Preparation of Volborthite by a Facile Synthetic Chemical Solvent Extraction Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Experiments
2.2. Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Q.; Shao, M.W.; Que, R.H.; Cheng, L.; Zhuo, S.J.; Tong, Y.H.; Lee, S.T. Silver vanadate nanoribbons: A label-free bioindicator in the conversion between human serum transferrin and apotransferrin via surface-enhanced Raman scattering. Appl. Phys. Lett. 2011, 98, 193110. [Google Scholar] [CrossRef]
- Pan, G.T.; Lai, M.H.; Juang, R.C.; Chung, T.W.; Yang, T.C.K. Preparation of visible-light-driven silver vanadates by a microwave-assisted hydrothermal method for the photodegradation of volatile organic vapors. Ind. Eng. Chem. Res. 2011, 50, 2807–2814. [Google Scholar] [CrossRef]
- Pitale, S.S.; Gohain, M.; Nagpure, I.M.; Ntwaeaborwa, O.M.; Bezuidenhoudt, B.C.B.; Swart, H.C. A comparative study on structural, morphological and luminescence characteristics of Zn3(VO4)2 phosphor prepared via hydrothermal and citrate-gel combustion routes. Phys. B 2012, 407, 1485–1488. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Sun, Y.; Li, C.S.; Hua, R.S. Rational synthesis of copper vanadates/polypyrrole nanowires with enhanced electrochemical property. Mater. Lett. 2013, 91, 154–157. [Google Scholar] [CrossRef]
- Hao, M.F.; Xiao, M.S.; Qian, L.H.; Miao, Y.Q. Synthesis of cobalt vanadium nanomaterials for efficient electrocatalysis of oxygen evolution. Front. Chem. Sci. Eng. 2018, 12, 409–416. [Google Scholar] [CrossRef]
- Pulipaka, S.; Boni, N.; Meduri, P. Copper vanadate (Cu3V2O8):(Mo, W) doping insights to enhance performance as an anode for photoelectrochemical water splitting. ACS Appl. Energy Mater. 2020, 3, 6060–6064. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Li, W.Y.; Li, C.S.; Chen, J. Synthesis, characterization, and electrochemical properties of Ag2V4O11 and AgVO3 1-D nano/microstructures. J. Phys. Chem. B 2006, 110, 24855–24863. [Google Scholar] [CrossRef]
- Chen, Z.J.; Gao, S.K.; Li, R.H.; Wei, M.D.; Wei, K.M.; Zhou, H.S. Lithium insertion in ultra-thin nanobelts of Ag2V4O11/Ag. Electrochim. Acta 2008, 53, 8134–8137. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, S.Y.; Ji, W.Q.; Tao, Z.L.; Chen, J. CuV2O6 Nanowires: Hydrothermal synthesis and primary lithium battery application. J. Am. Chem. Soc. 2008, 130, 5361–5367. [Google Scholar] [CrossRef]
- Sauvage, F.; Bodenez, V.; Tarascon, J.M.; Poeppelmeier, K.R. Room-temperature synthesis leading to nanocrystalline Ag2V4O11. J. Am. Chem. Soc. 2010, 132, 6778–6782. [Google Scholar] [CrossRef]
- Kaur, P.; Khanna, A. Structural, electrical and luminescence properties of M2V2O7 (M = Mg, Ca, Sr, Ba, Zn). J. Mater. Sci. Mater. Electron. 2021, 32, 21813–21823. [Google Scholar] [CrossRef]
- Hillel, T.; Ein-Eli, Y. Copper vanadate as promising high voltage cathodes for Li thermal batteries. J. Power Sources 2013, 229, 112–116. [Google Scholar] [CrossRef]
- Lin, N.; Pei, L.Z.; Wei, T.; Yu, H.Y. Synthesis of Cu vanadate nanorods for visible-light photocatalytic degradation of gentian violet. Cryst. Res. Technol. 2015, 50, 255–262. [Google Scholar] [CrossRef]
- Zhang, S.; Ci, L.; Liu, H. Synthesis, characterization, and electrochemical properties of Cu3V2O7(OH)2·2H2O nanostructures. J. Phys. Chem. C 2009, 113, 8624–8629. [Google Scholar] [CrossRef]
- Wei, Y.J.; Nam, K.W.; Chen, G.; Ryu, C.W.; Kim, K.B. Synthesis and structural properties of stoichiometric and oxygen deficient CuV2O6 prepared via co-precipitation method. Solid State Ion. 2005, 176, 2243–2249. [Google Scholar] [CrossRef]
