Rapid Microwave-Assisted Synthesis of ZnIn2S4 Nanosheets for Highly Efficient Photocatalytic Hydrogen Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of In2S3, ZnIn2S4, and ZnS Nanosheets
2.3. Characterization
2.4. Photocatalytic Hydrogen Production Experiment
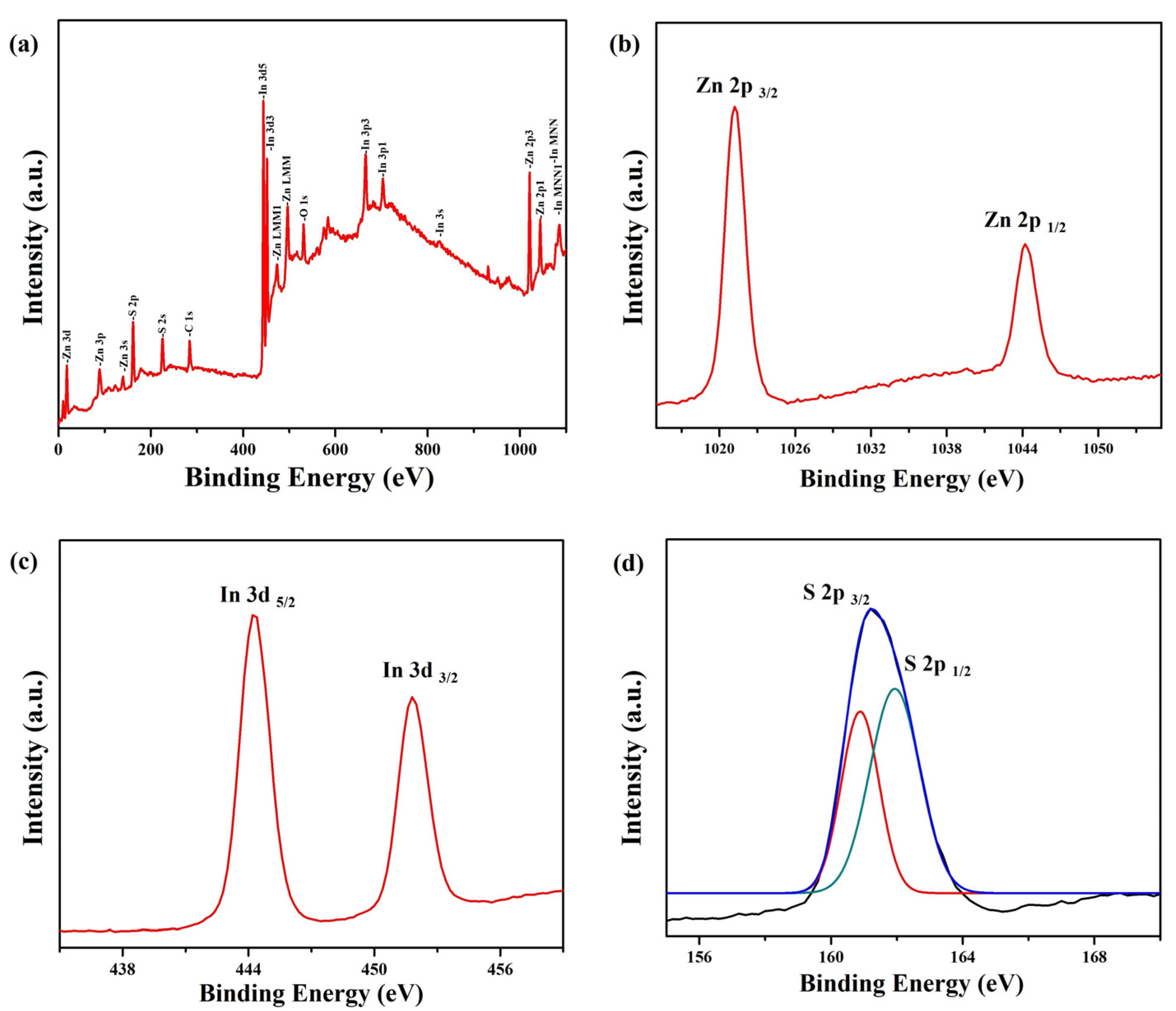
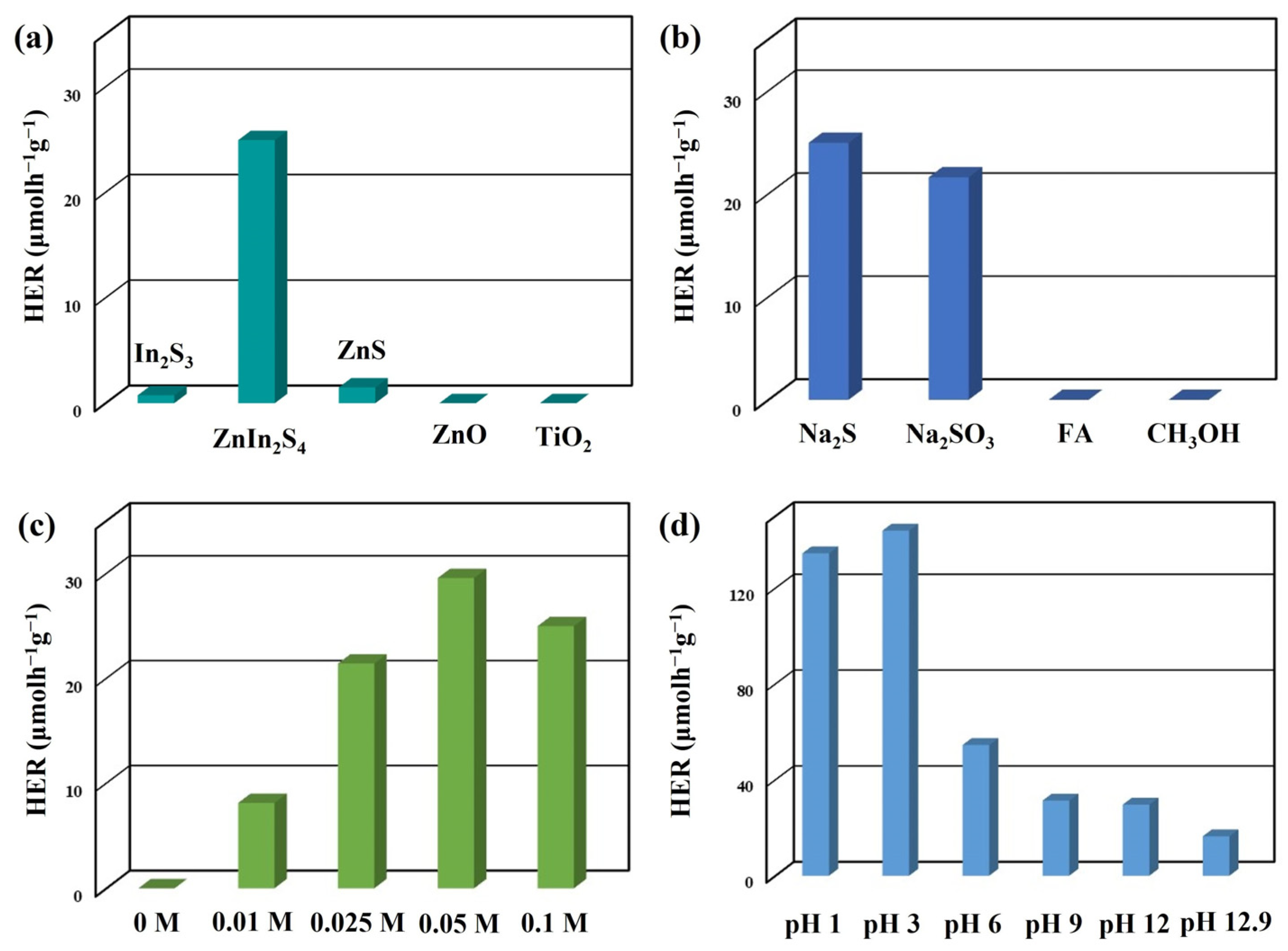
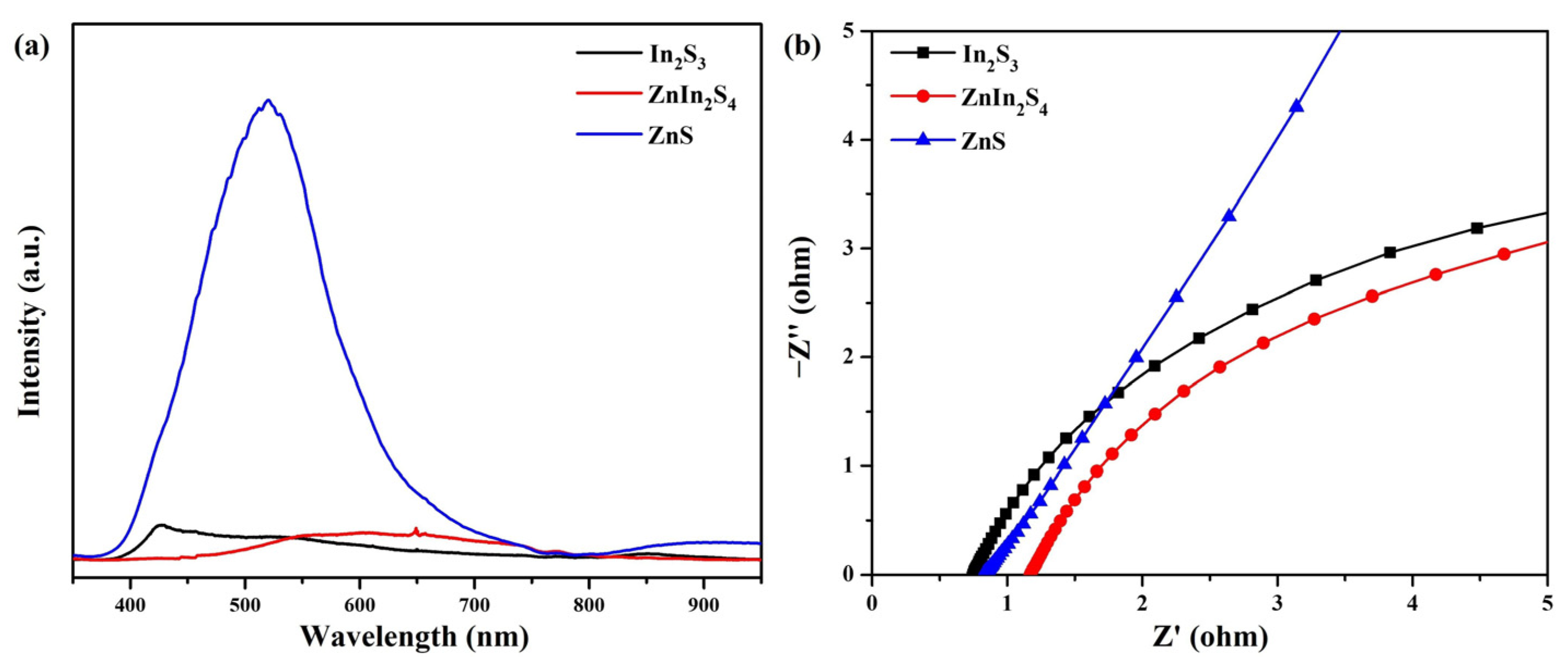
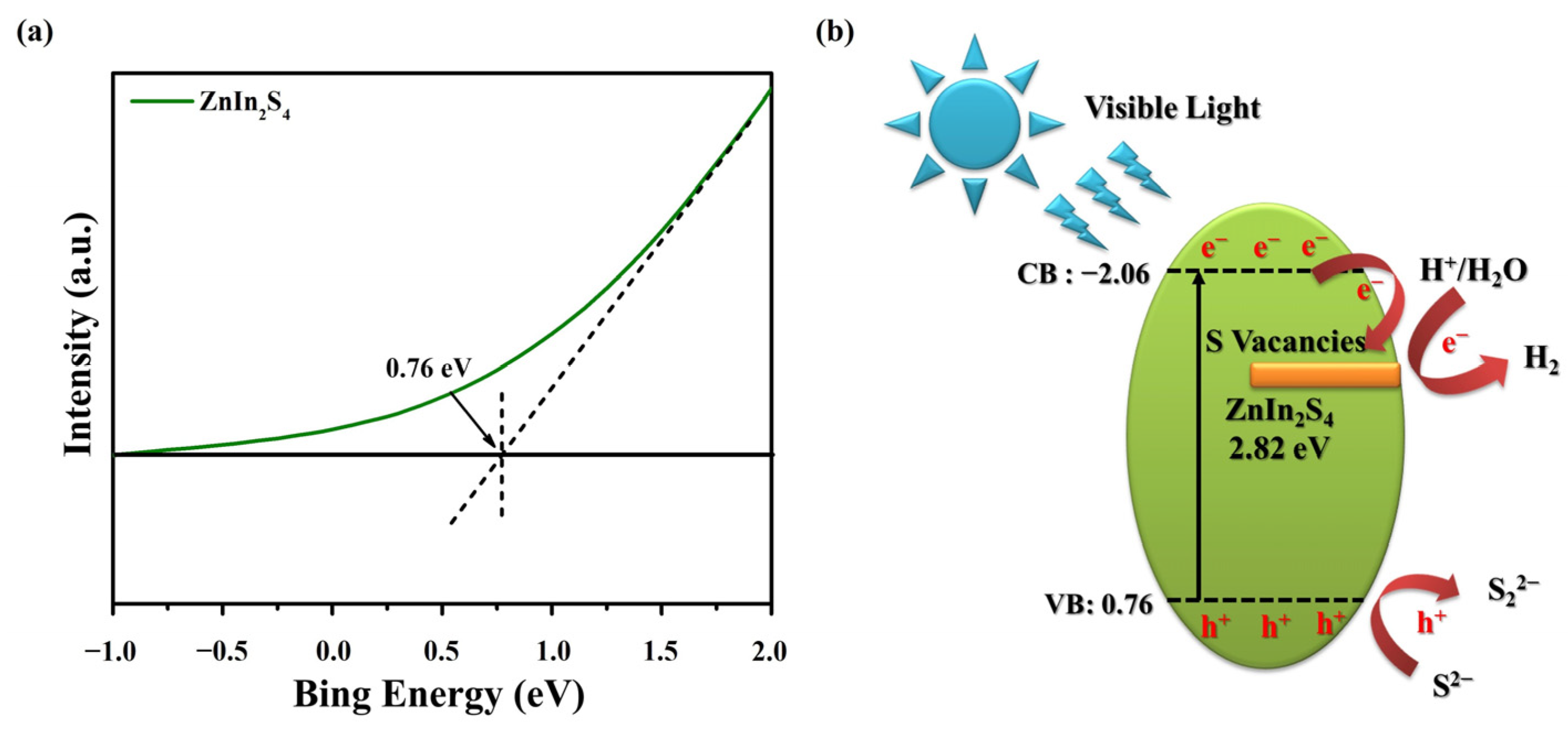
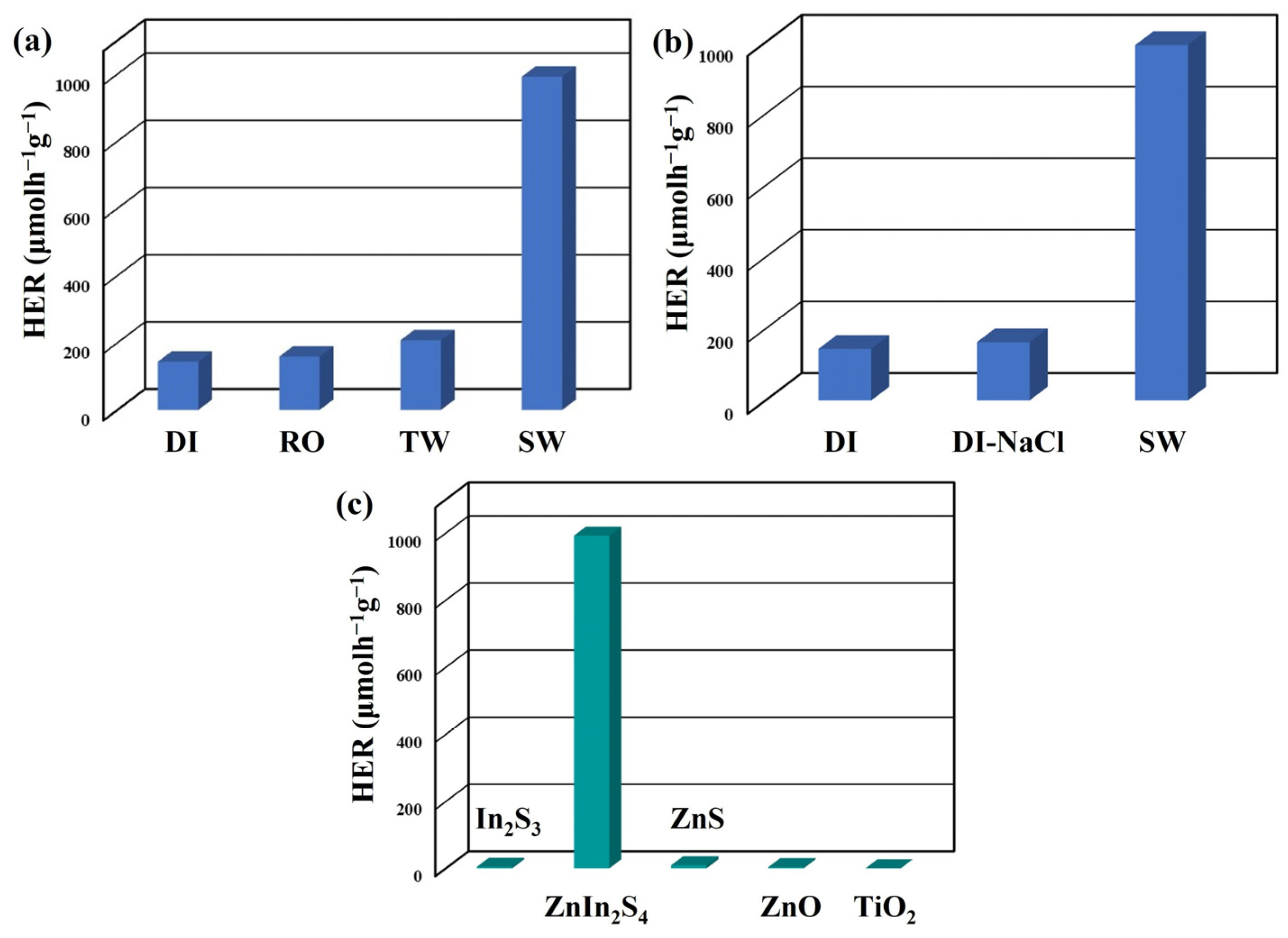
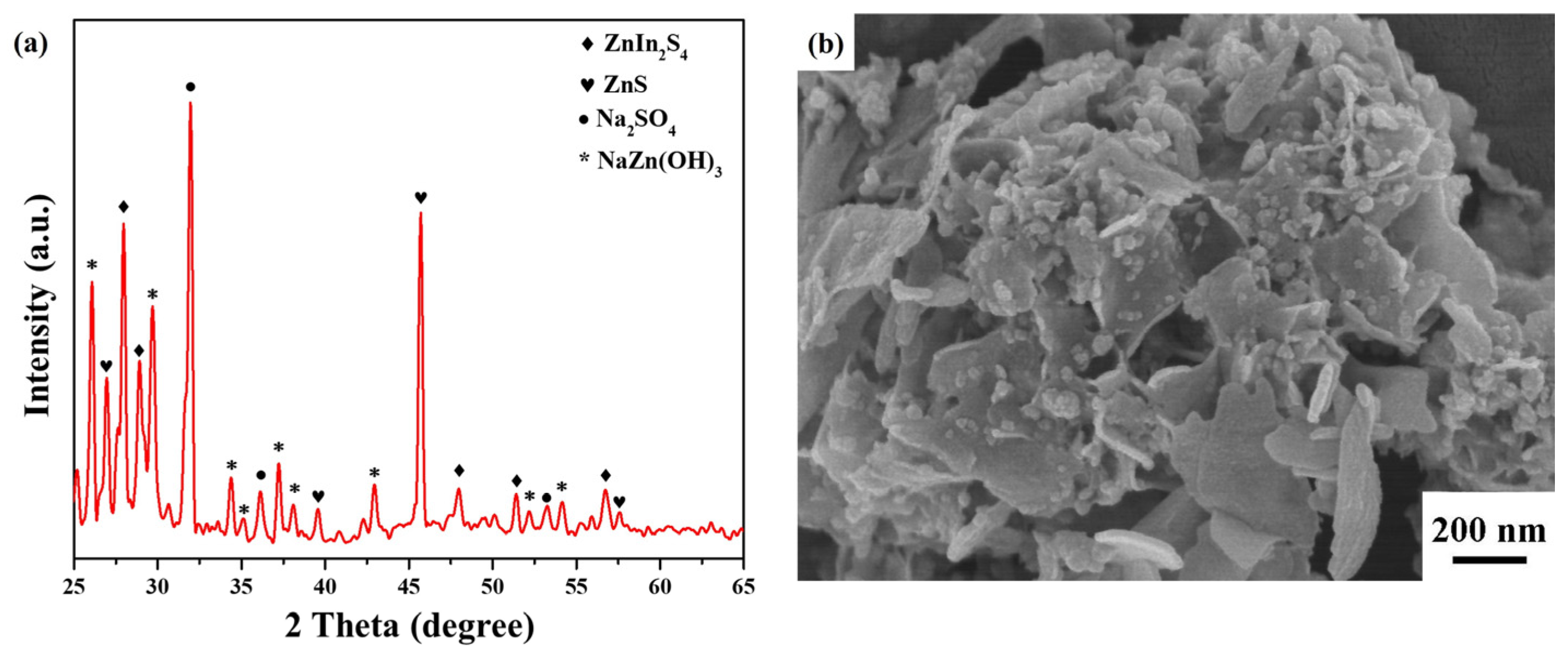
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-Assisted Chemistry: Synthetic Applications for Rapid Assembly of Nanomaterials and Organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef]
- Wang, J.; Wu, W.; Kondo, H.; Fan, T.; Zhou, H. Recent progress in microwave-assisted preparations of 2D materials and catalysis applications. Nanotechnology 2022, 33, 342002. [Google Scholar] [CrossRef]
- Chang, T.-H.; Chang, Y.-C.; Lee, C.-I.; Lin, Y.-R.; Ko, F.-H. Optimization Temperature Programming of Microwave-Assisted Synthesis ZnO Nanoneedle Arrays for Optical and Surface-Enhanced Raman Scattering Applications. Nanomaterials 2022, 12, 3989. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-J.; Chen, F. Microwave-Assisted Preparation of Inorganic Nanostructures in Liquid Phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.G.; Choudhari, K.S.; Shivashankar, S.A.; Chidangil, S.; Kulkarni, S.D. Microwave solution route to ceramic ZnAl2O4 nanoparticles in 10 minutes: Inversion and photophysical changes with thermal history. New J. Chem. 2017, 41, 5420–5428. [Google Scholar] [CrossRef]
- Tompsett, G.A.; Conner, W.C.; Yngvesson, K.S. Microwave Synthesis of Nanoporous Materials. ChemPhysChem 2006, 7, 296–319. [Google Scholar] [CrossRef]
- Mirzaei, A.; Neri, G. Microwave-assisted synthesis of metal oxide nanostructures for gas sensing application: A review. Sens. Actuators B Chem. 2016, 237, 749–775. [Google Scholar] [CrossRef]
