Barium Titanate/Gadolinium Ferrite: A New Material Composite to Store Energy
Abstract
:1. Introduction
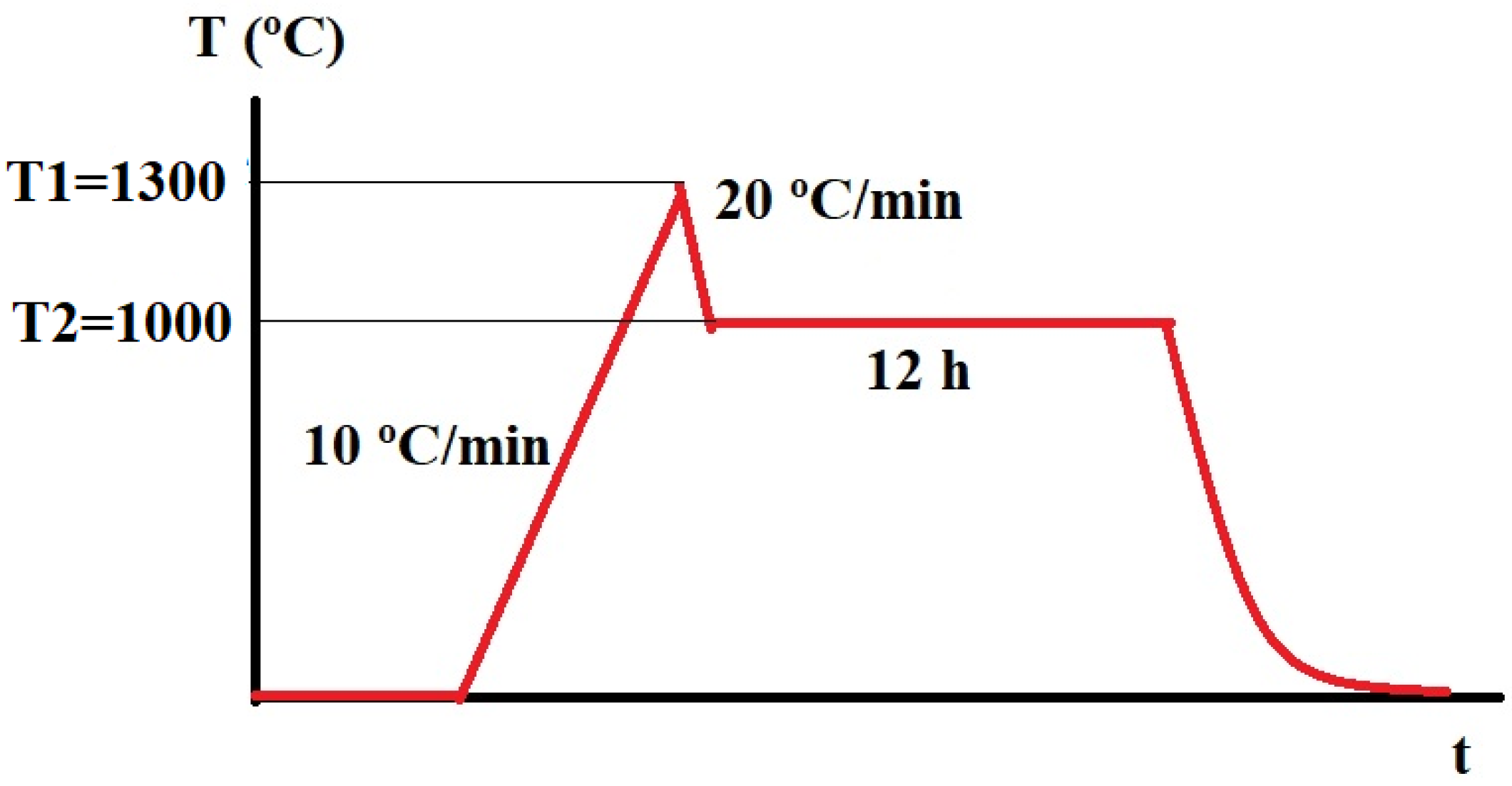
2. Materials and Methods
3. Results
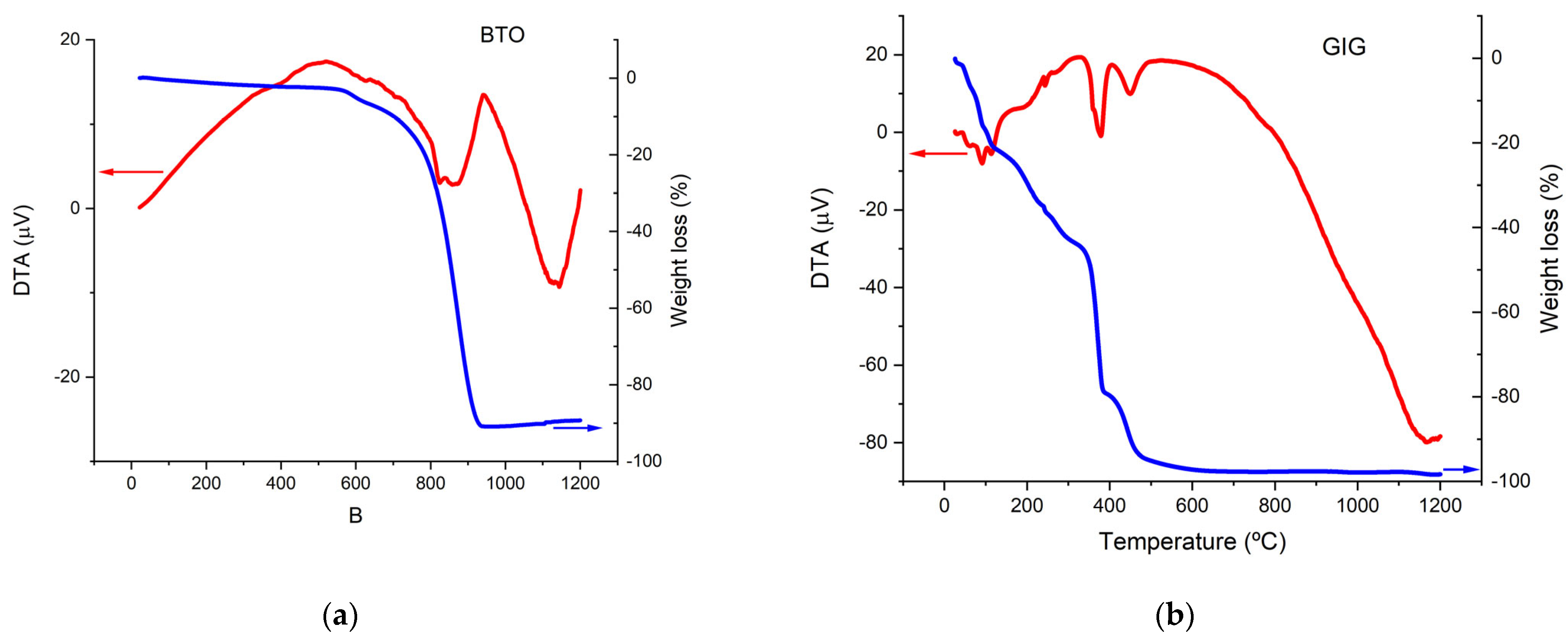
3.1. Thermo Analysis
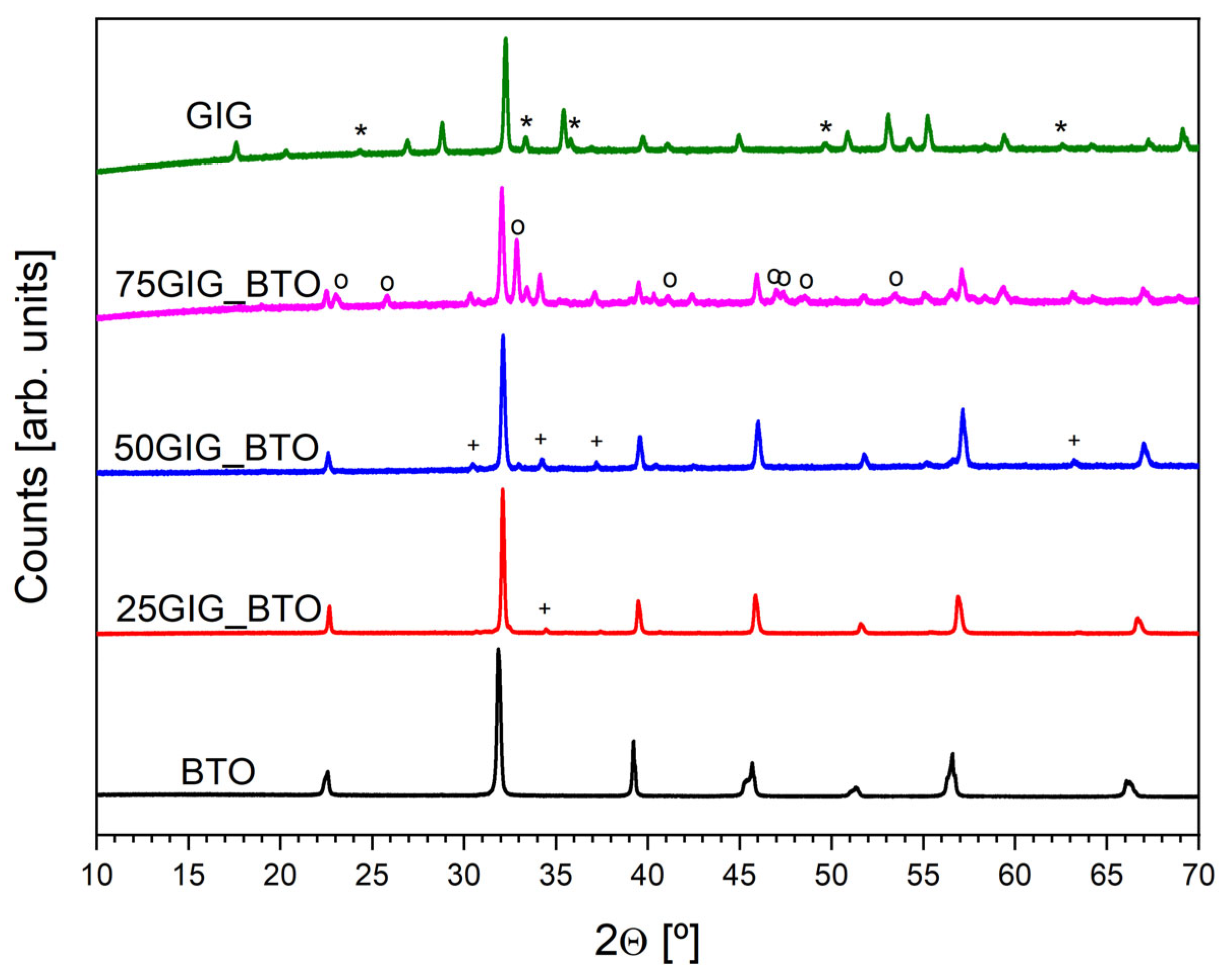
3.2. Structural Analysis
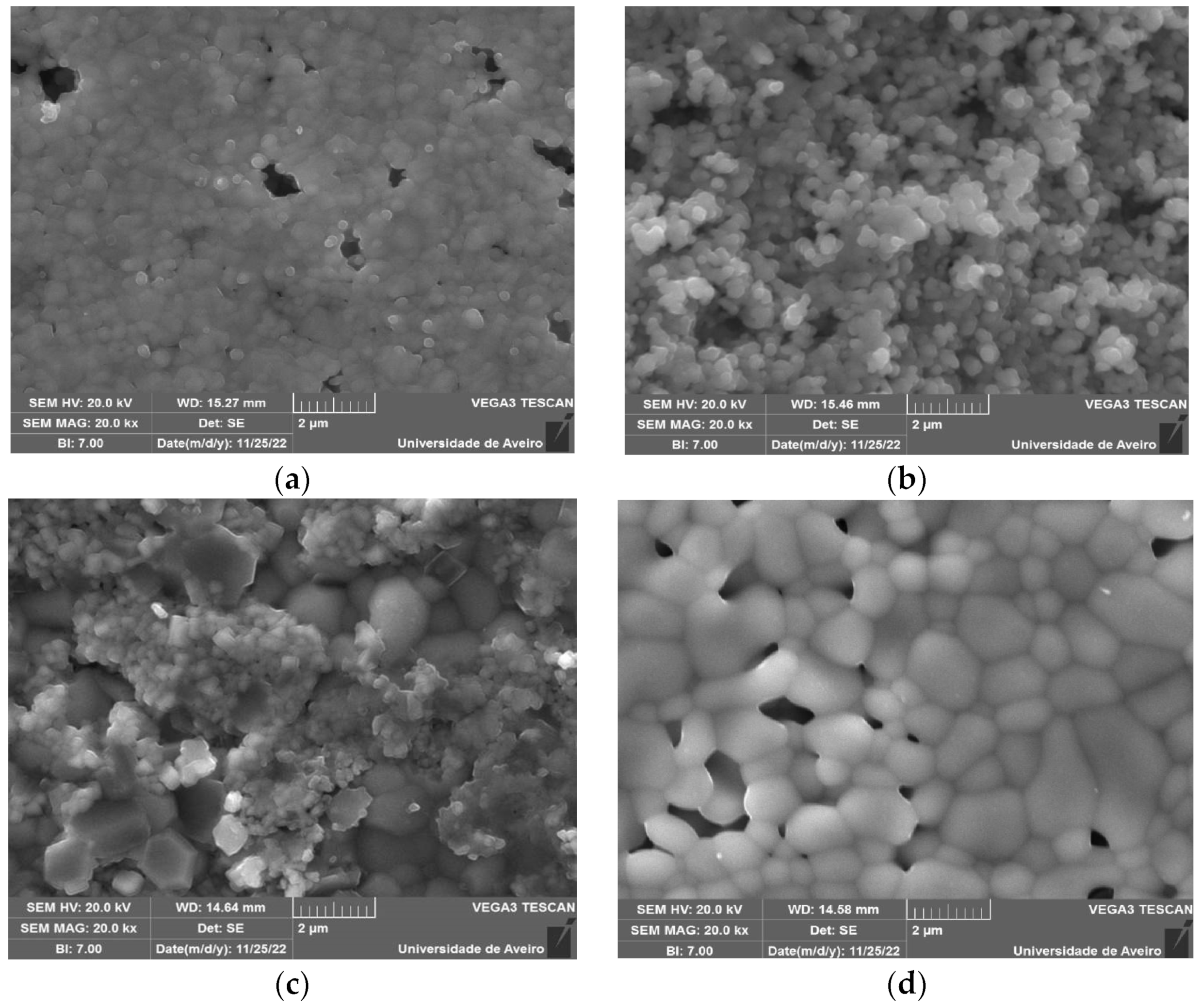
3.3. Morphological Analysis
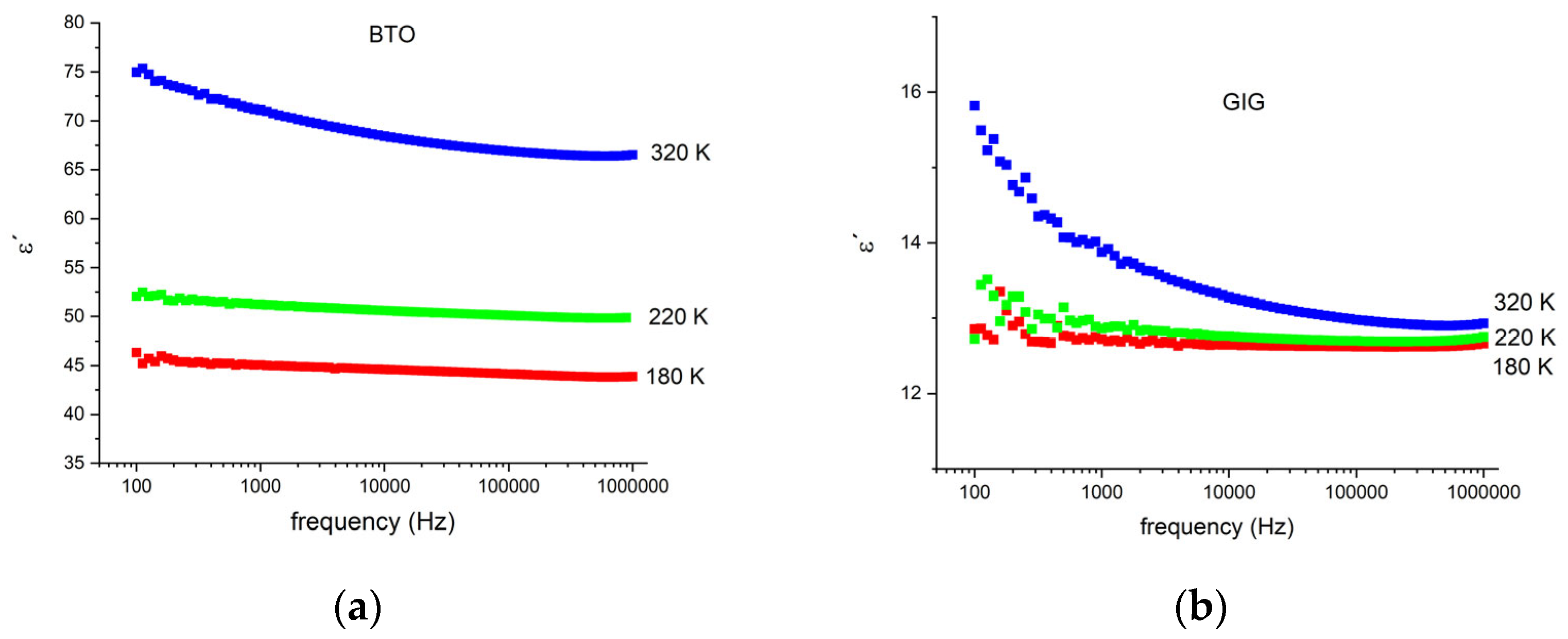
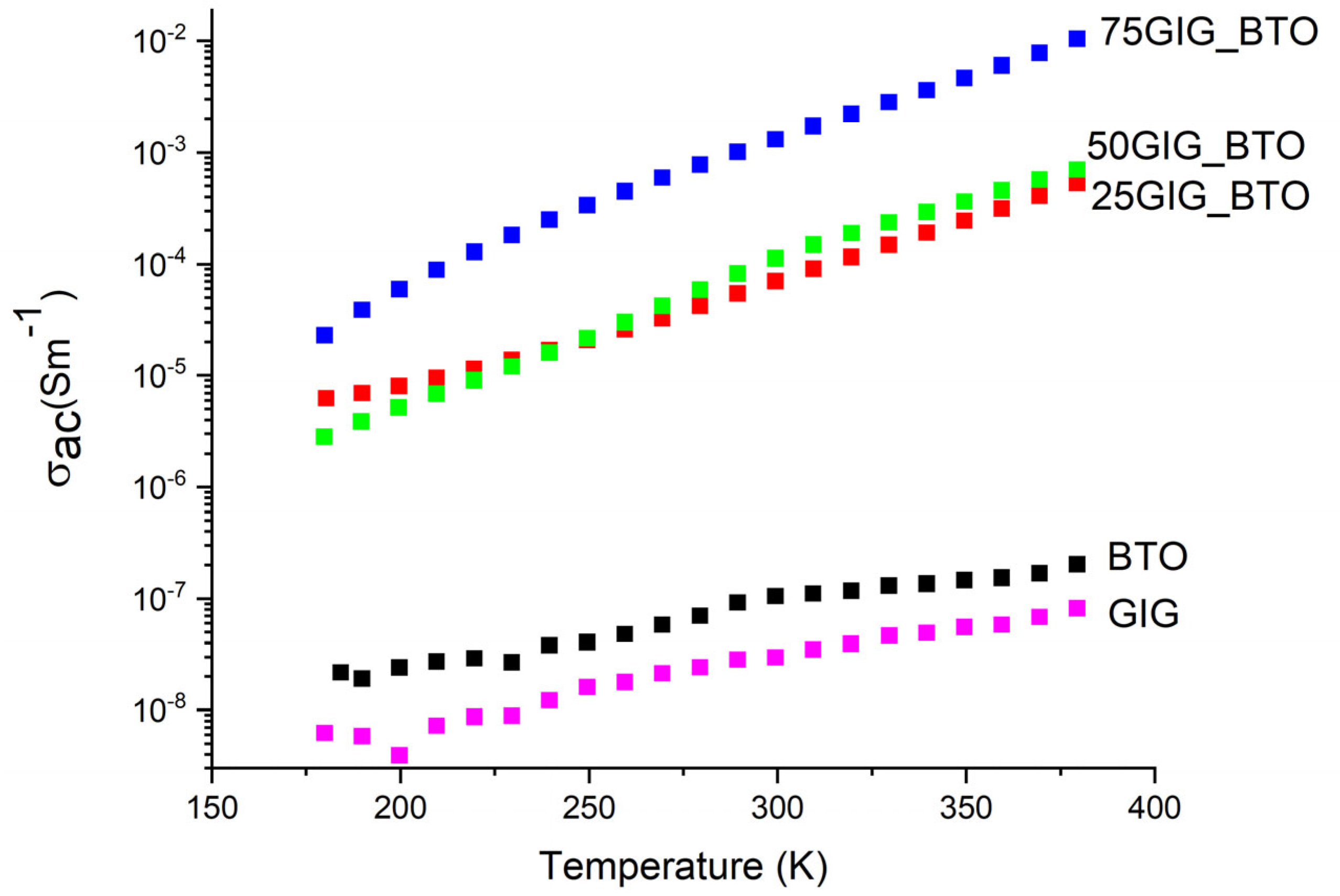
3.4. Electrical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ogihara, H.; Randall, C.A.; Trolier-McKinstry, S. Weakly coupled relaxor behavior of BaTiO3-BiScO3. J. Am. Ceram. Soc. 2009, 92, 110–118. [Google Scholar] [CrossRef]
- Wang, T.; Jin, L.; Hu, Q.; Wei, X. Relaxor Ferroelectric BaTiO3–Bi(Mg2/3Nb1/3)O3 Ceramics for Energy Storage Application. J. Am. Ceram. Soc. 2015, 98, 559–566. