Research Progress of Polymer Biomaterials as Scaffolds for Corneal Endothelium Tissue Engineering
Abstract
:1. Introduction
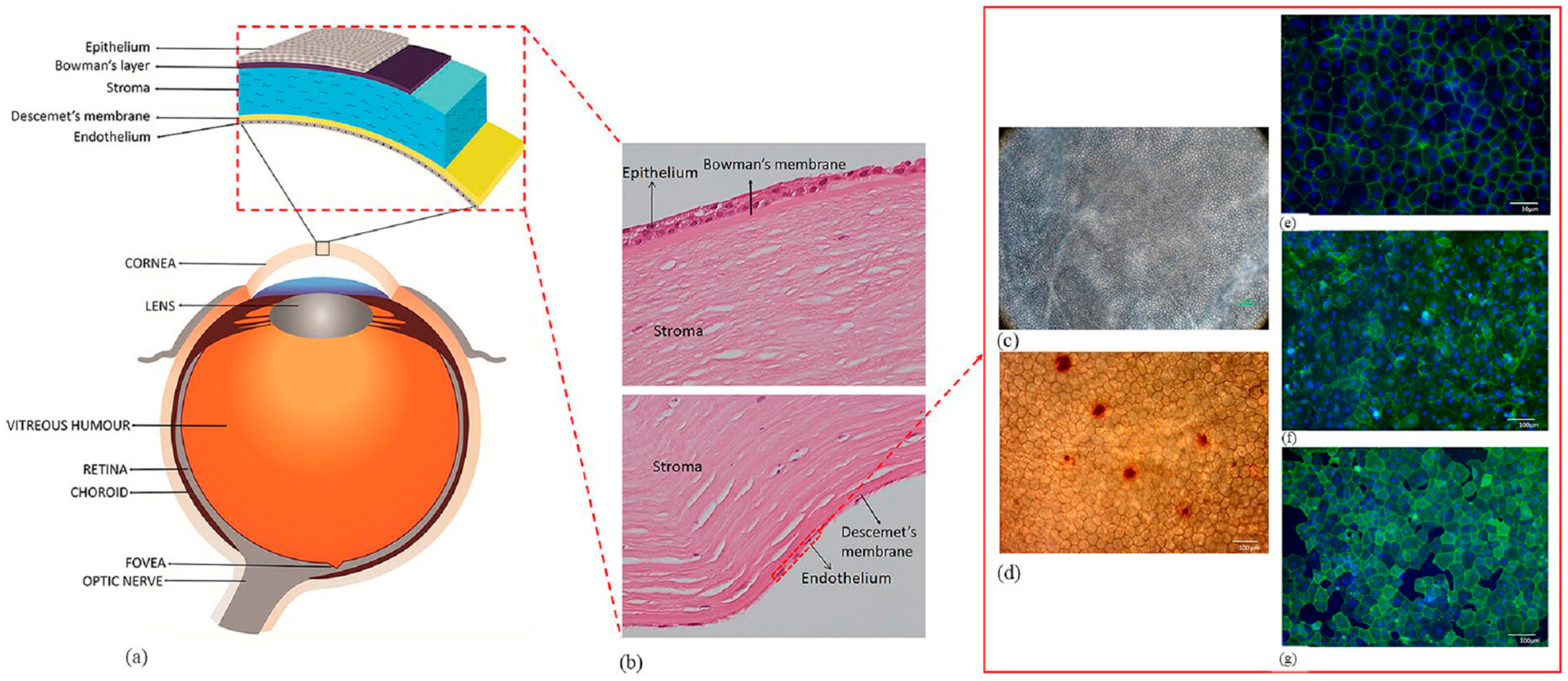
2. Required Properties of the Corneal Endothelial Implants
3. Materials for Producing Scaffoldless CEC Sheets
3.1. Biologically Derived Materials
3.2. Synthetic Materials
4. Materials for Producing Scaffolds of CECs
4.1. Substrates
4.1.1. Natural Substrates
4.1.2. Synthetic Substrates
| Category | Type | Advantages | Limitation | Ref. |
|---|---|---|---|---|
| natural substrates | amniotic membrane | biocompatibility in ocular applications | semi-opaque; scarcity from a donor bank; biological variability; unpredictable degradation rates | [29,30] |
| decellularized human cornea | mechanical support, and transparency | donor dependency | [31,32] | |
| collagen | biocompatibility and biodegradability; mimic the ECM | transparency not optimal | [34] | |
| hydroxyethyl chitosan, gelatin, and chondroitin sulfate | promote cell proliferation | inflammation | [35] | |
| agarose | ultrathin; strong; cell-adhesive | no use with human CECs | [36] | |
| synthetic substrates | poly (DL-lactide-co-glycolide) | a positive impact on CECs | a faster degradation rate resulting in a more acidic pH in the culture media; no in vivo studies | [37] |
| polyvinylidene fluoride | BCECs exhibited well-developed expressions of specific markers | no in vivo studies | [38] | |
| polyethylene glycol | high tensile strength and transparency; facilitate the proliferation of CECs | need further evaluation of the biological activity of the biodegradable system | [15] | |
| poly-ε-lysine | enhanced adhesion and growth of pCECs | no in vivo studies | [39] | |
| poly(methyl-methacrylate) | inexpensive; modifiability of transparency | cytotoxicity; lowest viscosity | [19] |
4.2. Modifications of the Substrates
4.2.1. Changes in the Composition
4.2.2. Regulation of Topography
4.3. Scaffolds with Drug Delivery System
5. Challenges and Prospects of Scaffolds for Corneal Endothelium Tissue Engineering
- (i)
- It is important to note that meeting all requirements for a tissue-engineered corneal endothelial graft remains a challenging task. It should be acknowledged that the important properties of a graft are interdependent. For instance, altering the thickness of a scaffold can impact its permeability and mechanical properties. The increase in mechanical strength may be accompanied by a decrease in water content, thus affecting cytocompatibility. Researchers need to balance as many performances as possible through exquisite scaffold design.