- Meng, L.-Y.; Wang, B.; Ma, M.-G.; Lin, K.-L. The progress of microwave-assisted hydrothermal method in the synthesis of functional nanomaterials. Mater. Today Chem. 2016, 1–2, 63–83. [Google Scholar] [CrossRef]
- Shen, P.-S.; Tseng, C.-M.; Kuo, T.-C.; Shih, C.-K.; Li, M.-H.; Chen, P. Microwave-assisted synthesis of titanium dioxide nanocrystalline for efficient dye-sensitized and perovskite solar cells. Sol. Energy 2015, 120, 345–356. [Google Scholar] [CrossRef]
- Schwenke, A.M.; Hoeppener, S.; Schubert, U.S. Synthesis and Modification of Carbon Nanomaterials utilizing Microwave Heating. Adv. Mater. 2015, 27, 4113–4141. [Google Scholar] [CrossRef] [PubMed]
- Architha, N.; Ragupathi, M.; Shobana, C.; Selvankumar, T.; Kumar, P.; Lee, Y.S.; Kalai Selvan, R. Microwave-assisted green synthesis of fluorescent carbon quantum dots from Mexican Mint extract for Fe3+ detection and bio-imaging applications. Environ. Res. 2021, 199, 111263. [Google Scholar] [CrossRef]
- Chin, C.D.; Treadwell, L.J.; Wiley, J.B. Microwave Synthetic Routes for Shape-Controlled Catalyst Nanoparticles and Nanocomposites. Molecules 2021, 26, 3647. [Google Scholar] [CrossRef]
- Sharifvaghefi, S.; Zheng, Y. Microwave vs conventional heating in hydrogen production via catalytic dry reforming of methane. Resour. Chem. Mater. 2022, 1, 290–307. [Google Scholar] [CrossRef]
- Motasemi, F.; Ani, F.N. A review on microwave-assisted production of biodiesel. Renew. Sustain. Energy Rev. 2012, 16, 4719–4733. [Google Scholar] [CrossRef]
- Devi, N.; Sahoo, S.; Kumar, R.; Singh, R.K. A review of the microwave-assisted synthesis of carbon nanomaterials, metal oxides/hydroxides and their composites for energy storage applications. Nanoscale 2021, 13, 11679–11711. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.N.; Bhasin, C.P.; Nayak, M.G. A review on microwave-assisted transesterification processes using various catalytic and non-catalytic systems. Renew. Energ. 2019, 143, 1366–1387. [Google Scholar] [CrossRef]
