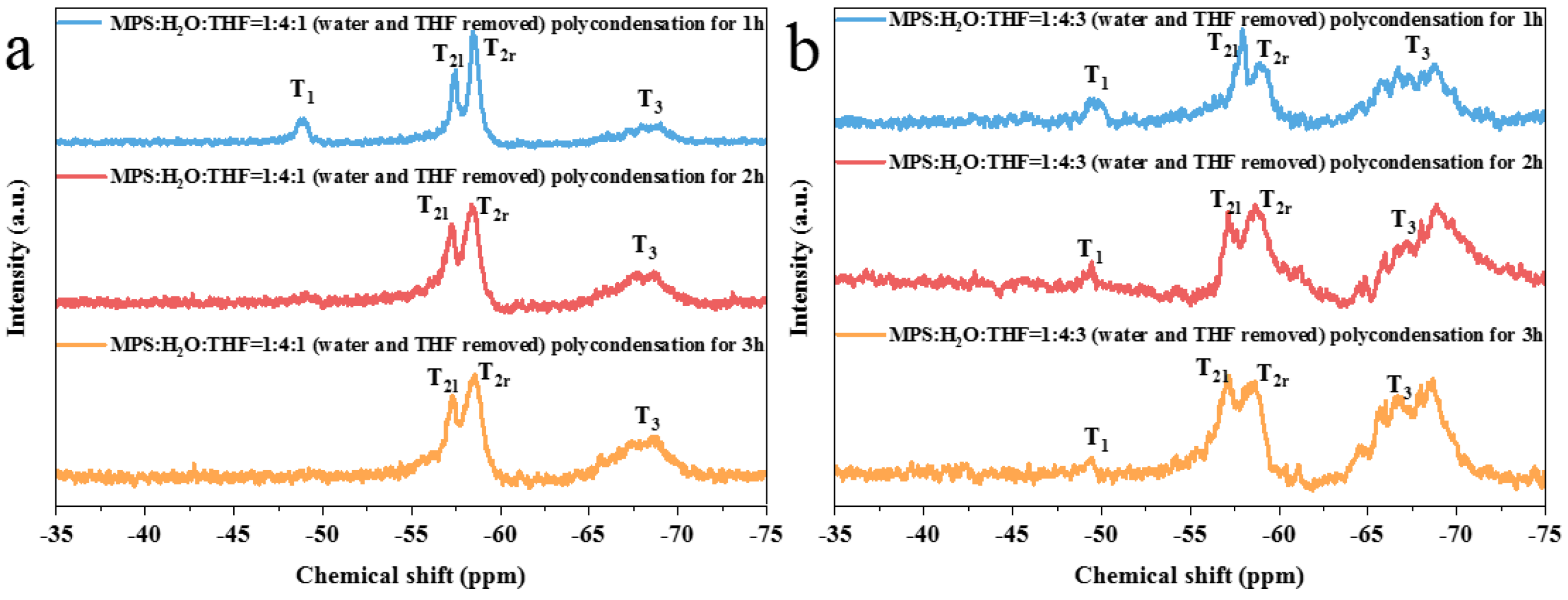
3.1. Study on the Controllable Hydrolysis of MPS Monomer
3.2. Preparation of Prepolymers with Different Crosslinking Structures
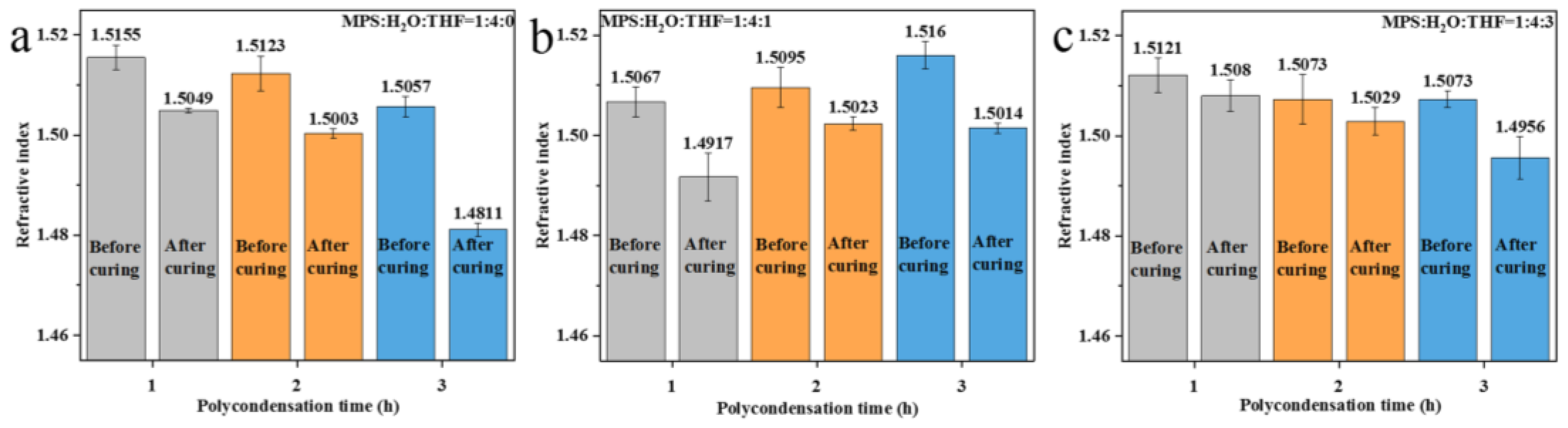
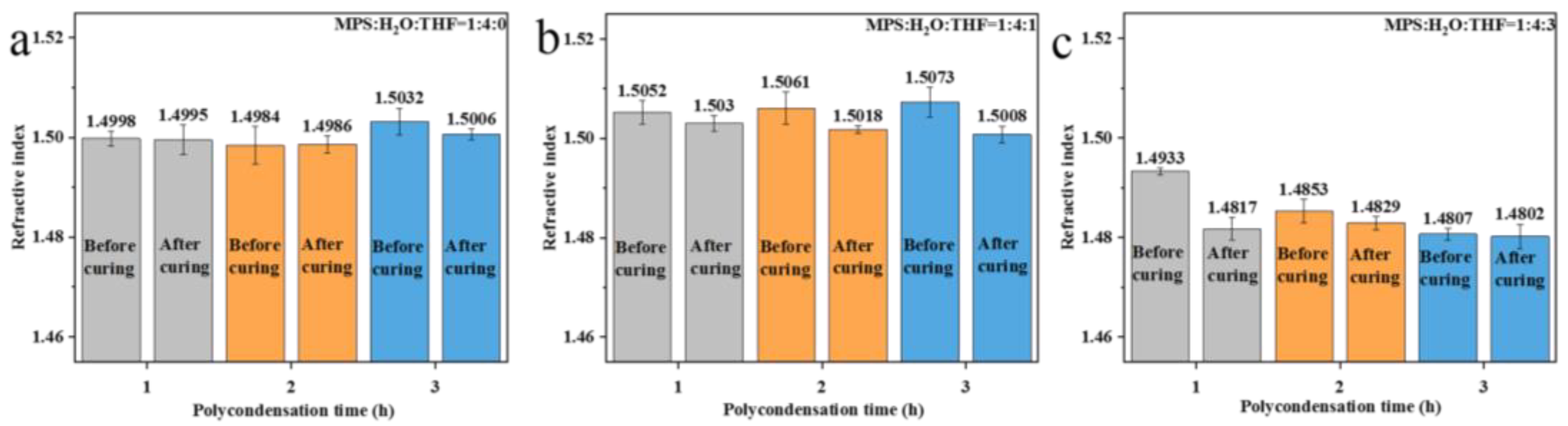
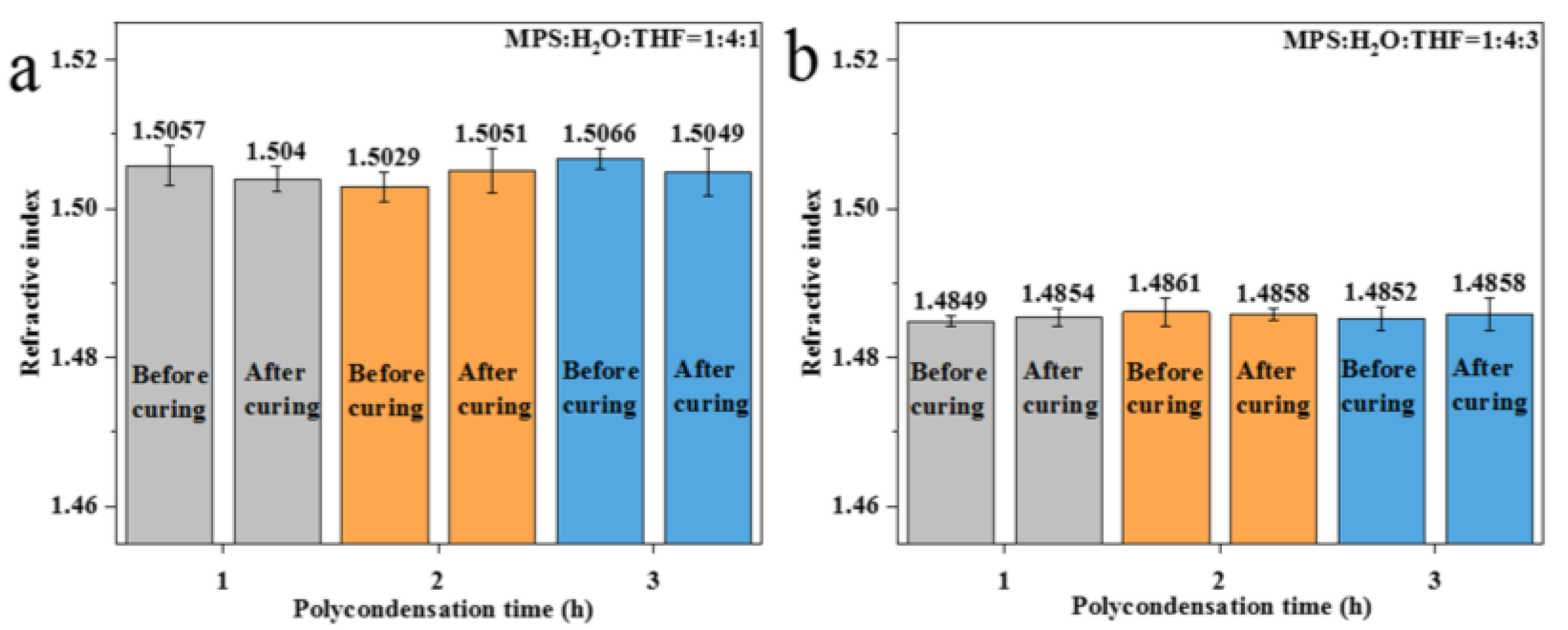
3.3. Effect of Different Crosslinking Structures on the Refractive Index of Polysiloxane Coating
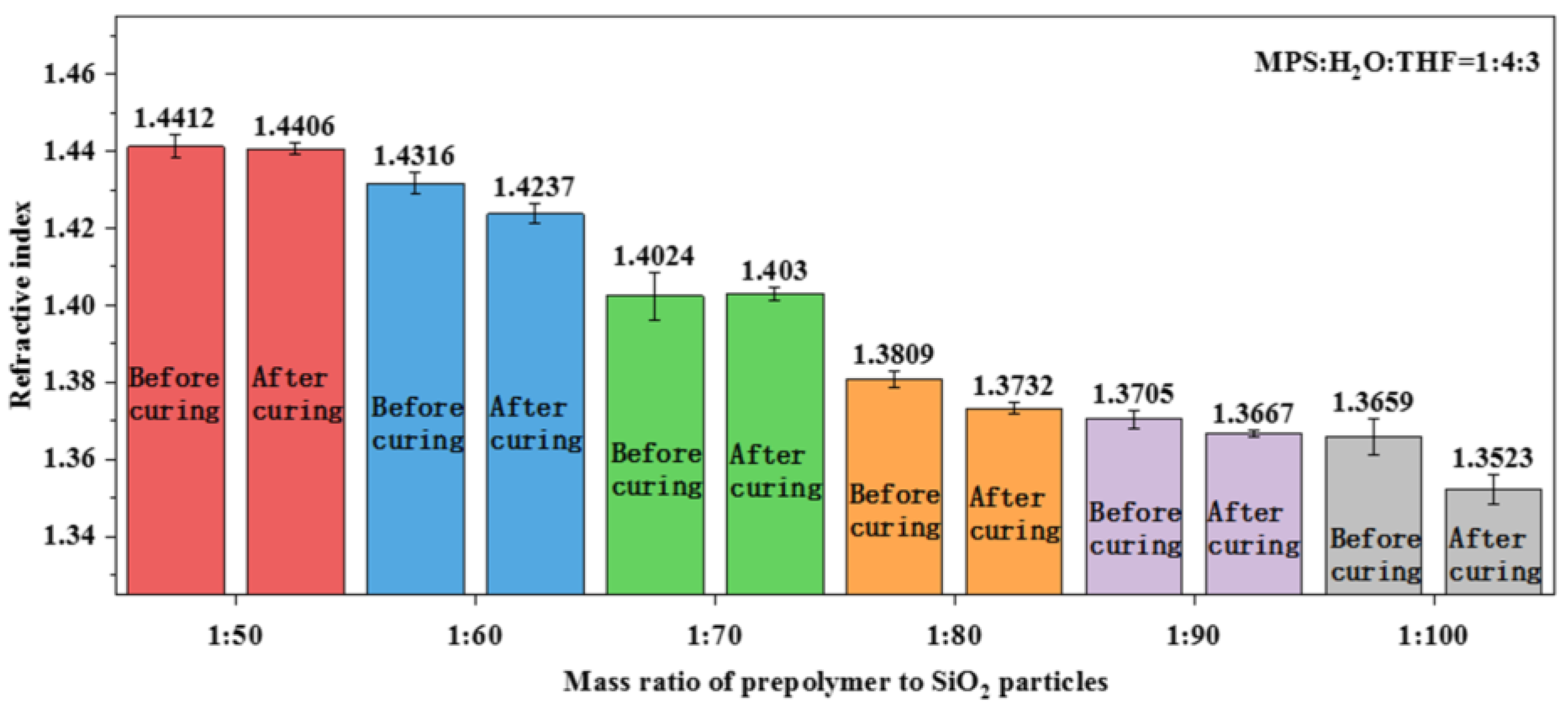
3.4. Fabrication of Polysiloxane Coating with Precisely Controlled Refractive Index
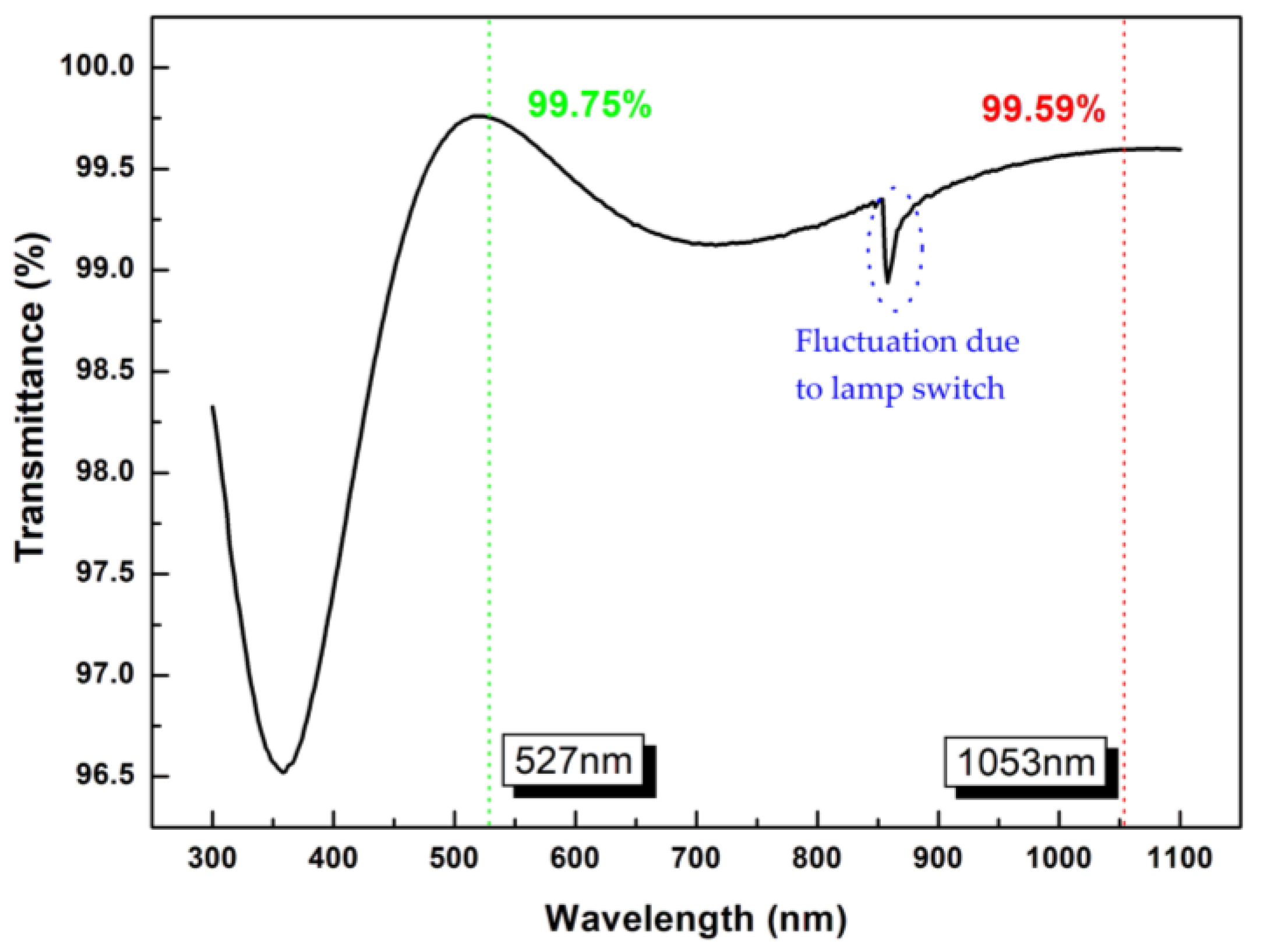
3.5. Optical Properties of bi-Layer Antireflection Coating System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.; Jiang, Y.; Xu, Z.; Wang, S.; Liu, W.; Meng, C.; Tan, Y.; Gao, Z. Simulation and measurement of external electromagnetic environment of Tokamak device. Radiat. Prot. Dosim. 2021, 194, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Sovacool, B.K.; Schmid, P.; Stirling, A.; Walter, G.; Mackerron, G. Differences in carbon emissions reduction between countries pursuing renewable electricity versus nuclear power. Nat. Energy 2020, 5, 928–935. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, S.; You, D.; Yan, L.; Lv, H.; Yuan, X.; Jiang, B. Template-free sol-gel preparation of superhydrophobic ORMOSIL coatings for double-wavelength broadband antireflection coatings. Adv. Funct. Mater. 2013, 23, 4361–4365. [Google Scholar] [CrossRef]