- Cao, J.Q.; Wang, X.Y.; Tang, A.P.; Wang, X.; Wang, Y.; Wen, W. Sol–gel synthesis and electrochemical properties of CuV2O6 cathode material. J. Alloys Compd. 2009, 479, 875–878. [Google Scholar] [CrossRef]
- Sivakumar, V.; Suresh, R.; Giribabu, K.; Manigandan, R.; Munusamy, S.; Praveen Kumar, S.; Muthamizh, S.; Narayanan, V. Copper vanadate nanoparticles: Synthesis, characterization and its electrochemical sensing property. J. Mater. Sci. Mater. Electron. 2014, 25, 1485–1491. [Google Scholar] [CrossRef]
- Ponomarenko, L.; Vasil’ev, A.; Antipov, E.; Velikodny, Y.A. Magnetic properties of Cu2V2O7. Phys. B Condens. Matter 2000, 284, 1459–1460. [Google Scholar] [CrossRef]
- Seabold, J.A.; Neale, N.R. All first row transition metal oxide photoanode for water splitting based on Cu3V2O8. Chem. Mater. 2015, 27, 1005–1013. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, Y.; Li, C.; Ci, L. Cu3V2O8 hollow spheres in photocatalysis and primary lithium batteries. Solid State Sci. 2013, 25, 15–21. [Google Scholar] [CrossRef]
- Ni, S.B.; Wang, X.H.; Zhou, G.; Yang, F.; Wang, J.M.; He, D.Y. Hydrothermal synthesis and magnetic property of Cu3(OH)2V2O7·nH2O. Mater. Lett. 2010, 64, 516–519. [Google Scholar] [CrossRef]
- Yu, X.; Hu, F.; Guo, Z.-Q.; Liu, L.; Song, G.-H.; Zhu, K. High-performance Cu0.95V2O5 nanoflowers as cathode materials for aqueous zinc-ion batteries. Rare Met. 2022, 41, 29–36. [Google Scholar] [CrossRef]
- Bayat, A.; Reza Mahjoub, A.; Amini, M. Facile hydrothermal synthesis of the colloidal hierarchical Volborthite (Cu3V2O7(OH)2·2H2O) hollow sphere phosphors. J. Lumin. 2018, 204, 204382–204385. [Google Scholar] [CrossRef]
- Fu, X.; Hu, Z.S.; Gu, G.H.; Wang, D.B.; Zhu, H.T.; Zhou, X.D. Study on the preparation of nano-materials with extractant and extraction systems. In Proceedings of the International Solvent Extraction Conference 2005, Beijing, China, 19–23 September 2005; China Academic Journal (CD) Electronic Publishing House: Beijing, China, 2005; pp. 852–857. [Google Scholar]
- Shi, H.Q.; Fu, X.; Zhou, X.D.; Hu, Z.S. Preparation of organic fluids containing Ag2S nano-particles with the extractant Cyanex 301. In Proceedings of the International Solvent Extraction Conference 2005, Beijing, China, 19–23 September 2005; China Academic Journal (CD) Electronic Publishing House: Beijing, China, 2005; pp. 874–880. [Google Scholar]
- Zhang, S.L.; Shi, H.Q.; Fu, X.; Hu, Z.S. Preparation and characterisation of organic fluids containing Bi2S3 nano-particles. In Proceedings of the International Solvent Extraction Conference 2005, Beijing, China, 19–23 September 2005; China Academic Journal (CD) Electronic Publishing House: Beijing, China, 2005; pp. 858–863. [Google Scholar]
- Sánchez-Loredo, G.; Tovar-Tovar, R.; Aguilera-Mares, J.; Ruiz, F.; Martínez-Castañón, G. Stabilized metal and metal sulphide nanoparticles prepared by the two-phase liquid-liquid method. Solvent Extraction: Fundamentals to Industrial Applications ed B Moyer; The Canadian Institute of Mining, Metallurgy and Petroleum: Montreal, QC, Canada, 2008; pp. 1621–1626. [Google Scholar]
- Konishi, Y.; Asai, S.; Murai, T.; Takemori, H. Preparation of fine ceria powders by hydrolysis of cerium(IV) carboxylate solutions. Metall. Mater. Trans. B 1997, 28, 959–961. [Google Scholar] [CrossRef]
- Doyle, F.M. Integrating solvent extraction with the processing of advanced ceramic materials. Hydromet. 1992, 29, 527–545. [Google Scholar] [CrossRef]
- Palomares-Sánchez, S.A.; Ponce-Castañeda, S.; Martínez, J.R.; Ruiz, F.; Chumakov, Y.; Domínguez, O. Quantitative analysis of iron oxide particles embedded in an amorphous xerogel matrix. J. Non Cryst. Solids 2003, 325, 251–257. [Google Scholar] [CrossRef]
