Size Uncertainty in Individual Nanoparticles Measured by Single Particle Inductively Coupled Plasma Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
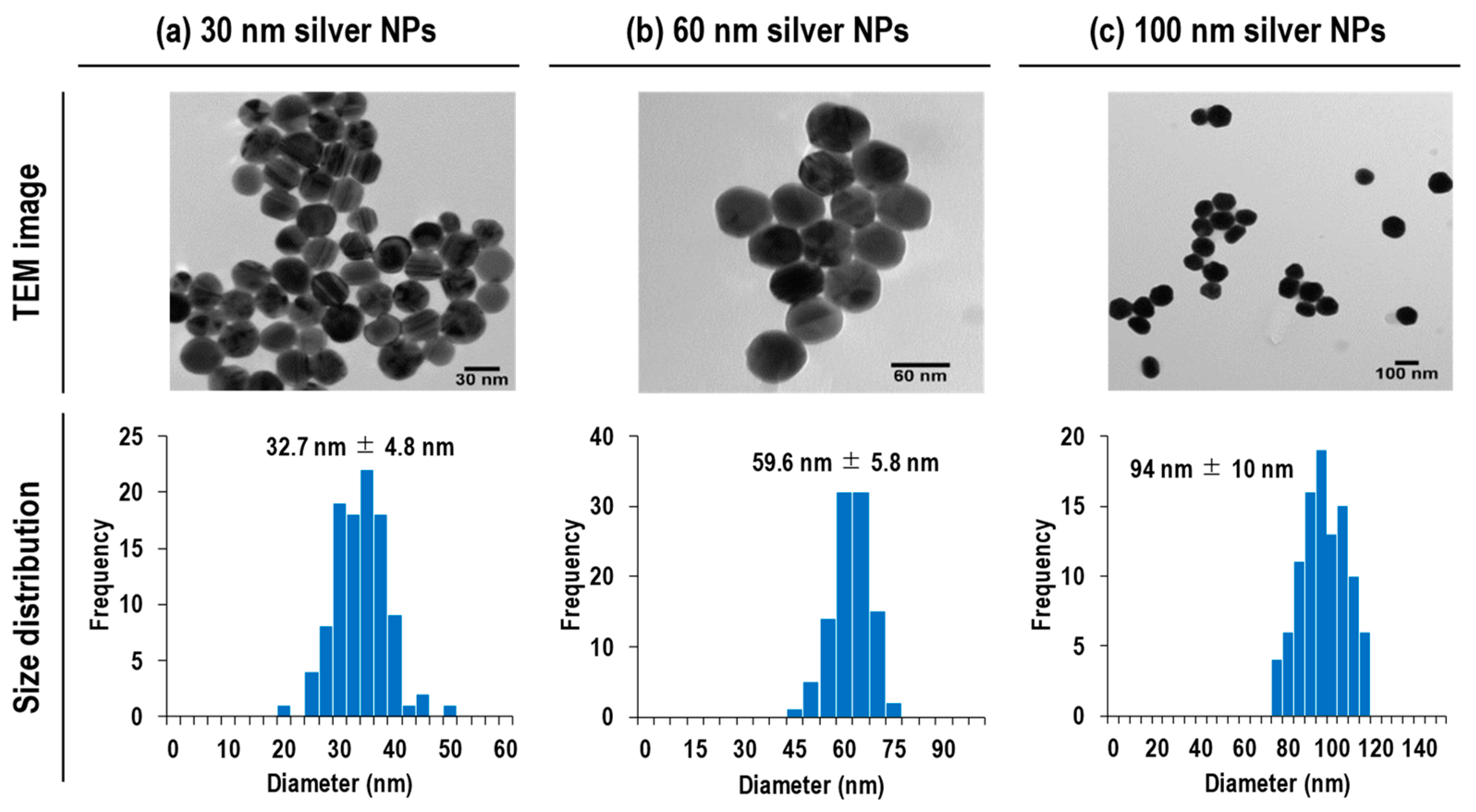
2.3. Size Analysis
- (i)
- Sizing with particle size standard
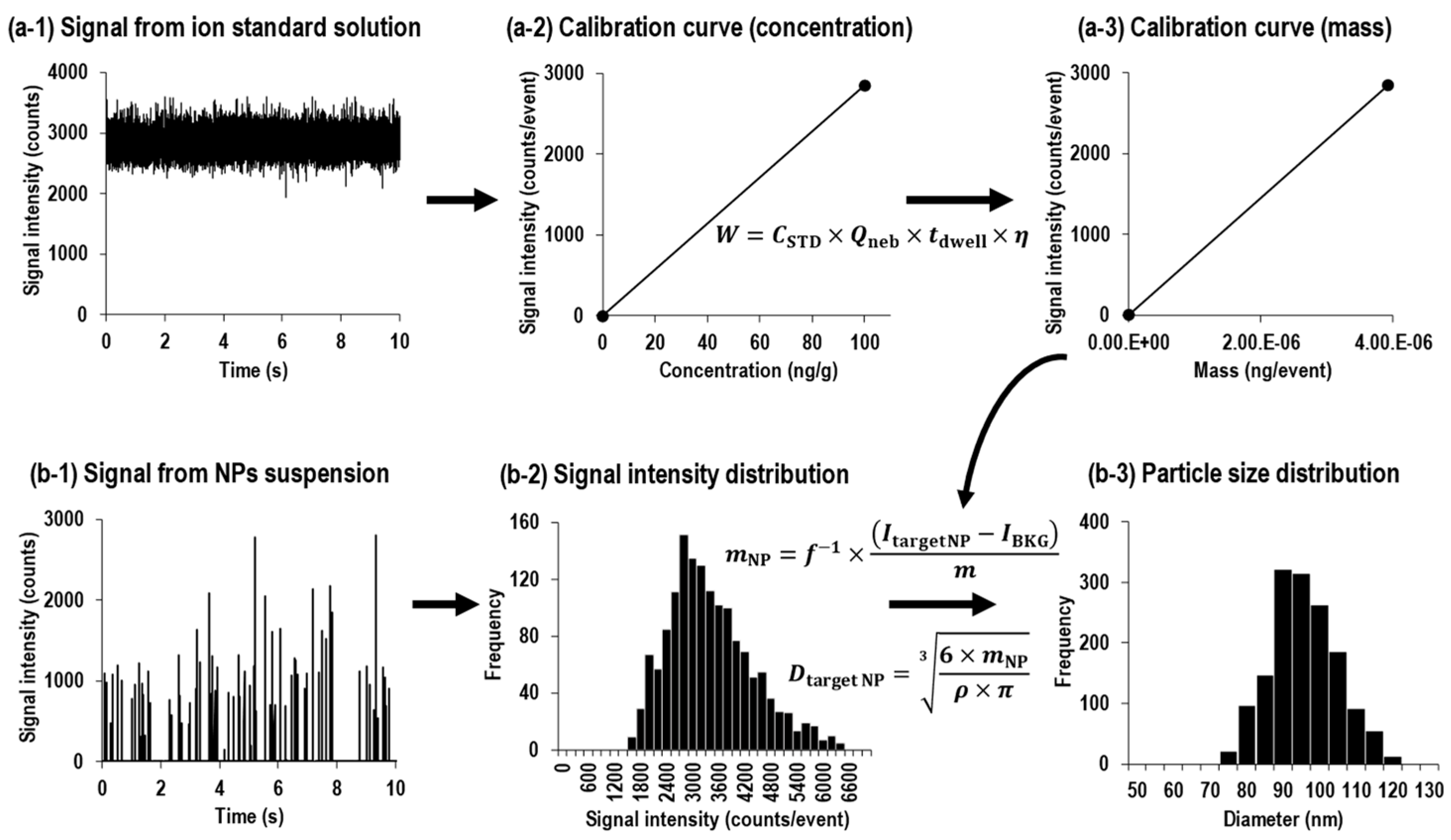
- (ii)
- Sizing with ion standard solution
2.4. Uncertainty Analysis
3. Results and Discussion
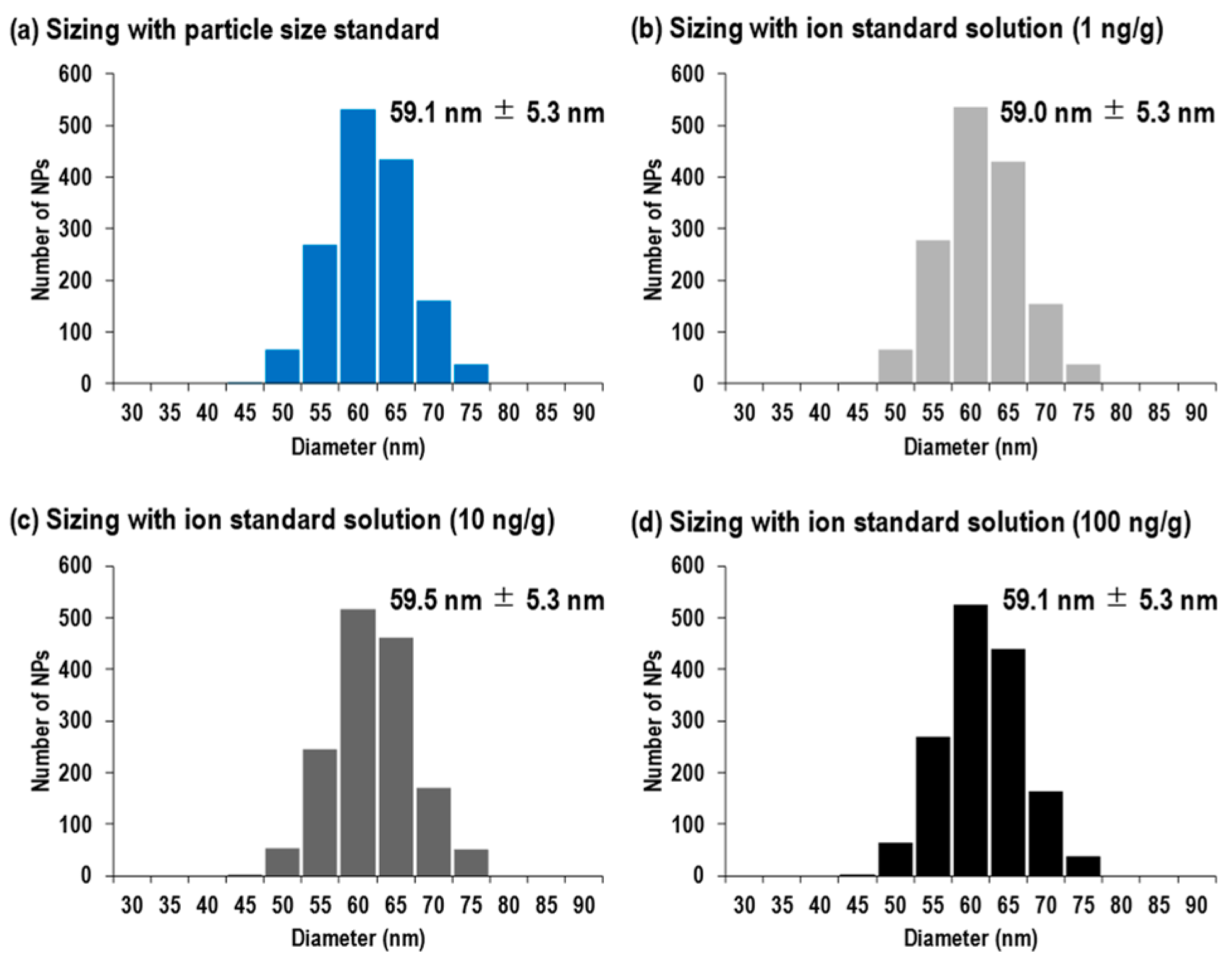
3.1. Comparison of Size Distributions between Two Size Calibration Approaches
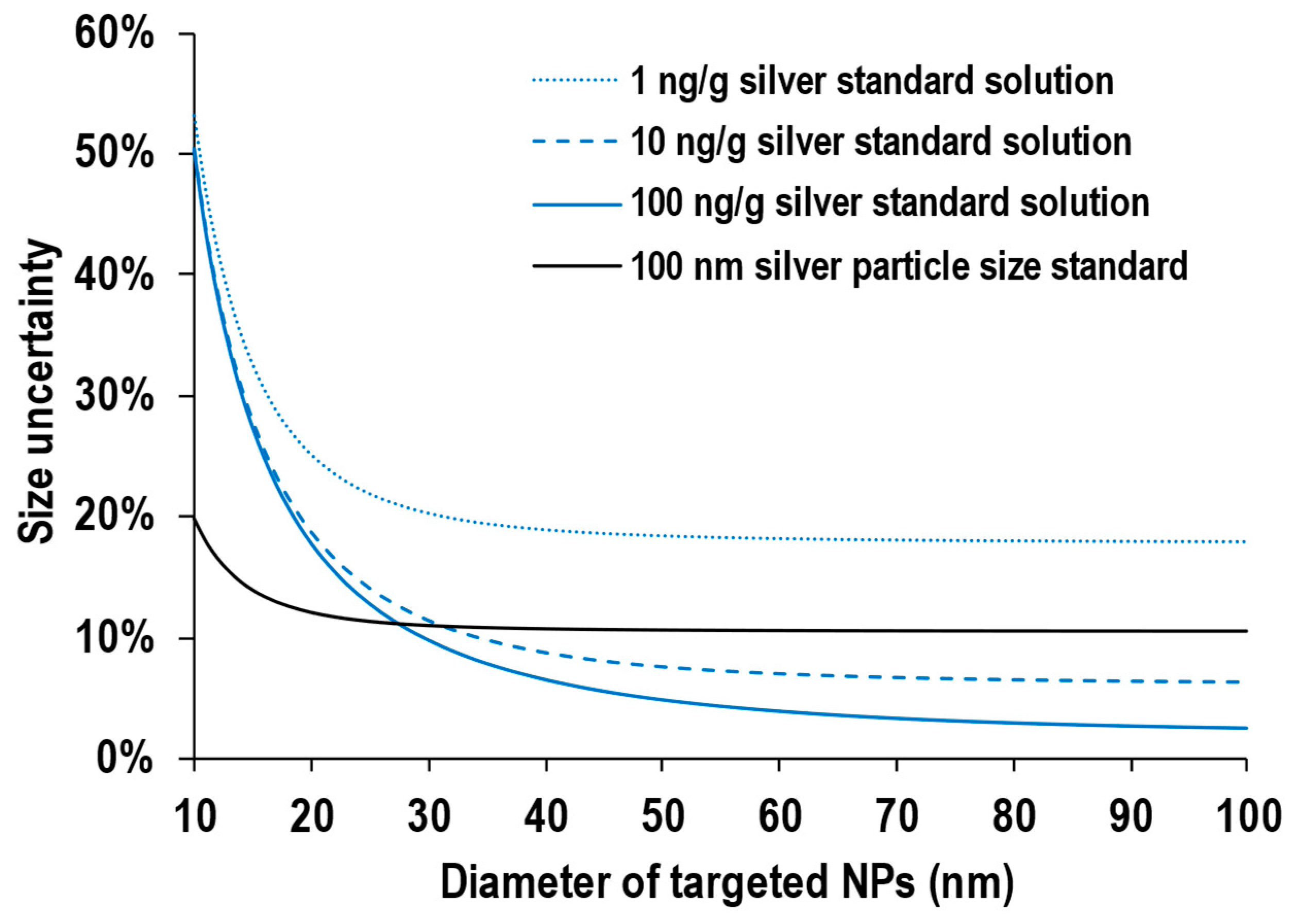
3.2. Uncertainty for Sizing with Particle Size Standard
3.3. Uncertainty for Sizing with Ion Standard Solution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Degueldre, C.; Favarger, P.Y. Colloid analysis by single particle inductively coupled plasma-mass spectroscopy: A feasibility study. Colloids Surf. A Physicochem. Eng. Asp. 2003, 217, 137–142. [Google Scholar] [CrossRef]
- Degueldre, C.; Favarger, P.Y.; Wold, S. Gold colloid analysis by inductively coupled plasma-mass spectrometry in a single particle mode. Anal. Chim. Acta 2006, 555, 263–268. [Google Scholar] [CrossRef]
- Hadioui, M.; Knapp, G.; Azimzada, A.; Jreije, I.; Frechette-Viens, L.; Wilkinson, K.J. Lowering the size detection limits of Ag and TiO2 nanoparticles by single particle ICP-MS. Anal. Chem. 2019, 91, 13275–13284. [Google Scholar] [CrossRef]