- Thomas, I.M. A two layer broadband antireflection coating prepared from a methyl silicone and porous silica. Proc. SPIE 1997, 3136, 215–219. [Google Scholar] [CrossRef]
- Deng, X.R.; Yang, W.; Wang, T.Y.; Hui, H.H.; Lei, X.Y.; Zhang, Q.H.; Xu, Q.; Fan, F. Antireflective laser coating with improved mechanical property and organic-contaminant resistance for high-power laser application. Prog. Org. Coat. 2021, 161, 106535. [Google Scholar] [CrossRef]
- Han, S.Y.; Pan, C.; Kim, D.H.; Chang, C.H. Low-cost & low-temperature curable solution-processed silica-based nanostructured antireflective coatings on CuIn1−xGaxSe2 thin film solar cells. RSC Adv. 2015, 5, 24712–24717. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, W.; Wu, Y.; Liu, G.; Wu, X. Micro/nano-structures transition and electrochemical response of Ti-6Al-4V alloy in simulated seawater. Surf. Topogr.-Metrol. Prop. 2018, 6, 034009–035010. [Google Scholar] [CrossRef]
- Salhi, H.; Mleiki, A.; M’nassri, R.; Rahmouni, H.; Ajili, L.; Khirouni, K. Study of electrical properties and conduction mechanisms in La0.4Bi0.3Sr0.2Ba0.1MnO3 Ceramic elaborated by sol gel method. J. Alloys Compd. 2022, 928, 167132. [Google Scholar] [CrossRef]