- Pan, J.; Guan, Z.; Yang, J.; Li, Q. Facile fabrication of ZnIn2S4/SnS2 3D heterostructure for efficient visible-light photocatalytic reduction of Cr(VI). Chin. J. Catal. 2020, 41, 200–208. [Google Scholar] [CrossRef]
- Janani, R.; Preethi, V.R.; Singh, S.; Rani, A.; Chang, C.-T. Hierarchical Ternary Sulfides as Effective Photocatalyst for Hydrogen Generation Through Water Splitting: A Review on the Performance of ZnIn2S4. Catalysts 2021, 11, 277. [Google Scholar] [CrossRef]
- Yang, R.; Mei, L.; Fan, Y.; Zhang, Q.; Zhu, R.; Amal, R.; Yin, Z.; Zeng, Z. ZnIn2S4-Based Photocatalysts for Energy and Environmental Applications. Small Methods 2021, 5, 2100887. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Balayeva, N.O. Metal Sulfide Photocatalysts for Hydrogen Generation: A Review of Recent Advances. Catalysts 2022, 12, 1316. [Google Scholar] [CrossRef]
- Wang, J.; Sun, S.; Zhou, R.; Li, Y.; He, Z.; Ding, H.; Chen, D.; Ao, W. A review: Synthesis, modification and photocatalytic applications of ZnIn2S4. J. Mater. Sci. Technol. 2021, 78, 1–19. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, L.; Shi, H.; Zhang, H.; Wang, Y.; Bai, S.; Yang, Y.; Li, Y.; Zhang, T.; Zhang, H. Highly efficient solar-driven photocatalytic hydrogen evolution by a ternary 3D ZnIn2S4–MoS2 microsphere/1D TiO2 nanobelt heterostructure. New J. Chem. 2021, 45, 14167–14176. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, H.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. A mini-review on ZnIn2S4-Based photocatalysts for energy and environmental application. Green Energy Environ. 2022, 7, 176–204. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, R.; Chen, D.; Wang, Y.; Liu, W.; Li, X.; Li, Z. Exploring the Different Photocatalytic Performance for Dye Degradations over Hexagonal ZnIn2S4 Microspheres and Cubic ZnIn2S4 Nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 2273–2279. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Tasi, C.-L.; Ko, F.-H. Construction of ZnIn2S4/ZnO heterostructures with enhanced photocatalytic decomposition and hydrogen evolution under blue LED irradiation. Int. J. Hydrogen Energy 2021, 46, 10281–10292. [Google Scholar] [CrossRef]
