Eco-Efficient Systems Based on Nanocarriers for the Controlled Release of Fertilizers and Pesticides: Toward Smart Agriculture
Abstract
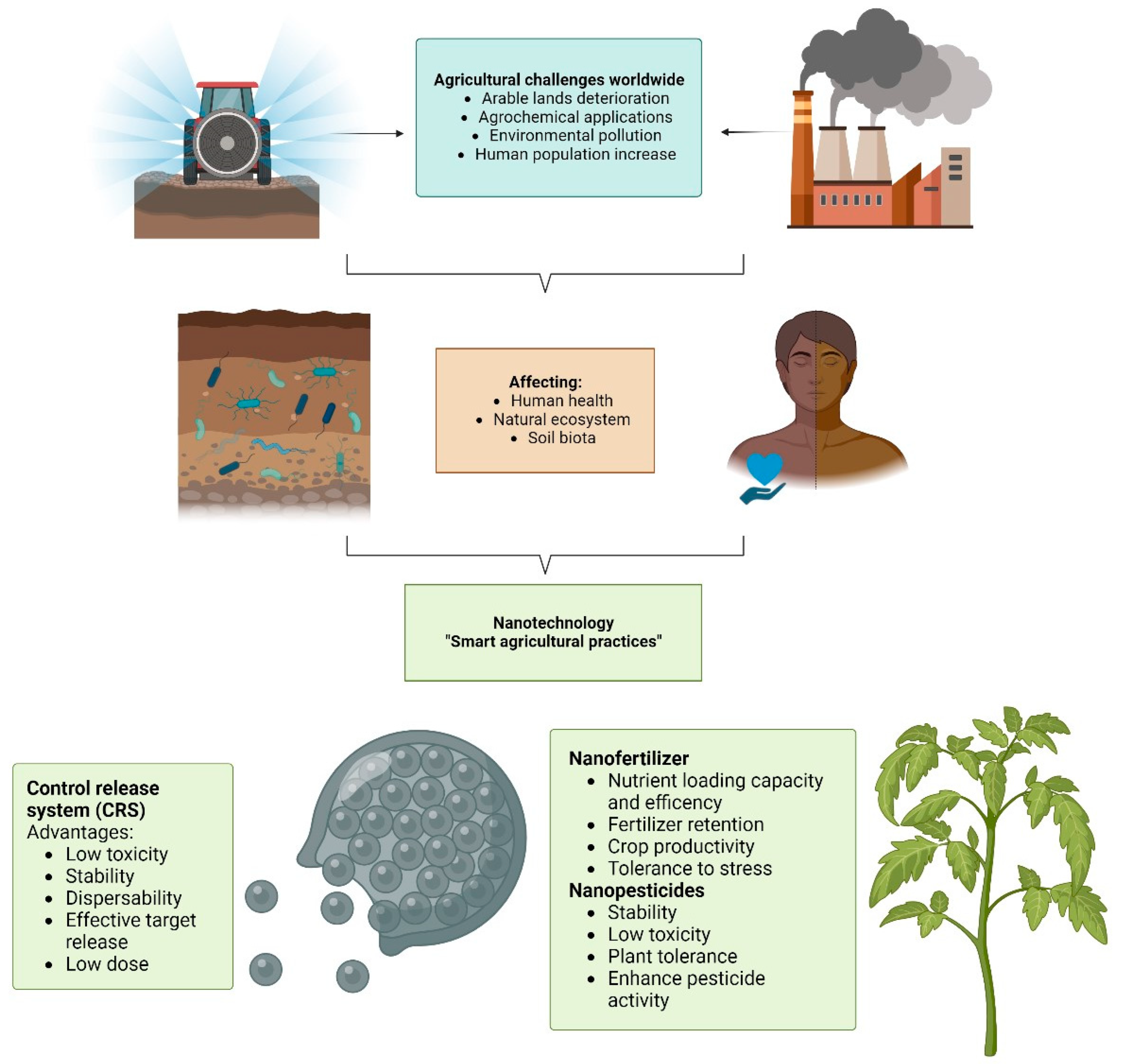
:1. Introduction
2. A Smart Agricultural Technology Based on Controlled Release Systems
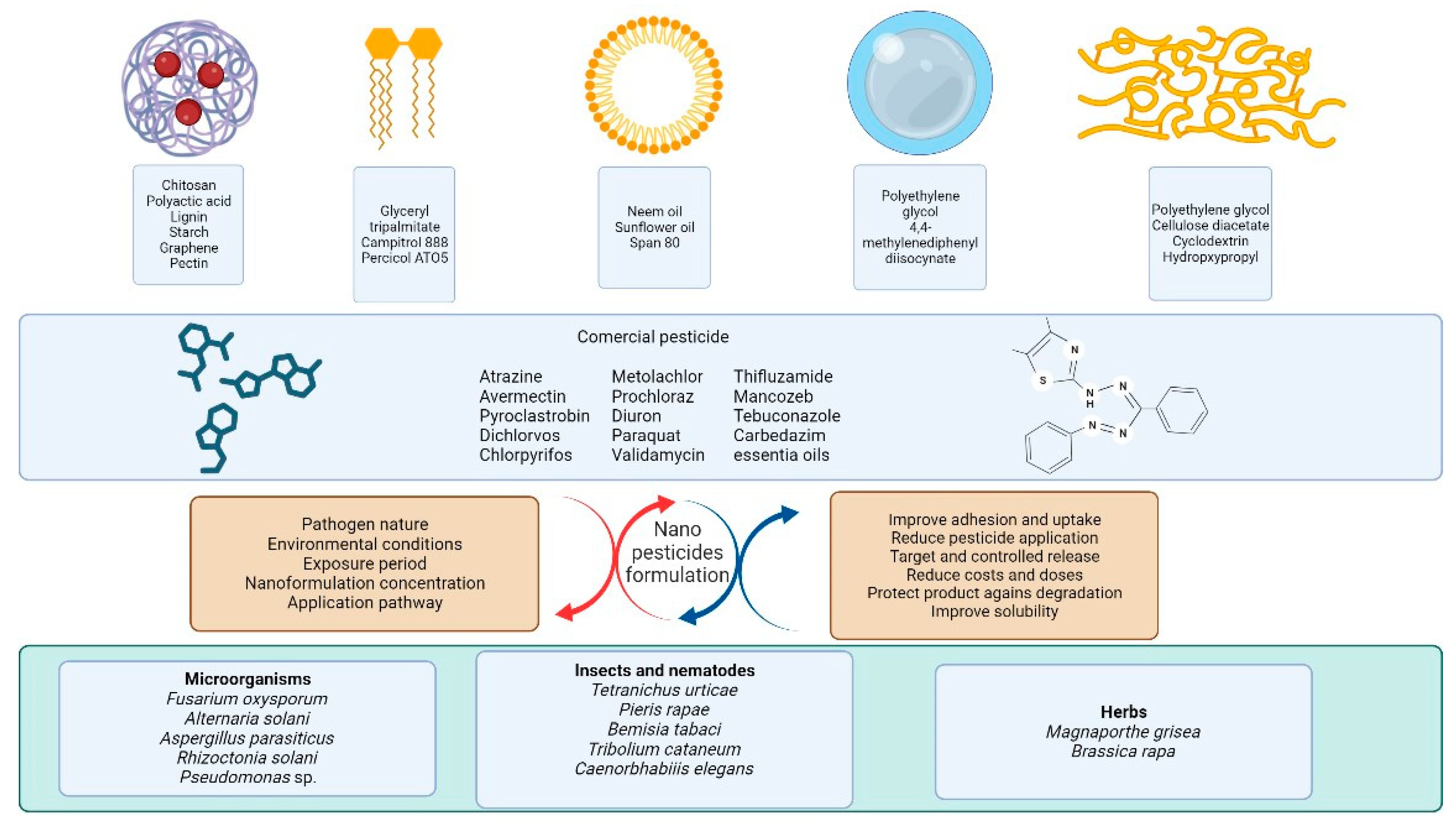
3. Nanopesticides
3.1. Polymeric Nanoparticles
3.2. Nanoemulsions
3.3. Lipid Nanoparticles
3.4. Nanogels
3.5. Nanofibers
3.6. Stimuli-Responsive Nano-Based Pesticides
3.6.1. Responsive to pH
3.6.2. Responses to Temperature
3.6.3. Response to Light
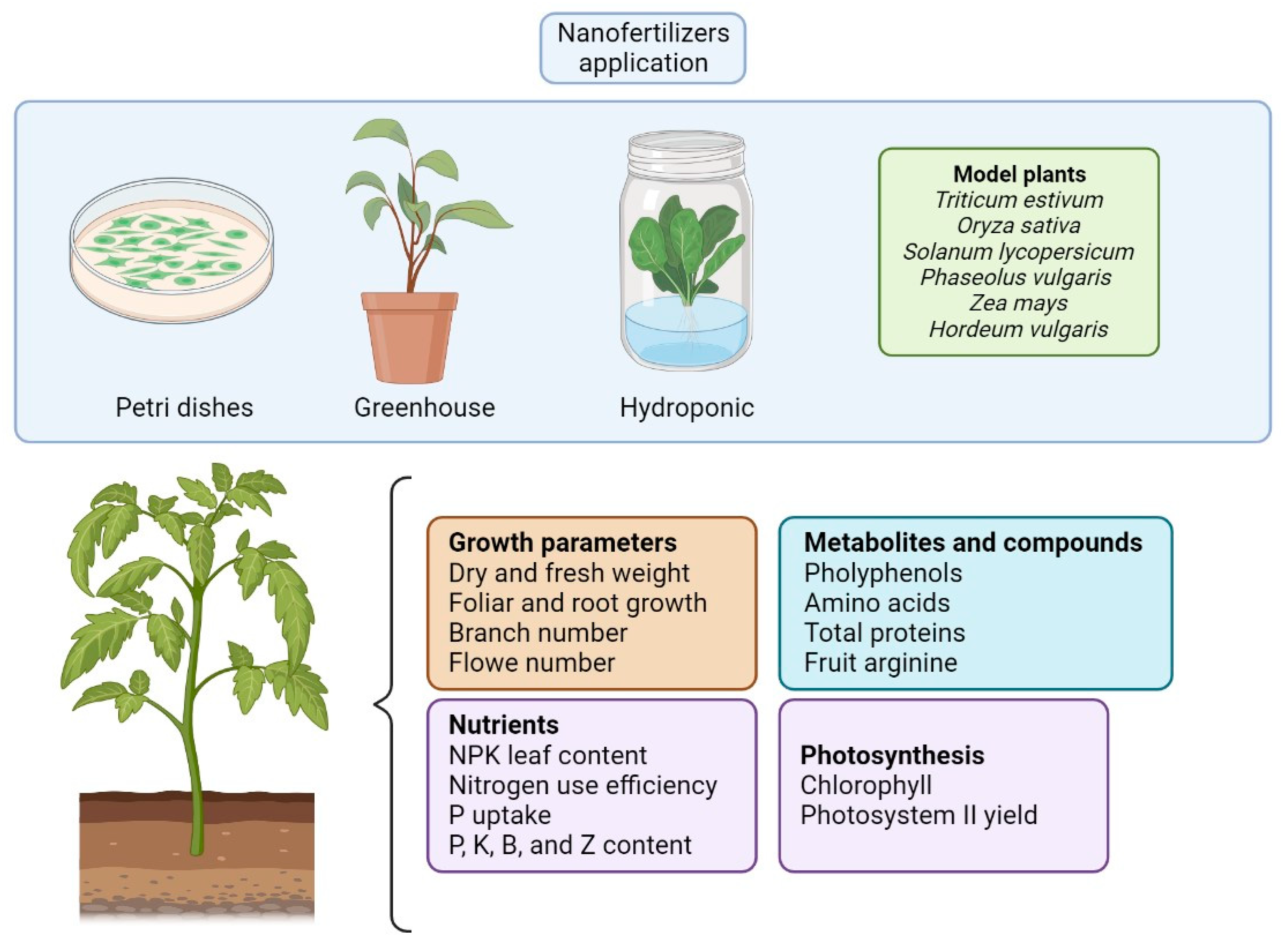
4. Nanofertilizers
4.1. Nanohydroxyapatite
4.2. Nanoclays
4.3. Chitosan Nanoparticles
4.4. Mesoporous Silica Nanoparticles
4.5. Amorphous Calcium Phosphate
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Moneim, D.A.; Dawood, M.F.A.; Moursi, Y.S.; Farghaly, A.A.; Afifi, M.; Sallam, A. Positive and negative effects of nanoparticles on agricultural crops. Nanotechnol. Environ. Eng. 2021, 6, 21. [Google Scholar] [CrossRef]
- Erkmen, O.; Bozoglu, F. Chapter 20: Spoilage of vegetables and fruits. In Food Microbiology: Principles into Practice; Wiley: Hoboken, NJ, USA, 2016; Volume 1. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, C.; Guo, Q.; Zhang, J.; Ruiz-Menjivar, J. The impact of agricultural chemical inputs on environment: Global evidence from informetrics analysis and visualization. Intl J. Low Carbon Technol. 2018, 13, 338–352. [Google Scholar] [CrossRef] [Green Version]
- Rajmohan, K.S.; Chandrasekaran, R.; Varjani, S. A review on occurrence of pesticides in environment and current technologies for their remediation and management. Indian J. Microbiol. 2020, 60, 125–138. [Google Scholar] [CrossRef]
- FAO. World Fertilizer Trends and Outlook to 2022; FAO: Rome, Italy, 2019. [Google Scholar]
- Nisar Pahalvi, H.; Rafya, L.; Rashid, S.; Nisar, B.; Kamili, A.N. Chapter 1: Chemical fertilizers and their impact on soil health. In Microbiota and Biofertilizers; Dar, G.H., Bhat, R.A., Mehmood, M.A., Hakeem, K.R., Eds.; Springer: Cham, Switzerland, 2021; Volume 2. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in griculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasi-Jorjandi, M.; Asadikaram, G.; Abolhassani, M.; Fallah, H.; Abdollahdokht, D.; Salimi, F.; Faramarz, S.; Pournamdari, M. Pesticide exposure and related health problems among family members of farmworkers in southeast Iran. A case-control study. Environ. Pollut. 2020, 267, 115424. [Google Scholar] [CrossRef]
- Alves Pedroso, T.M.; Benvindo-Souza, M.; de Araújo Nascimento, F.; Woch, J.; Gonçalves dos Reis, F.; de Melo e Silva, D. Cancer and occupational exposure to pesticides: A bibliometric study of the past 10 years. Environ. Sci. Pollut. Res. 2022, 29, 17464–17475. [Google Scholar] [CrossRef] [PubMed]
- Fierros-González, I.; López-Feldma, A. Farmers’ perception of climate change: A review of the literature for Latin America. Front. Environ. Sci. 2021, 9, 672399. [Google Scholar] [CrossRef]
- Bonanomi, G.; Lorito, M.; Vinale, F.; Woo, S.L. Organic amendments, beneficial microbes, and soil microbiota: Toward a unified framework for disease suppression. Annu. Rev. Phytopathol. 2018, 56, 1–20. [Google Scholar] [CrossRef]
- Chugh, G.; Siddique, K.H.M.; Solaiman, Z.M. Nanobiotechnology for agriculture: Smart technology for combating nutrient deficiencies with nanotoxicity challenges. Sustainability 2021, 13, 1781. [Google Scholar] [CrossRef]
- Candido Camara, M.; Ramos Campos, E.V.; Monteiro, R.A.; Santo Pereira, A.; de Freitas Proença, P.L.; Fraceto, L.F. Development of stimuli-responsive nano-based pesticides: Emerging opportunities for agriculture. J. Nanobiotechnol. 2019, 17, 100. [Google Scholar] [CrossRef] [Green Version]
- Iavicoli, I.; Leso, V.; Beezhold, D.H.; Shvedova, A.A. Nanotechnology in agriculture: Opportunities, toxicological implications, and occupational risks. Toxicol. Appl. Pharmacol. 2017, 329, 96–111. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, A.; Kaur, H.; Mehta, S.; Husen, A. Smart nanomaterial and nanocomposite with advanced agrochemical activities. Nanoscale Res. Lett. 2021, 16, 156. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Hassan, A.A.; Kim, K.H. Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J. Con. Rel. 2019, 294, 131–153. [Google Scholar] [CrossRef]
- An, C.; Sun, C.; Li, N.; Huang, B.; Jiang, J.; Shen, Y.; Wang, C.; Zhao, X.; Cui, B.; Wang, C.; et al. Nanomaterials and nanotechnology for the delivery of agrochemicals: Strategies towards sustainable agriculture. J. Nanobiotechnol. 2022, 20, 11. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Mahmudur Rahman, M.; Liu, Y.; Naidu, R. Nanoencapsulation, nano-guard for pesticides: A new window for safe application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Zhao, H.; Wang, P. Recent development in functional nanomaterials for sustainable and smart agricultural chemical technologies. Nano Converg. 2022, 9, 11. [Google Scholar] [CrossRef]
- Wu, L.; Pan, H.; Huang, W.; Hu, Z.; Wang, M.; Zhang, F. pH and redox dual-responsive mesoporous silica nanoparticle as nanovehicle for improving fungicidal efficiency. Materials 2022, 15, 2207. [Google Scholar] [CrossRef]
- Mishra, D.; Khare, P. Emerging nano-agrochemicals for sustainable agriculture: Benefits, challenges, and risk mitigation. In Sustainable Agriculture Reviews 50; Kumar Singh, V., Singh, R., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2021; Volume 50. [Google Scholar] [CrossRef]