- (ii)
- Although many designs of scaffold topography have been shown to promote endothelial cell growth, the mechanism of this effect is unclear. The functional implementation of bioengineered CECs is not yet ideal due to a limited understanding of the molecular mechanisms of endothelial cell proliferation and the related inter- and intra-signaling pathways that maintain the dynamic balance of corneal endothelial tissue.
- (iii)
- For biodegradable scaffolds, the match between their degradation rate and the regeneration rate of DM needs to be further investigated. However, there are few reports on the regeneration of human DM in vivo, making the study of degradation rates of scaffolds challenging.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zavala, J.; Lopez Jaime, G.R.; Rodriguez Barrientos, C.A.; Valdez-Garcia, J. Corneal endothelium: Developmental strategies for regeneration. Eye 2013, 27, 579–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. Jama Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parekh, M.; Romano, V.; Hassanin, K.; Testa, V.; Wongvisavavit, R.; Ferrari, S.; Haneef, A.; Willoughby, C.; Ponzin, D.; Jhanji, V.; et al. Biomaterials for corneal endothelial cell culture and tissue engineering. J. Tissue Eng. 2021, 12, 2041731421990536. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Kruse, F.E. DMEK: Descemet membrane endothelial keratoplasty. Ophthalmologe 2010, 107, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.S.; Ang, M.; Mehta, J.S. Evolution of therapies for the corneal endothelium: Past, present and future approaches. Br. J. Ophthalmol. 2021, 105, 454–467. [Google Scholar] [CrossRef]
- Kim, K.W.; Park, S.H.; Lee, S.J.; Kim, J.C. Ribonuclease 5 facilitates corneal endothelial wound healing via activation of PI3-kinase/Akt pathway. Sci. Rep. 2016, 6, 31162. [Google Scholar] [CrossRef] [Green Version]
- Meek, K.M.; Dennis, S.; Khan, S. Changes in the refractive index of the stroma and its extrafibrillar matrix when the cornea swells. Biophys. J. 2003, 85, 2205–2212. [Google Scholar] [CrossRef] [Green Version]
- Reinhart-King, C.A.; Dembo, M.; Hammer, D.A. Cell-Cell Mechanical Communication through Compliant Substrates. Biophys. J. 2008, 95, 6044–6051. [Google Scholar] [CrossRef] [Green Version]
- Mehta, J.S.; Kocaba, V.; Peh, G.S. A prognostic biomarker of corneal repair. Nat. Biomed. Eng. 2019, 3, 945–946. [Google Scholar] [CrossRef]
- Perez, R.A.; Choi, S.-J.; Han, C.-M.; Kim, J.-J.; Shim, H.; Leong, K.W.; Kim, H.-W. Biomaterials control of pluripotent stem cell fate for regenerative therapy. Prog. Mater. Sci. 2016, 82, 234–293. [Google Scholar] [CrossRef]
- Song, J.E.; Sim, B.R.; Jeon, Y.S.; Kim, H.S.; Shin, E.Y.; Carlomagno, C.; Khang, G. Characterization of surface modified glycerol/silk fibroin film for application to corneal endothelial cell regeneration. J. Biomater. Sci.-Polym. Ed. 2019, 30, 263–275. [Google Scholar] [CrossRef]
