Efficient Dual-Function Catalyst: Palladium–Copper Nanoparticles Immobilized on Co-Cr LDH for Seamless Aerobic Oxidation of Benzyl Alcohol and Nitrobenzene Reduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
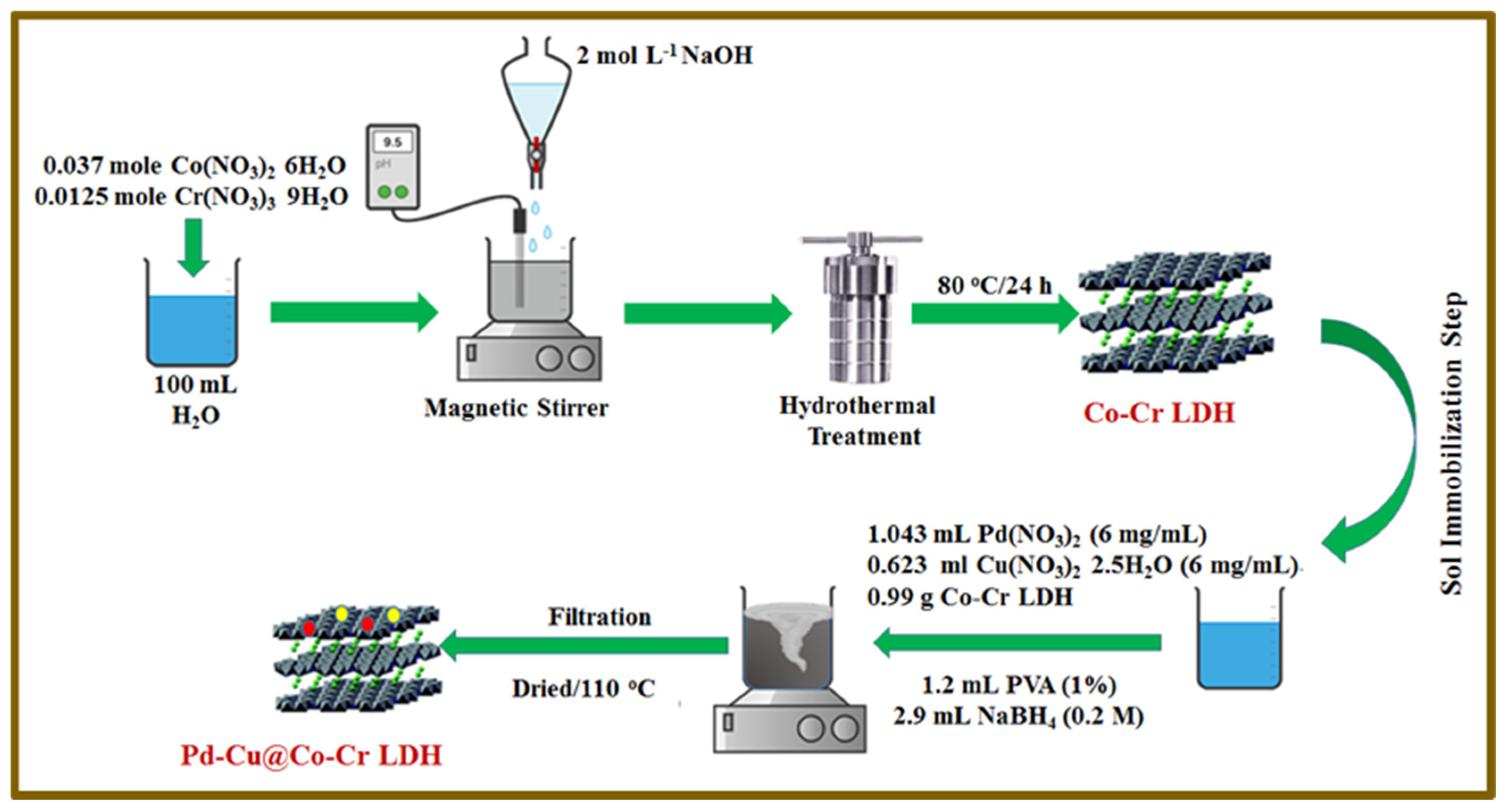
2.2. Materials Fabrication
2.2.1. Synthesis of Pristine Co-Cr LDH
2.2.2. Sol-Immobilization of Pd-Cu NPs over Co-Cr LDH
2.3. Characterizations
2.4. Catalytic Activity
2.4.1. Benzyl Alcohol Oxidation
2.4.2. Nitrobenzene Reduction
3. Results and Discussion
3.1. Materials Characterization
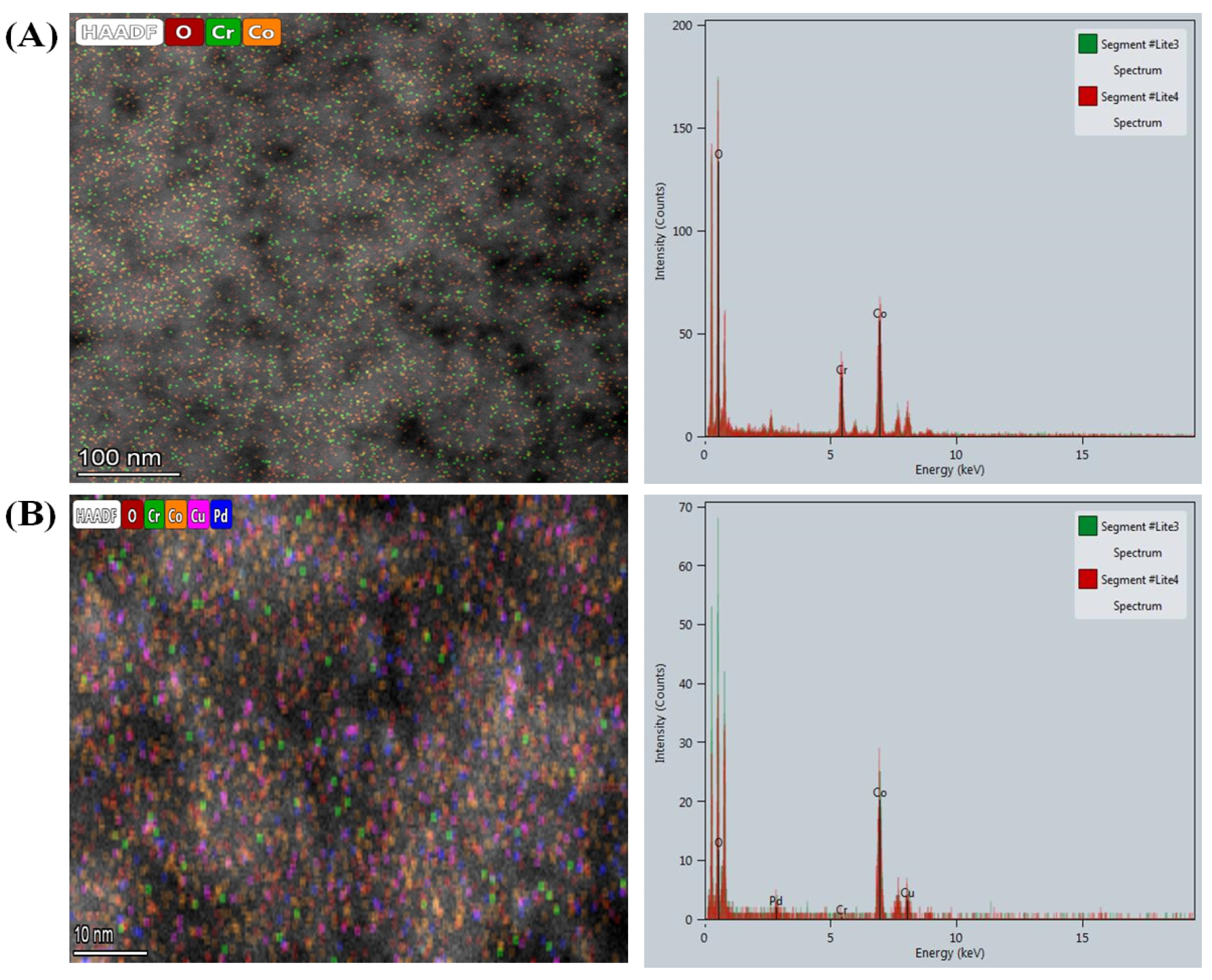
3.1.1. Elemental Assessment
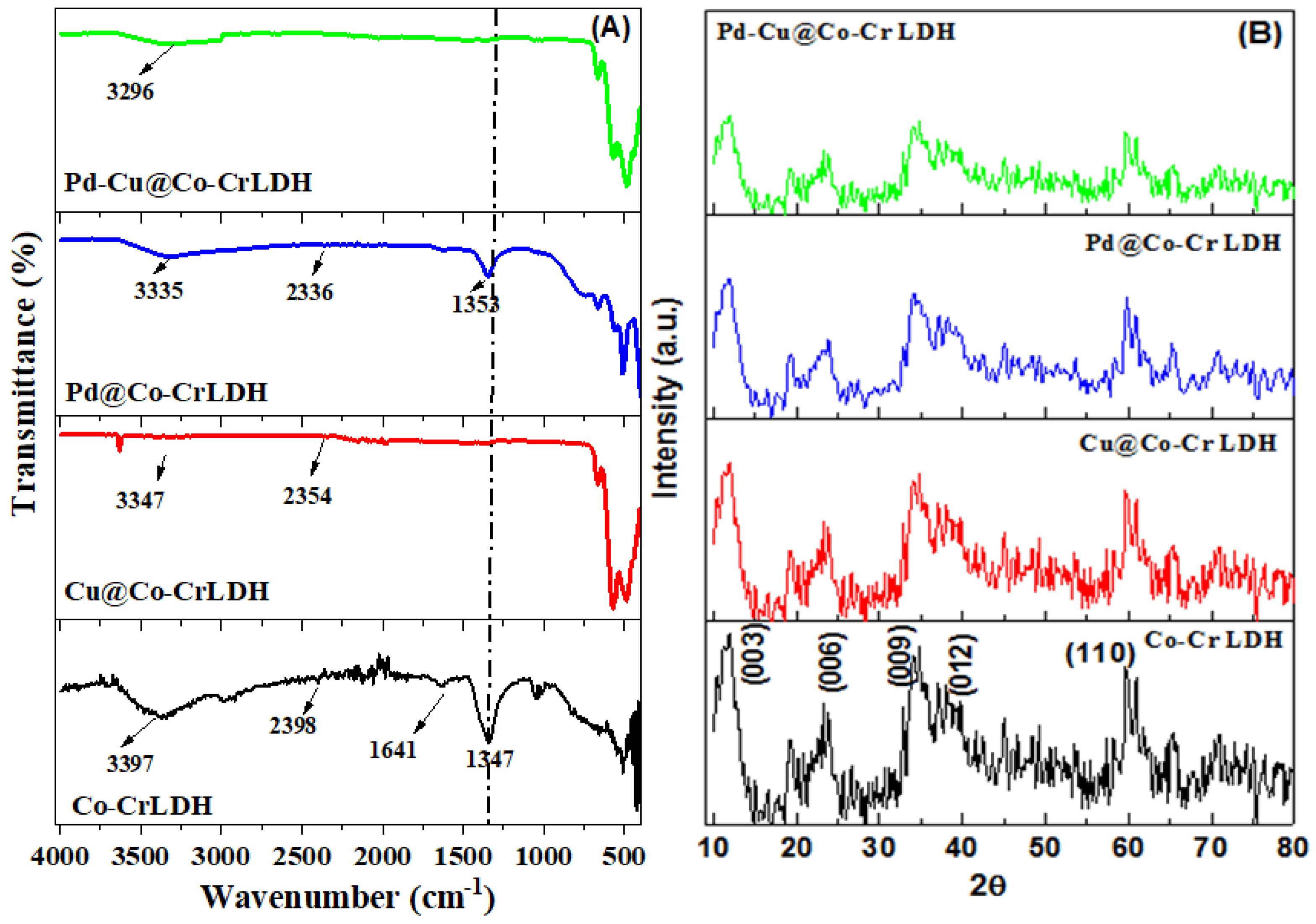
3.1.2. Spectroscopic Evaluation
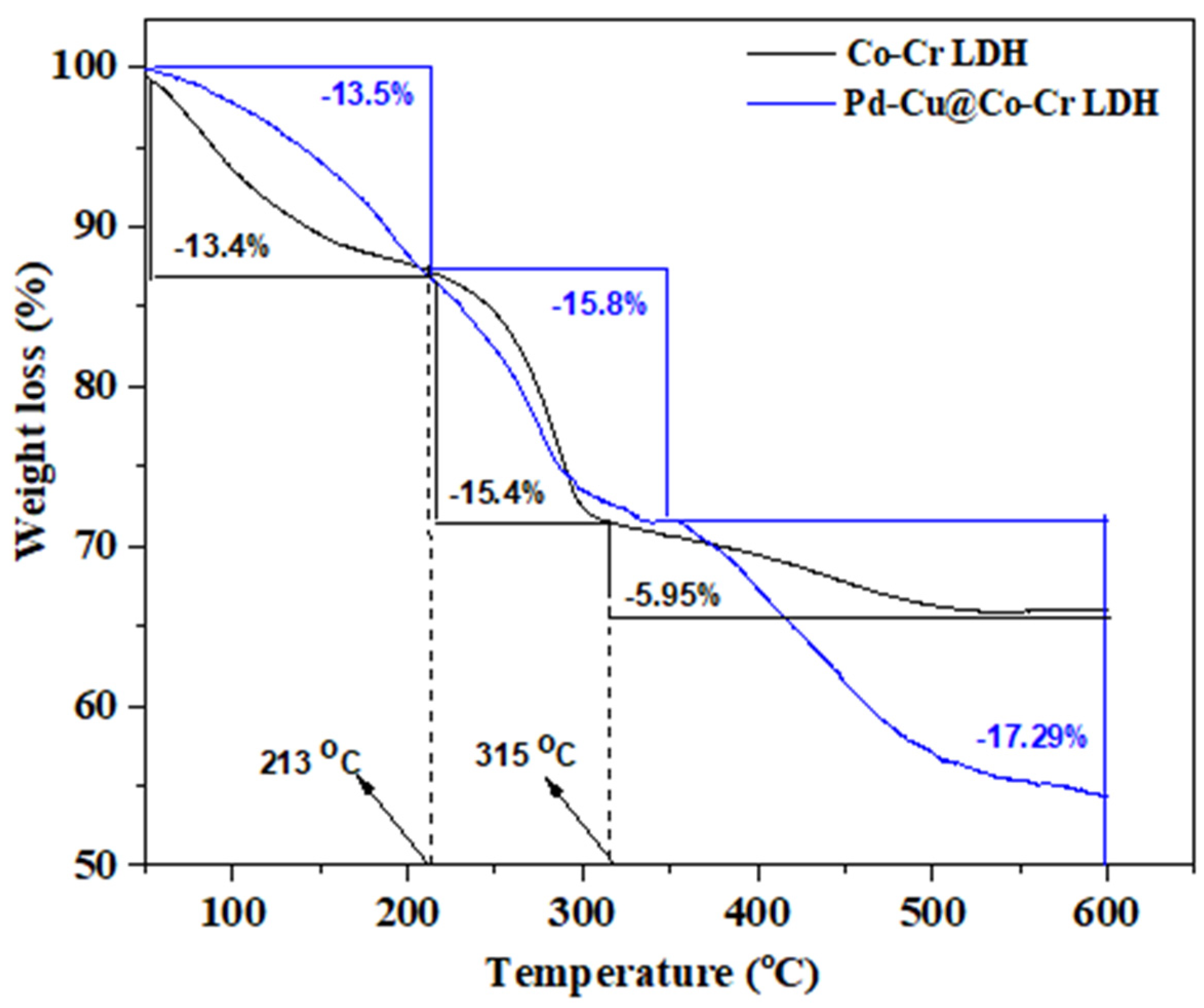
3.1.3. TGA Analysis
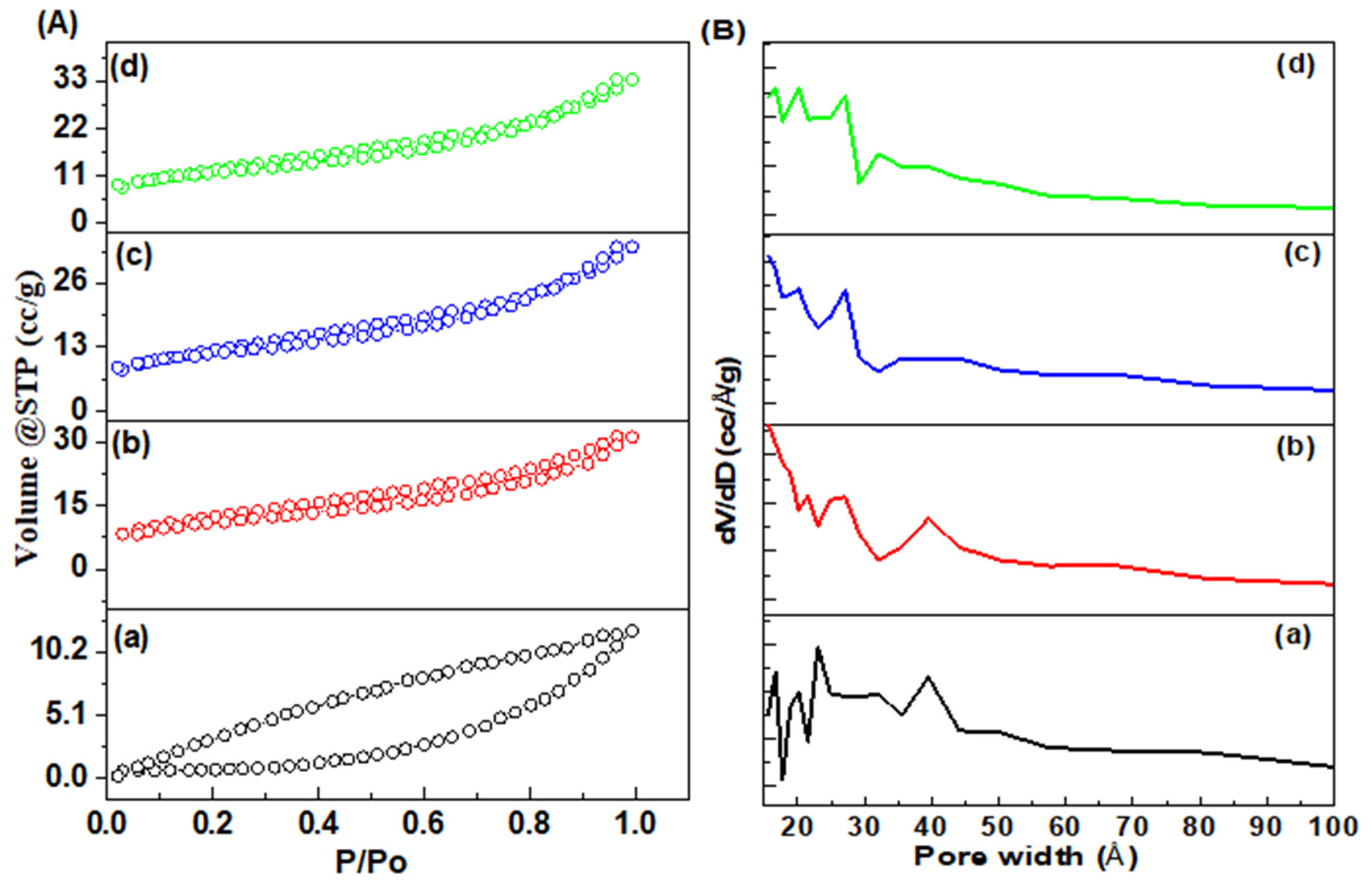
3.1.4. N2 Isotherms
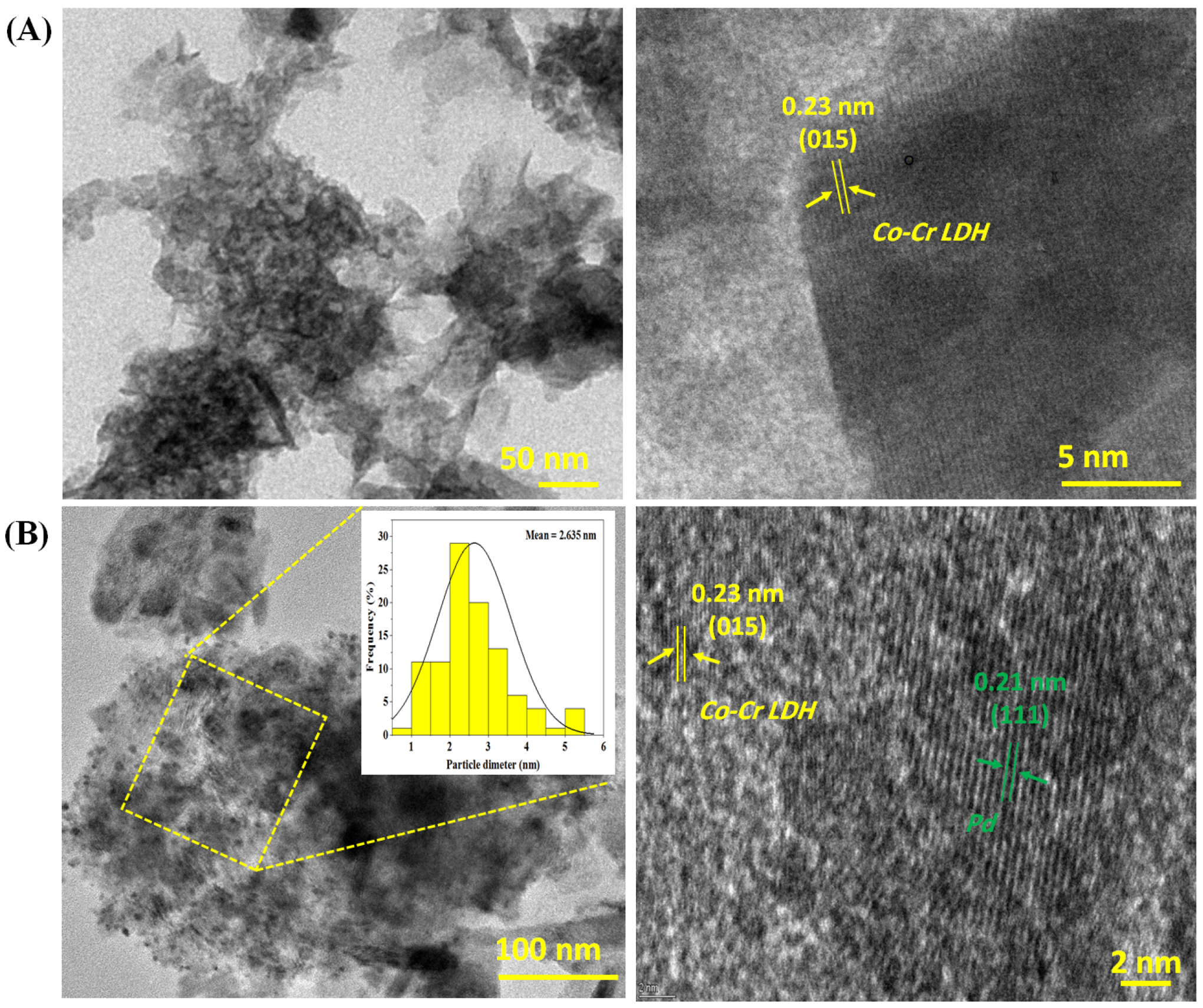
3.1.5. Surface Analyses
3.2. Catalytic Activity
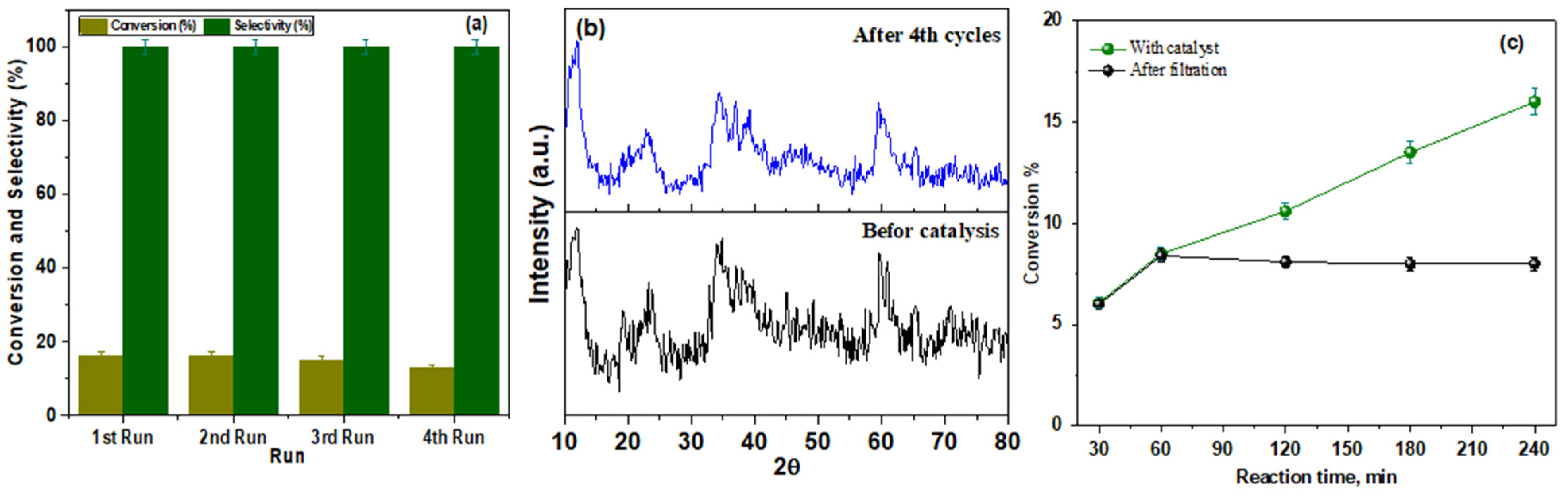
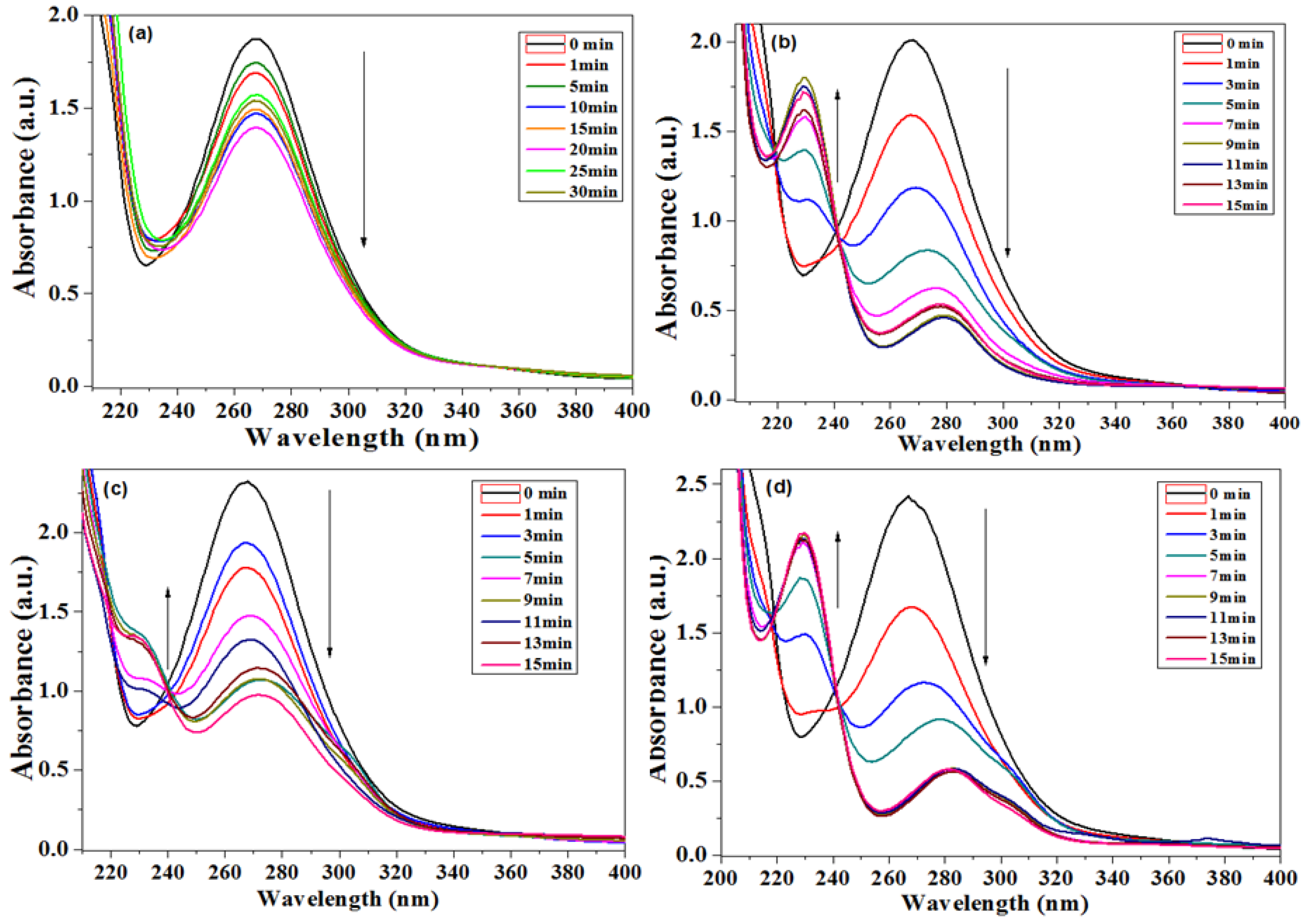
3.2.1. Benzyl Alcohol Oxidation
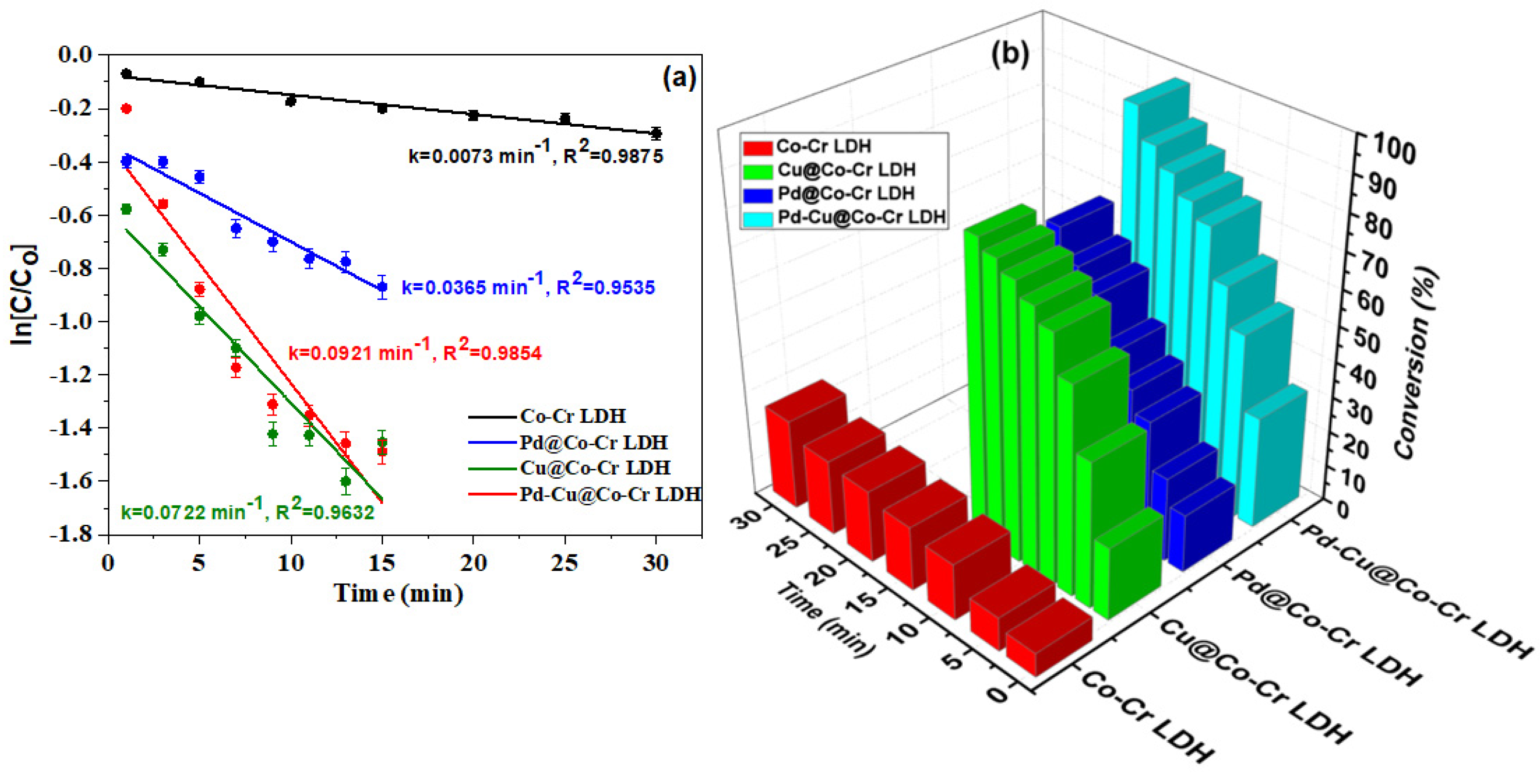
3.2.2. Catalytic Activity for Nitrobenzene (NB) Reduction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Constable, D.J.C.; Dunn, P.J.; Hayler, J.D.; Humphrey, G.R.; Leazer, J.L.; Linderman, R.J.; Lorenz, K.; Manley, J.; Pearlman, B.A.; Wells, A. Key green chemistry research areas—A perspective from pharmaceutical manufacturers. Green Chem. 2007, 9, 411. [Google Scholar] [CrossRef]
- Sheldon, R.A. Fundamentals of green chemistry: Efficiency in reaction design. Chem. Soc. Rev. 2012, 41, 1437. [Google Scholar] [CrossRef] [PubMed]
- Backvall, J.-E. (Ed.) Modern Oxidation Methods; Wiley-VCH: Weinhein, Germany, 2010. [Google Scholar]
- Tojo, G.; Fernandez, M. (Eds.) Oxidation of Alcohols to Aldehydes and Ketones; Springer: New York, NY, USA, 2010. [Google Scholar]
- Cardona, F.; Parmeggiani, C. (Eds.) Transition Metal Catalysis in Aerobic Alcohol Oxidation; RSC Green Chemistry: Cambridge, UK, 2015. [Google Scholar]
