Combustion Synthesis of Magnetic Nanomaterials for Biomedical Applications
Abstract
:1. Introduction
2. Solution Combustion Synthesis: Fundamentals
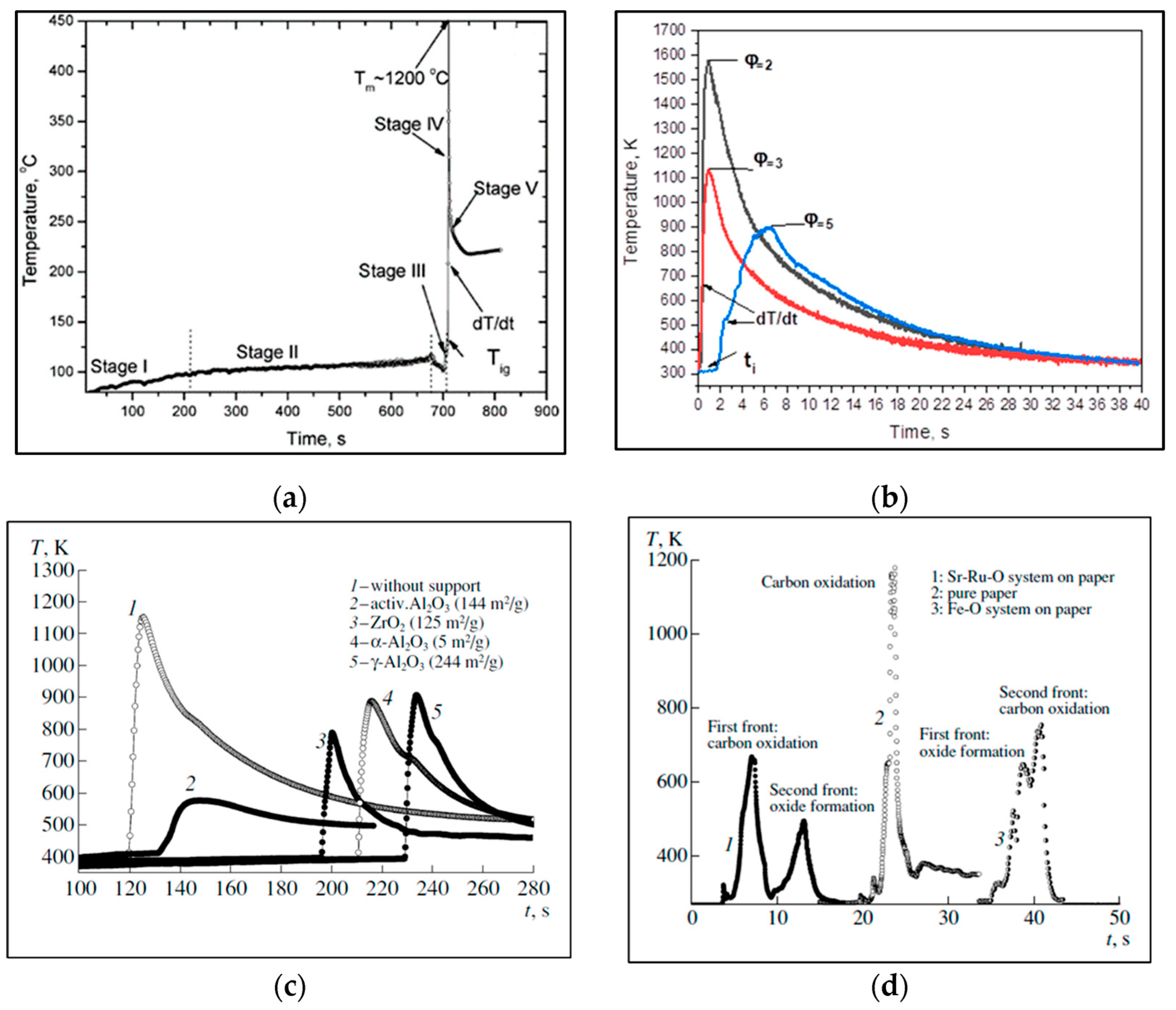
3. Solution Combustion Synthesis of Magnetic Compounds
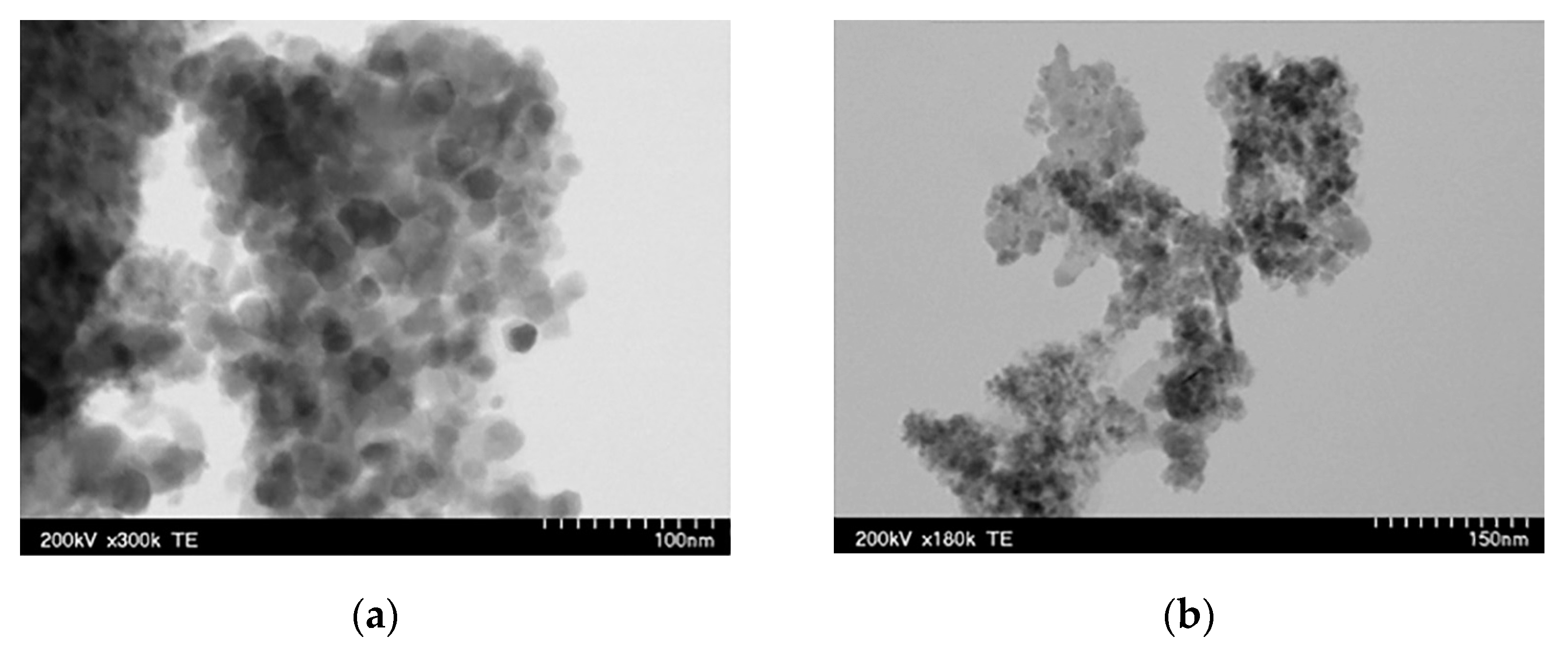
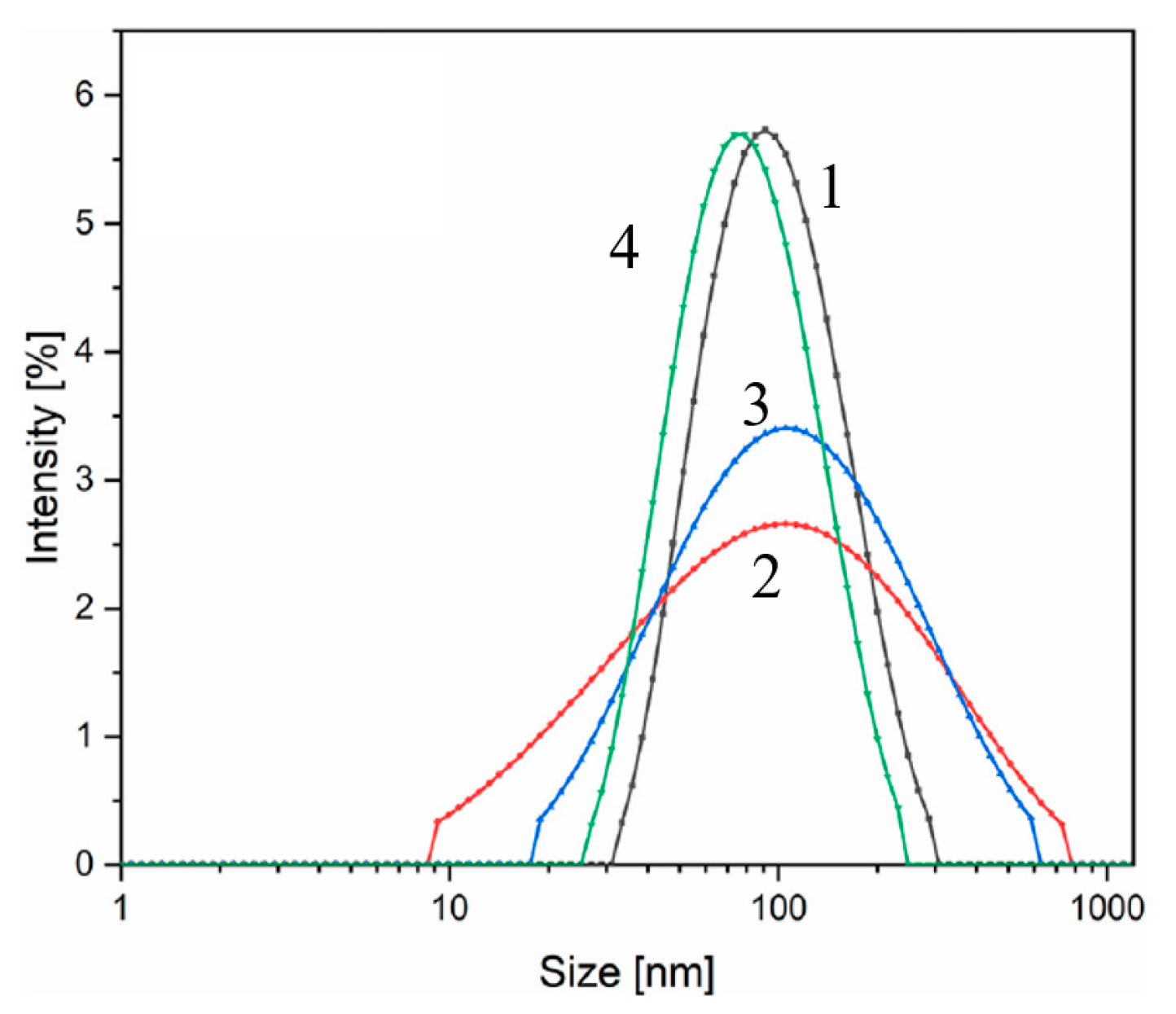
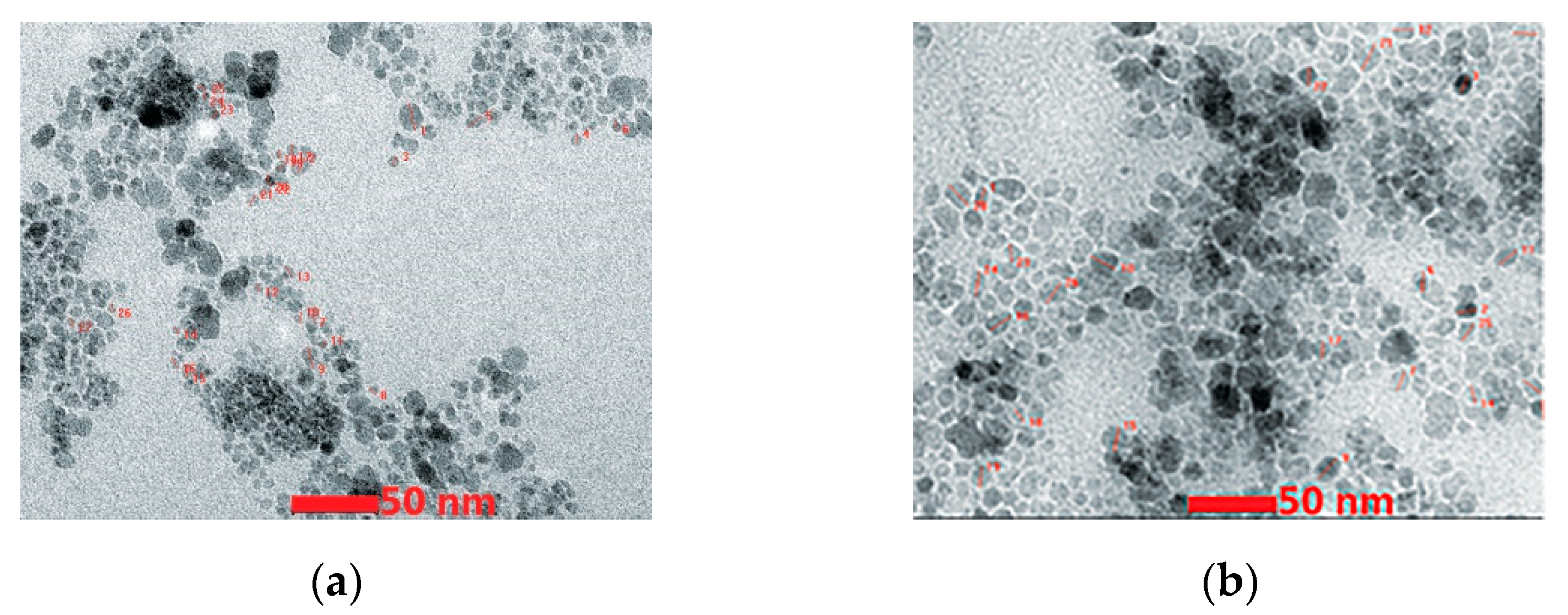
3.1. Iron-Oxide-Based Materials
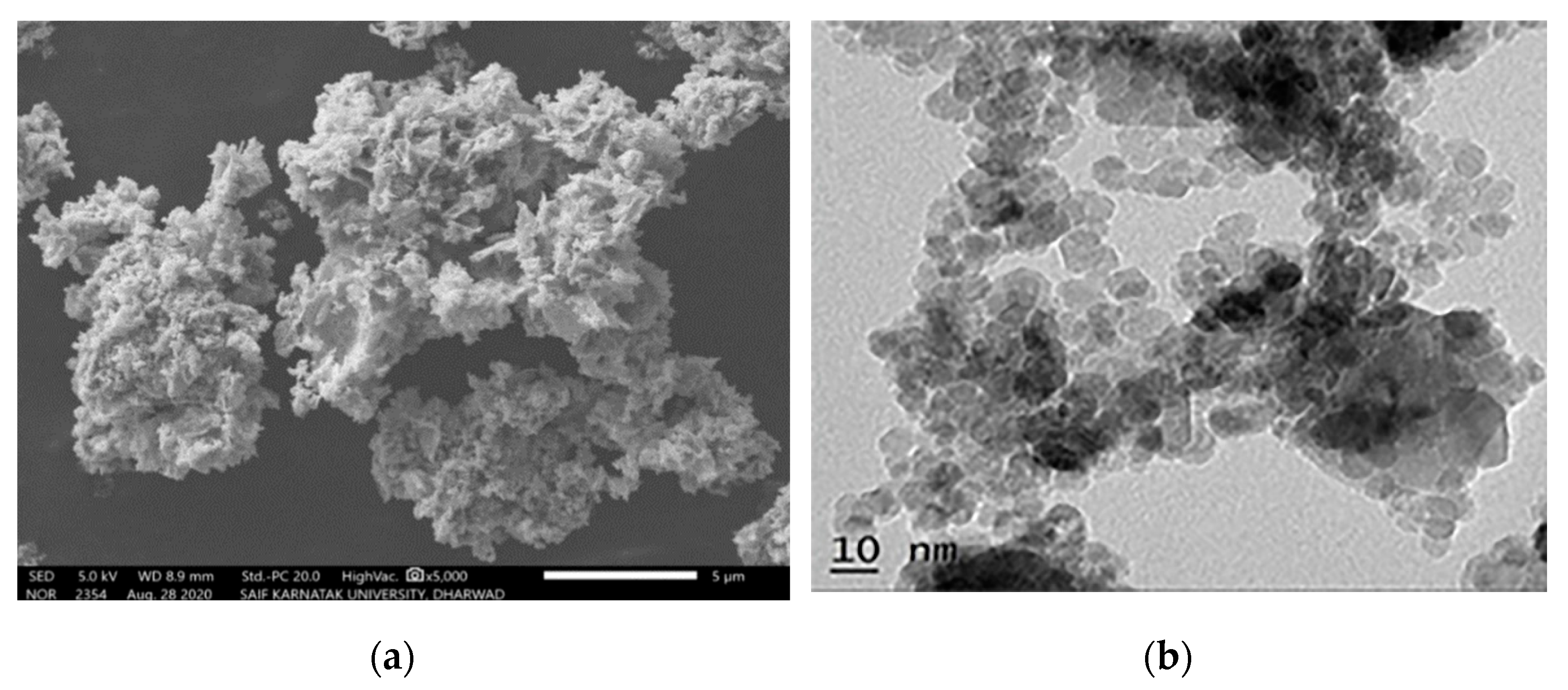
3.2. Nickel Oxide and Ni-Based Alloys
3.3. Complex Oxides
3.4. Zinc Oxide
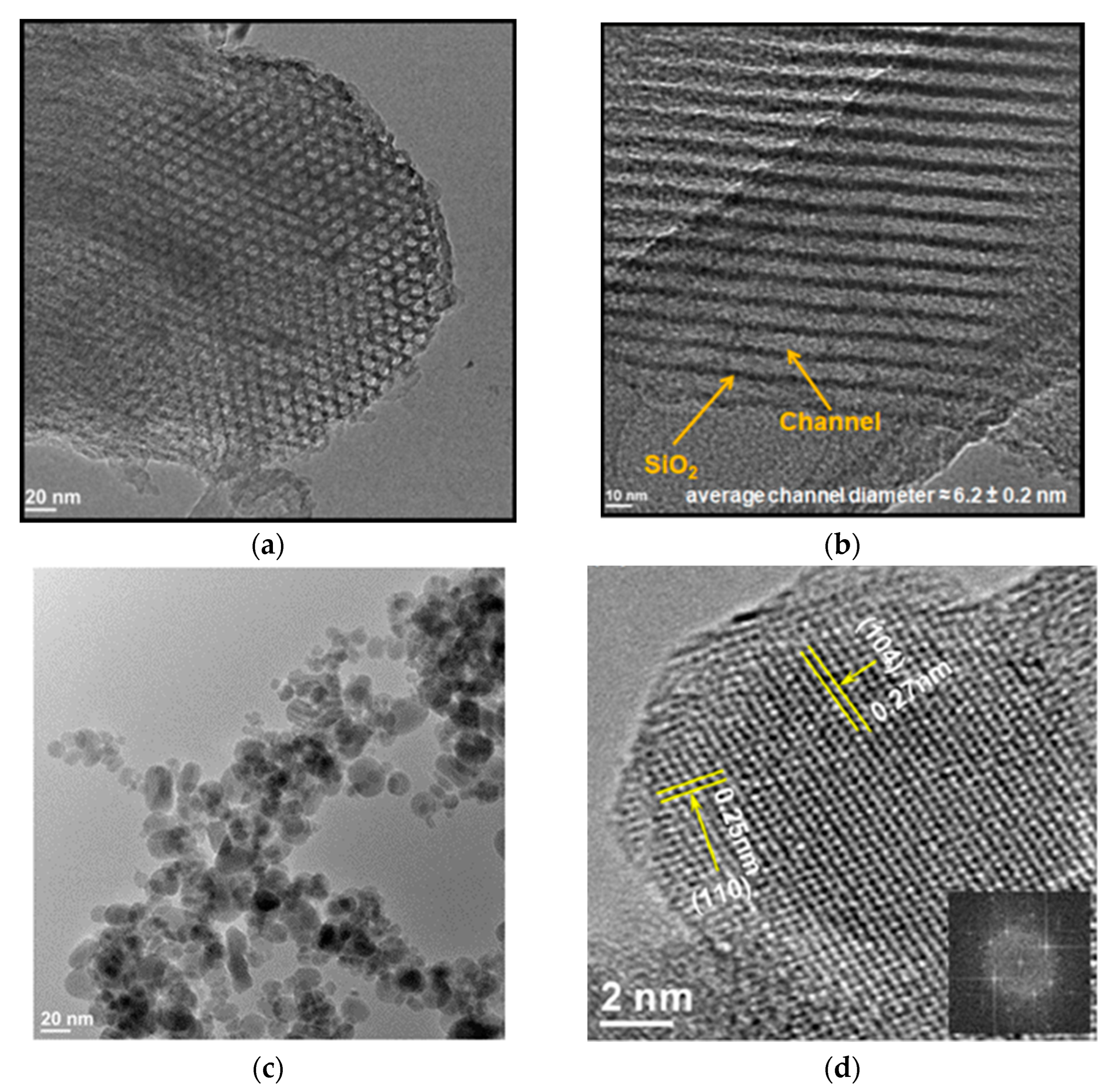
3.5. Calcium-Based Compounds
4. Challenges and Tasks
Funding
Data Availability Statement
Conflicts of Interest
References
- Materón, E.M.; Miyazaki, C.M.; Carr, O.; Joshi, N.; Picciani, P.H.S.; Dalmaschio, C.J.; Davis, F.; Shimizu, F.M. Magnetic Nanoparticles in Biomedical Applications: A Review. Appl. Surf. Sci. Adv. 2021, 6, 100163. [Google Scholar] [CrossRef]
- Binandeh, M. Performance of Unique Magnetic Nanoparticles in Biomedicine. EJMECH Rep. 2022, 6, 100072. [Google Scholar] [CrossRef]
- Khan, A.U.; Chen, L.; Ge, G. Recent Development for Biomedical Applications of Magnetic Nanoparticles. Inorg. Chem. Commun. 2021, 134, 108995. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.G.M.; Martín, M.J.; Otarola, J.; Vakarelska, E.; Simeonov, V.; Lassalle, V.; Nedyalkova, M. Biomedical Applications of Iron Oxide Nanoparticles: Current Insights Progress and Perspectives. Pharmaceutics 2022, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Yu, J.; Gao, S. Synthesis and Biomedical Applications of Magnetic Nanomaterials; EDP Sciences: Les Ulis, France, 2022; ISBN 978-2-7598-2715-2. [Google Scholar]
- Aram, E.; Moeni, M.; Abedizadeh, R.; Sabour, D.; Sadeghi-Abandansari, H.; Gardy, J.; Hassanpour, A. Smart and Multi-Functional Magnetic Nanoparticles for Cancer Treatment Applications: Clinical Challenges and Future Prospects. Nanomaterials 2022, 12, 3567. [Google Scholar] [CrossRef] [PubMed]
- Prijic, S.; Sersa, G. Magnetic Nanoparticles as Targeted Delivery Systems in Oncology. Radiol. Oncol. 2011, 45, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mody, V.V.; Cox, A.; Shah, S.; Singh, A.; Bevins, W.; Parihar, H. Magnetic Nanoparticle Drug Delivery Systems for Targeting Tumor. Appl. Nanosci. 2014, 4, 385–392. [Google Scholar] [CrossRef] [Green Version]
- Vangijzegem, T.; Stanicki, D.; Laurent, S. Magnetic Iron Oxide Nanoparticles for Drug Delivery: Applications and Characteristics. Expert Opin. Drug Deliv. 2019, 16, 69–78. [Google Scholar] [CrossRef]
- Anderson, S.D.; Gwenin, V.V.; Gwenin, C.D. Magnetic Functionalized Nanoparticles for Biomedical, Drug Delivery and Imaging Applications. Nanoscale Res. Lett. 2019, 14, 188. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron Oxide Based Nanoparticles for Multimodal Imaging and Magnetoresponsive Therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef]
- Luengo Morato, Y.; Marciello, M.; Lozano Chamizo, L.; Ovejero Paredes, K.; Filice, M. Hybrid Magnetic Nanoparticles for Multimodal Molecular Imaging of Cancer. In Magnetic Nanoparticle-Based Hybrid Materials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 343–386. ISBN 978-0-12-823688-8. [Google Scholar] [CrossRef]