- Lee, S.; Bi, X.; Reed, R.B.; Ranville, J.F.; Herckes, P.; Westerhoff, P. Nanoparticle size detection limits by single particle ICP-MS for 40 elements. Environ. Sci. Technol. 2014, 48, 10291–10300. [Google Scholar] [CrossRef]
- Laborda, F.; Jiménez-Lamana, J.; Bolea, E.; Castillo, J.R. Critical considerations for the determination of nanoparticle number concentrations, size and number size distributions by single particle ICP-MS. J. Anal. At. Spectrom. 2013, 28, 1220–1232. [Google Scholar] [CrossRef]
- Pace, H.E.; Rogers, N.J.; Jarolimek, C.; Coleman, V.A.; Gray, E.P.; Higgins, C.P.; Ranville, J.F. Single particle inductively coupled plasma-mass spectrometry: A performance evaluation and method comparison in the determination of nanoparticle size. Environ. Sci. Technol. 2012, 46, 12272–12280. [Google Scholar] [CrossRef] [PubMed]
- Montoro Bustos, A.R.; Purushotham, K.P.; Possolo, A.; Farkas, N.; Vladár, A.E.; Murphy, K.E.; Winchester, M.R. Validation of Single Particle ICP-MS for Routine Measurements of Nanoparticle Size and Number Size Distribution. Anal. Chem. 2018, 90, 14376–14386. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Nakazato, M.; Hirata, T. Size analysis of small metal nanoparticles using single particle ICP mass spectrometry. Anal. Sci. 2021, 37, 1637–1640. [Google Scholar] [CrossRef] [PubMed]
- Laborda, F.; Jiménez-Lamana, J.; Bolea, E.; Castillo, J.R. Selective identification, characterization and determination of dissolved silver (i) and silver nanoparticles based on single particle detection by inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2011, 26, 1362–1371. [Google Scholar] [CrossRef]