- Shi, Z.; Zhang, C.; Tang, C.; Jiao, N. For a critical review on other transition metal catalyzed reactions using molecular oxygen see. Chem. Soc. Rev. 2012, 41, 3381. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Arends, I.; Hanefeld, U. (Eds.) Green Chemistry and Catalysis; Wiley-VCH: Weinhein, Germany, 2007. [Google Scholar]
- Tidwell, T.T. Organic Reactions; Wiley: New York, NY, USA, 1990; Volume 39, p. 297. [Google Scholar]
- Steinhoff, B.A.; Fix, S.R.; Stahl, S.S. Mechanistic study of alcohol oxidation by the Pd (OAc) 2/O2/DMSO catalyst system and implications for the development of improved aerobic oxidation catalysts. J. Am. Chem. Soc. 2002, 124, 766. [Google Scholar] [CrossRef]
- Brink, G.J.T.; Arends, I.W.C.E.; Sheldon, R.A. Green, catalytic oxidation of alcohols in water. Science 2000, 287, 1636. [Google Scholar] [CrossRef]
- Brink, G.J.T.; Arends, I.W.C.E.; Sheldon, R.A. Catalytic Conversions in Water. Part 21: Mechanistic Investigations on the Palladium-Catalysed Aerobic Oxidation of Alcohols in Water. Adv. Synth. Catal. 2002, 344, 355. [Google Scholar] [CrossRef]
- Neumann, R.; Gara, M. The manganese-containing polyoxometalate,[WZnMnII2 (ZnW9O34)2] 12-, as a remarkably effective catalyst for hydrogen peroxide mediated oxidations. J. Am. Chem. Soc. 1995, 117, 5066. [Google Scholar] [CrossRef]
- Choudary, B.M.; Kantam, M.L.; Rahman, A.; Reddy, C.V.; Rao, K.K. The First Example of Activation of Molecular Oxygen by Nickel in Ni-Al Hydrotalcite: A Novel Protocol for the Selective Oxidation of Alcohols. Angew. Chem. Int. Ed. 2001, 40, 763. [Google Scholar] [CrossRef]
- Riahi, A.; Hénin, F.; Muzart, J. Homogeneous chromium (VI)-catalyzed oxidations of allylic alcohols by alkyl hydroperoxides: Influence of the nature of the alkyl group on the product distribution. Tetrahedron Lett. 1999, 40, 2303–2306. [Google Scholar] [CrossRef]
- Musawir, M.; Davey, P.N.; Kelly, G.; Kozhevnikov, I.V. Highly efficient liquid-phase oxidation of primary alcohols to aldehydes with oxygen catalysed by Ru–Co oxide. Chem. Commun. 2003, 1414–1415. [Google Scholar] [CrossRef]
- Mallat, T.; Baiker, A. Oxidation of alcohols with molecular oxygen on solid catalysts. Chem. Rev. 2004, 104, 3037. [Google Scholar] [CrossRef] [PubMed]