- Alphandéry, E. Iron Oxide Nanoparticles as Multimodal Imaging Tools. RSC Adv. 2019, 9, 40577–40587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, B.P.; Cawthorne, C.; Archibald, S.J. Multimodal Nanoparticle Imaging Agents: Design and Applications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20170261. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.; Zhang, H.; Zhang, L.; Liu, L.; Cao, Y.; Ran, H.; Tian, J. A Non-Invasive Nanoparticles for Multimodal Imaging of Ischemic Myocardium in Rats. J. Nanobiotechnol. 2021, 19, 82. [Google Scholar] [CrossRef] [PubMed]
- Angelakeris, M. Magnetic Nanoparticles: A Multifunctional Vehicle for Modern Theranostics. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Dutz, S.; Hergt, R. Magnetic Particle Hyperthermia—A Promising Tumour Therapy? Nanotechnology 2014, 25, 452001. [Google Scholar] [CrossRef]
- Zhao, L.-Y.; Liu, J.-Y.; Ouyang, W.-W.; Li, D.-Y.; Li, L.; Li, L.-Y.; Tang, J.-T. Magnetic-Mediated Hyperthermia for Cancer Treatment: Research Progress and Clinical Trials. Chin. Phys. B 2013, 22, 108104. [Google Scholar] [CrossRef]
- Périgo, E.A.; Hemery, G.; Sandre, O.; Ortega, D.; Garaio, E.; Plazaola, F.; Teran, F.J. Fundamentals and Advances in Magnetic Hyperthermia. Appl. Phys. Rev. 2015, 2, 041302. [Google Scholar] [CrossRef] [Green Version]
- Thanh, B.T.; Sau, N.V.; Ju, H.; Bashir, M.J.K.; Jun, H.K.; Phan, T.B.; Ngo, Q.M.; Tran, N.Q.; Hai, T.H.; Van, P.H.; et al. Immobilization of Protein A on Monodisperse Magnetic Nanoparticles for Biomedical Applications. J. Nanomater. 2019, 2019, 2182471. [Google Scholar] [CrossRef] [Green Version]
- Unni, M.; Zhang, J.; George, T.J.; Segal, M.S.; Fan, Z.H.; Rinaldi, C. Engineering Magnetic Nanoparticles and Their Integration with Microfluidics for Cell Isolation. J. Colloid Interface Sci. 2020, 564, 204–215. [Google Scholar] [CrossRef]
- Xing, Z.-C.; Chang, Y.; Kang, I.-K. Immobilization of Biomolecules on the Surface of Inorganic Nanoparticles for Biomedical Applications. Sci. Technol. Adv. Mater. 2010, 11, 014101. [Google Scholar] [CrossRef]
- Li, D.; Teoh, W.Y.; Gooding, J.J.; Selomulya, C.; Amal, R. Functionalization Strategies for Protease Immobilization on Magnetic Nanoparticles. Adv. Funct. Mater. 2010, 20, 1767–1777. [Google Scholar] [CrossRef]
- Rashid, Z.; Soleimani, M.; Ghahremanzadeh, R.; Vossoughi, M.; Esmaeili, E. Effective Surface Modification of MnFe2O4@SiO2 @PMIDA Magnetic Nanoparticles for Rapid and High-Density Antibody Immobilization. Appl. Surf. Sci. 2017, 426, 1023–1029. [Google Scholar] [CrossRef]
- Liu, Z.; Li, M.; Yang, X.; Yin, M.; Ren, J.; Qu, X. The Use of Multifunctional Magnetic Mesoporous Core/Shell Heteronanostructures in a Biomolecule Separation System. Biomaterials 2011, 32, 4683–4690. [Google Scholar] [CrossRef] [PubMed]
- Corot, C.; Robert, P.; Idee, J.; Port, M. Recent Advances in Iron Oxide Nanocrystal Technology for Medical Imaging. Adv. Drug Deliv. Rev. 2006, 58, 1471–1504. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, Y.; Chen, Z.; Zhang, Y.; Wu, J.; Gu, N. Superparamagnetic Iron Oxide Nanoparticle-Embedded Encapsulated Microbubbles as Dual Contrast Agents of Magnetic Resonance and Ultrasound Imaging. Biomaterials 2009, 30, 3882–3890. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Scholz, R.; Maier-Hauff, K.; Johannsen, M.; Wust, P.; Nadobny, J.; Schirra, H.; Schmidt, H.; Deger, S.; Loening, S.; et al. Presentation of a New Magnetic Field Therapy System for the Treatment of Human Solid Tumors with Magnetic Fluid Hyperthermia. J. Magn. Magn. Mater. 2001, 225, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Balivada, S.; Rachakatla, R.S.; Wang, H.; Samarakoon, T.N.; Dani, R.K.; Pyle, M.; Kroh, F.O.; Walker, B.; Leaym, X.; Koper, O.B.; et al. A/C Magnetic Hyperthermia of Melanoma Mediated by Iron(0)/Iron Oxide Core/Shell Magnetic Nanoparticles: A Mouse Study. BMC Cancer 2010, 10, 119. [Google Scholar] [CrossRef] [Green Version]