- Tregulov, V.V.; Litvinov, V.G.; Ermachikhin, A.V. Deep-Level defects in a photovoltaic converter with an antireflection porous silicon coating formed by chemical stain etching. Technol. Phys. Lett. 2019, 45, 145–148. [Google Scholar] [CrossRef]
- Mohite, K.C.; Khollam, Y.B.; Mandale, A.B.; Patil, K.R.; Takwale, M.G. Characterization of silicon oxynitride thin coatings deposited by electron beam physical vapor deposition technique. Mater. Lett. 2003, 57, 4170–4175. [Google Scholar] [CrossRef]
- Lim, K.P.; Ng, D.K.T.; Wang, Q. Broadband antireflection for a high-index substrate using SiNx/SiO2 by inductively coupled plasma chemical vapour deposition. J. Phys. D-Appl. Phys. 2016, 49, 085302. [Google Scholar] [CrossRef]
- Miller, G.H.; Moses, E.I.; Wuest, C.R. The national ignition facility. Opt. Eng. 2004, 43, 2841–2853. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.R.; Yang, W.; Zhang, Q.H.; Hui, H.H.; Wei, Y.W.; Wang, J.; Xu, Q.; Lei, X.Y.; Chen, J.J.; Zhu, J.L. Fabrication of UV-curable silicone coating with high transmittance and laser-induced damage threshold for high-power laser system. J. Sol.-Gel. Sci. Technol. 2018, 88, 249–254. [Google Scholar] [CrossRef]