- Mallat, T.; Baiker, A. Oxidation of alcohols with molecular oxygen on platinum metal catalysts in aqueous solutions. Catal. Today 1994, 19, 247. [Google Scholar] [CrossRef]
- Gallezot, P. Selective oxidation with air on metal catalysts. Catal. Today 1997, 37, 405. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P. Selective oxidation of alcohols and aldehydes on metal catalysts. Catal. Today 2000, 57, 127. [Google Scholar] [CrossRef]
- Lee, A.F.; Gee, J.J.; Theyers, H.J. Aspects of allylic alcohol oxidation—A bimetallic heterogeneous. Green Chem. 2000, 2, 279. [Google Scholar] [CrossRef]
- Abad, A.; Conception, P.; Corma, A.; Garcia, H. A collaborative effect between gold and a support induces the selective oxidation of alcohols. Angew. Chem. Int. Ed. 2005, 44, 4066. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Hutchings, G.J. Gold catalysis. Chem. Int. Ed. 2006, 45, 7896. [Google Scholar] [CrossRef]
- Dimitratos, N.; Villa, A.; Wang, D.; Porta, F.; Su, D.L. Pd and Pt catalysts modified by alloying with Au in the selective oxidation of alcohols. Prati J. Catal. 2006, 244, 113. [Google Scholar] [CrossRef]
- Hutchings, G.J. Nanocrystalline gold and gold palladium alloy catalysts for chemical synthesis. Chem. Commun. 2008, 1148–1164. [Google Scholar] [CrossRef]
- Della Pina, C.; Falletta, E.; Prati, L.; Rossi, M. Selective oxidation using gold. Chem. Soc. Rev. 2008, 37, 2077. [Google Scholar] [CrossRef]
- Miyamura, H.; Matsubara, R.; Kobayashi, S. Gold–platinum bimetallic clusters for aerobic oxidation of alcohols under ambient conditions. Chem. Commun. 2008, 2031–2033. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.H.; Ikeda, S.; Harada, T.; Morita, Y.; Matsumura, M. An efficient and reusable carbon-supported platinum catalyst for aerobic oxidation of alcohols in water. Chem. Commun. 2008, 3181–3183. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Janjic, N.; Spontoni, P.; Wang, D.; Su, D.S.; Prati, L. Au–Pd/AC as catalysts for alcohol oxidation: Effect of reaction parameters on catalytic activity and selectivity. Appl. Catal. A 2009, 364, 221. [Google Scholar] [CrossRef]