- Tartaj, P.; del Puerto Morales, M.; Veintemillas-Verdaguer, S.; González-Carreño, T.; Serna, C.J. The Preparation of Magnetic Nanoparticles for Applications in Biomedicine. J. Phys. D Appl. Phys. 2003, 36, R182–R197. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and Surface Engineering of Iron Oxide Nanoparticles for Biomedical Applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Thapa, D.; Palkar, V.R.; Kurup, M.B.; Malik, S.K. Properties of Magnetite Nanoparticles Synthesized through a Novel Chemical Route. Mater. Lett. 2004, 58, 2692–2694. [Google Scholar] [CrossRef] [Green Version]
- Petcharoen, K.; Sirivat, A. Synthesis and Characterization of Magnetite Nanoparticles via the Chemical Co-Precipitation Method. Mater. Sci. Eng. B 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Daoush, W.M. Co-Precipitation and Magnetic Properties of Magnetite Nanoparticles for Potential Biomedical Applications. J. Nanomed. Res. 2017, 5, 00118. [Google Scholar] [CrossRef]
- Pei, W.; Kumada, H.; Natusme, T.; Saito, H.; Ishio, S. Study on Magnetite Nanoparticles Synthesized by Chemical Method. J. Magn. Magn. Mater. 2007, 310, 2375–2377. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Viñas, S.L.; Simeonidis, K.; Li, Z.-A.; Ma, Z.; Myrovali, E.; Makridis, A.; Sakellari, D.; Angelakeris, M.; Wiedwald, U.; Spasova, M.; et al. Tuning the Magnetism of Ferrite Nanoparticles. J. Magn. Magn. Mater. 2016, 415, 20–23. [Google Scholar] [CrossRef]
- Angelakeris, M.; Li, Z.-A.; Hilgendorff, M.; Simeonidis, K.; Sakellari, D.; Filippousi, M.; Tian, H.; Tendeloo, G.V.; Spasova, M.; Acet, M.; et al. Enhanced Biomedical Heat-Triggered Carriers via Nanomagnetism Tuning in Ferrite-Based Nanoparticles. J. Magn. Magn. Mater. 2015, 381, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Yang, H.; Fu, W.; Du, K.; Sui, Y.; Chen, J.; Zeng, Y.; Li, M.; Zou, G. Preparation and Magnetic Properties of Magnetite Nanoparticles by Sol–Gel Method. J. Magn. Magn. Mater. 2007, 309, 307–311. [Google Scholar] [CrossRef]
- Biddlecombe, G.B.; Gun’ko, Y.K.; Kelly, J.M.; Pillai, S.C.; Coey, J.M.D.; Venkatesan, M.; Douvalis, A.P. Preparation of Magnetic Nanoparticles and Their Assemblies Using a New Fe(II) Alkoxide Precursor. J. Mater. Chem. 2001, 11, 2937–2939. [Google Scholar] [CrossRef]
- Corr, S.A.; Gun’ko, Y.K.; Douvalis, A.P.; Venkatesan, M.; Gunning, R.D. Magnetite Nanocrystals from a Single Source Metallorganic Precursor: Metallorganic Chemistry vs. Biogeneric Bacteria. J. Mater. Chem. 2004, 14, 944–946. [Google Scholar] [CrossRef] [Green Version]
- Kayani, Z.N.; Arshad, S.; Riaz, S.; Naseem, S. Synthesis of Iron Oxide Nanoparticles by Sol–Gel Technique and Their Characterization. IEEE Trans. Magn. 2014, 50, 2200404. [Google Scholar] [CrossRef]
- Rasheed, R.T.; Al-Algawi, S.D.; Kareem, H.H.; Mansoor, H.S. Preparation and Characterization of Hematite Iron Oxide (α-Fe2O3) by Sol-Gel Method. Chem. Sci. J. 2018, 9, 4. [Google Scholar] [CrossRef]