- Shen, S.; Zhao, L.; Guan, X.; Guo, L. Improving visible-light photocatalytic activity for hydrogen evolution over ZnIn2S4: A case study of alkaline-earth metal doping. J. Phys. Chem. Solids 2012, 73, 79–83. [Google Scholar] [CrossRef]
- Shen, J.; Zai, J.; Yuan, Y.; Qian, X. 3D hierarchical ZnIn2S4: The preparation and photocatalytic properties on water splitting. Int. J. Hydrogen Energy 2012, 37, 16986–16993. [Google Scholar] [CrossRef]
- Sabbah, A.; Shown, I.; Qorbani, M.; Fu, F.-Y.; Lin, T.-Y.; Wu, H.-L.; Chung, P.-W.; Wu, C.-I.; Santiago, S.R.M.; Shen, J.-L.; et al. Boosting photocatalytic CO2 reduction in a ZnS/ZnIn2S4 heterostructure through strain-induced direct Z-scheme and a mechanistic study of molecular CO2 interaction thereon. Nano Energy 2022, 93, 106809. [Google Scholar] [CrossRef]
- Chen, J.; Li, K.; Cai, X.; Zhao, Y.; Gu, X.; Mao, L. Sulfur vacancy-rich ZnIn2S4 nanosheet arrays for visible-light-driven water splitting. Mater. Sci. Semicond. Process. 2022, 143, 106547. [Google Scholar] [CrossRef]
- Jiao, X.; Chen, Z.; Li, X.; Sun, Y.; Gao, S.; Yan, W.; Wang, C.; Zhang, Q.; Lin, Y.; Luo, Y.; et al. Defect-Mediated Electron–Hole Separation in One-Unit-Cell ZnIn2S4 Layers for Boosted Solar-Driven CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 7586–7594. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Liu, C.; Luo, S.; Wang, L.; Cai, T.; Zeng, Y.; Yuan, J.; Dong, W.; Pei, Y.; et al. MoS2 Quantum Dot Growth Induced by S Vacancies in a ZnIn2S4 Monolayer: Atomic-Level Heterostructure for Photocatalytic Hydrogen Production. ACS Nano 2018, 12, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Chen, J.; Wang, X.; Zhao, L.; Guo, L. Microwave-assisted hydrothermal synthesis of transition-metal doped ZnIn2S4 and its photocatalytic activity for hydrogen evolution under visible light. J. Power Sources 2011, 196, 10112–10119. [Google Scholar] [CrossRef]