- Lutterotti, L.; Matthies, S.; Wenk, H.-R. MAUD: A friendly Java program for Material Analysis Using Diffraction. Newsl. CPD 1999, 21, 14–15. [Google Scholar]
- Bergmann, J.; Friedel, P.; Kleeberg, R. BGMN—A new fundamental parameters-based Rietveld program for laboratory X-ray sources, its use in quantitative analysis and structure investigations. CPD Newsl. 1998, 20, 5–8. [Google Scholar]
- Döbelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Lafontaine, M.A.; Le Bail, A.; Ferey, G. Copper-containing minerals—I. Cu3V2O7(OH)2.2H2O: The synthetic homolog of volborthite; crystal structure determination from X-ray and neutron data; structural correlations. J. Solid State Chem. 1990, 85, 220–227. [Google Scholar] [CrossRef]
- Ishikawa, H.; Yamaura, J.; Okamoto, Y.; Yoshida, H.; Nilsen, G.J.; Hiroi, Z. A novel crystal polymorph of volborthite, Cu3V2O7(OH)2·2H2O. Acta Cryst. 2012, C68, i41–i44. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D.; Calvo, C. Crystal structure of Cu5V2O10. Acta Cryst. 1973, B29, 1338–1345. [Google Scholar] [CrossRef]
- Leblanc, M.; Ferey, G. Room-temperature structures of oxocopper(II) vanadate(V) hydrates, Cu3V2O8(H2O) and CuV2O6(H2O)2. Acta Cryst. 1990, C46, 15–18. [Google Scholar] [CrossRef]
- Ghiyasiyan-Arani, M.; Masjedi-Arani, M.; Ghanbari, D.; Bagheri, S.; Salavati-Niasari, M. Novel chemical synthesis and characterization of copper pyrovanadate nanoparticles and its influence on the flame retardancy of polymeric nanocomposites. Sci. Rep. 2016, 6, 25231. [Google Scholar] [CrossRef]
- Naz, G.; Othaman, Z.; Shamsuddin, M.; Krishna Ghoshal, S. Aliquat 336 stabilized multi-faceted gold nanoparticles with minimal ligand density. Appl. Surf. Sci. 2016, 363, 74–82. [Google Scholar] [CrossRef]
- Gole, A.; Murphy, C.J. Seed-mediated synthesis of gold nanorods: Role of the size and nature of the seed. Chem. Mater. 2004, 16, 3633–3640. [Google Scholar] [CrossRef]
- Xu, Z.X.; Zhuang, X.D.; Yang, C.Q.; Cao, J.; Yao, Z.Q.; Tang, Y.P.; Jiang, J.Z.; Wu, D.Q.; Feng, X.L. Nitrogen-doped porous carbon superstructures derived from hierarchical assembly of polyimide nanosheets. Adv. Mater. 2016, 28, 1981–1987. [Google Scholar] [CrossRef]
- Xia, H.C.; Xu, Q.; Zhang, J.N. Recent progress on two-dimensional nanoflake ensembles for energy storage applications. Nano Micro Lett. 2018, 10, 66. [Google Scholar] [CrossRef]
- Mahmoud, S.A.; Bendary, S.H.; Salem, A.A.; Fouad, O.A. Facile synthesis of high yield two-dimensional zinc vanadate nanoflakes, SN. Appl. Sci. 2019, 1, 497. [Google Scholar]
- Le, M.N.; Son, S.H.; Lee, M.S. Extraction behavior of hydrogen ion by an ionic liquid mixture of Aliquat 336 and Cyanex 272 in chloride solution. Korean J. Met. Mater. 2019, 57, 162–169. [Google Scholar] [CrossRef]
- Nguyen, V.N.H.; Le, M.N.; Lee, M.S. Comparison of extraction ability between a mixture of Alamine 336/Aliquat 336 and D2EHPA and ionic liquid ALi-D2 from weak hydrochloric acid solution. Metals 2020, 10, 1678. [Google Scholar] [CrossRef]
- Mireles, L.K.; Wu, M.-R.; Saadeh, N.; Yahia, L.H.; Sacher, E. Physicochemical characterization of polyvinyl pyrrolidone: A tale of two polyvinyl pyrrolidones. ACS Omega 2020, 5, 30461–30467. [Google Scholar] [CrossRef] [PubMed]
- Frost, R.L.; Palmer, S.J.; Čejka, J.; Sejkora, J.; Plášil, J.; Bahfenne, S.; Keeffe, E.C. A Raman spectroscopic study of the different vanadate groups in solid-state compounds—Model case: Mineral phases vésigniéite [BaCu3(VO4)2(OH)2] and volborthite [Cu3V2O7(OH)2·2H2O]. J. Raman Spectrosc. 2011, 42, 1701–1710. [Google Scholar] [CrossRef]
- Mineral Data Publishing. Available online: http://www.handbookofmineralogy.org/pdfs/volborthite.pdf (accessed on 10 June 2022).