- Perez, R.A.; Won, J.-E.; Knowles, J.C.; Kim, H.-W. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv. Drug Deliv. Rev. 2013, 65, 471–496. [Google Scholar] [CrossRef]
- Gutermuth, A.; Maassen, J.; Harnisch, E.; Kuhlen, D.; Sauer-Budge, A.; Skazik-Voogt, C.; Engelmann, K. Descemet’s Membrane Biomimetic Microtopography Differentiates Human Mesenchymal Stem Cells into Corneal Endothelial-Like Cells. Cornea 2019, 38, 110–119. [Google Scholar] [CrossRef]
- Rizwan, M.; Peh, G.S.; Adnan, K.; Naso, S.L.; Mendez, A.R.; Mehta, J.S.; Yim, E.K.F. In Vitro Topographical Model of Fuchs Dystrophy for Evaluation of Corneal Endothelial Cell Monolayer Formation. Adv. Healthc. Mater. 2016, 5, 2896–2910. [Google Scholar] [CrossRef]
- Ozcelik, B.; Brown, K.D.; Blencowe, A.; Ladewig, K.; Stevens, G.W.; Scheerlinck, J.P.Y.; Abberton, K.; Daniell, M.; Qiao, G.G. Biodegradable and Biocompatible Poly(Ethylene Glycol)-based Hydrogel Films for the Regeneration of Corneal Endothelium. Adv. Healthc. Mater. 2014, 3, 1496–1507. [Google Scholar] [CrossRef]
- Auffarth, G.U.; Son, H.S.; Koch, M.; Weindler, J.; Merz, P.; Daphna, O.; Marcovich, A.L.; Augustin, V.A. Implantation of an Artificial Endothelial Layer for Treatment of Chronic Corneal Edema. Cornea 2021, 40, 1633–1638. [Google Scholar] [CrossRef]
- Wang, L.; Li, A.F.; Zhang, D.; Zhang, M.; Ma, L.Y.; Li, Y.; Wang, W.W.; Nan, K.H.; Chen, H.; Li, L.L. Injectable double-network hydrogel for corneal repair. Chem. Eng. J. 2023, 455, 140698. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, B.; Zhang, W.J.; Yan, C.X.; Yao, Q.K.; Shao, C.Y.; Yu, F.; Li, F.; Fu, Y. Electrospun gelatin/PCL and collagen/PCL scaffolds for modulating responses of bone marrow endothelial progenitor cells. Exp. Ther. Med. 2019, 17, 3717–3726. [Google Scholar] [CrossRef] [Green Version]
- Kruse, M.; Walter, P.; Bauer, B.; Ruetten, S.; Schaefer, K.; Plange, N.; Gries, T.; Jockenhoevel, S.; Fuest, M. Electro-spun Membranes as Scaffolds for Human Corneal Endothelial Cells. Curr. Eye Res. 2018, 43, 1–11. [Google Scholar] [CrossRef]
- Tayebi, T.; Baradaran-Rafii, A.; Hajifathali, A.; Rahimpour, A.; Zali, H.; Shaabani, A.; Niknejad, H. Biofabrication of chitosan/chitosan nanoparticles/polycaprolactone transparent membrane for corneal endothelial tissue engineering. Sci. Rep. 2021, 11, 7060. [Google Scholar] [CrossRef]
- Song, E.S.; Park, J.H.; Ha, S.S.; Cha, P.H.; Kang, J.T.; Park, C.Y.; Park, K. Novel Corneal Endothelial Cell Carrier Couples a Biodegradable Polymer and a Mesenchymal Stem Cell-Derived Extracellular Matrix. ACS Appl. Mater. Interfaces 2022, 14, 12116–12129. [Google Scholar] [CrossRef] [PubMed]
- Walshe, J.; Abdulsalam, N.A.K.; Suzuki, S.; Chirila, T.V.; Harkin, D.G. Growth of Human and Sheep Corneal Endothelial Cell Layers on Biomaterial Membranes. Jove-J. Vis. Exp. 2020, 156, e60762. [Google Scholar] [CrossRef]
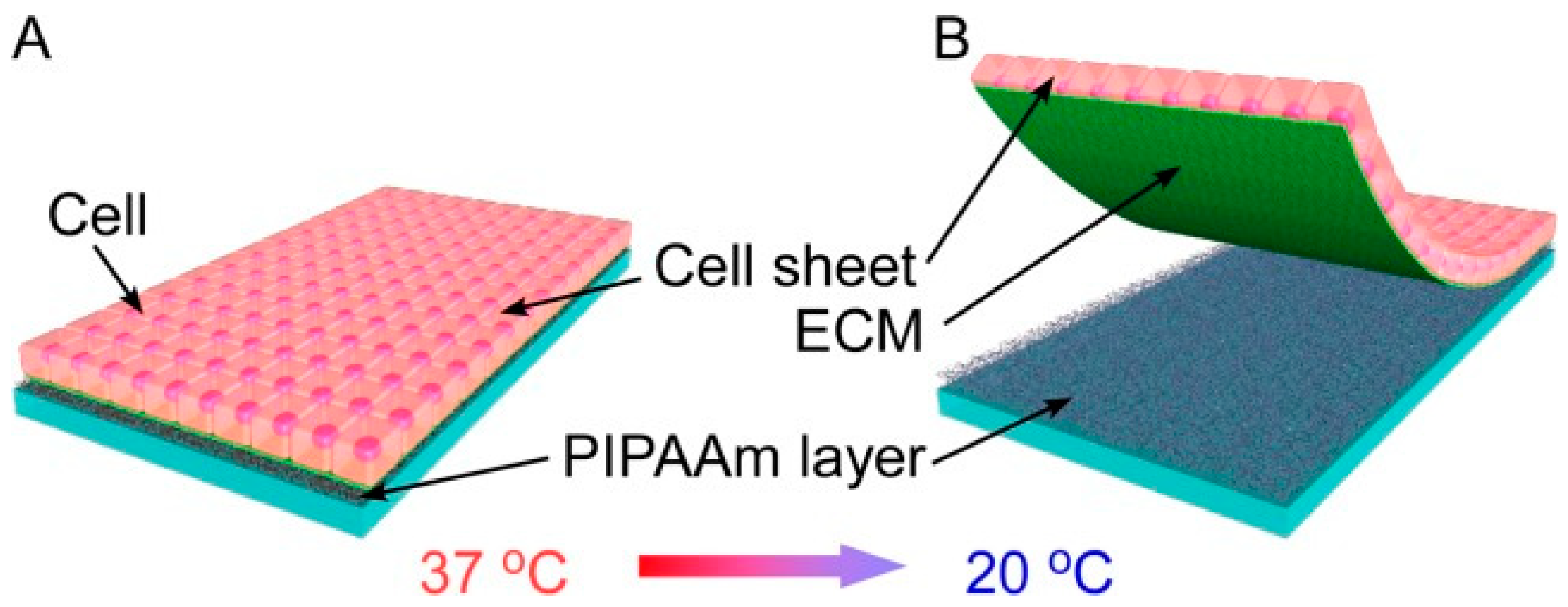