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, M.; Chen, Y.; Luo, Z.; Zhao, P.; Su, H.; Wang, J.; Wang, H.; Yang, L.; Pan, H.; et al. High-entropy assisted BaTiO3-based ceramic capacitors for energy storage. Cell Rep. Phys. Sci. 2022, 3, 10111. [Google Scholar] [CrossRef]
- Hu, J.-M.; Cheng, L.-Q.; Nan, C.-W. Multiferroic Heterostructures Integrating Ferroelectric and Magnetic Materials. Adv. Mater. 2016, 28, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Soreto Teixeira, S.; Sales, A.J.M.; Graça, M.P.F.; Costa, L.C. Yttrium ferrites with enhanced dielectric properties. Mater. Sci. Eng. B 2018, 232–235, 41–47. [Google Scholar] [CrossRef]
- Saini, A.; Sharma, A.; Sharma, M.; Kuanr, B.K. Yttrium iron garnet (YIG)/barium titanate (BTO) an engineered multiferroic nanocomposite. J. Alloy. Compd. 2021, 879, 160422. [Google Scholar] [CrossRef]
- Paiva, D.V.M.; Silva, M.A.S.; Ribeiro, T.S.; Vasconcelos, I.F.; Sombra, A.S.B.; Góes, J.C.; Fechine, P.B.A. Novel magnetic–dielectric composite ceramic obtained from Y3Fe5O12 and CaTiO3. J. Alloy. Compd. 2015, 644, 763–769. [Google Scholar] [CrossRef]
- Kulkarni, D.D.; Joshi, V.R.; Shetty, S.N.; Patel, S.B.; Khalfe, M.S. Comparative Study between Co-Precipitation and Sol Gel Method for the Preparation of Ferrites. Int. J. Adv. Res. Sci. Com. Techn. 2022, 2, 4. [Google Scholar]
- Trukhanov, S.V.; Troyanchuk, I.O.; Hervieu, M.; Szymczak, H.; Bärner, K. Magnetic and electrical properties of LBaMn2O6-γ (L = Pr, Nd, Sm, Eu, Gd, Tb) manganites. Phys. Rev. B 2002, 66, 184424. [Google Scholar] [CrossRef]
- Zhumatayeva, I.Z.; Kenzhina, I.E.; Kozlovskiy, A.L.; Zdorovets, M.V. The study of the prospects for the use of Li0.15Sr0.85TiO3 ceramics. J. Mater. Sci. Mater. Electron. 2020, 31, 6764–6772. [Google Scholar] [CrossRef]