- Deng, X.R.; Lei, X.Y.; Yang, W.; Hui, H.H.; Wang, T.Y.; Chen, J.J.; Zhu, J.L.; Zhang, Q.H. Template-free fabrication of refractive index tunable polysiloxane coating using homogeneous embedding strategy: Application in high-power laser system. Nanomaterials 2020, 10, 381. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Chen, G.; Liang, Q.; Yang, Z.; Shen, H. Multifunctional cellulose nanocrystal structural colored film with good flexibility and water-resistance. Int. J. Biol. Macromol. 2020, 149, 819–825. [Google Scholar] [CrossRef]
- Coady, M.J.; Getangama, N.N.K.; Khalili, A.; Wood, M.; Nielsen, K.E.; de Bruyn, J.R.; Hutter, J.L.; Klassen, R.J.; Kietzig, A.; Ragogna, P.J. Highly cross-linked UV-cured siloxane copolymer networks as icephobic coatings. J. Polym. Sci. 2020, 58, 1022–1029. [Google Scholar] [CrossRef]
- Yan, B.; Chen, Z.; Wang, Y.; Wang, G. Fast UV-curing encapsulation for GaN-based light-emitting diodes. IEEE Trans. Compon. Packag. Technol. 2019, 9, 1759–1764. [Google Scholar] [CrossRef]
- Li, Q.F.; Du, X.; Chen, S.; Zhang, S.D. Research on UV-cured composite films of epoxy with superparamagnetic, hollow nickel-zinc ferrite nanospheres and their microwave absorption. J. Mater. Sci.-Mater. Electron. 2018, 29, 3286–3295. [Google Scholar] [CrossRef]