- Marx, S.; Baiker, A. Beneficial interaction of gold and palladium in bimetallic catalysts for the selective oxidation of benzyl alcohol. J. Phys. Chem. C 2009, 113, 6191. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, L.; Zhang, B.; Jin, J.; Su, D.S.; Wang, S.; Sun, G. Controllable Synthesis of Cobalt Monoxide Nanoparticles and the Size-Dependent Activity for Oxygen Reduction Reaction. ACS Catal. 2014, 4, 2998–3001. [Google Scholar] [CrossRef]
- Papaefthimiou, V.; Dintzer, T.; Dupuis, V.; Tamion, A.; Tournus, F.; Hilion, A.; Teschner, D.; Hävecker, M.; Knop-Gericke, A.; Schlögl, R.; et al. Nontrivial Redox Behavior of Nanosized Cobalt: New Insights from Ambient Pressure X-ray Photoelectron and Absorption Spectroscopies. ACS Nano 2011, 5, 2182–2190. [Google Scholar] [CrossRef]
- Khasu, M.; Nyathi, T.; Morgan, D.J.; Hutchings, G.J.; Claeys, M.; Fischer, N. Co3O4 Morphology in the Preferential Oxidation of CO. Catal. Sci. Technol. 2017, 7, 4806–4817. [Google Scholar] [CrossRef]
- Mohseni, N.; Haghighi, M.; Shabani, M. Bimetallic Co2+ Cr3+ LDH Anchored AgCl as Excellent Solar-light-Responsive Ag-Decorated Type II Nano-Heterojunction for Photodegradation of Various Dyes. Process Saf. Environ. Prot. 2022, 168, 668–688. [Google Scholar] [CrossRef]
- Qian, L.; Lu, Z.; Xu, T.; Wu, X.; Tian, Y.; Li, Y.; Huo, Z.; Sun, X.; Duan, X. Electrocatalysts: From water oxidation to reduction: Homologous Ni–Co based nanowires as complementary water splitting electrocatalysts. Adv. Energy Mater. 2015, 5, 1402031. [Google Scholar]
- Xie, Z.H.; Zhou, H.Y.; He, C.S.; Pan, Z.C.; Yao, G.; Lai, B. Synthesis, application and catalytic performance of layered double hydroxide based catalysts in advanced oxidation processes for wastewater decontamination: A review. Chem. Eng. J. 2021, 414, 128713. [Google Scholar] [CrossRef]
- Bahranowski, K.; Bielańska, E.; Janik, R.; Machej, T.; Serwicka, E.M. LDH-derived catalysts for complete oxidation of volatile organic compounds. Clay Miner. 1999, 34, 67–77. [Google Scholar] [CrossRef]