- Nitschke, M.; Gramm, S.; Goetze, T.; Valtink, M.; Drichel, J.; Voit, B.; Engelmann, K.; Werner, C. Thermo-responsive poly(NiPAAm-co-DEGMA) substrates for gentle harvest of human corneal endothelial cell sheets. J. Biomed. Mater. Res. Part A 2007, 80, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Nishida, K.; Yamato, M.; Sumide, T.; Utsumi, M.; Nozaki, T.; Kikuchi, A.; Okano, T.; Tano, Y. Structural characterization of bioengineered human corneal endothelial cell sheets fabricated on temperature-responsive culture dishes. Biomaterials 2006, 27, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Akiyama, Y.; Okano, T. Recent Development of Temperature-Responsive Cell Culture Surface Using Poly(N-isopropylacrylamide). J. Polym. Sci. Part B-Polym. Phys. 2014, 52, 917–926. [Google Scholar] [CrossRef]
- Teichmann, J.; Valtink, M.; Nitschke, M.; Gramm, S.; Funk, R.H.W.; Engelmann, K.; Werner, C. Tissue Engineering of the Corneal Endothelium: A Review of Carrier Materials. J. Funct. Biomater. 2013, 4, 178–208. [Google Scholar] [CrossRef] [Green Version]
- Soh, W.W.M.; Zhu, J.L.; Song, X.; Jain, D.; Yim, E.K.F.; Li, J. Detachment of bovine corneal endothelial cell sheets by cooling-induced surface hydration of poly (R)-3-hydroxybutyrate -based thermoresponsive copolymer coating. J. Mater. Chem. B 2022, 10, 8407–8418. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Chen, K.-H.; Hsu, W.-M.; Hsiue, G.-H.; Lee, Y.-H. Bioengineered human corneal endothelium for transplantation. Arch. Ophthalmol. 2006, 124, 1441–1448. [Google Scholar] [CrossRef]
- Wu, W.; Ye, M.; Yan, W.; Lu, F.; Qu, J.; Wang, Q.; Zhou, X. Using basement membrane of human amniotic membrane as a cell carrier for cultivated cat corneal endothelial cell transplantation. Curr. Eye Res. 2007, 32, 199–215. [Google Scholar] [CrossRef]
- Ishino, Y.; Sano, Y.; Nakamura, T.; Connon, C.J.; Rigby, H.; Fullwood, N.J.; Kinoshita, S. Amniotic membrane as a carrier for cultivated human corneal endothelial cell transplantation. Investig. Ophthalmol. Vis. Sci. 2004, 45, 800–806. [Google Scholar] [CrossRef] [Green Version]
- Mimura, T.; Amano, S.; Usui, T.; Araie, M.; Ono, K.; Akihiro, H.; Yokoo, S.; Yamagami, S. Transplantation of corneas reconstructed with cultured adult human corneal endothelial cells in nude rats. Exp. Eye Res. 2004, 79, 231–237. [Google Scholar] [CrossRef]
- Fan, T.; Zhao, J.; Ma, X.; Xu, X.; Zhao, W.; Xu, B. Establishment of a continuous untransfected human corneal endothelial cell line and its biocompatibility to denuded amniotic membrane. Mol. Vis. 2011, 17, 469–480. [Google Scholar]
- Yoeruek, E.; Saygili, O.; Spitzer, M.S.; Tatar, O.; Bartz-Schmidt, K.U.; Szurman, P. Human Anterior Lens Capsule as Carrier Matrix for Cultivated Human Corneal Endothelial Cells. Cornea 2009, 28, 416–420. [Google Scholar] [CrossRef]