- Olesik, J.W.; Gray, P.J. Considerations for measurement of individual nanoparticles or microparticles by ICP-MS: Determination of the number of particles and the analyte mass in each particle. J. Anal. At. Spectrom. 2012, 27, 1143–1155. [Google Scholar] [CrossRef]
- Pace, H.E.; Rogers, N.J.; Jarolimek, C.; Coleman, V.A.; Higgins, C.P.; Ranville, J.F. Determining transport efficiency for the purpose of counting and sizing nanoparticles via single particle inductively coupled plasma mass spectrometry. Anal. Chem. 2011, 83, 9361–9369. [Google Scholar] [CrossRef]
- Montaño, M.D.; Olesik, L.W.; Barber, A.G.; Challis, K.; Ranville, J.F. Single particle ICP-MS: Advances toward routine analysis of nanomaterials. Anal. Bioanal. Chem. 2016, 408, 5053–5074. [Google Scholar] [CrossRef] [PubMed]
- Mozhayeva, D.; Engelhard, C. A critical review of single particle inductively coupled plasma mass spectrometry-A step towards an ideal method for nanomaterial characterization. J. Anal. At. Spectrom. 2020, 35, 1740–1783. [Google Scholar] [CrossRef]
- Bolea, E.; Jimenez, M.S.; Perez-Arantegui, J.; Vidal, J.C.; Bakir, M.; Ben-Jeddou, K.; Gimenez-Ingalaturre, A.C.; Ojeda, D.; Trujillo, C.; Laborda, F. Analytical applications of single particle inductively coupled plasma mass spectrometry: A comprehensive and critical review. Anal. Methods 2021, 13, 2742–2795. [Google Scholar] [CrossRef]