- Hiroi, Z.; Hanawa, M.; Kobayashi, N.; Nohara, M.; Takagi, H.; Kato, Y.; Takigawa, M. Spin-1/2 Kagomé-like lattice in Volborthite Cu3V2O7(OH)2.2H2O. J. Phys. Soc. Jpn. 2001, 70, 3377–3384. [Google Scholar] [CrossRef]
- Yan, H.; Luo, Y.; Xu, X.; He, L.; Tan, J.; Li, Z.; Hong, X.; He, P.; Mai, L. Facile and scalable synthesis of Zn3V2O7(OH)2·2H2O microflowers as a high-performance anode for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 27707–27714. [Google Scholar] [CrossRef]
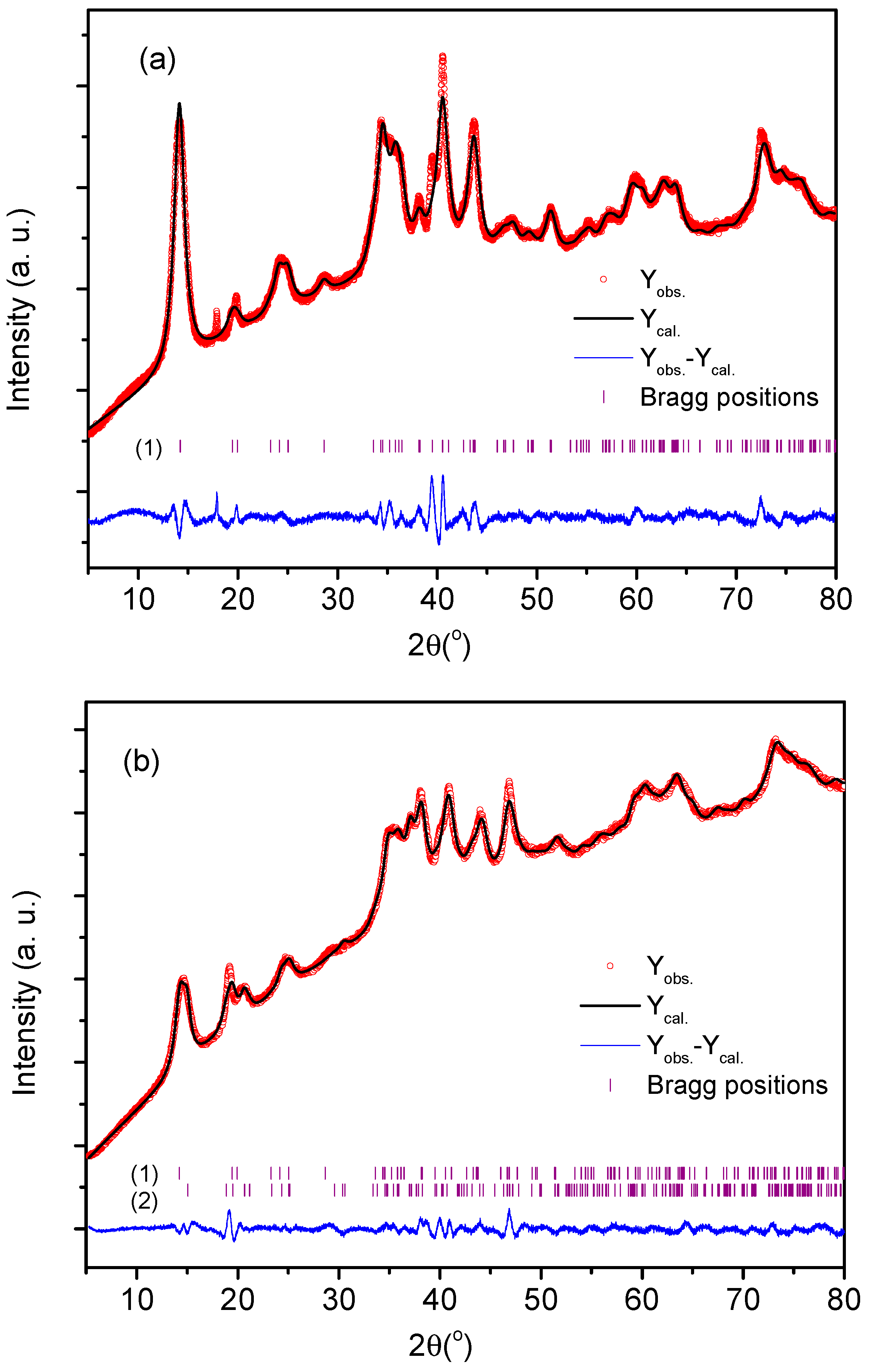
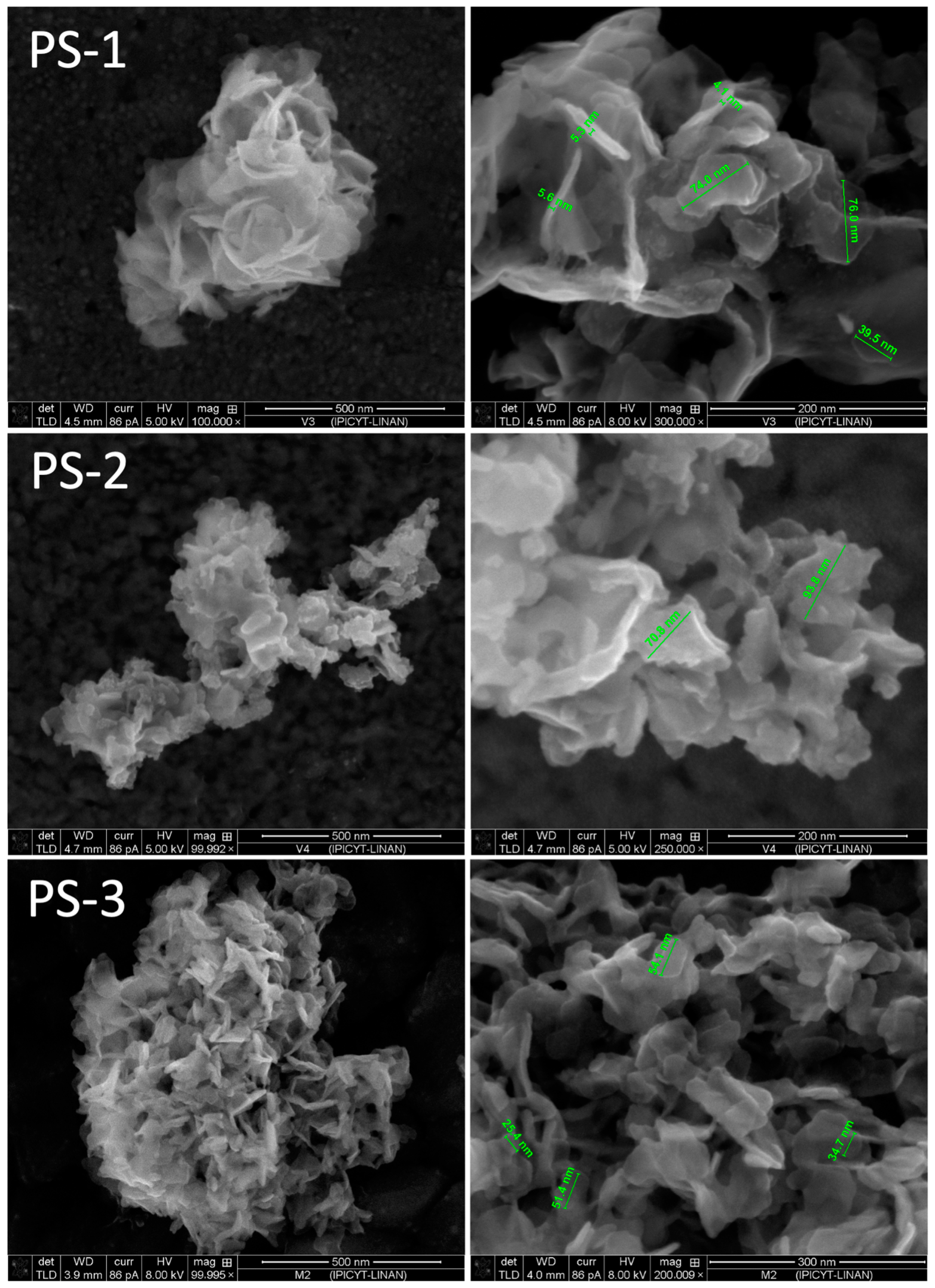

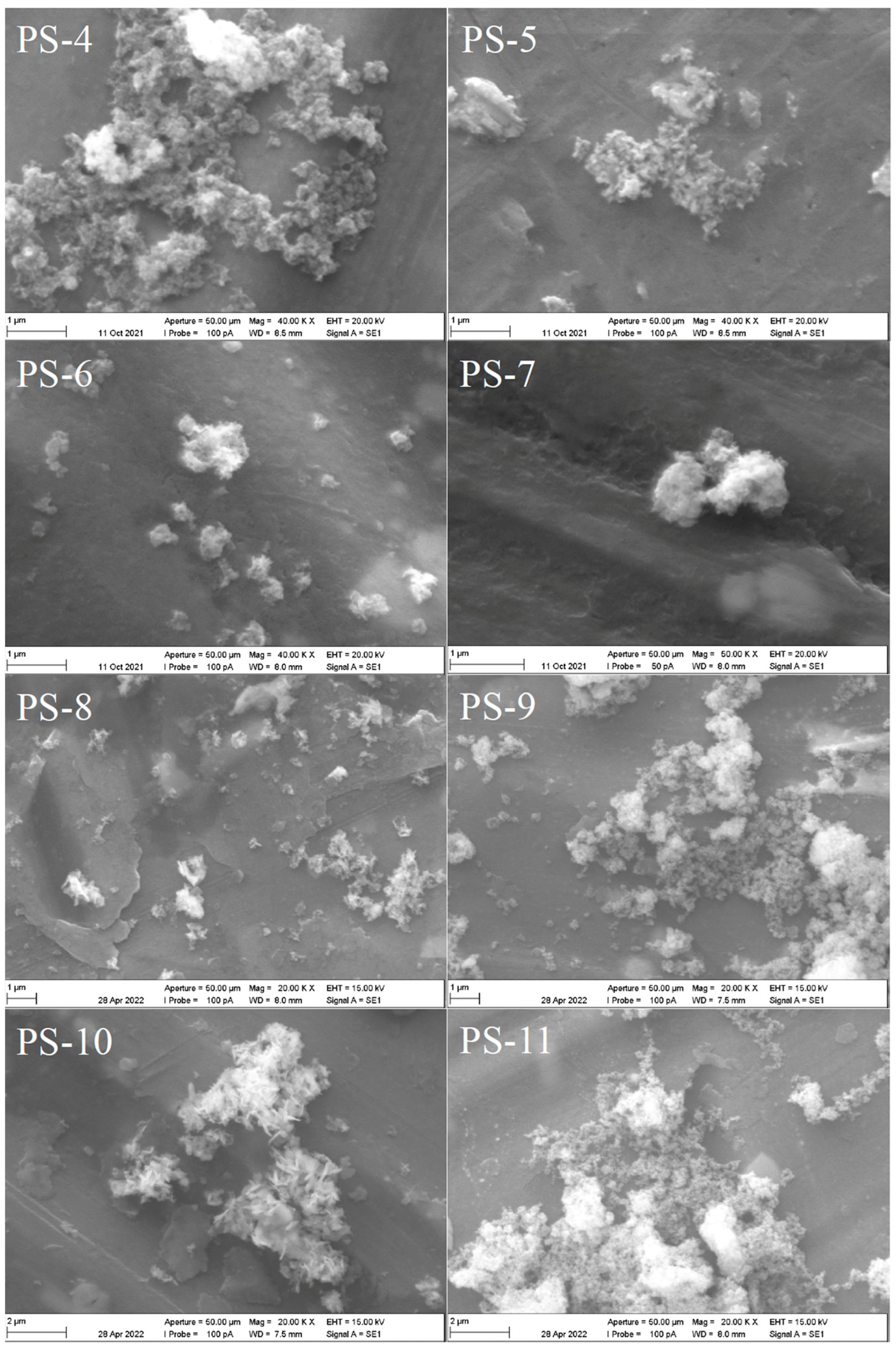
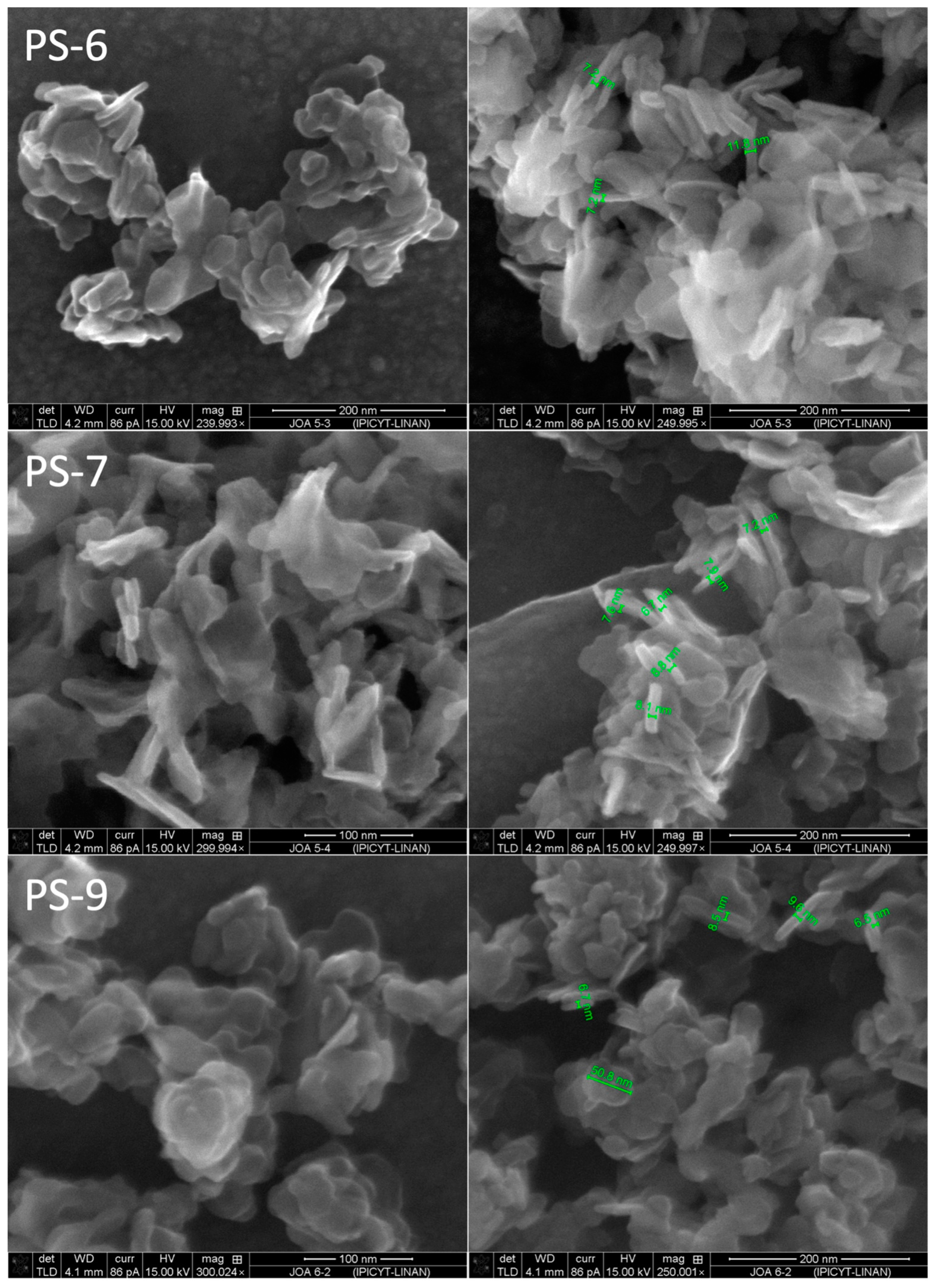
| Experiment | Cu(II), M | Cl−, M | PVP, g/L | O/A |
|---|---|---|---|---|
| Set 1 | ||||
| PS-1 | 0.05 | 4 | 0 | 1/1 |
| PS-2 | 0.1 | 4 | 0 | 1/1 |
| PS-3 | 0.05 | 4 | 20 | 1/1 |
| CP-1 * | 0.1 | 4 | 20 | - |
| Set 2 | ||||
| PS-4 | 0.1 | 4 | 0 | 1/1 |
| PS-5 | 0.05 | 4 | 0 | 1/1 |
| PS-6 | 0.05 | 2 | 20 | 1/1 |
| PS-7 | 0.05 | 2 | 40 | 1/1 |
| PS-8 | 0.05 | 4 | 0 | 1/2 |
