Lightweight Co3O4/CC Composites with High Microwave Absorption Performance
Abstract
:1. Introduction
2. Materials and Methods
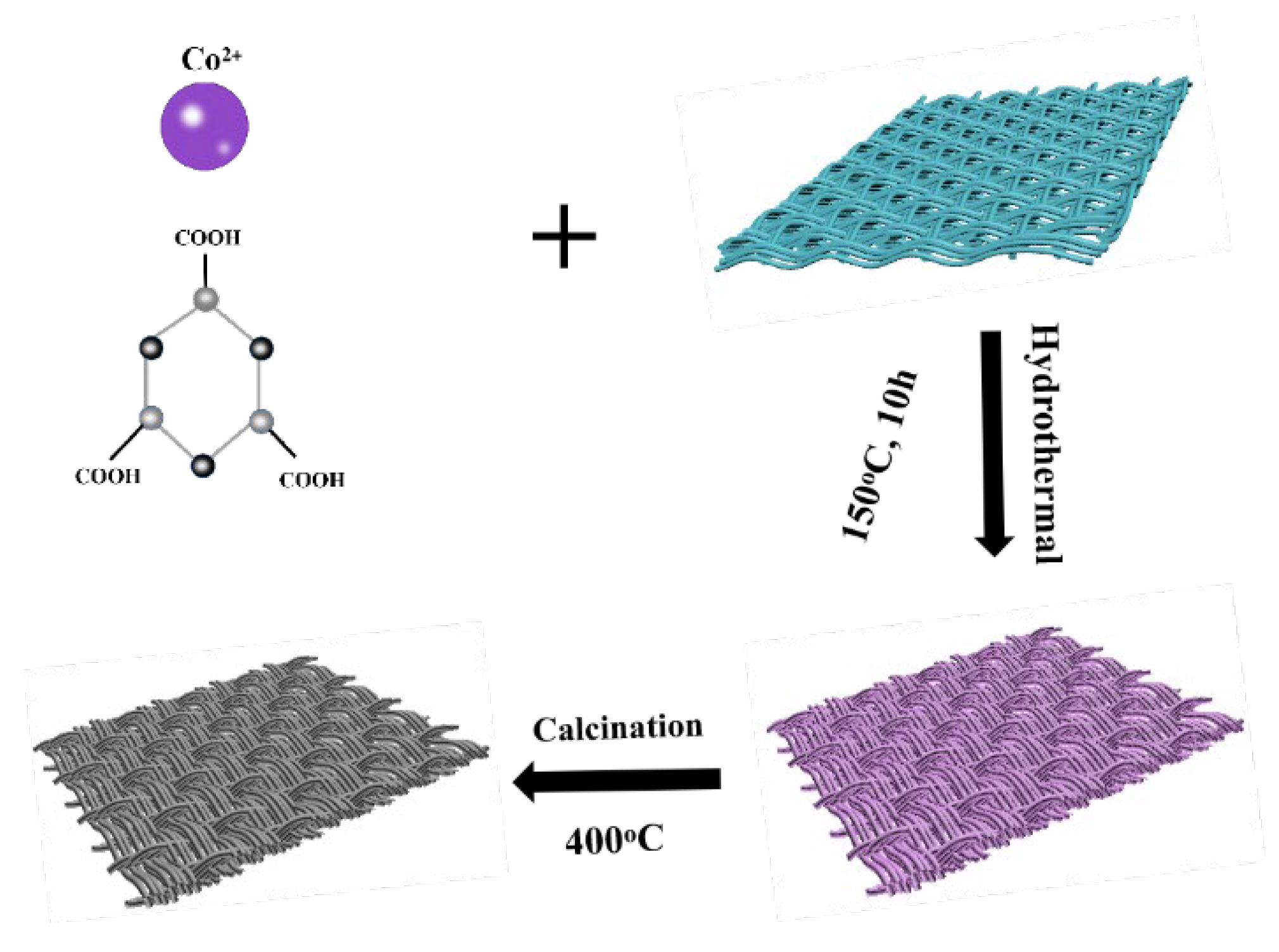
2.1. Preparation of the Co3O4/CC Precursor
2.2. Synthesis of Co3O4/CC Composites