- Kim, D.H.; Jang, W.; Choi, K.; Ji, S.C.; Im, S.G. One-step vapor-phase synthesis of transparent high refractive index sulfur-containing polymers. Sci. Adv. 2020, 6, eabb5320. [Google Scholar] [CrossRef]
- Thompson, C.H.; Soro, D. Fluoro silsesquioxanes as low index optical materials. Silicon 2021, 13, 4215–4222. [Google Scholar] [CrossRef]
- Wang, H.T.; Zhong, W.; Xu, P.; Du, Q.G. Properties of polyimide/silica nanohybrids from silicic acid oligomer. Macromol. Mater. Eng. 2004, 289, 793–799. [Google Scholar] [CrossRef]
- Witoon, T.; Tatan, N.; Rattanavichian, P.; Chareonpanich, M. Preparation of silica xerogel with high silanol content from sodium silicate and its application as CO2 adsorbent. Ceram. Int. 2011, 37, 2297–2303. [Google Scholar] [CrossRef]
- Wang, D.; Liu, N.; Zhang, J.; Zhao, X.; Zhang, W.; Zhang, M. Oxidative desulfurization using ordered mesoporous silicas as catalysts. J. Mol. Catal. A-Chem. 2014, 393, 47–55. [Google Scholar] [CrossRef]
- Yamamoto, E.; Shimojima, A.; Wada, H.; Kuroda, K. Mesoporous silica nanoparticles with dispersibility in organic solvents and their versatile surface modification. Langmuir 2022, 36, 5571–5578. [Google Scholar] [CrossRef]