- Kah, M.; Singh Kookana, R.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Singh, A.; Minkina, T.; Rawat, S.; Mandzhieva, S.; Sushkova, S.; Shuvaeva, V.; Nazarenko, O.; Rajput, P.; Komariah; et al. Nano-enabled products: Challenges and opportunities for sustainable agricultura. Plants 2021, 10, 2727. [Google Scholar] [CrossRef]
- Singh, H.; Sharma, A.; Bhardwaj, S.K.; Kumar Arya, S.; Bhardwaj, N.; Khatri, M. Recent advances in the applications of nano-agrochemicals for sustainable agricultural development. Environ. Sci. Process. Impacts 2021, 23, 213. [Google Scholar] [CrossRef]
- Xin, X.; Judy, J.D.; Sumerlin, B.B.; He, Z. Nano-enabled agriculture: From nanoparticles to smart nanodelivery systems. Environ. Chem. 2020, 17, 413–425. [Google Scholar] [CrossRef]
- Chaud, M.; Souto, E.B.; Zielinska, A.; Severino, P.; Batain, F.; Oliveira-Junior, J.; Alves, T. Nanopesticides in agriculture: Benefits and challenge in agricultural productivity, toxicological risks to human health and environment. Toxics 2021, 9, 131. [Google Scholar] [CrossRef]
- Adisa, I.O.; Reddy Pullagurala, V.L.; Peralta-Videa, J.R.; Dimkpa, C.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environ. Sci. Nano J. 2019, 6, 2002–2030. [Google Scholar] [CrossRef]
- Caixeta Oliveira, H.; Stolf-Moreira, R.; Bueno Reis Martinez, C.; Grillo, R.; Bispo de Jesus, M.; Fraceto, L.F. Nanoencapsulation enhances the post-emergence herbicidal activity of atrazine against mustard plants. PloS ONE 2015, 10, e0132971. [Google Scholar] [CrossRef]
- Xing, K.; Chen, X.G.; Liu, C.S.; Cha, D.S.; Park, H.J. Oleoyl-chitosan nanoparticles inhibits Escherichia coli and Staphylococcus aureus by damaging the cell membrane and putative binding to extracellular or intracellular targets. Int. J. Food Microbiol. 2009, 132, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Yu, A.; Wang, G.; Zheng, F.; Hu, P.; Jia, J.; Xu, H. A novel water-based chitosan-La pesticide nanocarrier enhancing defense responses in rice (Oryza sativa L.) growth. Carbohydr. Polym. 2018, 199, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhi, H.; Liang, J.; Yu, M.; Cui, B.; Zhao, X.; Sun, S.; Wang, Y.; Cui, H.; Zeng, Z. Development of leaf-adhesive pesticide nanocapsules with pH-responsive release to enhance retention time on crop leaves and improve utilization efficiency. J. Mater. Chem. B 2021, 9, 783–792. [Google Scholar] [CrossRef]
- Sousa, G.F.M.; Gomes, D.G.; Campos, E.V.R.; Oliveira, J.L.; Fraceto, L.F.; Stolf-Moreira, R.; Oliveira, H.C. Post-emergence herbicidal activity of nanoatrazine against susceptible weeds. Front. Environ. Sci. 2018, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, I.F.; Hussein, M.Z. Synthesis and technology of nanoemulsion-based pesticide formulation. Nanomaterials 2020, 10, 1608. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.L.; Campos, E.V.R.; Pereira, A.E.S.; Pasquoto, T.; Lima, R.; Grillo, R.; de Andrade, D.J.; Aparecido dos Santos, F.; Fraceto, L. Zein nanoparticles as eco-friendly carrier systems for botanical repellents aiming sustainable agriculture. J. Agric. Food Chem. 2018, 66, 1330–1340. [Google Scholar] [CrossRef]
- Tong, Y.; Wu, Y.; Zhao, C.; Xu, Y.; Lu, J.; Xiang, S.; Zong, F.; Wu, X. Polymeric nanoparticles as a metolachlor carrier: Water-based formulation for hydrophobic pesticides and absorption by plants. J. Agric. Food Chem. 2017, 65, 7371–7378. [Google Scholar] [CrossRef] [PubMed]
- Jacques, M.T.; Oliveira, J.L.; Campos, E.V.R.; Fraceto, L.F.; Silva Ávila, D. Safety assessment of nanopesticides using the roundworm Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2017, 139, 245–253. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.L.; Campos, E.V.R.; Germano-Costa, G.; Lima, R.; Della Vechia, J.F.; Soares, S.T.; de Andrade, D.J.; Gonçalves, K.C.; do Nascimento, J.; Polanczyk, R.A.; et al. Association of zein nanoparticles with botanical compounds for effective pest control systems. Pest Manag. Sci. 2019, 75, 1855–1865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhao, C.; Liu, Y.; Cao, L.; Wu, Y.; Huang, Q. Size-dependent effect of prochloraz-loaded PEG-PLGA micro- and nanoparticles. J. Nanosci. Nanotechnol. 2016, 16, 6231–6237. [Google Scholar] [CrossRef]
- da Silva Gündel, S.; Rodrigues dos Reis, T.; MarquezanCopetti, P.; Reis Favarin, F.; RoratoSagrillo, M.; Schafer da Silva, A.; CoráSegat, J.; Baretta, D.; Ferreira Ourique, A. Evaluation of cytotoxicity, genotoxicity and ecotoxicity of nanoemulsions containing Mancozeb and Eugenol. Ecotoxicol. Environ. Saf. 2019, 169, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Panwar, D.; Singh Panesar, P.; Bandhu Bera, M. Encapsulation of functional ingredients in lipidic nanocarriers and antimicrobial applications: A review. Environ. Chem. Lett. 2021, 19, 1107–1134. [Google Scholar] [CrossRef]
- Cui, J.; Sun, C.; Wang, A.; Wang, Y.; Zhu, H.; Shen, Y.; Li, N.; Zhao, X.; Cui, B.; Wang, C.; et al. Dual-functionalized pesticide nanocapsule delivery system with improved spreading behavior and enhanced bioactivity. Nanomaterials 2020, 10, 220. [Google Scholar] [CrossRef] [Green Version]
- Heydari, M.; Amirjani, A.; Bagheri, M.; Sharifian, I.; Sabahi, Q. Eco-friendly pesticide based on peppermint oil nanoemulsion: Preparation, physicochemical properties, and its aphicidal activity against cotton aphid. Environ. Sci. Pollut. Res. 2020, 27, 6667–6679. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar Sharma, N.; Srivastava, A.; Kataria, A.; Dubey, S.; Sharma, S.; Kundu, B. Clove and lemongrass oil based non-ionic nanoemulsion for suppressing the growth of plant pathogenic Fusarium oxysporum f. sp. lycopersici. Ind. Crops Prod. 2018, 123, 353–362. [Google Scholar] [CrossRef]