- Scavetta, E.; Berrettoni, M.; Giorgetti, M.; Tonelli, D. Electrochemical characterisation of Ni/Al– X hydrotalcites and their electrocatalytic behaviour. Electrochim. Acta 2002, 47, 2451–2461. [Google Scholar] [CrossRef]
- Alhumaimess, M.S.; Alsohaimi, I.H.; Hassan, H.M.; El-Sayed, M.Y.; Alshammari, M.S.; Aldosari, O.F.; Alshammari, H.M.; Kamel, M.M. Synthesis of ionic liquid intercalated layered double hydroxides of magnesium and aluminum: A greener catalyst of Knoevenagel condensation. J. Saudi Chem. Soc. 2020, 24, 321–333. [Google Scholar] [CrossRef]
- Kamela, M.M.; Alhumaimessa, M.S.; Alotaibib, M.H.; Alsohaimia, I.H.; Hassana, H.M.A.; Alshammarid, H.M.; Aldosari, O.F. Decomposition and removal of hydrazine by Mn/MgAl-layered double hydroxides. Desalination Water Treat. 2020, 205, 242–251. [Google Scholar] [CrossRef]
- Alshammari, H.M.; Humaidi, J.R.; Alhumaimess, M.S.; Aldosari, O.F.; Alotaibi, M.H.; Hassan, H.M.A.; Wawata, I. Bimetallic Au:Pd nanoparticle supported on MgO for the oxidation of benzyl alcohol. React. Kinet. Mech. Catal. 2019, 128, 97–108. [Google Scholar] [CrossRef]
- Goebbert, D.J.; Garand, E.; Wende, T.; Bergmann, R.; Meijer, G.; Asmis, K.R.; Neumark, D.M. Infrared spectroscopy of the microhydrated nitrate ions NO3-(H2O) 1-6. J. Phys. Chem. A 2009, 113, 7584–7592. [Google Scholar] [CrossRef] [PubMed]
- Asiabi, H.; Yamini, Y.; Shamsayei, M. Using cobalt/chromium layered double hydroxide nano-sheets as a novel packed in-tube solid phase microextraction sorbent for facile extraction of acidic pesticides from water samples. New J. Chem. 2018, 42, 9935–9944. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000. [Google Scholar]
- Aisawa, S.; Takahashi, S.; Ogasawara, W.; Umetsu, Y.; Narita, E. Direct intercalation of amino acids into layered double hydroxides by coprecipitation. J. Solid State Chem. 2001, 162, 52–62. [Google Scholar] [CrossRef]
- Music, S.; Maljkovic, M.; Popovic, S.; Trojko, R. Formation of chromia from amorphous chromium hydroxide, Croat. Chem. Acta 1999, 72, 789–802. [Google Scholar]
- Lidin, R.A.; Molochko, V.A.; Andreeva, L.L. Chemical Properties of Inorganic Substances; Khimiya: Moscow, Russia, 2000. [Google Scholar]