- Zhang, R.; Gong, K.; Cao, S.; Du, F. Amorphous sulfur-rich CoSx nanodots as highly efficient cocatalyst to promote photocatalytic hydrogen evolution over TiO2. Int. J. Hydrogen Energy 2022, 47, 39875–39885. [Google Scholar] [CrossRef]
- Chen, L.; Wang, D.; Xia, Y.; Liang, R.; Huang, R.; Yan, G. Decoration of nicale phosphide nanoparticles on CdS nanorods for enhanced photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 28486–28494. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Zeng, C.-J.; Chen, C.-Y.; Tsay, C.-Y.; Lee, G.-J.; Wu, J.J. NiS/Pt loaded on electrospun TiO2 nanofiber with enhanced visible-light-driven photocatalytic hydrogen production. Mater. Res. Bull. 2023, 157, 112041. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Wan, S.; Wang, F.; Dou, W.; Xu, X.; Li, S.; Ma, R.; Qi, L. A long short-term memory-based model for greenhouse climate prediction. Int. J. Intell. Syst. 2022, 37, 135–151. [Google Scholar] [CrossRef]
- Gruda, N.; Bisbis, M.; Tanny, J. Influence of climate change on protected cultivation: Impacts and sustainable adaptation strategies—A review. J. Clean. Prod. 2019, 225, 481–495. [Google Scholar] [CrossRef]
- Levy, K.; Woster, A.P.; Goldstein, R.S.; Carlton, E.J. Untangling the Impacts of Climate Change on Waterborne Diseases: A Systematic Review of Relationships between Diarrheal Diseases and Temperature, Rainfall, Flooding, and Drought. Environ. Sci. Technol. 2016, 50, 4905–4922. [Google Scholar] [CrossRef] [Green Version]
- Guan, X.; Chowdhury, F.A.; Pant, N.; Guo, L.; Vayssieres, L.; Mi, Z. Efficient Unassisted Overall Photocatalytic Seawater Splitting on GaN-Based Nanowire Arrays. J. Phys. Chem. C 2018, 122, 13797–13802. [Google Scholar] [CrossRef]
- Chen, H.; Li, M.; Gao, J.; Yang, D.; Li, Z.; Liu, H.; Xie, Y.; Guo, L.; Zhou, W. Synergism of sulfur vacancy and Schottky junction in Ni/ZnIn2S4 nanosheet assembly for efficient charge separation and photocatalytic hydrogen evolution. Appl. Surf. Sci. 2023, 628, 157385. [Google Scholar] [CrossRef]