- Golden, G.; Quinn, E.; Shaaya, E.; Kostyukovsky, M.; Poverenov, E. Coarse and nano emulsions for effective delivery of natural pest control agent pulegone for stored grain protection. Pest Manag. Sci. 2018, 74, 820–827. [Google Scholar] [CrossRef]
- de Castro e Silva, P.; Silva Pereira, L.A.; de Rezende, E.M.; Valquíria dos Reis, M.; Teixeira Lago, A.M.; Ribeiro Carvalho, G.; Paiva, R.; Oliveira, J.E.; Marconcini, J.M. Production and efficacy of neem nanoemulsion in the control of Aspergillus flavus and Penicillium citrinum in soybean seeds. Eur. J. Plant Pathol. 2019, 155, 1105–1116. [Google Scholar] [CrossRef]
- Keskin, D.; Zu, G.; Forson, A.M.; Tromp, L.; Sjollema, J.; van Rijn, P. Nanogels: A novel approach in antimicrobial delivery systems and antimicrobial coatings. Bioact. Mater. 2021, 6, 3634–3657. [Google Scholar] [CrossRef]
- Czarnobai De Jorge, B.; Bisotto-de-Oliveira, R.; Nunes Pereira, C.; Sant’Ana, J. Novel nanoscale pheromone dispenser for more accurate evaluation of Grapholita molesta (Lepidoptera: Tortricidae) attract-and-kill strategies in the laboratory. Pest Manag. Sci. 2017, 73, 1921–1926. [Google Scholar] [CrossRef]
- Kikionis, S.; Ioannou, E.; Konstantopoulou, M.; Roussis, V. Electrospun micro/nanofibers as controlled release systems for pheromones of Bactroceraoleae and Prays oleae. J. Chem. Ecol. 2017, 43, 254–262. [Google Scholar] [CrossRef]
- Christofoli, M.; Candida Costa, E.C.; Bicalho, K.U.; de Cássia Domingues, V.; Fernandes Peixoto, M.; Fernandes Alves, C.C.; Araújo, W.L.; de Melo Cazal, C. Insecticidal effect of nanoencapsulated essential oils from Zanthoxylum rhoifolium (Rutaceae) in Bemisia tabaci populations. Ind. Crops Prod. 2015, 70, 301–308. [Google Scholar] [CrossRef]
- Maluin, F.N.; Hussein, M.Z. Chitosan-based agronanochemicals as a sustainable alternative in crop protection. Molecules 2020, 25, 1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luque-Alcaraz, A.G.; Cortez-Rocha, M.O.; Velázquez-Contreras, C.A.; Acosta-Silva, A.L.; Santacruz-Ortega, H.C.; Burgos-Hernández, A.; Argüelles-Monal, W.M.; Plascencia-Jatomea, M. Enhanced antifungal effect of chitosan/pepper tree (Schinusmolle) essential oil bionanocomposites on the viability of Aspergillus parasiticus spores. J. Nanomater. 2016, 6060137. [Google Scholar] [CrossRef] [Green Version]
- Campos, E.V.R.; Proença, P.L.F.; Oliveira, J.L.; Melville, C.C.; Della Vechia, J.F.; de Andrade, D.J.; Fraceto, L.F. Chitosan nanoparticles functionalized with β-cyclodextrin: A promising carrier for botanical pesticides. Sci. Rep. 2017, 8, 2067. [Google Scholar] [CrossRef] [Green Version]
- Rao Yearla, S.; Padmasree, K. Exploitation of subabul stem lignin as a matrix in controlled release agrochemical nanoformulations: A case study with herbicide diuron. Environ. Sci. Pollut. Res. 2016, 23, 18085–18098. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, D.X.; Jing, T.; Liu, G.; Cao, H.; Li, B.X.; Hou, Y.; Liu, F. Pyraclostrobin loaded lignin-modified nanocapsules: Delivery efficiency enhancement in soil improved control efficacy on tomato Fusarium crown and root rot. Chem. Eng. J. 2020, 394, 124854. [Google Scholar] [CrossRef]
- Pavoni, L.; Pavela, R.; Cespi, M.; Bonacucina, G.; Maggi, F.; Zeni, V.; Canale, A.; Lucchi, A.; Bruschi, F.; Benelli, G. Green micro- and nanoemulsions for managing parasites, vectors and pests. Nanomaterials 2019, 9, 1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; Zhang, Q.; Liu, Q.; Zhu, Z.; McClements, D.J.; Mahdi Jafari, S. Chapter 12: Application of nanoemulsions in formulation of pesticides. In Nanoemulsions Formulation, Applications, and Characterization; Academic Press: Cambridge, MA, USA, 2018; pp. 379–413. [Google Scholar] [CrossRef]
- Nenaah, G.E.; Ibrahim, S.I.A.; Al-Assiuty, B.A. Chemical composition, insecticidal activity and persistence of three Asteraceae essential oils and their nanoemulsions against Callosobruchus maculatus (F.). J. Stored Prod. Res. 2015, 61, 9–16. [Google Scholar] [CrossRef]
- Ramos Campos, E.V.; de Oliveira, J.L.; Gonçalves daSilva, C.M.; Pascoli, M.; Pasquoto, T.; Lima, R.; Abhilash, P.C.; Fernandes Fraceto, L. Polymeric and solid lipid nanoparticles for sustained release of carbendazim and tebuconazole in agricultural applications. Sci. Rep. 2015, 5, 13809. [Google Scholar] [CrossRef] [Green Version]
- Hosseinpour Jajarm, F.; Moravvej, G.; Modarres Awal, M.; Golmohammadzadeh, S. Insecticidal activity of solid lipid nanoparticle loaded by Ziziphora clinopodioides Lam. against Tribolium castaneum (Herbst, 1797) (Coleoptera: Tenebrionidae). Int. J. Pest Manag. 2020, 67, 147–154. [Google Scholar] [CrossRef]
- Luo, J.; Gao, Y.; Liu, Y.; Huang, X.; Zhang, D.X.; Cao, H.; Jing, T.; Liu, F.; Li, B. Self-assembled degradable nanogels provide foliar affinity and pinning for pesticide delivery by flexibility and adhesiveness adjustment. ACS Nano 2021, 15, 14598–14609. [Google Scholar] [CrossRef]
- Farías, B.V.; Pirzada, T.; Mathew, R.; Sit, T.L.; Opperman, C.; Khan, S.A. Electrospun polymer nanofibers as seed coatings for crop protection. ACS Sustain. Chem. Eng. 2019, 7, 19848–19856. [Google Scholar] [CrossRef]
- Bruneau, M.; Bennici, S.; Brendle, J.; Dutournie, P.; Limousy, L.; Pluchon, S. Systems for stimuli-controlled release: Materials and applications. J. Control. Release. 2019, 294, 355–371. [Google Scholar] [CrossRef]
- Feng, S.; Wang, J.; Zhang, L.; Chen, Q.; Yue, W.; Ke, N.; Xie, H. Coumarin-containing light-responsive carboxymethyl chitosan micelles as nanocarriers for controlled release of pesticide. Polymers 2020, 12, 2268. [Google Scholar] [CrossRef]
- Hill, M.R.; MacKrell, E.J.; Forsthoefel, C.P.; Jensen, S.P.; Chen, M.; Moore, G.A.; He, Z.L.; Sumerlin, B.S. Biodegradable and pH-responsive nanoparticles designed for sitespecific delivery in agriculture. Biomacromolecules 2015, 16, 1276–1282. [Google Scholar] [CrossRef]