- Lemine, O.M.; Omri, K.; Zhang, B.; Mir, L.E.; Sajieddine, M.; Alyamani, A.; Bououdina, M. Sol–Gel Synthesis of 8 nm Magnetite (Fe3O4) Nanoparticles and Their Magnetic Properties. Superlattices Microstruct. 2012, 52, 793–799. [Google Scholar] [CrossRef]
- Ang, C.B.; Yaacob, I.I.; Chew, C.S. Effect of FeCl2 Concentration on the Properties of Magnetic Nanoparticles by Using Massart’s Procedure. Sains Malays. 2014, 43, 611–616. [Google Scholar]
- Chin, A.B.; Yaacob, I.I. Synthesis and Characterization of Magnetic Iron Oxide Nanoparticles via w/o Microemulsion and Massart’s Procedure. J. Mater. Process. Technol. 2007, 191, 235–237. [Google Scholar] [CrossRef]
- Pérez, J.A.L.; Quintela, M.A.L.; Mira, J.; Rivas, J.; Charles, S.W. Advances in the Preparation of Magnetic Nanoparticles by the Microemulsion Method. J. Phys. Chem. B 1997, 101, 8045–8047. [Google Scholar] [CrossRef]
- Salvador, M.; Gutiérrez, G.; Noriega, S.; Moyano, A.; Blanco-López, M.C.; Matos, M. Microemulsion Synthesis of Superparamagnetic Nanoparticles for Bioapplications. Int. J. Mol. Sci. 2021, 22, 427. [Google Scholar] [CrossRef]
- Koutzarova, T.; Kolev, S.; Ghelev, C.; Paneva, D.; Nedkov, I. Microstructural Study and Size Control of Iron Oxide Nanoparticles Produced by Microemulsion Technique. Phys. Status Solidi C 2006, 3, 1302–1307. [Google Scholar] [CrossRef]
- Chen, D.; Xu, R. Hydrothermal Synthesis and Characterization of Nanocrystalline Fe3O4 Powders. Mater. Res. Bull. 1998, 33, 1015–1021. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, M.G.; Guo, J.D.; Zhang, B.H.; Xu, X.H. The Reaction Mechanism in the Hydrothermal Synthesis of Nd2Fe14B Magnetic Particles. J. Solid State Chem. 2021, 296, 122003. [Google Scholar] [CrossRef]
- Ge, S.; Shi, X.; Sun, K.; Li, C.; Uher, C.; Baker, J.R.; Holl, M.M.B.; Orr, B.G. Facile Hydrothermal Synthesis of Iron Oxide Nanoparticles with Tunable Magnetic Properties. J. Phys. Chem. C 2009, 113, 13593–13599. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Sun, K.; Liang, Y. Hydrothermal Synthesis of Magnetite: Investigation of Influence of Aging Time and Mechanism. Micro Nano Lett. 2015, 10, 99–104. [Google Scholar] [CrossRef]
- Aruna, S.T.; Mukasyan, A.S. Combustion Synthesis and Nanomaterials. Curr. Opin. Solid State Mater. Sci. 2008, 12, 44–50. [Google Scholar] [CrossRef]
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K.V. Solution Combustion Synthesis of Nanoscale Materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef] [PubMed]
- Mukasyan, A.S.; Roslyakov, S.; Pauls, J.M.; Gallington, L.C.; Orlova, T.; Liu, X.; Dobrowolska, M.; Furdyna, J.K.; Manukyan, K.V. Nanoscale Metastable ε-Fe3N Ferromagnetic Materials by Self-Sustained Reactions. Inorg. Chem. 2019, 58, 5583–5592. [Google Scholar] [CrossRef] [PubMed]
- Mukasyan, A.S.; Moskovskikh, D.O.; Nepapushev, A.A.; Pauls, J.M.; Roslyakov, S.I. Ceramics from Self-Sustained Reactions: Recent Advances. J. Eur. Ceram. Soc. 2020, 40, 2512–2526. [Google Scholar] [CrossRef]
- Deshpande, K.; Mukasyan, A.; Varma, A. Direct Synthesis of Iron Oxide Nanopowders by the Combustion Approach: Reaction Mechanism and Properties. Chem. Mater. 2004, 16, 4896–4904. [Google Scholar] [CrossRef]
- Yermekova, Z.; Roslyakov, S.I.; Kovalev, D.Y.; Danghyan, V.; Mukasyan, A.S. One-Step Synthesis of Pure γ-FeNi Alloy by Reactive Sol–Gel Combustion Route: Mechanism and Properties. J. Sol-Gel Sci. Technol. 2020, 94, 310–321. [Google Scholar] [CrossRef]