- Kuniyoshi, M.; Takahashi, M.; Tokuda, Y.; Yoko, T. Hydrolysis and polycondensation of acid-catalyzed Phenyltriethoxysilane (PhTES). J. Sol.-Gel. Sci. Technol. 2006, 39, 175–183. [Google Scholar] [CrossRef]
- Soppera, O.; Croutxé-Barghorn, C. Real-time fourier transform infrared study of free-radical UV-induced polymerization of hybrid sol-gel. I. effect of silicate backbone on photopolymerization kinetics. J. Polym. Sci. Pol. Chem. 2003, 41, 831–840. [Google Scholar] [CrossRef]
- Paquet, O.; Brochier Salon, M.C.; Zeno, E.; Belgacem, M.N. Hydrolysis-condensation kinetics of 3-(2-amino-ethylamino) propyl-trimethoxysilane. Mater. Sci. Eng. C-Mater. Biol. Appl. 2012, 32, 487–493. [Google Scholar] [CrossRef]

| MPS | H2O | THF | CH3COOH | Notes |
|---|---|---|---|---|
| 0.1 mol | 0.2 mol | / | 0.1 mol/L | Effect of catalyst content |
| 0.1 mol | 0.2 mol | / | 1 mol/L | |
| 0.1 mol | 0.2 mol | / | 2 mol/L | |
| 0.1 mol | 0.1 mol | / | 1 mol/L | Effect of water content without THF |
| 0.1 mol | 0.2 mol | / | 1 mol/L | |
| 0.1 mol | 0.3 mol | / | 1 mol/L | |
| 0.1 mol | 0.4 mol | / | 1 mol/L | |
| 0.1 mol | 0.4 mol | 0.1 mol | 1 mol/L | Effect of inhibitor content in water-rich system |
| 0.1 mol | 0.4 mol | 0.2 mol | 1 mol/L | |
| 0.1 mol | 0.4 mol | 0.3 mol | 1 mol/L | |