- Singh, A.; Dhiman, N.; Kumar Kar, A.; Singh, D.; Prasad Purohit, M.; Ghosh, D.; Patnaik, S. Advances in controlled release pesticide formulations: Prospects to safer integrated pest management and sustainable agriculture. J. Hazard Mater. 2020, 385, 121525. [Google Scholar] [CrossRef]
- Chauhan, N.; Dilbaghi, N.; Gopal, M.; Kumar, R.; Kim, K.H.; Kumar, S. Development of chitosan nanocapsules for the controlled release of hexaconazole. Int. J. Biol. Macromol. 2017, 97, 616–624. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, G.; Chen, C.; Liu, B.; Cai, D.; Wu, Z. Fabrication of a pH-responsively controlled-release pesticide using an attapulgite-based hydrogel. ACS Sust. Chem. Eng. 2018, 6, 1192–1201. [Google Scholar] [CrossRef]
- Liang, W.; Yu, A.; Wang, G.; Zheng, F.; Jia, J.; Xu, H. Chitosan-based nanoparticles of avermectin to control pine wood nematodes. Int. J. Biol. Macromol. 2018, 112, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Fellet, G.; Pilotto, L.; Marchiol, L.; Braidot, E. Tools for nano-enabled agriculture: Fertilizers based on calcium phosphate, silicon, and chitosan nanostructures. Agronomy 2021, 11, 1239. [Google Scholar] [CrossRef]
- Wu, H.; Li, Z. Recent advances in nano-enabled agriculture for improving plantperformance. Crop J. 2022, 10, 1–12. [Google Scholar] [CrossRef]
- Xu, X.; Bai, B.; Wang, H.; Suo, Y. A near-infrared and temperature-responsive pesticide release platform through core−shell polydopamine@PNIPAm nanocomposites. ACS Appl. Mater. Interfaces 2017, 9, 6424–6432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, W.; Jing, M.; Liu, S.; Feng, J.; Wu, H.; Zhou, Y.; Zhang, X.; Ma, Z. Self-assembled mixed micelle loaded with natural pyrethrins as an intelligent nano-insecticide with a novel temperature-responsive release mode. Chem. Eng. J. 2019, 361, 1381–1391. [Google Scholar] [CrossRef]
- Atta, S.; Paul, A.; Banerjee, R.; Bera, M.; Ikba, M.; Dhara, D.; Pradeep Singh, N.D. Photoresponsive polymers based on a coumarin moiety for the controlled release of pesticide 2,4-D. RSC Adv. 2015, 5, 99968–99975. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, Z.; Shao, X. Light-triggered release of insecticidally active spirotetramat-enol. Chin. Chem. Lett. 2018, 29, 1648–1650. [Google Scholar] [CrossRef]
- Ye, Z.; Guo, J.; Wu, D.; Tan, M.; Xiong, X.; Yin, Y.; He, G. Photo-responsive shell cross-linked micelles based on carboxymethyl chitosan and their application in controlled release of pesticide. Carbohyd. Polym. 2015, 132, 520–528. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, G.; Dai, Z.; Xiang, Y.; Liu, B.; Bian, P.; Zheng, K.; Wu, Z.; Cai, D. Fabrication of light-responsively controlled-release herbicide using a nanocomposite. J. Chem. Eng. 2018, 349, 101–110. [Google Scholar] [CrossRef]
- Hao, L.; Gong, L.; Chen, L.; Guan, M.; Zhou, H.; Qiu, S.; Wen, H.; Chen, H.; Zhou, X.; Akbulut, M. Composite pesticide nanocarriers involving functionalized boron nitride nanoplatelets for pH-responsive release and enhanced UV stability. Chem. Eng. J. 2020, 396, 125233. [Google Scholar] [CrossRef]
- Tong, Y.; Shao, L.; Li, X.; Lu, J.; Sun, H.; Xiang, S.; Zhang, Z.; Wu, Y.; Wu, X. Adhesive and stimuli-responsive polydopaminecoated graphene oxide system for pesticide loss control. J. Agricul. Food Chem. 2018, 66, 2616–2622. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Xie, Z.; Cheng, J.; Xiao, D.; Xiong, Q.; Wang, Q.; Zhao, J.; Gui, W. A light-triggered pH-responsive metal −organic framework for smart delivery of fungicide to control Sclerotinia diseases of oilseed rape. ACS Nano 2021, 15, 6987–6997. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Zhang, G.; Xiang, Y.; Cai, D.; Wu, Z. Fabrication of a temperature-controlled-release herbicide using a nanocomposite. ACS Sustain. Chem. Eng. 2017, 5, 4969–4975. [Google Scholar] [CrossRef]
- Wang, Y.; Song, S.; Chu, X.; Feng, W.; Li, J.; Huang, X.; Zhou, N.; Shen, J. A new temperature-responsive controlled-release pesticide formulation - poly(N-isopropylacrylamide) modified graphene oxide as the nanocarrier for lambda-cyhalothrin delivery and their application in pesticide transportation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125987. [Google Scholar] [CrossRef]
- Ding, K.; Shi, L.; Zhang, L.; Zeng, T.; Yin, Y.; Yi, Y. Synthesis of photoresponsive polymeric propesticide micelles based on PEG for the controlled release of herbicide. Polym. Chem. 2016, 7, 899–904. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef]
- Mikula, K.; Izydorczyk, G.; Skrzypczak, D.; Mironiuk, M.; Moustakas, K.; Witek-Krowiak, A.; Chojnacka, K. Controlled release micronutrient fertilizers for precision agricultura—A review. Sci. Total Environ. 2020, 712, 136365. [Google Scholar] [CrossRef]
- Singh Duhan, J.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Surekha. Nanotechnology: The new perspective in precision agricultura. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef]
- Guo, H.; White, J.C.; Wang, Z.; Xing, B. Nano-enabled fertilizers to control the release and use efficiency of nutrients. Curr. Opin. Environ. Sustain. 2018, 6, 77–83. [Google Scholar] [CrossRef]
- Marchiol, L.; Filippi, A.; Adamiano, A.; DegliEsposti, L.; Iafisco, M.; Mattiello, A.; Petrussa, E.; Braidot, E. Influence of hydroxyapatite nanoparticles on germination and plant metabolism of tomato (Solanum lycopersicum L.): Preliminary evidence. Agronomy 2019, 9, 161. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, S.; Durgam, M.; Rao Mailapalli, D. Urea loaded hydroxyapatite nanocarrier for efficient delivery of plant nutrients in rice. Arch. Agron. Soil Sci. 2020, 67, 371–382. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, P.; Kopittke, P.M. Tailoring hydroxyapatite nanoparticles to increase their efficiency as phosphorus fertilisers in soils. Geoderma 2018, 323, 116–125. [Google Scholar] [CrossRef]