- Dinka, P.; Mukasyan, A.S. In Situ Preparation of Oxide-Based Supported Catalysts by Solution Combustion Synthesis. J. Phys. Chem. B 2005, 109, 21627–21633. [Google Scholar] [CrossRef]
- Wolf, E.E.; Kumar, A.; Mukasyan, A.S. Combustion Synthesis: A Novel Method of Catalyst Preparation. In Catalysis; Spivey, J., Han, Y.-F., Shekhawat, D., Eds.; Royal Society of Chemistry: Cambridge, UK, 2019; Volume 31, pp. 297–346. ISBN 978-1-78801-454-0. [Google Scholar]
- Mukasyan, A.S.; Dinka, P. Novel Method for Synthesis of Nano-Materials: Combustion of Active Impregnated Layers. Adv. Eng. Mater. 2007, 9, 653–657. [Google Scholar] [CrossRef]
- Danghyan, V.; Orlova, T.; Roslyakov, S.; Wolf, E.E.; Mukasyan, A.S. Cellulose Assisted Combustion Synthesis of High Surface Area Ni-MgO Catalysts: Mechanistic Studies. Combust. Flame 2020, 221, 462–475. [Google Scholar] [CrossRef]
- Lennon, E.M.; Tanzy, M.C.; Volpert, V.A.; Mukasyan, A.S.; Bayliss, A. Combustion of Reactive Solutions Impregnated into a Cellulose Carrier: Modeling of Two Combustion Fronts. Chem. Eng. J. 2011, 174, 333–340. [Google Scholar] [CrossRef]
- Manukyan, K.V.; Chen, Y.-S.; Rouvimov, S.; Li, P.; Li, X.; Dong, S.; Liu, X.; Furdyna, J.K.; Orlov, A.; Bernstein, G.H.; et al. Ultrasmall α-Fe2O3 Superparamagnetic Nanoparticles with High Magnetization Prepared by Template-Assisted Combustion Process. J. Phys. Chem. C 2014, 118, 16264–16271. [Google Scholar] [CrossRef]
- Trusov, G.V.; Tarasov, A.B.; Goodilin, E.A.; Rogachev, A.S.; Roslyakov, S.I.; Rouvimov, S.; Podbolotov, K.B.; Mukasyan, A.S. Spray Solution Combustion Synthesis of Metallic Hollow Microspheres. J. Phys. Chem. C 2016, 120, 7165–7171. [Google Scholar] [CrossRef]
- Yu, X.; Smith, J.; Zhou, N.; Zeng, L.; Guo, P.; Xia, Y.; Alvarez, A.; Aghion, S.; Lin, H.; Yu, J.; et al. Spray-Combustion Synthesis: Efficient Solution Route to High-Performance Oxide Transistors. Proc. Natl. Acad. Sci. USA 2015, 112, 3217–3222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Căpraru, A.; Moacă, E.-A.; Păcurariu, C.; Ianoş, R.; Lazău, R.; Barbu-Tudoran, L. Development and Characterization of Magnetic Iron Oxide Nanoparticles Using Microwave for the Combustion Reaction Ignition, as Possible Candidates for Biomedical Applications. Powder Technol. 2021, 394, 1026–1038. [Google Scholar] [CrossRef]
- AlSalhi, M.S.; Devanesan, S.; Shanmugam, P.; Kim, Y.O.; Kwon, J.-T.; Kim, H.-J. Synthesis and Biocompatible Role of Hierarchical Structured Carbon Nanoplates Incorporated α-Fe2O3 Nanocomposites for Biomedical Applications with Respect to Cancer Treatment. Saudi J. Biol. Sci. 2020, 27, 588–593. [Google Scholar] [CrossRef]
- Coricovac, D.-E.; Moacă, E.-A.; Pinzaru, I.; Cîtu, C.; Soica, C.; Mihali, C.-V.; Păcurariu, C.; Tutelyan, V.A.; Tsatsakis, A.; Dehelean, C.-A. Biocompatible Colloidal Suspensions Based on Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Toxicological Profile. Front. Pharmacol. 2017, 8, 154. [Google Scholar] [CrossRef] [Green Version]
- Varma, A.; Mukasyan, A.S.; Deshpande, K.T.; Pranda, P.; Erri, P.R. Combustion Synthesis of Nanoscale Oxide Powders: Mechanism, Characterization and Properties. In Proceedings of the Materials Research Society Symposium Proceedings, Boston, MA, USA, 29 November–3 December 2004; Ronald, A., Ed.; Materials Research Society: Warrendale, PA, USA, 2004; pp. 113–124. [Google Scholar]