| PS-9 | 0.05 | 2 | 20 | 1/2 |
| PS-10 | 0.05 | 4 | 0 | 2/1 |
| PS-11 | 0.05 | 2 | 20 | 2/1 |
| Sample | PS-1 | PS-2 | |
|---|---|---|---|
| Compound | Tricopper divanadate dihydroxide dihydrate Volborthite [34] | Tricopper divanadate dihydroxide dihydrate Volborthite [34] | Tricopper divanadate dihydroxide dihydrate Volborthite [35] |
| Molecular formula | Cu3V2O7(OH)2·2(H2O) | Cu3V2O7(OH)2·2(H2O) | Cu3V2O7(OH)2·2(H2O) |
| Molecular weight (g/mol) | 474.56 | 474.56 | 474.56 |
| wt. (%) | 100 | 57.69 | 42.30 |
| Symmetry | Monoclinic | Monoclinic | Monoclinic |
| Space group (H. M.) | C2/m | C2/m | C2/c |
| a (Å) | 10.610(3) | 10.716(7) | 10.947(3) |
| b (Å) | 5.912(1) | 5.837(5) | 6.023(2) |
| c (Å) | 7.242(1) | 7.160(3) | 13.683(5) |
| β (°) | 94.23(2) | 93.78(6) | 94.01(3) |
| Crystallite size (Å) | 99.6(5) | 105(1) | 151(3) |
| ρX-ray (g/cm3) | 3.47 | 3.52 | 3.47 |
| Rwp (%) | 1.561 | 1.001 | |
| Rb (%) | 1.112 | 0.706 | |
| Rexp (%) | 0.540 | 0.405 | |