| 0.1 mol | 0.1 mol | 0.4 mol | 1 mol/L | Effect of water content in THF-rich system |
| 0.1 mol | 0.2 mol | 0.4 mol | 1 mol/L | |
| 0.1 mol | 0.3 mol | 0.4 mol | 1 mol/L |
| Polycondensation Method | Reactant Ratio of MPS:H2O:THF | Polycondensation Product | Refractive Index | ||
|---|---|---|---|---|---|
| T2l | T2r | T3 | |||
| Direct polycondensation | 1:4:0 (RT) | Moderate | Strong | Weak | 1.4811~1.5049 |
| 1:4:1 (IT) | Moderate | Strong | Weak | 1.4917~1.5023 | |
| 1:4:3 (IT) | Strong | Strong | Weak | 1.4956~1.5080 | |
| Polycondensation after the removal of water | 1:4:0 (RT) | Moderate | Strong | Moderate | 1.4986~1.5006 |
| 1:4:1 (IT) | Moderate | Strong | Moderate | 1.5008~1.5030 | |
| 1:4:3 (IT) | Moderate | Strong | Weak | 1.4802~1.4817 | |
| Polycondensation after THF removal | 1:4:1 (IT) | Moderate | Strong | Weak | 1.5029~1.5049 |
| 1:4:3 (IT) | Moderate | Moderate | Moderate | 1.4854~1.4858 | |
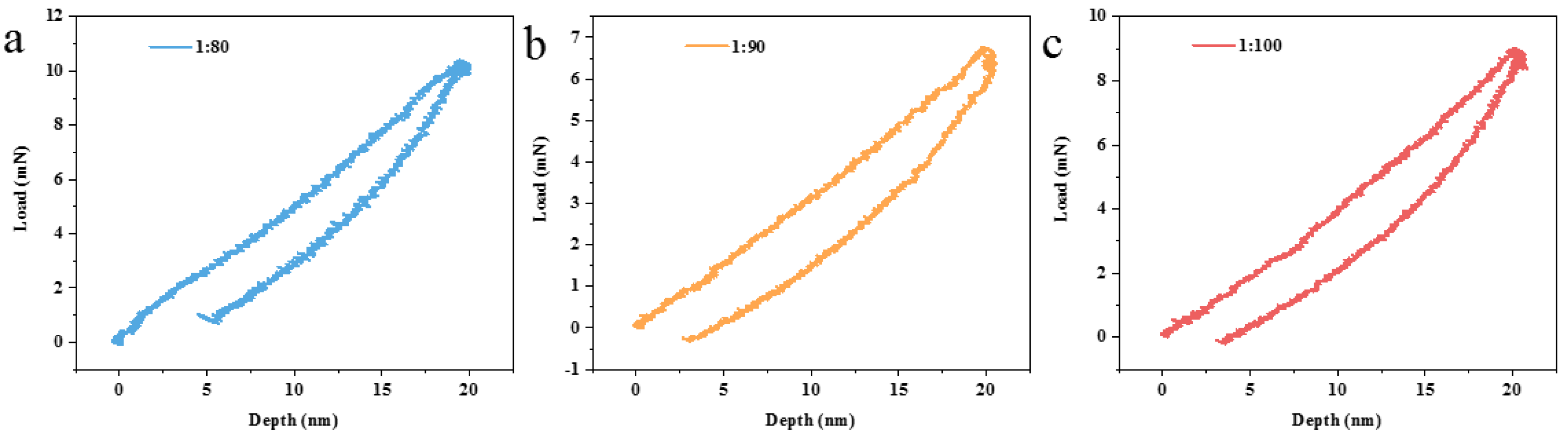
| Ratio of Prepolymer to SiO2 Nanoparticles | Elastic Modulus (GPa) | Hardness (MPa) |
|---|---|---|
| 1:80 | 5.45 | 424.91 |
| 1:90 | 3.34 | 243.65 |
| 1:100 | 2.44 | 181.82 |
| Ratio of Prepolymer to SiO2 Nanoparticles | 9.5 ns Pulsed Laser-Induced Damage Threshold(J/cm2) | 5 ns Pulsed Laser-Induced Damage Threshold(J/cm2) |