- Raguraj, S.; Wijayathunga, W.M.S.; Gunaratne, G.P.; Amali, R.K.A.; Priyadarshana, G.; Sandaruwan, C.; Karunaratne, V.; Hettiarachchi, L.S.K.; Kottegoda, N. Urea–hydroxyapatite nanohybrid as an efficient nutrient source in Camellia sinensis (L.) Kuntze (tea). J. Plant Nut. 2020, 43, 2383–2394. [Google Scholar] [CrossRef]
- Rop, K.; Karuku, G.N.; Mbui, D.; Michira, I.; Njomo, N. Formulation of slow release NPK fertilizer (cellulose-graft-poly(acrylamide)/nano-hydroxyapatite/soluble fertilizer) composite and evaluating its N mineralization potential. Ann. Agric. Sci. 2018, 63, 163–172. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Lee, J.G.; Esposti, L.D.; Iafisco, M.; Kim, P.J.; Shin, S.G.; Jeon, J.R.; Adamiano, A. Synergistic release of crop nutrients and stimulants from hydroxyapatite nanoparticles functionalized with humic substances: Toward a multifunctional nanofertilizer. ACS Omega 2020, 5, 6598–6610. [Google Scholar] [CrossRef] [Green Version]
- Everaert, M.; Warrinnier, R.; Baken, S.; Gustafsson, J.P.; De Vos, D.; Smolders, E. Phosphate-exchanged Mg − Al layered double hydroxides: A new slow-release phosphate fertilizer. ACS Sustain. Chem. Eng. 2016, 4, 4280–4287. [Google Scholar] [CrossRef]
- Figueredo Benício, B.; Leopoldo Constantino, V.R.; Garcia Pinto, F.; Vergütz, L.; Tronto, J.; da Costa, L.M. Layered double hydroxides: New technology in phosphate fertilizers based on nanostructured materials. ACS Sustain. Chem. Eng. 2017, 5, 399–409. [Google Scholar] [CrossRef]
- Sarkar, S.; Datta, S.C.; Biswas, D.R. Effect of fertilizer loaded nanoclay/superabsorbent polymer composites on nitrogen and phosphorus release in soil. Proc. Natl. Acad. Sci. India Sect. B-Biol. Sci. 2015, 85, 415–421. [Google Scholar] [CrossRef]
- Bernardo, M.P.; Guimarães, G.G.F.; Majaron, V.F.; Ribeiro, C. Controlled release of phosphate from LDH structures: Dynamics in soil and application as smart fertilizer. ACS Sustain. Chem. Eng. 2018, 6, 5152–5161. [Google Scholar] [CrossRef]
- Songkhum, P.; Wuttikhun, T.; Chanlek, N.; Khemthong, P.; Laohhasurayotin, K. Controlled release studies of boron and zinc from layered double hydroxides as the micronutrient hosts for agricultural application. App. Clay Sci. 2018, 152, 311–322. [Google Scholar] [CrossRef]
- Lateef, A.; Nazir, R.; Jamil, N.; Alam, S.; Shah, R.; Naeem Khan, M.; Saleem, M. Synthesis and characterization of zeolite based nanoecomposite: An environment friendly slow release fertilizer. Microporous Mesoporous Mater. 2016, 232, 174–183. [Google Scholar] [CrossRef]
- Choudhary, R.C.; Kumaraswamy, R.V.; Kumari, S.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Zinc encapsulated chitosan nanoparticle to promote maize crop yield. Int. J. Biol. Macromol. 2019, 127, 126–135. [Google Scholar] [CrossRef]
- Ha, N.M.C.; Nguyen, T.H.; Wang, S.L.; Nguyen, A.D. Preparation of NPK nanofertilizer based on chitosan nanoparticles and its effect on biophysical characteristics and growth of coffee in green house. Res. Chem. Intermed. 2019, 45, 51–63. [Google Scholar] [CrossRef]
- Choudhary, R.C.; Kumaraswamy, R.V.; Kumari, S.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Cu-chitosan nanoparticle boosts defense responses and plant growth in maize (Zea mays L.). Sci. Rep. 2018, 7, 9754. [Google Scholar] [CrossRef] [PubMed]
- Dhlamini, B.; Kamdem Paumo, H.; Katata-Seru, L.; Kutu, F.R. Sulphate-supplemented NPK nanofertilizer and its effect on maize growth. Mater. Res. Express 2020, 7, 095011. [Google Scholar] [CrossRef]
- Pereira, A.E.S.; Sandoval-Herrera, I.E.; Zavala-Betancourt, S.A.; Oliveira, H.C.; Ledezma-Pérez, A.S.; Romero, J.; Fraceto, L.F. γ -polyglutamic acid/chitosan nanoparticles for the plant growth regulator gibberellic acid: Characterization and evaluation of biological activity. Carbohyd. Polym. 2016, 157, 1862–1873. [Google Scholar] [CrossRef] [Green Version]
- Yi, Z.; Hussain, H.I.; Feng, C.; Sun, D.; She, F.; Rookes, J.E.; Cahill, D.M.; Kong, L. Functionalized mesoporous silica nanoparticles with redox-responsive short-chain gatekeepers for agrochemical delivery. ACS Appl. Mater. Interfaces 2015, 7, 9937–9946. [Google Scholar] [CrossRef]
- Kokina, I.; Jahundovica, I.; Mickevica, I.; Jermaonoka, M.; Strautinš, J.; Popovs, S.; Ogurcovs, A.; Sledevskis, E.; Polyakov, B.; Gerbreders, V. Target transportation of auxin on mesoporous Au/SiO2 nanoparticles as a method for somaclonal variation increasingin flax (L. usitatissimum L.). J. Nanomater. 2017, 2017, 7143269. [Google Scholar] [CrossRef] [Green Version]
- Naseem, F.; Zhi, Y.; Akhyar Farrukh, M.; Hussain, F.; Yin, Z. Mesoporous ZnAl2Si10O24 nanofertilizers enable high yield of Oryza sativa L. Sci. Rep. 2020, 10, 10841. [Google Scholar] [CrossRef]
- Rane, M.; Bawskar, M.; Rathod, D.; Nagaonkar, D.; Rai, M. Influence of calcium phosphate nanoparticles, Piriformospora indica and Glomus mosseae on growth of Zea mays. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 045014. [Google Scholar] [CrossRef]