- Vazquez, N.; Chacon, M.; Rodriguez-Barrientos, C.A.; Merayo-Lloves, J.; Naveiras, M.; Baamonde, B.; Alfonso, J.F.; Zambrano-Andazol, I.; Riestra, A.C.; Meana, A. Human Bone Derived Collagen for the Development of an Artificial Corneal Endothelial Graft. In Vivo Results in a Rabbit Model. PLoS ONE 2016, 11, e0167578. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Liu, W.; Han, B.; Yang, C.; Ma, Q.; Zhao, W.; Rong, M.; Li, H. Fabrication and characters of a corneal endothelial cells scaffold based on chitosan. J. Mater. Sci.-Mater. Med. 2011, 22, 175–183. [Google Scholar] [CrossRef]
- Seow, W.Y.; Kandasamy, K.; Peh, G.S.L.; Mehta, J.S.; Sun, W. Ultrathin, Strong, and Cell-Adhesive Agarose-Based Membranes Engineered as Substrates for Corneal Endothelial Cells. Acs Biomater. Sci. Eng. 2019, 5, 4067–4076. [Google Scholar] [CrossRef]
- Huhtala, A.; Pohjonen, T.; Salminen, L.; Salminen, A.; Kaarniranta, K.; Uusitalo, H. In vitro biocompatibility of degradable biopolymers in cell line cultures from various ocular tissues: Extraction studies. J. Mater. Sci.-Mater. Med. 2008, 19, 645–649. [Google Scholar] [CrossRef]
- Wang, T.J.; Wang, I.J.; Chen, Y.H.; Lu, J.N.; Young, T.H. Polyvinylidene fluoride for proliferation and preservation of bovine corneal endothelial cells by enhancing type IV collagen production and deposition. J. Biomed. Mater. Res. Part A 2012, 100, 252–260. [Google Scholar] [CrossRef]
- Kennedy, S.; Lace, R.; Carserides, C.; Gallagher, A.G.; Wellings, D.A.; Williams, R.L.; Levis, H.J. Poly-epsilon-lysine based hydrogels as synthetic substrates for the expansion of corneal endothelial cells for transplantation. J. Mater. Sci.-Mater. Med. 2019, 30, 102. [Google Scholar] [CrossRef] [Green Version]
- Vijayasekaran, S.; Fitton, J.H.; Hicks, C.R.; Chirila, T.V.; Crawford, G.J.; Constable, I.J. Cell viability and inflammatory response in hydrogel sponges implanted in the rabbit cornea. Biomaterials 1998, 19, 2255–2267. [Google Scholar] [CrossRef]
- Gao, X.; Liu, W.; Han, B.; Wei, X.; Yang, C. Preparation and properties of a chitosan-based carrier of corneal endothelial cells. J. Mater. Sci. Mater. Med. 2008, 19, 3611–3619. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Kim, D.-K.; Lee, O.J.; Kim, J.-H.; Ju, H.W.; Lee, J.M.; Moon, B.M.; Park, H.J.; Kim, D.W.; Kim, S.H.; et al. Fabrication of silk fibroin film using centrifugal casting technique for corneal tissue engineering. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2016, 104, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Sun, W.; Chen, G.; Tang, S.; Li, M.; Shao, Z.; Mi, S. Tissue-engineered cornea constructed with compressed collagen and laser-perforated electrospun mat. Sci. Rep. 2017, 7, 970. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.K.; Sim, B.R.; Kim, J.I.; Khang, G. Functionalized silk fibroin film scaffold using beta-Carotene for cornea endothelial cell regeneration. Colloids Surf. B-Biointerfaces 2018, 164, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.Y.; Chen, K.H.; Hsiue, G.H. Tissue-engineered human corneal endothelial cell sheet transplantation in a rabbit model using functional biomaterials. Transplantation 2007, 84, 1222–1232. [Google Scholar] [CrossRef]