- Resano, M.; Aramendia, M.; García-Ruiz, E.; Bazo, A.; Bolea-Fernandez, E.; Vanhaecke, F. Living in a transient world: ICP-MS reinvented via time-resolved analysis for monitoring single events. Chem. Sci. 2022, 13, 4436–4473. [Google Scholar] [CrossRef] [PubMed]
- Waegeneers, N.; De Vos, S.; Verleysen, E.; Ruttens, A.; Mast, J. Estimation of the uncertainties related to the measurement of the size and quantities of individual silver nanoparticles in confectionery. Materials 2019, 12, 2677. [Google Scholar] [CrossRef] [PubMed]
- Kaynarova, L.I.; Georgieva, D.L.; Stefanova, V.M. An approach to estimate the contribution of signal noise to the diameter uncertainty of individual silver nanoparticles and resolution of spICP-MS analysis. J. Anal. At. Spectrom. 2022, 37, 1484–1500. [Google Scholar] [CrossRef]
- Yamashita, S.; Yoshikuni, Y.; Obayashi, H.; Suzuki, T.; Green, D.; Hirata, T. Simultaneous determination of size and position of silver and gold nanoparticles in onion cells using laser ablation-ICP-MS. Anal. Chem. 2019, 91, 4544–4551. [Google Scholar] [CrossRef] [PubMed]
- Kato, K. Indirect measuring method of aerosol transport efficiency in inductively coupled plasma spectrometry. Fresenius’ J. Anal. Chem. 1988, 329, 861–863. [Google Scholar] [CrossRef]
- Smith, D.D.; Browner, R.F. Measurement of aerosol transport efficiency in atomic spectrometry. Anal. Chem. 1982, 54, 533–537. [Google Scholar] [CrossRef]