| Distribution of V(V), % | Chemical Composition, % | V/Cu Ratio | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cu(II) mol/L | Cl− mol/L | PVP g/L | O/A | V(V) Org | V(V) Solid | V(V) Aq | V(V) | Cu(II) | ||
| PS-1 | 0.05 | 4 | 0 | 1/1 | 71.7 | 28.3 | 0.0 | 18 | 42 | 0.43 |
| PS-2 | 0.1 | 4 | 0 | 1/1 | 87.5 | 12.5 | 0.0 | 13.6 | 44 | 0.31 |
| PS-3 | 0.05 | 4 | 20 | 1/1 | 33.4 | 66.0 | 0.6 | 24.8 | 54.4 | 0.45 |
| Cu(II) mol/L | Cl− mol/L | PVP g/L | O/A | % V Org | % V Solid | % V Aq | % Cu Org | % Cu Solid | % Cu Aq | pH eq Aq | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PS-4 | 0.1 | 4 | 0 | 1/1 | 61.2 | 38.7 | 0.1 | 43.6 | 19.5 | 36.9 | 3.39 |
| PS-5 | 0.05 | 4 | 0 | 1/1 | 60.9 | 38.9 | 0.2 | 78.1 | 13.1 | 8.7 | 3.54 |
| PS-6 | 0.05 | 2 | 20 | 1/1 | 37.1 | 62.8 | 0.1 | 55.8 | 21.0 | 23.2 | 3.84 |
| PS-7 | 0.05 | 2 | 40 | 1/1 | 38.4 | 61.5 | 0.1 | 52.3 | 24.4 | 23.3 | 3.89 |
| PS-8 | 0.05 | 4 | 0 | 1/2 | 68.8 | 31.0 | 0.3 | 72.1 | 4.1 | 23.8 | 3.41 |
| PS-9 | 0.05 | 2 | 20 | 1/2 | 32.8 | 66.8 | 0.4 | 51.2 | 9.8 | 39.0 | 3.61 |