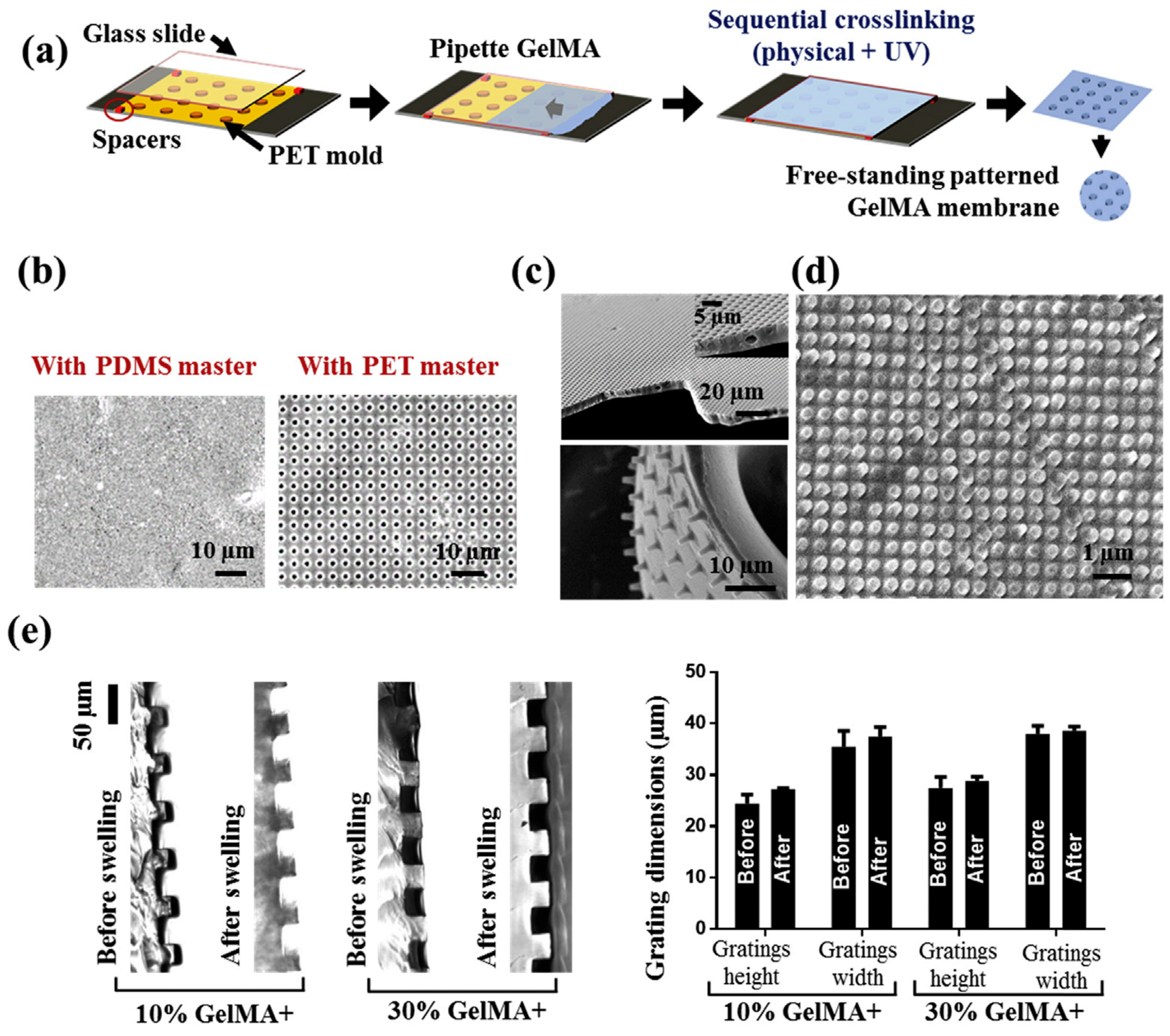
- Rizwan, M.; Peh, G.S.L.; Ang, H.-P.; Lwin, N.C.; Adnan, K.; Mehta, J.S.; Tan, W.S.; Yim, E.K.F. Sequentially-crosslinked bioactive hydrogels as nano-patterned substrates with customizable stiffness and degradation for corneal tissue engineering applications. Biomaterials 2017, 120, 139–154. [Google Scholar] [CrossRef]
- Van Hoorick, J.; Delaey, J.; Vercammen, H.; Van Erps, J.; Thienpont, H.; Dubruel, P.; Zakaria, N.; Koppen, C.; Van Vlierberghe, S.; van den Bogerd, B. Designer Descemet Membranes Containing PDLLA and Functionalized Gelatins as Corneal Endothelial Scaffold. Adv. Healthc. Mater. 2020, 9, e2000760. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Young, T.-H.; Wang, T.-J. Investigating the effect of chitosan/polycaprolactone blends in differentiation of corneal endothelial cells and extracellular matrix compositions. Exp. Eye Res. 2019, 185, 107679. [Google Scholar] [CrossRef]
- Ozcelik, B.; Brown, K.D.; Blencowe, A.; Daniell, M.; Stevens, G.W.; Qiao, G.G. Ultrathin chitosan-poly(ethylene glycol) hydrogel films for corneal tissue engineering. Acta Biomater. 2013, 9, 6594–6605. [Google Scholar] [CrossRef]
- Jiang, X.; Peng, Y.; Yang, C.; Liu, W.; Han, B. The feasibility study of an in situ marine polysaccharide-based hydrogel as the vitreous substitute. J. Biomed. Mater. Res. Part A 2018, 106, 1997–2006. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, W.; Han, B.; Yang, C.; Ma, Q.; Song, F.; Bi, Q. An in situ formed biodegradable hydrogel for reconstruction of the corneal endothelium. Colloids Surf. B-Biointerfaces 2011, 82, 1–7. [Google Scholar] [CrossRef]
- De Hoon, I.; Barras, A.; Swebocki, T.; Vanmeerhaeghe, B.; Bogaert, B.; Muntean, C.; Abderrahmani, A.; Boukherroub, R.; De Smedt, S.; Sauvage, F.; et al. Influence of the Size and Charge of Carbon Quantum Dots on Their Corneal Penetration and Permeation Enhancing Properties. Acs Appl. Mater. Interfaces 2023, 15, 3760–3771. [Google Scholar] [CrossRef]
- Tsai, M.C.; Daniels, J.T. The impact of biomechanics on corneal endothelium tissue engineering. Exp. Eye Res. 2021, 209, 108690. [Google Scholar] [CrossRef]
- Noro, A.; Kaneko, M.; Murata, I.; Yoshinari, M. Influence of surface topography and surface physicochemistry on wettability of zirconia (tetragonal zirconia polycrystal). J. Biomed. Mater. Res. Part B-Appl. Biomater. 2013, 101, 355–363. [Google Scholar] [CrossRef]
- Medilanski, E.; Kaufmann, K.; Wick, L.Y.; Wanner, O.; Harms, H. Influence of the surface topography of stainless steel on bacterial adhesion. Biofouling 2002, 18, 193–203. [Google Scholar] [CrossRef]