- Farino, J.; Miller, J.R.; Smith, D.D.; Browner, R.F. Influence of solution uptake rate on signals and interferences in inductively coupled plasma optical emission spectrometry. Anal. Chem. 1987, 59, 2303–2309. [Google Scholar] [CrossRef]
- Gustavsson, A. The determination of some nebulizer characteristics. Spectrochim. Acta Part B 1984, 39, 743–746. [Google Scholar] [CrossRef]

| Description | Value | Standard Uncertainty | Relative Uncertainty |
|---|---|---|---|
| Concentration of silver standard stock solution | 1003 μg/g | 3.5 μg/g 1 | 0.35% |
| Weight of standard stock solution | 0.1 g (1 μg/g) | 0.0002 g 2 | 0.2% |
| Weight of 1 μg/g standard solution | 0.1 g (1 ng/g), 1.0 g (10 ng/g), 10 g (100 ng/g) | 0.0002 g 2 | 0.2% (1 ng/g), 0.02% (10 ng/g), 0.002% (100 ng/g) |
| Weight of dilution | 100 g | 0.0002 g 2 | 0.0002% |
| Combined standard uncertainty | – | – | 0.45% (1 ng/g), 0.40% (10 ng/g), 0.40% (100 ng/g) |
| Instrumental Parameters | |
| ICP-MS Instrument | Agilent 8900 |
| RF power | 1550 W |
| Argon gas flow rate | |
| Plasma gas | 15.0 L/min |
| Auxiliary gas | 0.90 L/min |
| Nebulizer gas | 1.20 L/min |
| Nebulizer type | MicroMist nebulizer (for 8900 Standard and #100) |
| Spray chamber type | Double-pass |
| Spray chamber temperature | 2 °C |
| Gas mode | No gas |
| Data acquisition parameters | |
| Acquisition mode | SQ |
| Dwell time | 100 μs |
| Settling time | - |
| Monitored isotopes | 107Ag |
| Source | Relative Uncertainty |
|---|---|
| Concentration of working standard solutions (Cstd) 1 | 0.45% (1 ng/g), 0.40% (10 ng/g), 0.40% (100 ng/g) |