| PS-10 | 0.05 | 4 | 0 | 2/1 | 70.9 | 23.8 | 5.3 | 85.6 | 14.2 | 0.1 | 4.82 |
| PS-11 | 0.05 | 2 | 20 | 2/1 | 30.9 | 68.9 | 0.2 | 56.2 | 37.2 | 6.6 | 3.73 |
| Cu(II) mol/L | Cl− mol/L | PVP g/L | O/A | % V | % Cu | % Cl− | V/Cu | |
|---|---|---|---|---|---|---|---|---|
| PS-4 | 0.1 | 4 | 0 | 1/1 | 13.2 | 33.9 | 4.7 | 0.39 |
| PS-5 | 0.05 | 4 | 0 | 1/1 | 17.8 | 30.5 | 5.4 | 0.58 |
| PS-6 | 0.05 | 2 | 20 | 1/1 | 18.5 | 31.5 | 3.3 | 0.59 |
| PS-7 | 0.05 | 2 | 40 | 1/1 | 18.1 | 36.3 | 6.3 | 0.50 |
| PS-8 | 0.05 | 4 | 0 | 1/2 | 17.1 | 22.8 | 3.2 | 0.75 |
| PS-9 | 0.05 | 2 | 20 | 1/2 | 19.3 | 28.2 | 3.2 | 0.68 |
| PS-10 | 0.05 | 4 | 0 | 2/1 | 10.4 | 15.5 | 25.1 | 0.67 |
| PS-11 | 0.05 | 2 | 20 | 2/1 | 25.5 | 34.3 | 1.7 | 0.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Loredo, M.G.; Palomares-Sánchez, S.A.; Labrada-Delgado, G.J.; Helbig, T.; Chekhonin, P.; Ebert, D.; Möckel, R.; Owusu Afriyie, J.; Kelly, N. Preparation of Volborthite by a Facile Synthetic Chemical Solvent Extraction Method. Nanomaterials 2023, 13, 1977. https://doi.org/10.3390/nano13131977