- Shafiee, F.N.; Mustaffa, M.S.; Abdullah, N.H.; Hamidon, M.N.; Ismail, I.; Nazlan, R.; Ibrahim, I.R.; Idris, F.M.; Shafie, M.S.E. Effect of microstructural evolution from nano to micron grain size regime towards structural, magnetic, electrical and microwave properties of gadolinium iron garnet (Gd3Fe5O12). J. Mater. Sci. Mater. Electron. 2021, 32, 10160–10179. [Google Scholar] [CrossRef]
- Soreto Teixeira, S.; Amaral, F.; Graça, M.P.F.; Costa, L.C. Comparison of lithium ferrite powders prepared by sol-gel and solid state reaction methods. Mater. Sci. Eng. B 2020, 255, 114529. [Google Scholar] [CrossRef]
- Graça, M.P.F.; Costa, L.C.; Amaral, F.; Valente, M.A.; Barcellos, W.M.; Freire, F.N.A.; Sabóia, K.D.A.; Sombra, A.S.B. Dielectric and magnetic properties of a yttrium ferrite/calcium copper titanate composite. Spectrosc. Lett. 2017, 50, 206–213. [Google Scholar] [CrossRef]
- Ciomaga, C.E.; Neagu, A.M.; Pop, M.V.; Airimioaei, M.; Tascu, S.; Schileo, G.; Galassi, C.; Mitoseriu, L. Ferroelectric and dielectric properties of ferrite-ferroelectric ceramic composites. J. Appl. Phys. 2013, 113, 074103. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Turchenko, V.A.; Kostishin, V.G.; Panina, L.V.; Kazakevich, I.S.; Balagurov, A.M. Crystal structure and magnetic properties of the BaFe12-xInxO19 (x=0.1-1.2) solid solutions. J. Magn. Magn. Mater. 2016, 417, 130–136. [Google Scholar] [CrossRef]
- Zdorovets, M.V.; Kozlovskiy, A.L.; Shlimas, D.I.; Borgekov, D.B. Phase transformations in FeCo—Fe2CoO4/Co3O4-spinel nanostructures as a result of thermal annealing and their practical application. J. Mater. Sci. Mater. Electron. 2021, 32, 16694–16705. [Google Scholar] [CrossRef]
- Lóh, N.J.; Simão, L.; Faller, C.A.; De Noni, A.; Montedo, O.R.K. A review of two-step sintering for ceramics. Ceram. Int. 2016, 42, 12556–12572. [Google Scholar] [CrossRef]