- Pérez-Álvarez, E.P.; Ramírez-Rodríguez, G.B.; Carmona, F.J.; Martínez-Vidaurre, J.M.; Masciocchi, N.; Guagliardi, A.; Garde-Cerdán, T.; Delgado-López, J.M. Towards a more sustainable viticulture: Foliar application of N-doped calcium phosphate nanoparticles on Tempranillo grapes. J. Sci. Food Agric. 2020, 101, 1307–1313. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.B.; DalSasso, G.; Carmona, F.J.; Miguel-Rojas, C.; Pérez-de-Luque, A.; Masciocchi, N.; Guagliardi, A.; Delgado-López, J.M. Engineering biomimetic calcium phosphate nanoparticles: A green synthesis of slow-release multinutrient (NPK) nanofertilizers. ACS Appl. Bio Mater. 2020, 3, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Gaiotti, F.; Lucchetta, M.; Rodegher, G.; Lorenzoni, D.; Longo, E.; Boselli, E.; Cesco, S.; Belfiore, N.; Lovat, L.; Delgado-López, J.M.; et al. Urea-doped calcium phosphate nanoparticles as sustainable nitrogen nanofertilizers for viticulture: Implications on yield and quality of pinot gris grapevines. Agronomy 2021, 11, 1026. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.B.; Miguel-Rojas, C.; Montanha, G.S.; Carmona, F.J.; Dal Sasso, G.; Sillero, J.C.; Skov Pedersen, J.; Masciocchi, N.; Guagliardi, A.; Pérez-de-Luque, A.; et al. Reducing nitrogen dosage in Triticum durum plants with urea-doped nanofertilizers. Nanomaterials 2020, 10, 1043. [Google Scholar] [CrossRef]
- Carmona, F.J.; Dal Sasso, G.; Ramírez-Rodríguez, G.B.; Pii, Y.; Delgado-López, J.M.; Guagliardi, A.; Masciocchi, N. Urea-functionalized amorphous calcium phosphate nanofertilizers: Optimizing the synthetic strategy towards environmental sustainability and manufacturing costs. Sci. Rep. 2021, 11, 3419. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Azmal Ali, S. Nanoparticle-based sustainable agriculture and food science: Recent advances and future outlook. Front. Nanotechnol. 2020, 2, 579954. [Google Scholar] [CrossRef]
- Kottegoda, N.; Sandaruwan, C.; Priyadarshana, G.; Siriwardhana, A.; Rathnayake, U.A.; BerugodaArachchige, D.M.; Kumarasinghe, A.R.; Dahanayake, D.; Karunaratne, V.; Amaratunga, G.A.J. Urea-hydroxyapatite nanohybrids for slow release of nitrogen. ACS Nano 2017, 11, 1214–1221. [Google Scholar] [CrossRef] [Green Version]
- Tarafder, C.; Daizy, M.; Alam, M.; Ali, R.; Islam, J.; Islam, R.; Ahommed, S.; Saad Aly, M.A.; Hossain Khan, Z. Formulation of a hybrid nanofertilizer for slow and sustainable release of micronutrients. ACS Omega 2020, 5, 23960–23966. [Google Scholar] [CrossRef]
- Mikhak, A.; Sohrabi, A.; Zaman Kassaee, M.; Feizian, M. Synthetic nanozeolite/nanohydroxyapatite as a phosphorus fertilizer for German chamomile (Matricaria chamomilla L.). Ind. Crops Prod. 2016, 95, 444–452. [Google Scholar] [CrossRef]
- Deshpande, P.; Dapkekar, A.; Oak, M.D.; Paknikar, K.M.; Rajwade, J.M. Zinc complexed chitosan/TPP nanoparticles: A promising micronutrient nanocarrier suited for foliar application. Carbohydr. Polym. 2017, 165, 394–401. [Google Scholar] [CrossRef]
- Kazemi, N.M.; Salimi, A.A. Chitosan nanoparticle for loading and release of nitrogen, potassium, and phosphorus nutrients. Iran J. Sci. Technol. Trans. A Sci. 2019, 43, 2781–2786. [Google Scholar] [CrossRef]
- Kubavat, D.; Trivedi, K.; Vaghela, P.; Prasad, K.; Vijay Anand, G.K.; Trivedi, H.; Patidar, R.; Chaudhari, J.; Andhariya, B.; Ghosh, A. Characterization of a chitosan-based sustained release nanofertilizer formulation used as a soil conditioner while simultaneously improving biomass production of Zea mays L. Land Deg. Dev. 2020, 31, 2734–2746. [Google Scholar] [CrossRef]
- Kondal, R.; Kalia, A.; Krejcar, O.; Kuca, K.; Sharma, S.P.; Luthra, K.; Singh Dheri, G.; Vikal, Y.; Sachdeva Taggar, M.; Abd-Elsalam, K.A.; et al. Chitosan-urea nanocomposite for improved fertilizer applications: The effect on the soil enzymatic activities and microflora dynamics in N cycle of potatoes (Solanum tuberosum L.). Polymers 2021, 13, 2887. [Google Scholar] [CrossRef]
- Leonardi, M.; Caruso, G.M.; Carroccio, S.C.; Boninelli, S.; Curcuruto, G.; Zimbone, M.; Allegra, M.; Torrisi, B.; Ferlito, F.; Miritello, M. Smart nanocomposites of chitosan/alginate nanoparticles loaded with copper oxide as alternative nanofertilizers. Environ. Sci. Nano 2021, 8, 174. [Google Scholar] [CrossRef]
- Sun, D.; Hussain, H.I.; Yi, Z.; Rookes, J.E.; Kong, L.; Cahill, D.M. Mesoporous silica nanoparticles enhance seedling growth and photosynthesis in wheat and lupin. Chemosphere 2016, 152, 81–91. [Google Scholar] [CrossRef]
- Qazi, G.; Dar, F.A. Nano-agrochemicals: Economic Potential and Future Trends. In Nanobiotechnology in Agriculture; Hakeem, K., Pirzadah, T., Eds.; Nanotechnology in the Life Sciences; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Kah, M. Nanopesticides and nanofertilizers: Emerging contaminants or opportunities for risk mitigation? Front. Chem. 2015, 3, 64. [Google Scholar] [CrossRef] [Green Version]
- Gomez, A.; Naraya, M.; Zhao, L.; Jia, X.; Bernal, R.A.; Lopez-Moreno, M.L.; Peralta-Videa, J.R. Effects of nano-enabled agricultural strategies on food quality: Current knowledge and future research needs. J. Hazard Mater. 2021, 401, 123385. [Google Scholar] [CrossRef]
- Pravin Shahane, S.; Kumar, A. Estimation of health risks due to copper-based nanoagrochemicals. Environ. Sci. Poll. Res. 2022, 29, 25046–25059. [Google Scholar] [CrossRef]
- Amenta, V.; Aschberger, K.; Arena, M.; Bouwmeester, H.; Botelho Moniz, F.; Brandhoff, P.; Gottardo, S.; Marvin, H.J.P.; Mech, A.; QuirosPesudo, L.; et al. Regulatory aspects of nanotechnology in the agri/feed/food sector in EU and non-EU countries. Regul. Toxicol.Pharmacol. 2015, 73, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Das, S. Regulatory requirements for nanopesticides and nanofertilizers. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture; Elsevier: Amsterdam, The Netherlands, 2021; pp. 145–152. [Google Scholar]
- Jain, A.; Ranjan, S.; Dasgupta, N.; Ramalingam, C. Nanomaterials in Food and Agriculture: An overview on their safety concerns and regulatory issues. Crit. Rev. Food Sci. Nut. 2016, 58, 297–317. [Google Scholar] [CrossRef] [PubMed]