- Kumar, A.; Wolf, E.E.; Mukasyan, A.S. Solution Combustion Synthesis of Metal Nanopowders: Nickel-Reaction Pathways. AIChE J. 2011, 57, 2207–2214. [Google Scholar] [CrossRef]
- Shwetha, U.R.; Rajith Kumar, C.R.; Kiran, M.S.; Betageri, V.S.; Latha, M.S.; Veerapur, R.; Lamraoui, G.; Al-Kheraif, A.A.; Elgorban, A.M.; Syed, A.; et al. Biogenic Synthesis of NiO Nanoparticles Using Areca Catechu Leaf Extract and Their Antidiabetic and Cytotoxic Effects. Molecules 2021, 26, 2448. [Google Scholar] [CrossRef]
- Roslyakov, S.; Yermekova, Z.; Trusov, G.; Khort, A.; Evdokimenko, N.; Bindiug, D.; Karpenkov, D.; Zhukovskyi, M.; Degtyarenko, A.; Mukasyan, A. One-Step Solution Combustion Synthesis of Nanostructured Transition Metal Antiperovskite Nitride and Alloy. Nano-Struct. Nano-Objects 2021, 28, 100796. [Google Scholar] [CrossRef]
- Ortiz-Quiñonez, J.-L.; Pal, U.; Villanueva, M.S. Effects of Oxidizing/Reducing Agent Ratio on Phase Purity, Crystallinity, and Magnetic Behavior of Solution-Combustion-Grown BiFeO3 Submicroparticles. Inorg. Chem. 2018, 57, 6152–6160. [Google Scholar] [CrossRef]
- Ortiz-Quiñonez, J.-L.; Pal, U.; Villanueva, M.S. Structural, Magnetic, and Catalytic Evaluation of Spinel Co, Ni, and Co–Ni Ferrite Nanoparticles Fabricated by Low-Temperature Solution Combustion Process. ACS Omega 2018, 3, 14986–15001. [Google Scholar] [CrossRef] [PubMed]
- Bera, P.; Lakshmi, R.V.; Prakash, B.H.; Tiwari, K.; Shukla, A.; Kundu, A.K.; Biswas, K.; Barshilia, H.C. Solution Combustion Synthesis, Characterization, Magnetic, and Dielectric Properties of CoFe2O4 and Co0.5M0.5Fe2O4 (M = Mn, Ni, and Zn). Phys. Chem. Chem. Phys. 2020, 22, 20087–20106. [Google Scholar] [CrossRef]
- Vasei, H.V.; Masoudpanah, S.M.; Habibollahzadeh, M. Different Morphologies of ZnO via Solution Combustion Synthesis: The Role of Fuel. Mater. Res. Bull. 2020, 125, 110784. [Google Scholar] [CrossRef]
- Vasei, H.V.; Masoudpanah, S.M.; Adeli, M.; Aboutalebi, M.R. Solution Combustion Synthesis of ZnO Powders Using CTAB as Fuel. Ceram. Int. 2018, 44, 7741–7745. [Google Scholar] [CrossRef]
- Amosov, A.P.; Novikov, V.A.; Kachkin, E.M.; Kryukov, N.A.; Titov, A.A.; Sosnin, I.M.; Merson, D.L. The Solution Combustion Synthesis of ZnO Powder for the Photodegradation of Phenol. Ceramics 2022, 5, 928–946. [Google Scholar] [CrossRef]
- Pathak, T.K.; Kroon, R.E.; Swart, H.C. Photocatalytic and Biological Applications of Ag and Au Doped ZnO Nanomaterial Synthesized by Combustion. Vacuum 2018, 157, 508–513. [Google Scholar] [CrossRef]
- Prashanth, G.K.; Prashanth, P.A.; Nagabhushana, B.M.; Ananda, S.; Krishnaiah, G.M.; Nagendra, H.G.; Sathyananda, H.M.; Singh, C.R.; Yogisha, S.; Anand, S.; et al. Comparison of Anticancer Activity of Biocompatible ZnO Nanoparticles Prepared by Solution Combustion Synthesis Using Aqueous Leaf Extracts of Abutilon indicum, Melia azedarach and Indigofera tinctoria as Biofuels. Artif. Cells Nanomed. Biotechnol. 2018, 46, 968–979. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.; Ahmed, E.; Zhang, Y.; Khalid, N.R.; Xu, J.; Ullah, M.; Hong, Z. Preparation of Highly Efficient Al-Doped ZnO Photocatalyst by Combustion Synthesis. Curr. Appl. Phys. 2013, 13, 697–704. [Google Scholar] [CrossRef]
- Bhatia, S.; Verma, N.; Bedi, R.K. Effect of Aging Time on Gas Sensing Properties and Photocatalytic Efficiency of Dye on In-Sn Co-Doped ZnO Nanoparticles. Mater. Res. Bull. 2017, 88, 14–22. [Google Scholar] [CrossRef]