- Kadyrzhanov, K.K.; Shlimas, D.I.; Kozlovskiy, A.L.; Zdorovets, M.V. Research of the shielding effect and radiation resistance of composite CuBi2O4 films as well as their practical applications. J. Mater. Sci. Mater. Electron. 2020, 31, 11729–11740. [Google Scholar] [CrossRef]
- Semaida, A.M.; Darwish, M.A.; Salem, M.M.; Zhou, D.; Zubar, T.I.; Trukhanov, S.V.; Trukhanov, A.V.; Menushenkov, V.P.; Savchenko, A.G. Impact of Nd3+ substitutions on the structure and magnetic properties of nanostructured SrFe12O19 hexaferrite. Nanomaterials 2022, 12, 3452. [Google Scholar] [CrossRef]
- Melnikov, P.; Nascimento, V.A.; Consolo, L.Z.Z.; Silva, A.F. Mechanism of thermal decomposition of yttrium nitrate hexahydrate, Y(NO3)36H2O and modeling of intermediate oxynitrates. J. Therm. Anal. Calor. 2013, 111, 115–119. [Google Scholar] [CrossRef]
- Grosseau, P. Synthèse et Étude du Grenat de fer et D’yttrium. PhD Thesis, Génie des Proceeds, Ecole Nationale Supérieure des Mines de Saint-Etienne, Saint-Étienne, France, 1993. [Google Scholar]
- Naghib-zadeh, H.; Glitzk, C.; Dörfel, I.; Rabe, T. Low temperature sintering of barium titanate ceramics assisted by addition of lithium fluoride-containing sintering additives. J. Eur. Ceram. Soc. 2010, 30, 81–86. [Google Scholar] [CrossRef]
- Hsiang, H.I.; His, C.S.; Huang, C.C.; Fu, S.L. Low temperature sintering and dielectric properties of BaTiO3 with glass addition. Mater. Chem. Phys. 2009, 113, 658–663. [Google Scholar] [CrossRef]
- Macdonald, J.R.; Johnson, W.B. Fundamentals of impedance spectroscopy. In Impedance Spectroscopy, Theory, Experiment and Applications, 3rd ed.; Macdonald, B.E., Ed.; Wiley: Hoboken, NJ, USA, 2018; pp. 195–200. [Google Scholar]
- Table of Dielectric Constant of Substances; Yamamodo Electric Industrial Co., Ltd.: Tainan, Taiwan, 2007.