| Formulation | Active Ingredient | Size (nm) | Target Organism | Suppresion Effect | Compared to Control | Reference |
|---|---|---|---|---|---|---|
| Nanocapsules | ||||||
| Chitosan | Pepper tree essential oil | 20–100 | Aspergillus parasiticus | Viability | 40–50% | [29] |
| Chitosan funcionalized with β-cyclodextrin | Carvacrol linalool | 175.2–245.8 | Tetranychus urticae | Repellency | >80% | [30] |
| Chitosan | Avermectin | 310 | Magnaporthe grisea | Blast fungus | 2-fold | [31] |
| Poly(ε-caprolactone) | Atrazine | 240.7 | Brassica juncea | Dry weight | 10-fold | [32] |
| Lignin | Pyraclostrobin | 162.4 | Fusarium oxysporum f.sp. radicis-lycopersici | EC50 | 3.8-fold | [33] |
| mPpeg-PLGA | Metolachlor | 90.49–128.7 | Oryza sativa-Digitaria sanguinalis | Seedling height | ~5.5-fold | [34] |
| Root length | ~10-fold | |||||
| mPEG-PLGA | Prochloraz | 190.7 | Fusarium graminearum | Germicidal efficacy | 7.7% | [35] |
| Poly(ε-caprolactone) | Atrazine | 260 | Bidens pilosa Amaranthus viridis | Inhibitory growth | 10-fold | [36] |
| Zein | Essential oil of citronella | 142.5–172.3 | Tetranychus urticae Koch mite | Repellency | 200% | [37] |
| PCL | Essential oil of Zanthoxylum rhoifolium | 500 | Bemisia tabaci | Number of eggs and nymphs | 95% | [38] |
| Nanoemulsions | ||||||
| Neem oil | Azadirachta indica | 59 | Aspergillus flavus Penicillium citrinum | Growth inhibition | 71.4% | [39] |
| Polylactide | Validamycin Thifluzamide | 260 | Rhizoctonia solani | Toxicity | 4.2-times | [40] |
| Span 80 | Mancozeb Eugenol | 200–300 | Glomerella cingulata | Number of juveniles | 1-fold | [41] |
| Sunflower oil | R-(+)-pulgone | 131–558 | Sitophilus oryzae L. Tribolium castaneum | Mortality rates | >90% | [42] |
| Mentha piperita oil and Tween 80 | Mentha piperita essential oil | 20–60 | Cotton aphid | Contact toxicity | LC50: ~3879 | [43] |
| - | Essential oil of Ageratum conyzoides, Achillea fragrantissima and Tagetes minuta | 48.6–136.3 | Callosobruchus maculatus | Egg toxicity | LC50:16.1–40.5 µL L−1 | [44] |
| Propylene glycol | Clove and lemongrass oil | 76.73 | Fusarium oxysporum f.sp. lycopersici | Severity | 70.6% | [45] |
| Lipid nanoparticles | ||||||
| Percirol ATO5 + campritol 888 | Essential oil of Ziziphora clinopodioides Lam. | 241.1 | Tribolium castaneum | Mortality | 100% | [46] |
| Nanogels | ||||||
| Polyethylene glycol 4,4-Methylenediphenyl diisocyanate | λ-cyhalothrine | 120 | Athetis dissimilis | Mortality | ~60% | [47] |
| Nanofibers | ||||||
| Poly-ε-caprolactone Polyethylene glycol | Cypermethrin (Z)-8-Dodecenyl acetate (Z)-8-Dodecanol | - | Grapholita molesta (Lepidoptera: Tortricidae) | Mortality | >87% | [48] |
| Response to | Polymer | AI | Condition Release | Size (nm) | Organism Target | Suppression Effect | Compared to Control | Reference |
|---|---|---|---|---|---|---|---|---|
| pH | ||||||||
| Chitosan/tripolyphosphate | Hexaconazole | pH 4 | 100 | Rhizoctonia solani | [67] | |||
| Polydopamine-modified attapulgite- calcium alginate hydrogel nanosphere | Chlorpyrifos | pH 5.5–8.5 | 20 | Grubs | Mortality | 42–100% | [68] | |
| Poly-γ-glutamic acid/chitosan | Avermectin | pH 8.5 | 56–61 | Pine wood nematode | Blast fungus | 2-fold | [31] | |
| Chitosan | Avermectin | Low pH | 251.5–258.5 | Aphids | Toxicity | LC50: 8.1 mg L−1 | [69] | |
| Zeoliticimidazolate (2-methylimidazole/2,4-dinitrobenzaldehyde/Zn(NO3)2·6H2O | Prochloraz | pH 5 | 129.6 | Sclerotinia sclerotiorum | Antifungal effectivity | 70.8% | [70] | |
| Bimodal mesoporous silica modified with a silane coupling agent | Prochloraz | pH 5 | 546.4 | Rhizoctonia solani | Inhibition rate | 80% | [21] | |
| Temperature | ||||||||
| Attapulgite/NH4HCO3/ amino silicon oil/ poly(vinyl alcohol) | Glyphosate | 40 °C | Zoysia matrella | Control efficiency | ~70% | [71] | ||
| Polydopamine/PNIPAm | Imidacloprid | 15–40°C | ~250 | - | - | - | [72] | |
| Poly[2-(2-Methoxyethoxy) ethyl methacrylate-co-Octadecyl methacrylate] /monomethoxy (polyethylene glycol) 13 -poly(D, L-l actide-co-glycolide) and monomethoxy (polyethylene glycol) 45 -poly(D, L-Lactide) | Pyrethrins | 26 °C | 60–120 | Culex pipienspallens Aedes albopictus | Toxicity | LC50: 0.06–0.12 µg a.i mL−1 | [3] | |
| Light | ||||||||
| Poly(ethylene glycol)/photolabile o-nitrobenzyl | Dichlorophenoxyacetic acid | After 365 nm UV light | 40 | - | - | - | [73] | |
| Carboxymethyl chitosan/photolabile 2-nitrobenzyl side groups | Diuron | 365 nm UV light | 140 | - | - | - | [74] | |
| Coumarin | 2,4-D | UV light | Cucurbita maxima | Root length | 25–50% | [75]) | ||
| Coumarin | Spirotetramat-enol | Blue light (420 nm) irradiation or sunlight | Aphis craccivora Koch | Toxicity | LC50:0.08–0.11 mmolL−1 | [76] | ||
| Attapulgite/biochar/azobenzene/amino silicon oil | Glyphosate | UV–Vis light (365 and 435 nm) | 0.5–1 μm | Bermuda weeds | Control efficiency | ~90% | [69] |
| Nanocarrier Nature | Fertilizer | Size (nm) | Plant | Exposure Period | Condition | Effect | Compared to Control | Reference |
|---|---|---|---|---|---|---|---|---|
| Hydroxyapatite | ||||||||
| Urea | 15–20 | Oryza sativa | 4 weeks | Field | Yield | ~41.8% | [88] | |
| NK leaf content | 5.9–10.9% | |||||||
| - | 35–45 | Solanum lycopersicum | 2 weeks | Hydroponic (controlled conditions) | Root elongation | 100% | [89] | |
| Urea | 40–60 | Oryza sativa | 5 days | Petri dishes (controlled conditions) | Amilase content | ~153% | [90] | |
| Starch content | ~100% | |||||||
| Urea | - | Camellia sinensis | Field | Yield increase | 10–17% | [91] | ||