- Sranko, D.; Pallagi, A.; Kuzmann, E.; Canton, S.E.; Walczak, M.; Sapi, A.; Kukovecz, A.; Sipos, P.; Palinko, I. Synthesis and properties of novel Ba(II)Fe(III) layered double hydroxides. Appl. Clay Sci. 2010, 48, 214–217. [Google Scholar] [CrossRef]
- Cotton, F.A.; Wilkinson, G. Advanced Inorganic Chemistry, 5th ed.; Wiley Interscience: New York, NY, USA, 1988. [Google Scholar]
- Miao, M.Y.; Feng, J.T.; Jin, Q.; He, Y.F.; Liu, Y.N.; Du, Y.Y.; Zhang, N.; Li, D.Q. Hybrid Ni–Al layered double hydroxide/graphene composite supported gold nanoparticles for aerobic selective oxidation of benzyl alcohol. RSC Adv. 2015, 5, 36066–36074. [Google Scholar] [CrossRef]
- Ding, X.; Liang, Y.; Zhang, H.; Zhao, M.; Wang, J.; Chen, Y. Preparation of Reduced Pt-Based Catalysts with High Dispersion and Their Catalytic Performances for NO Oxidation. Acta Phys.-Chim. Sin. 2022, 3, 2005009. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, Y.; Li, J.; Liu, Z.; Shi, J.; Fan, L.; Cai, W. Surface-neutralization engineered NiCo-LDH/phosphate hetero-sheets toward robust oxygen evolution reaction. Green Energy Environ. 2022, in press. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Sun, Z.; Yuan, T.Q. Recent advances in the catalytic upgrading of biomass platform chemicals via hydrotalcite-derived metal catalysts. Trans. Tianjin Univ. 2022, 28, 89–111. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Q.; Liu, X.; Wu, L.; Hu, H.; Zhao, Y. One-step mechanochemical synthesis of plasmonic Ag/Zn–Al LDH with excellent photocatalytic activity. J. Mater. Sci. 2018, 53, 12795–12806. [Google Scholar] [CrossRef]
- Levitskaia, T.G.; Chatterjee, S.; Arey, B.W.; Campbell, E.L.; Hong, Y.; Kovarik, L.; Peterson, J.M.; Pence, N.K.; Romero, J.; Shutthanandan, V.; et al. RedOx-controlled sorption of iodine anions by hydrotalcite composites. RSC Adv. 2016, 6, 76042–76055. [Google Scholar] [CrossRef]
- Alhumaimess, M.S.; Alsohaimi, I.H.; Alshammari, H.M.; Aldosari, O.F.; Hassan, H. Synthesis of gold and palladium nanoparticles supported on CuO/rGO using imidazolium ionic liquid for CO oxidation. Res. Chem. Intermed. 2020, 46, 5499–5516. [Google Scholar] [CrossRef]