- Bharat, T.C.; Shubham; Mondal, S.; Gupta, H.S.; Singh, P.K.; Das, A.K. Synthesis of Doped Zinc Oxide Nanoparticles: A Review. Mater. Today Proc. 2019, 11, 767–775. [Google Scholar] [CrossRef]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium Phosphates in Biomedical Applications: Materials for the Future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Cacciotti, I. Multisubstituted Hydroxyapatite Powders and Coatings: The Influence of the Codoping on the Hydroxyapatite Performances. Int. J. Appl. Ceram. Technol. 2019, 16, 1864–1884. [Google Scholar] [CrossRef]
- Lopera, A.A.; Montoya, A.; Vélez, I.D.; Robledo, S.M.; Garcia, C.P. Synthesis of Calcium Phosphate Nanostructures by Combustion in Solution as a Potential Encapsulant System of Drugs with Photodynamic Properties for the Treatment of Cutaneous Leishmaniasis. Photodiagn. Photodyn. Ther. 2018, 21, 138–146. [Google Scholar] [CrossRef]
- Kermani, F.; Gharavian, A.; Mollazadeh, S.; Kargozar, S.; Youssefi, A.; Khaki, J.V. Silicon-Doped Calcium Phosphates; the Critical Effect of Synthesis Routes on the Biological Performance. Mater. Sci. Eng. C 2020, 111, 110828. [Google Scholar] [CrossRef] [PubMed]
- Kermani, F.; Mollazadeh, S.; Kargozar, S.; Khakhi, J.V. Solution Combustion Synthesis (SCS) of Theranostic Ions Doped Biphasic Calcium Phosphates; Kinetic of Ions Release in Simulated Body Fluid (SBF) and Reactive Oxygen Species (ROS) Generation. Mater. Sci. Eng. C 2021, 118, 111533. [Google Scholar] [CrossRef] [PubMed]
- Borovinskaya, I.P. (Ed.) Concise Encyclopedia of Self-Propagating High-Temperature Synthesis: History, Theory, Technology, and Products; Elsevier: Amsterdam, The Netherlands; Cambridge, MA, USA, 2017; ISBN 978-0-12-804173-4. [Google Scholar]
- Borovinskaya, I.P.; Manukyan, K.; Mukasyan, A.S. SHS Ceramics: History and Recent Advances. Ceram. Mod. Technol. 2019, 1, 3–19. [Google Scholar] [CrossRef]
- Merzhanov, A.G.; Khaikin, B.I. Theory of Combustion Waves in Homogeneous Media. Prog. Energy Combust. Sci. 1988, 14, 1–98. [Google Scholar] [CrossRef]
- Mukasyan, A.S.; Rogachev, A.S. Discrete Reaction Waves: Gasless Combustion of Solid Powder Mixtures. Prog. Energy Combust. Sci. 2008, 34, 377–416. [Google Scholar] [CrossRef]
- Kumar, A.; Mukasyan, A.S.; Wolf, E.E. Modeling Impregnated Layer Combustion Synthesis of Catalyts for Hydrogen Generation from Oxidative Reforming of Methanol. Ind. Eng. Chem. Res. 2010, 49, 11001–11008. [Google Scholar] [CrossRef]
- Rogachev, A.S.; Mukasyan, A.S. Combustion for Material Synthesis, 1st ed.; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-1-4822-3952-2. [Google Scholar]
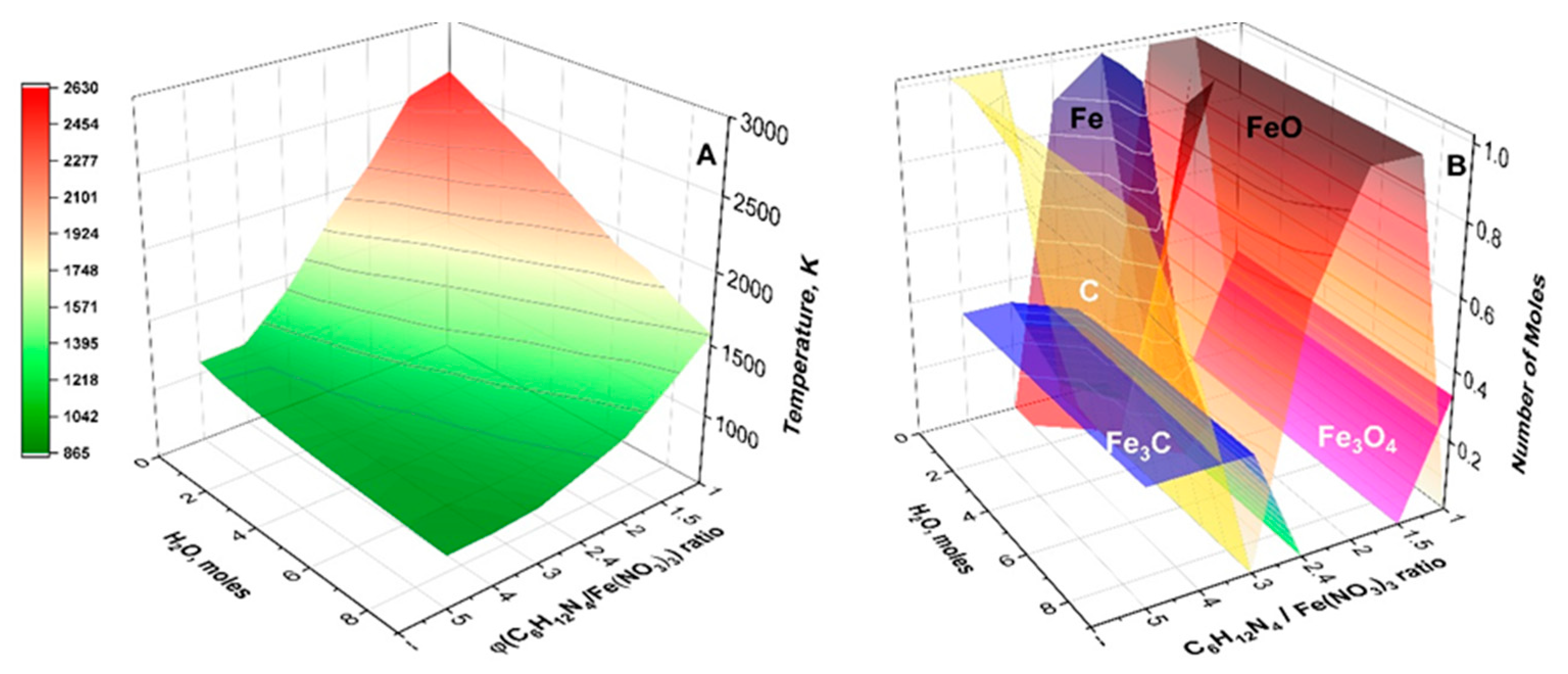

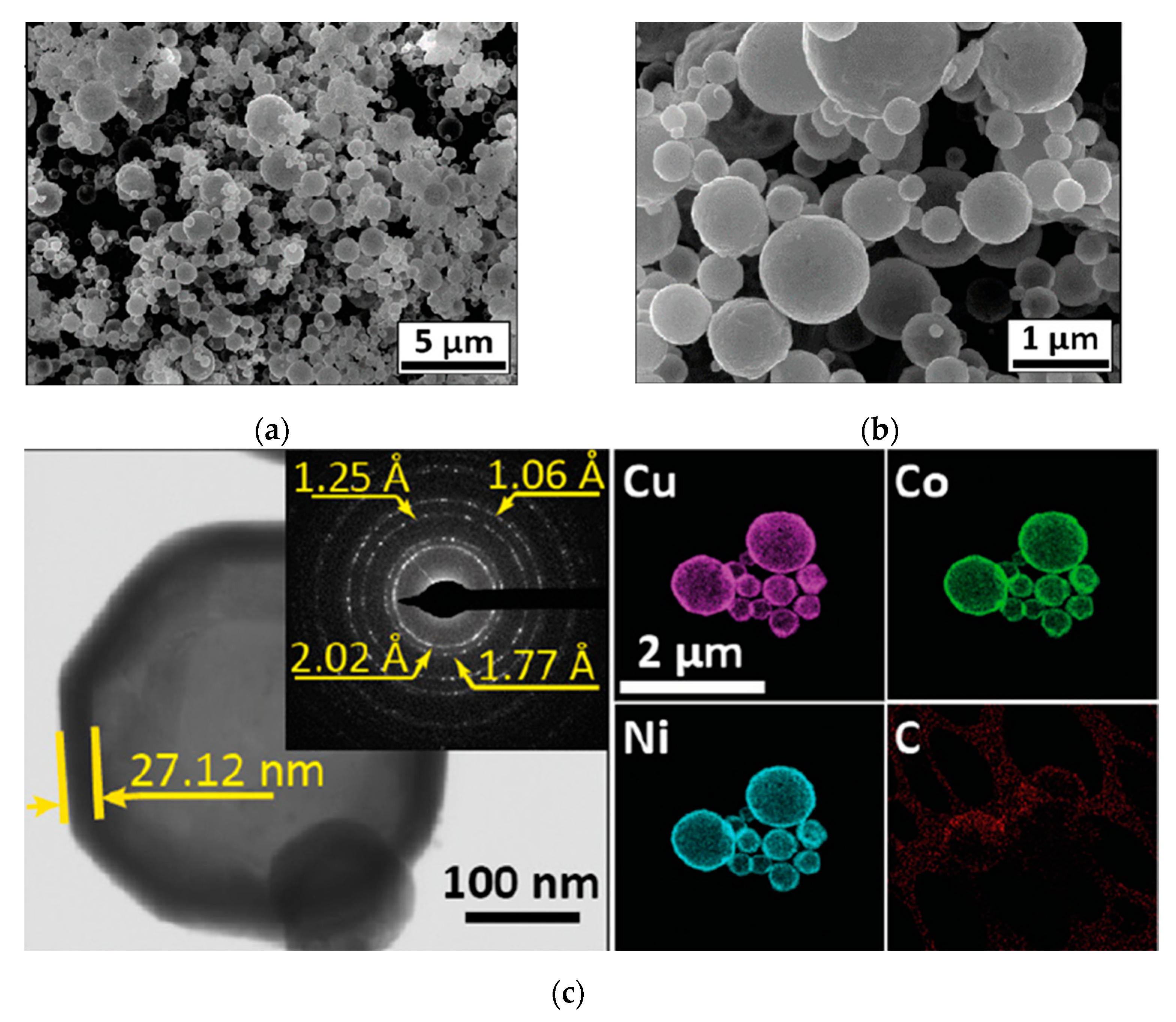
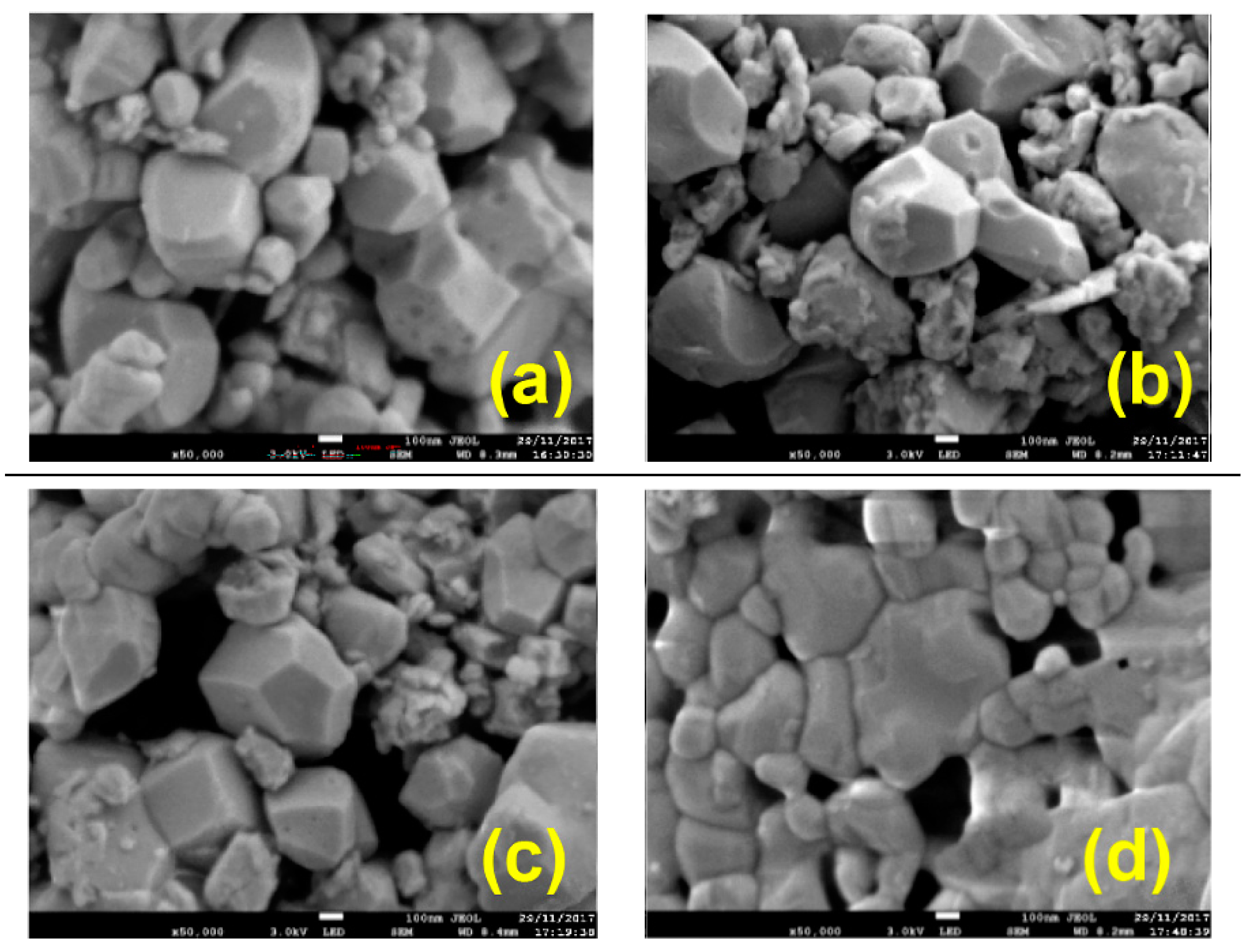


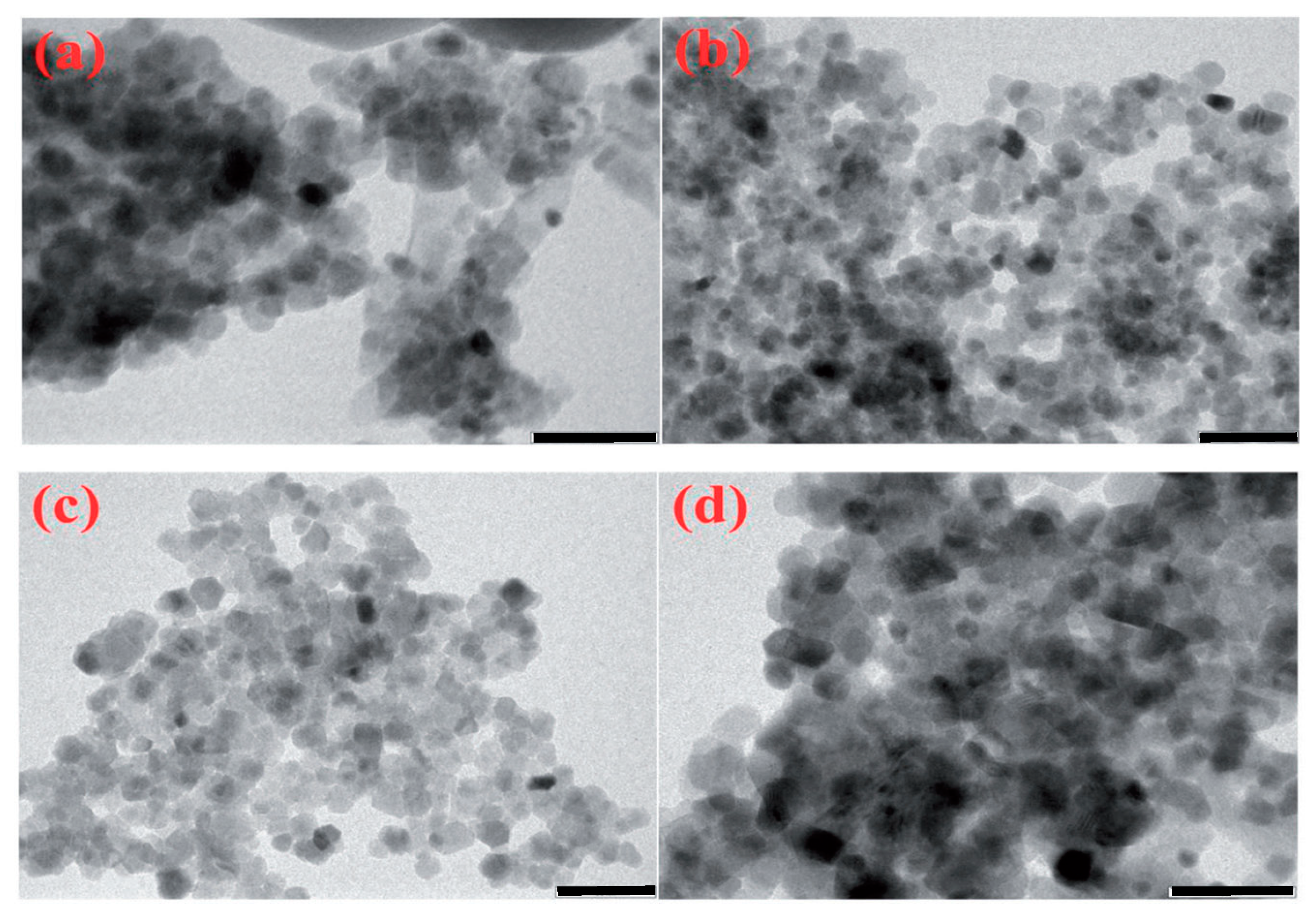
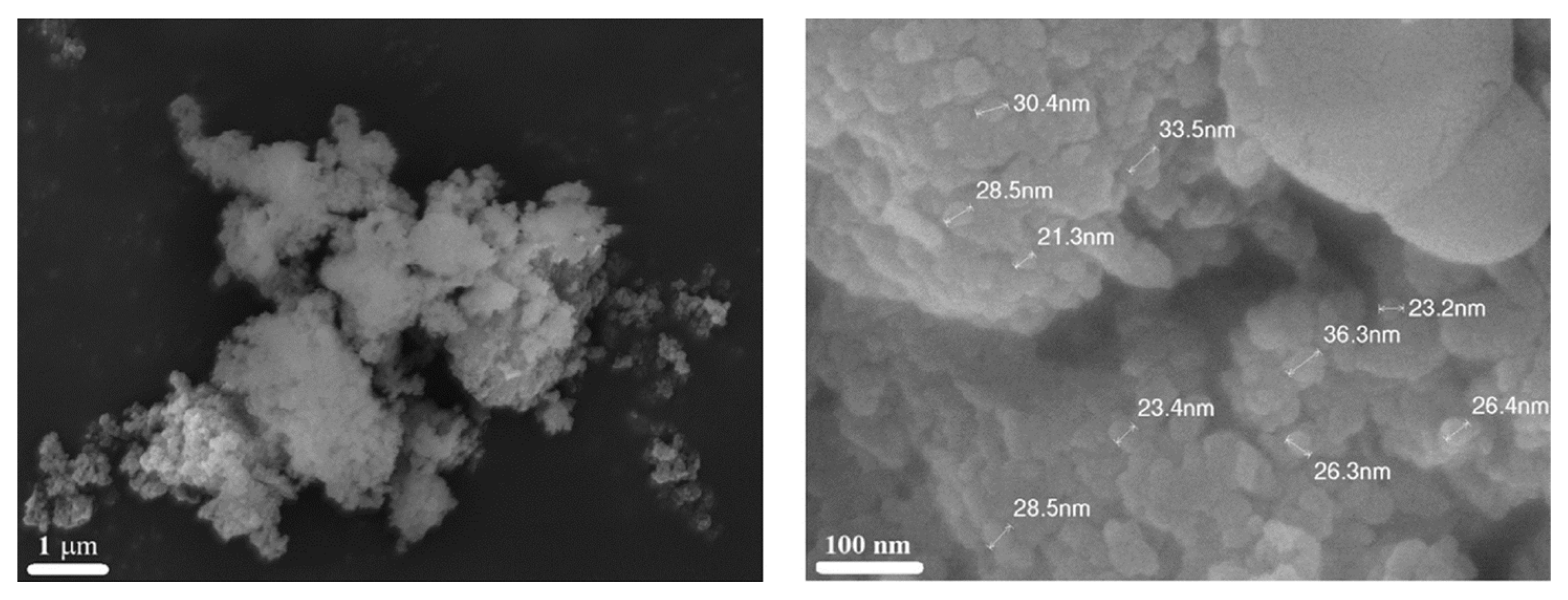
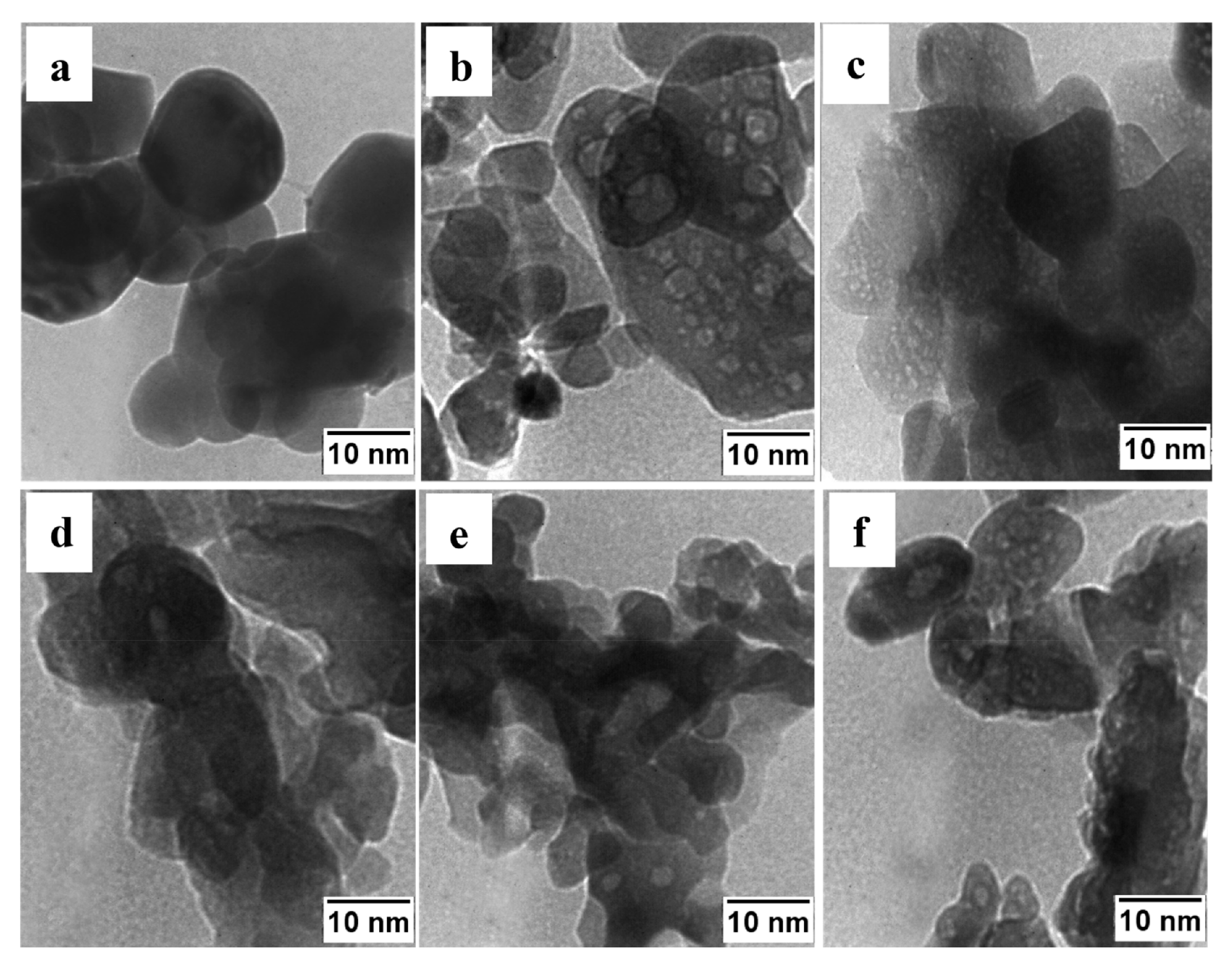
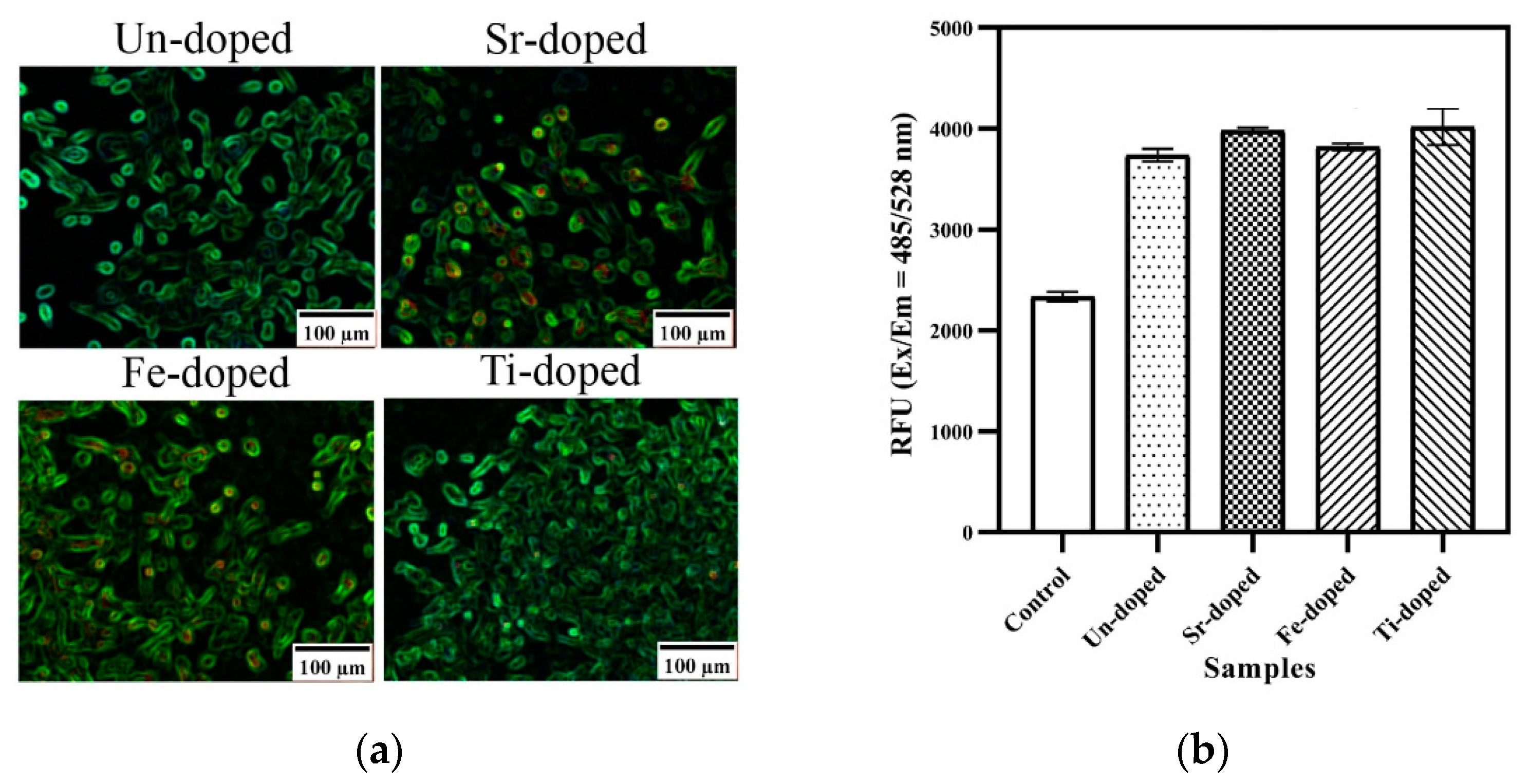
| Oxidizer | Fuel | Solvent | Atmosphere |
|---|---|---|---|
| Metal nitrate hydrates Meν(NO3)ν∙nH2O; (Me = Fe, Co, Ni, Mn, etc.) —metal valency Metal Oxalates hydrates: Me(C2O4)∙nH2O Ammonium Nitrate: NH4(NO3) Nitric acid (HNO3) | Glycine (CH4N2O) Citric Acid (C6H8O7) Urea (CH4N2O) Sucrose (C12H22O11) Glycose D-(+)-C6H12O6 Hydrazine (H2N-NH2) Carbohydrazide (CH6N4O) Oxalyhydrazide (C2H6N4O2) Metal hydrazinecarboxylates hydrates: (N2H5Me(N2H3COO)3·H2O, where Me = Fe, Co, Ni, Zn) Hexamethylenetetramine: (C6H12N4, HMTA) Acetylacetone (C5H8O2) | Water (H2O) Benzene (C6H6) Ethanol (C2H6O) Methanol (CH4O) Furfuryl alcohol: (C5H6O2) Formaldehyde: (CH2O) 2-methoxyethanol (C3H8O2) | Air Nitrogen Oxygen Inert gas: Argon Helios |