| Urea NPs of Cu, Fe, and Zn | 39.76 | Abelmoschus esculentus | 14 days | Field | Fe nutrient uptake | ~2-fold | [92] | |
| P | 75–125 | Zea mays | 3 months | Pot experiment (controlled conditions) | Dry weight/unit P | ~100% | [93] | |
| Corn grain productivity | ~35% | |||||||
| Resistance to NaCl stress (dry weight/unit P) | ~300% | |||||||
| Nanoclays | Phosphate | 20 | Hordeum vulgare | 17 days | Pot experiment | P efficiency | 4.5-times | [94] |
| Satured nano-zeolite with (NH4)2SO4 plus nano-HA and satured nano-zeolite with (NH4)2SO4 plus triple phosphate | <100 | Matricaria chamomilla | - | Greenhouse experiment | Height | 72.5% | [95] | |
| Branch number | 168.4% | |||||||
| Flower number | 292.9% | |||||||
| Phosphorus content | 85.7% | |||||||
| Fresh weight | ~180% | |||||||
| Dry weight | ~100% | |||||||
| Phosphate | - | Zea mays | 25 days after sowing | Growth chamber | Dry matter | ~11.5% | [96] | |
| P content | ~29% | |||||||
| Height | ~7.1% | |||||||
| Soil pH | ~18% | |||||||
| Phosphate | - | Triticum aestivum | 30 days | Pot experiment | Dry matter | 122.2% | [97] | |
| Phosphate content | ~10.3-fold | |||||||
| Available phosphate | ~24.6-fold | |||||||
| Zinc, boro | - | Solanum lycopersicum | 2 weeks | Pot experiment | Dry mass | ~6–10-fold | [98] | |
| P content | ~10–16-fold | |||||||
| K content | ~13–18-fold | |||||||
| B content | ~9–16-fold | |||||||
| Zn content | ~8–10-fold | |||||||
| Chitosan | ||||||||
| Zn | 250–300 | Wheat | 5 weeks | Pot experiment | Zn content | 27–42% | [99] | |
| Cu (0.01%) | 361.3 | Zea mays | 95 days | Field | Height | 7.8% | [100] | |
| Ear length | 15.3% | |||||||
| Zn (0.01%) | 200–300 | Zea mays | 95 days | Pot experiment | Grain yield | 19.3% | [101] | |
| Grain Zn | 20.9% | |||||||
| Height | 30.2% | |||||||
| Stem diameter | 87.5% | |||||||
| Plant defense | 14% | |||||||
| K (75% CNK) | 39–79 | Zea mays | 60 days after sowing | Pot experiment | Fresh and dry biomass | 47–51% | [102] | |
| Fresh shoot biomass | 8.4-fold | |||||||
| Dry shoot biomass | 10-fold | |||||||
| N uptake | 8.4-fold | |||||||
| P uptake | 11.4-fold | |||||||
| Urea (100%) | Solanum tuberosum | 90 days | Pot experiment | Fresh weight | 95.6% | [103] | ||
| Dry weight | 116% | |||||||
| CuO- chitosan/alginate NPs | ~300 | Fortunella margarita Swingle | Petri dishes | Germination seed | 10% | [104] | ||
| Mesoporous silica nanoparticle | - | 20 | Wheat | 6–14 days | Petri dishes (controlled conditions) | Germination rate | 12.8% | [105] |
| Shoot fresh weight | 30.4% | |||||||
| Root fresh weight | 50% | |||||||
| Chlorophyll content | 38.4% | |||||||
| Total proteins | 17.7% | |||||||
| Auxin on mesoporous Au/SiO2 | 40–60 | Linum usitatissimum | 3 weeks | Growth chamber (controlled conditions) | Embryogenesis | 65% | [106] | |
| Calli induction frequency | 6% | |||||||
| Calli length | 31.2% | |||||||
| Number of regeneration zones | 3.6-fold | |||||||
| Nanocomposite of ZnAl2Si10O24 + urea | 55.2 | Oryza sativa | 14 days | Pot experiment | Nitrogen recovery efficiency | ~10% | [107] | |
| Amorphous calcium phosphate | Glomus mosseae Piriformospora indica | 88 | Zea mays | 45 days | Pot experiment | Shoot length | 8.3% | [108] |
| Root length | 17.2% | |||||||
| Shoot dry weight | 14.6% | |||||||
| Shoot fresh weight | 39.44% | |||||||
| Root fresh weight | 54.3% | |||||||
| Urea | 30–100 | Tempranillo grapevine | 7 weeks | Field condition | Arginine | ~70% | [109] | |
| Amino N | ~21% | |||||||
| YAN (N content) | ~64% | |||||||
| NPK | 10–25 | Triticum durum | - | Pot experiment | Nitrogen efficiency | 40% | [110] | |
| Kernel weight | ~73% | |||||||
| Urea | 13.8 | Triticum durum | - | Growth chamber Field condition | Plant weight | ~40% | [111] | |
| Ear weight | ~60% | |||||||
| Ear number | ~50% | |||||||
| Kernel number | ~27% | |||||||
| Urea | ~10 | Vitis vinifera L. cv Pinot Gris | Two season of study (2019–2020) | Pot experiment (semi-controlled conditions) | Chlorophyll (SPAD) | ~10% | [112] | |
| Yield | ~40% | |||||||
| Bunch weight | ~46% | |||||||
| YAN | ~53% | |||||||
| Urea | ~10 | Cucumis sativus L | 7 days | Hydroponiccondition | Root biomass | ~120% | [113] | |
| Shoot biomass | ~25% | |||||||
| Root N concentration | ~32% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fincheira, P.; Hoffmann, N.; Tortella, G.; Ruiz, A.; Cornejo, P.; Diez, M.C.; Seabra, A.B.; Benavides-Mendoza, A.; Rubilar, O. Eco-Efficient Systems Based on Nanocarriers for the Controlled Release of Fertilizers and Pesticides: Toward Smart Agriculture. Nanomaterials 2023, 13, 1978. https://doi.org/10.3390/nano13131978
Fincheira P, Hoffmann N, Tortella G, Ruiz A, Cornejo P, Diez MC, Seabra AB, Benavides-Mendoza A, Rubilar O. Eco-Efficient Systems Based on Nanocarriers for the Controlled Release of Fertilizers and Pesticides: Toward Smart Agriculture. Nanomaterials. 2023; 13(13):1978. https://doi.org/10.3390/nano13131978
Chicago/Turabian StyleFincheira, Paola, Nicolas Hoffmann, Gonzalo Tortella, Antonieta Ruiz, Pablo Cornejo, María Cristina Diez, Amedea B. Seabra, Adalberto Benavides-Mendoza, and Olga Rubilar. 2023. "Eco-Efficient Systems Based on Nanocarriers for the Controlled Release of Fertilizers and Pesticides: Toward Smart Agriculture" Nanomaterials 13, no. 13: 1978. https://doi.org/10.3390/nano13131978
APA StyleFincheira, P., Hoffmann, N., Tortella, G., Ruiz, A., Cornejo, P., Diez, M. C., Seabra, A. B., Benavides-Mendoza, A., & Rubilar, O. (2023). Eco-Efficient Systems Based on Nanocarriers for the Controlled Release of Fertilizers and Pesticides: Toward Smart Agriculture. Nanomaterials, 13(13), 1978. https://doi.org/10.3390/nano13131978