- Tan, H.T.; Chen, Y.; Zhou, C.; Jia, X.; Zhu, J.; Chen, J.; Rui, X.; Yan, Q.; Yang, Y. Palladium nanoparticles supported on manganese oxide–CNT composites for solvent-free aerobic oxidation of alcohols: Tuning the properties of Pd active sites using MnOx. Appl. Catal B 2012, 119, 166–174. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, H.; Guo, Z.; Zhou, C.; Wang, C.; Borgna, A.; Yang, Y. Pd catalysts supported on MnCeOx mixed oxides and their catalytic application in solvent-free aerobic oxidation of benzyl alcohol: Support composition and structure sensitivity. J. Catal. 2011, 283, 34–44. [Google Scholar] [CrossRef]
- Miedziak, P.; Sankar, M.; Dimitratos, N.; Lopez-Sanchez, J.A.; Carley, A.F.; Knight, D.W.; Taylor, S.H.; Kiely, C.J.; Hutchings, G.J. Oxidation of benzyl alcohol using supported gold–palladium nanoparticles. Catal. Today 2011, 164, 315–319. [Google Scholar] [CrossRef]
- Sun, J.; Han, Y.; Fu, H.; Qu, X.; Xu, Z.; Zheng, S. Au@ Pd/TiO2 with atomically dispersed Pd as highly active catalyst for solvent-free aerobic oxidation of benzyl alcohol. Chem. Eng. J. 2017, 313, 1–9. [Google Scholar] [CrossRef]


| Catalyst | Pd (wt.%) | Cu (wt.%) | ||
|---|---|---|---|---|
| Calculated | Actual | Calculated | Actual | |
| Co-Cr LDH | - | - | - | - |
| Cu@Co-Cr LDH | - | - | 1.0 | 0.97 |
| Pd@Co-Cr LDH | 1.0 | 0.98 | - | - |
| Pd-Cu@Co-Cr LDH | 0.5 | 0.49 | 0.5 | 0.48 |
| Catalysts | d(003)/(nm) | d(006)/ (nm) | d(110)/ (nm) | Lattice Parameter (nm) | Particle Size (nm) | |
|---|---|---|---|---|---|---|
| a = 2d110 | c = 3d003 | |||||
| Co-Cr LDH | 0.7472 | 0.3846 | 0.1551 | 0.3102 | 2.2416 | 13.3 |
| Cu@Co-Cr LDH | 0.7951 | 0.3955 | 0.1553 | 0.3106 | 2.3853 | 13.1 |
| Pd@Co-Cr LDH | 0.8185 | 0.4211 | 0.1591 | 0.3182 | 2.4555 | 11.5 |
| Pd-Cu@Co-Cr LDH | 0.8621 | 0.4511 | 0.1611 | 0.3222 | 2.5863 | 10.4 |
| Catalysts | SBET a/m2 g−1 | DBJH b/nm | VBJH c/cm3 g−1 |
|---|---|---|---|
| Co-Cr LDH | 22.4 | 3.2 | 0.1232 |
| Cu@Co-Cr LDH | 30.1 | 3.9 | 0.1367 |
| Pd@Co-Cr LDH | 32.2 | 4.9 | 0.1389 |
| Pd-Cu@Co-Cr LDH | 37.8 | 5.5 | 0.1397 |
| Catalyst | Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|