- Brunette, D.M.; Chehroudi, B. The effects of the surface topography of micromachined titanium substrata on cell behavior in vitro and in vivo. J. Biomech. Eng.-Trans. Asme 1999, 121, 49–57. [Google Scholar] [CrossRef]
- Lord, M.S.; Foss, M.; Besenbacher, F. Influence of nanoscale surface topography on protein adsorption and cellular response. Nano Today 2010, 5, 66–78. [Google Scholar] [CrossRef]
- Mueller-Meskamp, L.; Kim, Y.H.; Roch, T.; Hofmann, S.; Scholz, R.; Eckardt, S.; Leo, K.; Lasagni, A.F. Efficiency Enhancement of Organic Solar Cells by Fabricating Periodic Surface Textures using Direct Laser Interference Patterning. Adv. Mater. 2012, 24, 906–910. [Google Scholar] [CrossRef]
- Lei, Y.; Yang, S.; Wu, M.; Wilde, G. Surface patterning using templates: Concept, properties and device applications. Chem. Soc. Rev. 2011, 40, 1247–1258. [Google Scholar] [CrossRef]
- Bao, L.R.; Cheng, X.; Huang, X.D.; Guo, L.J.; Pang, S.W.; Yee, A.F. Nanoimprinting over topography and multilayer three-dimensional printing. J. Vac. Sci. Technol. B 2002, 20, 2881–2886. [Google Scholar] [CrossRef]
- Chang-Yen, D.A.; Eich, R.K.; Gale, B.K. A monolithic PDMS waveguide system fabricated using soft-lithography techniques. J. Light. Technol. 2005, 23, 2088–2093. [Google Scholar] [CrossRef] [Green Version]
- Dai, Z.; Lu, L.; Sun, Y.; Tang, Z.; Lu, X. Wetting control through topography and surface hydrophilic/hydrophobic property changes by coarse grained simulation. Mol. Simul. 2017, 43, 1202–1208. [Google Scholar] [CrossRef]
- Ayala, R.; Zhang, C.; Yang, D.; Hwang, Y.; Aung, A.; Shroff, S.S.; Arce, F.T.; Lal, R.; Arya, G.; Varghese, S. Engineering the cell-material interface for controlling stem cell adhesion, migration, and differentiation. Biomaterials 2011, 32, 3700–3711. [Google Scholar] [CrossRef] [PubMed]
- Luensmann, D.; Jones, L. Protein deposition on contact lenses: The past, the present, and the future. Contact Lens Anterior Eye 2012, 35, 53–64. [Google Scholar] [CrossRef]
- Jones, L.; Senchyna, M.; Glasier, M.-A.; Schickler, J.; Forbes, I.; Louie, D.; May, C. Lysozyme and lipid deposition on silicone hydrogel contact lens materials. Eye Contact Lens 2003, 29, S75–S79; discussion S83–S74, S192–S194. [Google Scholar] [CrossRef]
- Schulte, V.A.; Diez, M.; Moeller, M.; Lensen, M.C. Surface Topography Induces Fibroblast Adhesion on Intrinsically Nonadhesive Poly(ethylene glycol) Substrates. Biomacromolecules 2009, 10, 2795–2801. [Google Scholar] [CrossRef]
- Schulte, V.A.; Diez, M.; Moeller, M.; Lensen, M.C. Topography-Induced Cell Adhesion to Acr-sP(EO-stat-PO) Hydrogels: The Role of Protein Adsorption. Macromol. Biosci. 2011, 11, 1378–1386. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, B.; Liu, Y.; Suo, X.; Li, H. Influence of surface topography on bacterial adhesion: A review. Biointerphases 2018, 13, 060801. [Google Scholar] [CrossRef] [Green Version]