Sánchez-Loredo MG, Palomares-Sánchez SA, Labrada-Delgado GJ, Helbig T, Chekhonin P, Ebert D, Möckel R, Owusu Afriyie J, Kelly N. Preparation of Volborthite by a Facile Synthetic Chemical Solvent Extraction Method. Nanomaterials. 2023; 13(13):1977. https://doi.org/10.3390/nano13131977
Chicago/Turabian StyleSánchez-Loredo, María Guadalupe, Salvador Antonio Palomares-Sánchez, Gladis Judith Labrada-Delgado, Toni Helbig, Paul Chekhonin, Doreen Ebert, Robert Möckel, Jones Owusu Afriyie, and Norman Kelly. 2023. "Preparation of Volborthite by a Facile Synthetic Chemical Solvent Extraction Method" Nanomaterials 13, no. 13: 1977. https://doi.org/10.3390/nano13131977
APA StyleSánchez-Loredo, M. G., Palomares-Sánchez, S. A., Labrada-Delgado, G. J., Helbig, T., Chekhonin, P., Ebert, D., Möckel, R., Owusu Afriyie, J., & Kelly, N. (2023). Preparation of Volborthite by a Facile Synthetic Chemical Solvent Extraction Method. Nanomaterials, 13(13), 1977. https://doi.org/10.3390/nano13131977