| Sample flow rate (Qneb) 2 | 0.69% |
| Transport efficiency (η) 2,3 | 0.34% |
| Slope of calibration curve (m) 2 | 0.13% (1 ng/g), 0.092% (10 ng/g), 0.045% (100 ng/g) |
| Signal intensity of working standard solutions (Isoln) 4 | 18% (1 ng/g), 6.0% (10 ng/g), 1.9% (100 ng/g) |
| Signal intensity of particles (INPs) 4 | 50% (4 counts/event = ca. 10 nm) − 1.6% (4000 counts/event = ca. 100 nm) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, S.; Miyashita, S.-i.; Hirata, T. Size Uncertainty in Individual Nanoparticles Measured by Single Particle Inductively Coupled Plasma Mass Spectrometry. Nanomaterials 2023, 13, 1958. https://doi.org/10.3390/nano13131958
Yamashita S, Miyashita S-i, Hirata T. Size Uncertainty in Individual Nanoparticles Measured by Single Particle Inductively Coupled Plasma Mass Spectrometry. Nanomaterials. 2023; 13(13):1958. https://doi.org/10.3390/nano13131958
Chicago/Turabian StyleYamashita, Shuji, Shin-ichi Miyashita, and Takafumi Hirata. 2023. "Size Uncertainty in Individual Nanoparticles Measured by Single Particle Inductively Coupled Plasma Mass Spectrometry" Nanomaterials 13, no. 13: 1958. https://doi.org/10.3390/nano13131958
APA StyleYamashita, S., Miyashita, S.-i., & Hirata, T. (2023). Size Uncertainty in Individual Nanoparticles Measured by Single Particle Inductively Coupled Plasma Mass Spectrometry. Nanomaterials, 13(13), 1958. https://doi.org/10.3390/nano13131958