| Sample | Heating | Phase Composition wt.% | Crystallite Size, nm | SBET m2/g | Average Pore Diameter, nm |
|---|---|---|---|---|---|
| P1 | Mantle | 97% Fe3O4 + 3% γ-F2O3 | 23 | 42 | 7.1 |
| P2 | Microwave | 85% γ-F2O3 + 11% Fe3O4 +2% FeO +2%Fe | 5 | 71 | 5.5 |
| Sample | Surfactant | Dispersion Media | Diameter, nm | PDI | Zeta Potential mV |
|---|---|---|---|---|---|
| O/W | Oleic acid | Water | 91 | 0.179 | −74 |
| O/PBS | Oleic acid | PBS | 105 | 0.326 | −47 |
| O/W | Oleic acid + Tween80 | Water | 105 | 0.315 | −14 |
| O/W | Oleic acid + Tween 80 | PBS | 79 | 0.189 | −12 |
| Sample | Crystallite Size, nm | S BET m2/g | Average Diameter Nm | Saturation Magnetization emu/g | Remnant Magnetization emu/g | Coercively kA/m |
|---|---|---|---|---|---|---|
| Fe3O4 | 18 | 56 | 21 | 57.7 | 4.5 | 5.2 |
| γ-F2O3 | 5 | 149 | 8 | 41.5 | 0.7 | 1.0 |
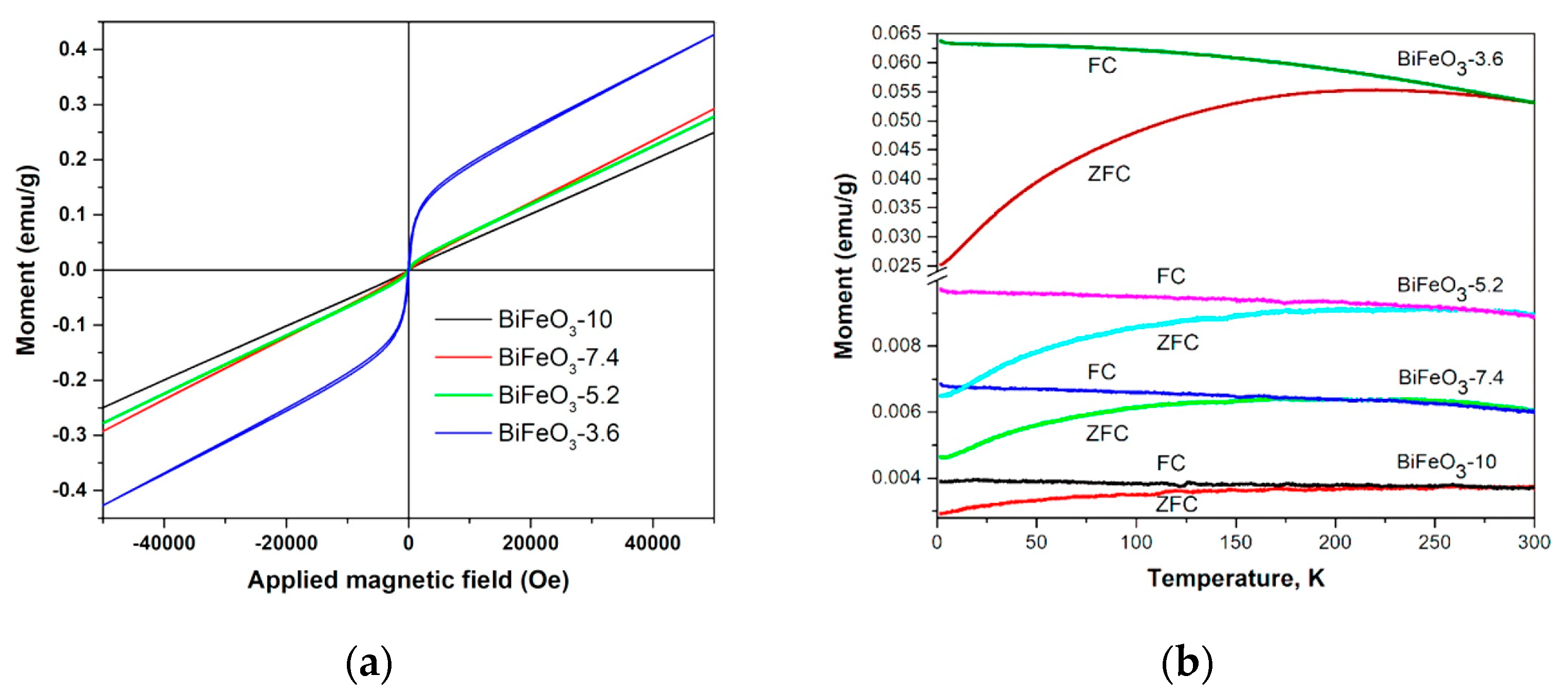
| Sample | 1.8 K | 60 K | 100 K | 200 K | 300 K |
|---|---|---|---|---|---|
| BiFeO3-10 | 734 | 330 | 195 | 108 | 80 |
| BiFeO3-7.4 | 1207 | 484 | 284 | 178 | 172 |
| BiFeO3-5.2 | 1226 | 692 | 529 | 353 | 288 |
| BiFeO3-3.6 | 1561 | 827 | 594 | 321 | 198 |
| Sample | Crystallite Size, nm | S BET m2/g | BJH, Average Pore Diameter, Å |
|---|---|---|---|
| ZnO(L) | 13 | 19 | 91 |
| ZnO(A) | 11 | 26 | 83 |
| ZnO(M) | 9 | 90 | 56 |
| ZnO(I) | 11 | 38 | 72 |
| Fuel | Amount of Si, Mole | Crystallinity % | HA wt.% | βTCP wt.% | Crystallite Size HA, nm | Crystallite Size βTCP, nm | Particle Size, nm |
|---|---|---|---|---|---|---|---|
| Glycine | 0 | 59 | 90 | 10 | 15.6 | 42.4 | 68 ± 6 |
| 0.4 | 52 | 86 | 14 | 15.6 | 42.4 | 60 ± 4 | |
| Citric Acid | 0 | 55 | 74 | 26 | 14.2 | 42.4 | 84 ± 12 |
| 0.4 | 50 | 94 | 6 | 14.2 | 42.4 | 54 ± 8 |
| Fuel | Amount Dopant, Mole | Crystallinity % | HA wt.% | βTCP wt.% | Crystallite Size, nm | BET. m2/g | Particle Size, nm |
|---|---|---|---|---|---|---|---|
| Un-doped | 0 | 59 | 90 | 10 | 60 ± 4 | 37 | 68 ± 6 |
| Sr-doped | 0.1 | 59 | 91 | 9 | 60 ± 4 | 79 | 39 ± 2 |
| 0.5 | 53 | 93 | 7 | 51 ± 6 | 23 | 69 ± 3 | |
| Fe-doped | 0.1 | 60 | 90 | 10 | 50 ± 3 | 65 | 24 ± 6 |
| 0.5 | 55 | 87 | 13 | 41 ± 5 | 23 | 60 ± 6 | |
| Ti-doped | 0.1 | 58 | 88 | 12 | 47 ± 9 | 106 | 29 ± 5 |
| 0.5 | 41 | 84 | 16 | 38 ± 13 | 66 | 74 ± 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyulasaryan, H.; Kuzanyan, A.; Manukyan, A.; Mukasyan, A.S. Combustion Synthesis of Magnetic Nanomaterials for Biomedical Applications. Nanomaterials 2023, 13, 1902. https://doi.org/10.3390/nano13131902
Gyulasaryan H, Kuzanyan A, Manukyan A, Mukasyan AS. Combustion Synthesis of Magnetic Nanomaterials for Biomedical Applications. Nanomaterials. 2023; 13(13):1902. https://doi.org/10.3390/nano13131902
Chicago/Turabian StyleGyulasaryan, Harutyun, Astghik Kuzanyan, Aram Manukyan, and Alexander S. Mukasyan. 2023. "Combustion Synthesis of Magnetic Nanomaterials for Biomedical Applications" Nanomaterials 13, no. 13: 1902. https://doi.org/10.3390/nano13131902
APA StyleGyulasaryan, H., Kuzanyan, A., Manukyan, A., & Mukasyan, A. S. (2023). Combustion Synthesis of Magnetic Nanomaterials for Biomedical Applications. Nanomaterials, 13(13), 1902. https://doi.org/10.3390/nano13131902