- Kozlovskiy, A.L.; Zdorovets, M.V. Effect of doping of Ce4+/3+ on optical, strength and shielding properties of (0.5-x)TeO2-0.25MoO-0.25Bi2O3-xCeO2 glasses. Mater. Chem. Phys. 2021, 263, 124444. [Google Scholar] [CrossRef]
- Khan, S.A.; Ali, I.; Hussain, A.; Javed, H.M.A.; Turchenko, V.A.; Trukhanov, A.V.; Trukhanov, S.V. Synthesis and characterization of composites with Y-hexaferrites for electromagnetic interference shielding applications. Magnetochemistry 2022, 8, 186. [Google Scholar] [CrossRef]
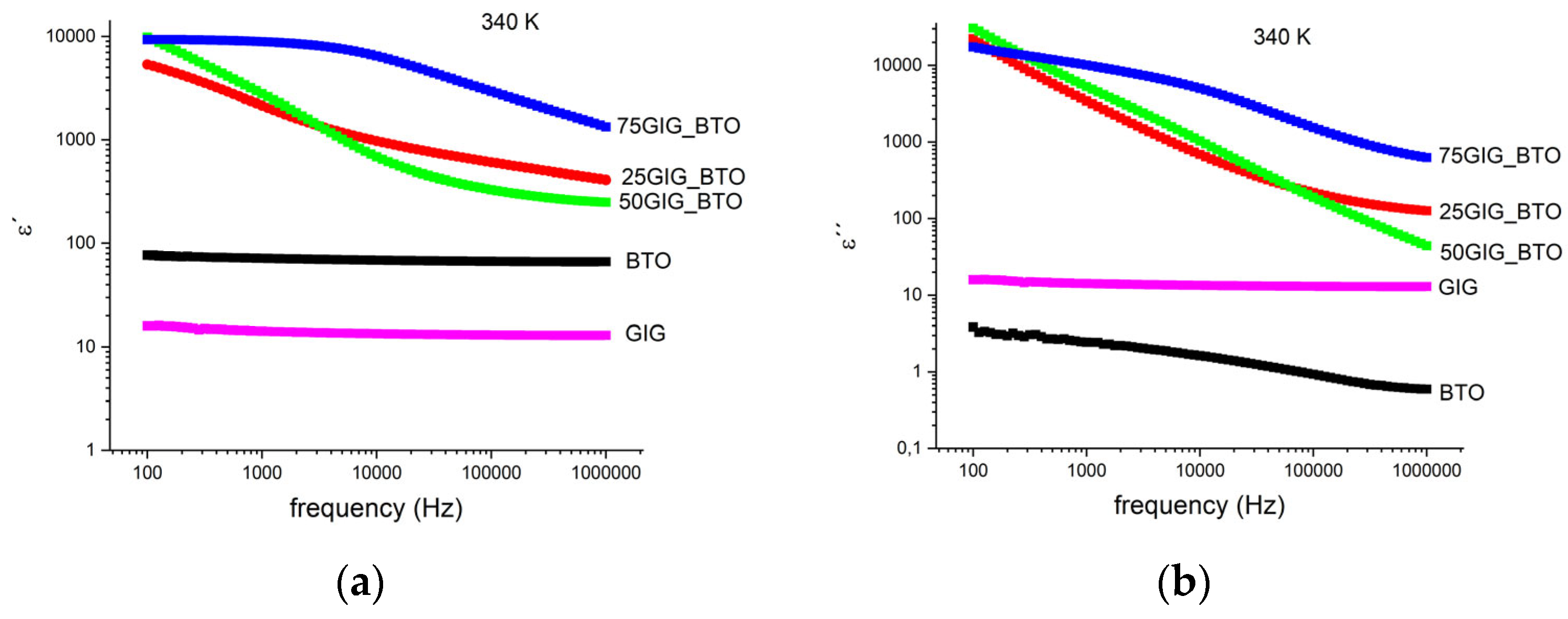

| Sample | ε′ | ε″ |
|---|---|---|
| BTO | 70 | 1.9 |
| 25GIG_BTO | 1380 | 1270 |
| 50GIG_BTO | 1100 | 2020 |
| 75GIG_BTO | 6800 | 23,700 |
| GIG | 14 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baivier, C.; Hammami, I.; Benzerga, R.; Graça, M.P.F.; Costa, L.C. Barium Titanate/Gadolinium Ferrite: A New Material Composite to Store Energy. Nanomaterials 2023, 13, 1955. https://doi.org/10.3390/nano13131955
Baivier C, Hammami I, Benzerga R, Graça MPF, Costa LC. Barium Titanate/Gadolinium Ferrite: A New Material Composite to Store Energy. Nanomaterials. 2023; 13(13):1955. https://doi.org/10.3390/nano13131955
Chicago/Turabian StyleBaivier, Clara, Imen Hammami, Ratiba Benzerga, Manuel P. F. Graça, and Luís C. Costa. 2023. "Barium Titanate/Gadolinium Ferrite: A New Material Composite to Store Energy" Nanomaterials 13, no. 13: 1955. https://doi.org/10.3390/nano13131955
APA StyleBaivier, C., Hammami, I., Benzerga, R., Graça, M. P. F., & Costa, L. C. (2023). Barium Titanate/Gadolinium Ferrite: A New Material Composite to Store Energy. Nanomaterials, 13(13), 1955. https://doi.org/10.3390/nano13131955