- Zhang, M.; Tan, P.; Yang, L.; Zhai, H.; Liu, H.; Chen, J.; Ren, R.; Tan, X.; Pan, J. Sulfur vacancy and p-n junction synergistically boosting interfacial charge transfer and separation in ZnIn2S4/NiWO4 heterostructure for enhanced photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2023, 634, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Chiao, Y.-C.; Fun, Y.-X. Cu2O/CuS/ZnS Nanocomposite Boosts Blue LED-Light-Driven Photocatalytic Hydrogen Evolution. Catalysts 2022, 12, 1035. [Google Scholar] [CrossRef]
- Corredor, J.; Rivero, M.J.; Rangel, C.M.; Gloaguen, F.; Ortiz, I. Comprehensive review and future perspectives on the photocatalytic hydrogen production. J. Chem. Technol. Biotechnol. 2019, 94, 3049–3063. [Google Scholar] [CrossRef] [Green Version]
- Kumaravel, V.; Imam, M.D.; Badreldin, A.; Chava, R.K.; Do, J.Y.; Kang, M.; Abdel-Wahab, A. Photocatalytic Hydrogen Production: Role of Sacrificial Reagents on the Activity of Oxide, Carbon, and Sulfide Catalysts. Catalysts 2019, 9, 276. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-R.; Chang, Y.-C.; Chiao, Y.-C.; Ko, F.-H. Au@CdS Nanocomposites as a Visible-Light Photocatalyst for Hydrogen Generation from Tap Water. Catalysts 2022, 13, 33. [Google Scholar] [CrossRef]
- Markovskaya, D.V.; Cherepanova, S.V.; Saraev, A.A.; Gerasimov, E.Y.; Kozlova, E.A. Photocatalytic hydrogen evolution from aqueous solutions of Na2S/Na2SO3 under visible light irradiation on CuS/Cd0.3Zn0.7S and NizCd0.3Zn0.7S1+z. Chem. Eng. J. 2015, 262, 146–155. [Google Scholar] [CrossRef]
- Bao, N.; Shen, L.; Takata, T.; Domen, K. Self-Templated Synthesis of Nanoporous CdS Nanostructures for Highly Efficient Photocatalytic Hydrogen Production under Visible Light. Chem. Mater. 2008, 20, 110–117. [Google Scholar] [CrossRef]
- Gomathisankar, P.; Hachisuka, K.; Katsumata, H.; Suzuki, T.; Funasaka, K.; Kaneco, S. Photocatalytic hydrogen production with CuS/ZnO from aqueous Na2S+Na2SO3 solution. Int. J. Hydrogen Energy 2013, 38, 8625–8630. [Google Scholar] [CrossRef]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Safizade, B.; Masoudpanah, S.M.; Hasheminiasari, M.; Ghasemi, A. Photocatalytic activity of BiFeO3/ZnFe2O4 nanocomposites under visible light irradiation. RSC Adv. 2018, 8, 6988–6995. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-C.; Syu, S.-Y.; Lu, M.-Y. Fabrication of In(OH)3–In2S3–Cu2O nanofiber for highly efficient photocatalytic hydrogen evolution under blue light LED excitation. Int. J. Hydrogen Energy 2023, 48, 9318–9332. [Google Scholar] [CrossRef]
- Khan, M.S.; Shi, L.; Zou, B.; Ullah, H. Effect of Vanadium doping on optoelectronic and magnetic properties of wurtzite ZnS crystal. Optik 2020, 204, 164162. [Google Scholar] [CrossRef]
- Hezam, A.; Wang, J.; Drmosh, Q.A.; Karthik, P.; Abdullah Bajiri, M.; Namratha, K.; Zare, M.; Lakshmeesha, T.R.; Shivanna, S.; Cheng, C.; et al. Rational construction of plasmonic Z-scheme Ag-ZnO-CeO2 heterostructures for highly enhanced solar photocatalytic H2 evolution. Appl. Surf. Sci. 2021, 541, 148457. [Google Scholar] [CrossRef]
- Munawar, K.; Mansoor, M.A.; Olmstead, M.M.; Zaharinie, T.; Mohd Zubir, M.N.; Haniffa, M.; Basirun, W.J.; Mazhar, M. Fabrication of Ag-ZnO composite thin films for plasmonic enhanced water splitting. Mater. Chem. Phys. 2020, 255, 123220. [Google Scholar] [CrossRef]
- Trang, T.N.Q.; Phan, T.B.; Nam, N.D.; Thu, V.T.H. In Situ Charge Transfer at the Ag@ZnO Photoelectrochemical Interface toward the High Photocatalytic Performance of H2 Evolution and RhB Degradation. ACS Appl. Mater. Interfaces 2020, 12, 12195–12206. [Google Scholar] [CrossRef] [PubMed]
- Motora, K.G.; Wu, C.-M.; Chala, T.F.; Chou, M.-H.; Kuo, C.-F.J.; Koinkar, P. Highly efficient photocatalytic activity of Ag3VO4/WO2.72 nanocomposites for the degradation of organic dyes from the ultraviolet to near-infrared regions. Appl. Surf. Sci. 2020, 512, 145618. [Google Scholar] [CrossRef]
- Zhang, D.; Liang, S.; Yao, S.; Li, H.; Liu, J.; Geng, Y.; Pu, X. Highly efficient visible/NIR photocatalytic activity and mechanism of Yb3+/Er3+ co-doped Bi4O5I2 up-conversion photocatalyst. Sep. Purif. Technol. 2020, 248, 117040. [Google Scholar] [CrossRef]