- Vivero-Lopez, M.; Pereira-da-Mota, A.F.; Carracedo, G.; Huete-Toral, F.; Parga, A.; Otero, A.; Concheiro, A.; Alvarez-Lorenzo, C. Phosphorylcholine-Based Contact Lenses for Sustained Release of Resveratrol: Design, Antioxidant and Antimicrobial Performances, and In Vivo Behavior. Acs Appl. Mater. Interfaces 2022, 14, 55431–55446. [Google Scholar] [CrossRef]
- Gruschwitz, R.; Friedrichs, J.; Valtink, M.; Franz, C.M.; Mueller, D.J.; Funk, R.H.W.; Engelmann, K. Alignment and Cell-Matrix Interactions of Human Corneal Endothelial Cells on Nanostructured Collagen Type I Matrices. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6303–6310. [Google Scholar] [CrossRef]
- Abdellah, M.M.; Ammar, H.G.; Anbar, M.; Mostafa, E.M.; Farouk, M.M.; Sayed, K.; Alsmman, A.H.; Elghobaier, M.G. Corneal Endothelial Cell Density and Morphology in Healthy Egyptian Eyes. J. Ophthalmol. 2019, 2019, 6370241. [Google Scholar] [CrossRef]
- Salehi, S.; Fathi, M.; Javanmard, S.H.; Bahners, T.; Gutmann, J.S.; Erguen, S.; Steuhl, K.P.; Fuchsluger, T.A. Generation of PGS/PCL Blend Nanofibrous Scaffolds Mimicking Corneal Stroma Structure. Macromol. Mater. Eng. 2014, 299, 455–469. [Google Scholar] [CrossRef]
- Niu, G.; Choi, J.-S.; Wang, Z.; Skardal, A.; Giegengack, M.; Soker, S. Heparin-modified gelatin scaffolds for human corneal endothelial cell transplantation. Biomaterials 2014, 35, 4005–4014. [Google Scholar] [CrossRef]
- Natu, M.V.; Gaspar, M.N.; Fontes Ribeiro, C.A.; Correia, I.J.; Silva, D.; de Sousa, H.C.; Gil, M.H. A poly(epsilon-caprolactone) device for sustained release of an anti-glaucoma drug. Biomed. Mater. 2011, 6, 025003. [Google Scholar] [CrossRef]
- Koda, S.; Okumura, N.; Kitano, J.; Koizumi, N.; Tabata, Y. Development of Poly Lactic/Glycolic Acid (PLGA) Microspheres for Controlled Release of Rho-Associated Kinase Inhibitor. J. Ophthalmol. 2017, 2017, 1598218. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; He, X.; Zhao, H.; Ma, J.; Tao, J.; Zhao, S.; Yan, Y.; Li, Y.; Zhu, S. Research Progress of Polymer Biomaterials as Scaffolds for Corneal Endothelium Tissue Engineering. Nanomaterials 2023, 13, 1976. https://doi.org/10.3390/nano13131976
Luo X, He X, Zhao H, Ma J, Tao J, Zhao S, Yan Y, Li Y, Zhu S. Research Progress of Polymer Biomaterials as Scaffolds for Corneal Endothelium Tissue Engineering. Nanomaterials. 2023; 13(13):1976. https://doi.org/10.3390/nano13131976
Chicago/Turabian StyleLuo, Xiaoying, Xin He, Hui Zhao, Jun Ma, Jie Tao, Songjiao Zhao, Yan Yan, Yao Li, and Shenmin Zhu. 2023. "Research Progress of Polymer Biomaterials as Scaffolds for Corneal Endothelium Tissue Engineering" Nanomaterials 13, no. 13: 1976. https://doi.org/10.3390/nano13131976
APA StyleLuo, X., He, X., Zhao, H., Ma, J., Tao, J., Zhao, S., Yan, Y., Li, Y., & Zhu, S. (2023). Research Progress of Polymer Biomaterials as Scaffolds for Corneal Endothelium Tissue Engineering. Nanomaterials, 13(13), 1976. https://doi.org/10.3390/nano13131976