|---|---|---|
| 1:80 | 74.86 | 59.79 |
| 1:90 | 70.56 | 56.36 |
| 1:100 | 54.56 | 43.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-L.; Shi, Q.-K.; Deng, Y.; Lei, X.-Y.; Zhang, Q.-H.; Chen, J.-J.; Deng, X.-R. Fabrication of UV-Curable Polysiloxane Coating with Tunable Refractive Index Based on Controllable Hydrolysis. Nanomaterials 2023, 13, 1985. https://doi.org/10.3390/nano13131985
Huang H-L, Shi Q-K, Deng Y, Lei X-Y, Zhang Q-H, Chen J-J, Deng X-R. Fabrication of UV-Curable Polysiloxane Coating with Tunable Refractive Index Based on Controllable Hydrolysis. Nanomaterials. 2023; 13(13):1985. https://doi.org/10.3390/nano13131985
Chicago/Turabian StyleHuang, Hong-Lan, Qi-Kai Shi, Yan Deng, Xiang-Yang Lei, Qing-Huang Zhang, Jin-Ju Chen, and Xue-Ran Deng. 2023. "Fabrication of UV-Curable Polysiloxane Coating with Tunable Refractive Index Based on Controllable Hydrolysis" Nanomaterials 13, no. 13: 1985. https://doi.org/10.3390/nano13131985
APA StyleHuang, H.-L., Shi, Q.-K., Deng, Y., Lei, X.-Y., Zhang, Q.-H., Chen, J.-J., & Deng, X.-R. (2023). Fabrication of UV-Curable Polysiloxane Coating with Tunable Refractive Index Based on Controllable Hydrolysis. Nanomaterials, 13(13), 1985. https://doi.org/10.3390/nano13131985