| Benzaldehyde | Toluene | Benzene | Benzyl Benzoate | ||
| Co-Cr LDH | 0.7 | 87 | 2.5 | 1 | 0.5 |
| Cu@Co-Cr LDH | 0.22 | 96 | 8 | 4 | 1 |
| Pd@Co-Cr LDH | 2.7 | 99 | 1 | - | - |
| Pd-Cu@Co-Cr LDH | 16 | 99.5 | 0.5 | - | - |
| Catalyst | Solvent | T (K) | Time (h) | Conversion (%) | Ref. |
|---|---|---|---|---|---|
| Pd/MnOx-CNT | Solvent of free | 433 | 1 | 15.4 | [56] |
| Pd/MnCeOx | Solvent of free | 433 | 1 | 18.4 | [57] |
| Pd-Au/TiO25Im | Solvent of free | 373 | 4 | 15 | [58] |
| Au@Pd/TiO2 | Solvent of free | 363 | 4 | 14.3 | [59] |
| Pd-Cu@Co-Cr LDH | Solvent of free | 393 | 4 | 16% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzarea, L.A.; Alhumaimess, M.S.; Alsohaimi, I.H.; Hassan, H.M.A.; El-Aassar, M.R.; Essawy, A.A.; Kalil, H. Efficient Dual-Function Catalyst: Palladium–Copper Nanoparticles Immobilized on Co-Cr LDH for Seamless Aerobic Oxidation of Benzyl Alcohol and Nitrobenzene Reduction. Nanomaterials 2023, 13, 1956. https://doi.org/10.3390/nano13131956
Alzarea LA, Alhumaimess MS, Alsohaimi IH, Hassan HMA, El-Aassar MR, Essawy AA, Kalil H. Efficient Dual-Function Catalyst: Palladium–Copper Nanoparticles Immobilized on Co-Cr LDH for Seamless Aerobic Oxidation of Benzyl Alcohol and Nitrobenzene Reduction. Nanomaterials. 2023; 13(13):1956. https://doi.org/10.3390/nano13131956
Chicago/Turabian StyleAlzarea, Linah A., Mosaed S. Alhumaimess, Ibrahim Hotan Alsohaimi, Hassan M. A. Hassan, M. R. El-Aassar, Amr A. Essawy, and Haitham Kalil. 2023. "Efficient Dual-Function Catalyst: Palladium–Copper Nanoparticles Immobilized on Co-Cr LDH for Seamless Aerobic Oxidation of Benzyl Alcohol and Nitrobenzene Reduction" Nanomaterials 13, no. 13: 1956. https://doi.org/10.3390/nano13131956
APA StyleAlzarea, L. A., Alhumaimess, M. S., Alsohaimi, I. H., Hassan, H. M. A., El-Aassar, M. R., Essawy, A. A., & Kalil, H. (2023). Efficient Dual-Function Catalyst: Palladium–Copper Nanoparticles Immobilized on Co-Cr LDH for Seamless Aerobic Oxidation of Benzyl Alcohol and Nitrobenzene Reduction. Nanomaterials, 13(13), 1956. https://doi.org/10.3390/nano13131956