- Cui, L.; Ding, X.; Wang, Y.; Shi, H.; Huang, L.; Zuo, Y.; Kang, S. Facile preparation of Z-scheme WO3/g-C3N4 composite photocatalyst with enhanced photocatalytic performance under visible light. Appl. Surf. Sci. 2017, 391, 202–210. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Li, H.; Wei, X.; Feng, J.; Liu, K.; Dang, Y.; Zhou, A. Efficient and Stable Photoelectrochemical Seawater Splitting with TiO2@g-C3N4 Nanorod Arrays Decorated by Co-Pi. J. Phys. Chem. C 2015, 119, 20283–20292. [Google Scholar] [CrossRef]
- Peng, H.; Liu, D.; Zheng, X.; Fu, X. N-Doped Carbon-Coated ZnS with Sulfur-Vacancy Defect for Enhanced Photocatalytic Activity in the Visible Light Region. Nanomaterials 2019, 9, 1657. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Zhao, J.; Liu, R. Defect engineering of zeolite imidazole framework derived ZnS nanosheets towards enhanced visible light driven photocatalytic hydrogen production. Appl. Catal. B Environ. 2020, 278, 119265. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-C.; Chiao, Y.-C.; Hsu, P.-C. Rapid Microwave-Assisted Synthesis of ZnIn2S4 Nanosheets for Highly Efficient Photocatalytic Hydrogen Production. Nanomaterials 2023, 13, 1957. https://doi.org/10.3390/nano13131957
Chang Y-C, Chiao Y-C, Hsu P-C. Rapid Microwave-Assisted Synthesis of ZnIn2S4 Nanosheets for Highly Efficient Photocatalytic Hydrogen Production. Nanomaterials. 2023; 13(13):1957. https://doi.org/10.3390/nano13131957
Chicago/Turabian StyleChang, Yu-Cheng, Yung-Chang Chiao, and Po-Chun Hsu. 2023. "Rapid Microwave-Assisted Synthesis of ZnIn2S4 Nanosheets for Highly Efficient Photocatalytic Hydrogen Production" Nanomaterials 13, no. 13: 1957. https://doi.org/10.3390/nano13131957
APA StyleChang, Y.-C., Chiao, Y.-C., & Hsu, P.-C. (2023). Rapid Microwave-Assisted Synthesis of ZnIn2S4 Nanosheets for Highly Efficient Photocatalytic Hydrogen Production. Nanomaterials, 13(13), 1957. https://doi.org/10.3390/nano13131957