2.3. Characterization and Measurement
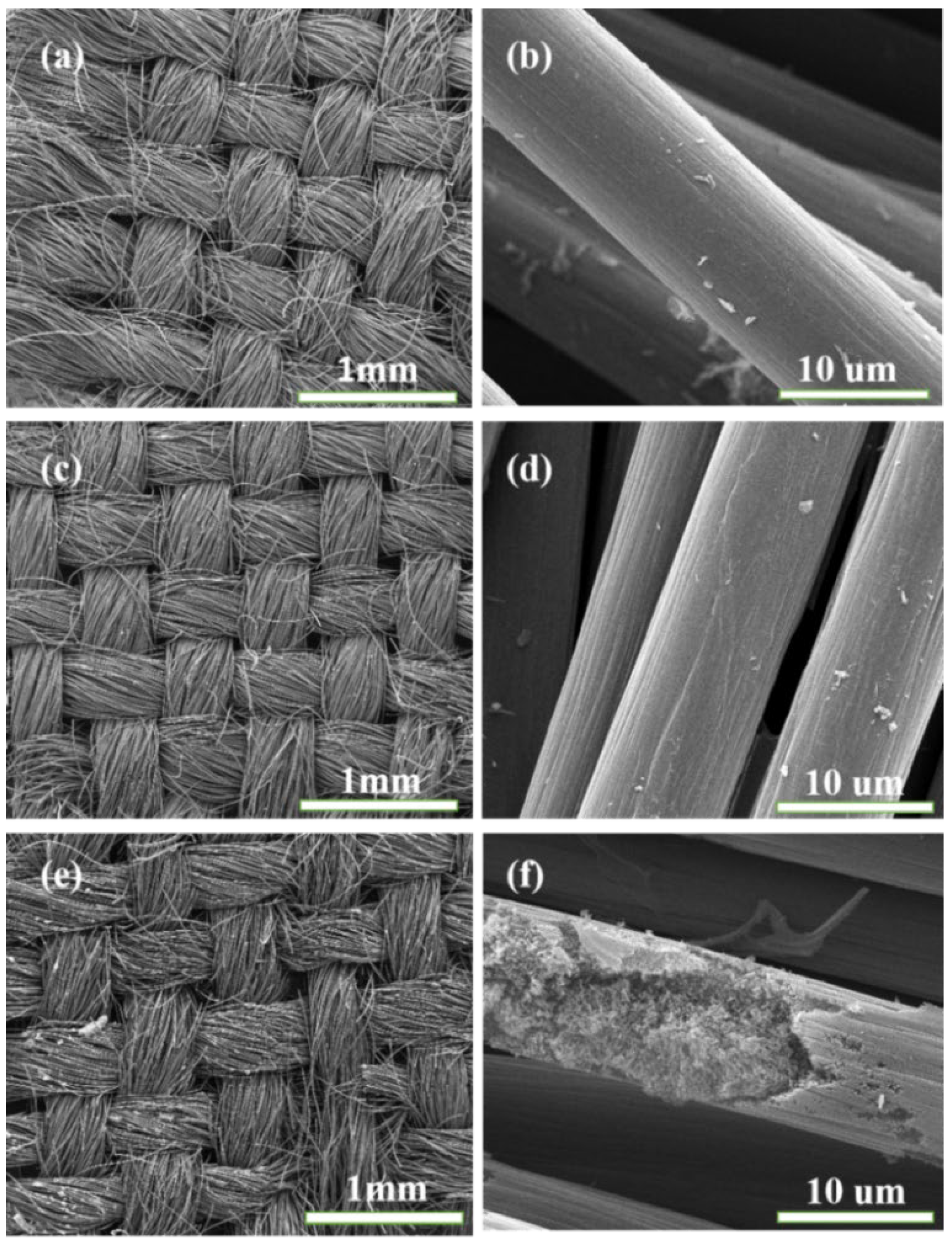
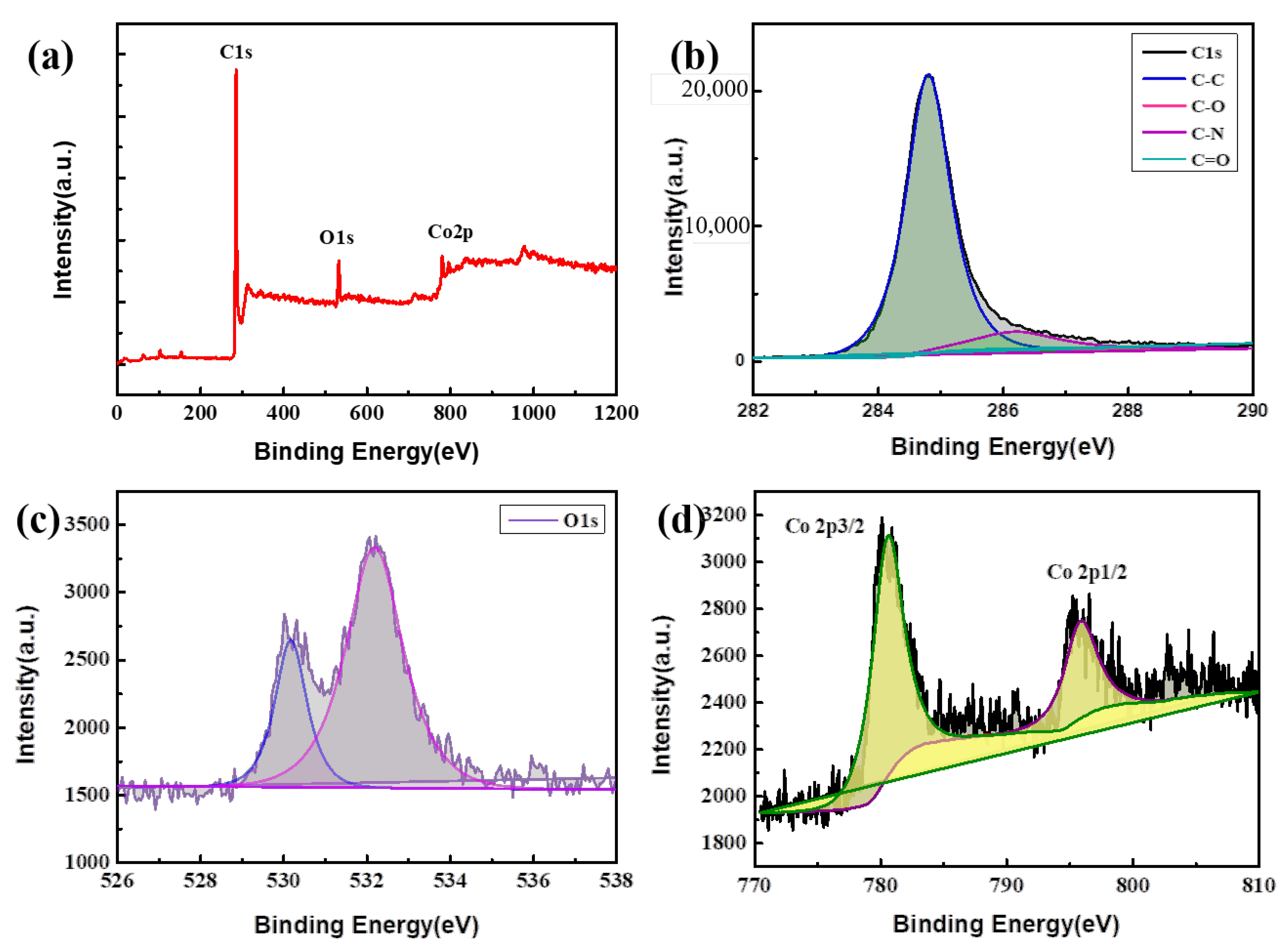
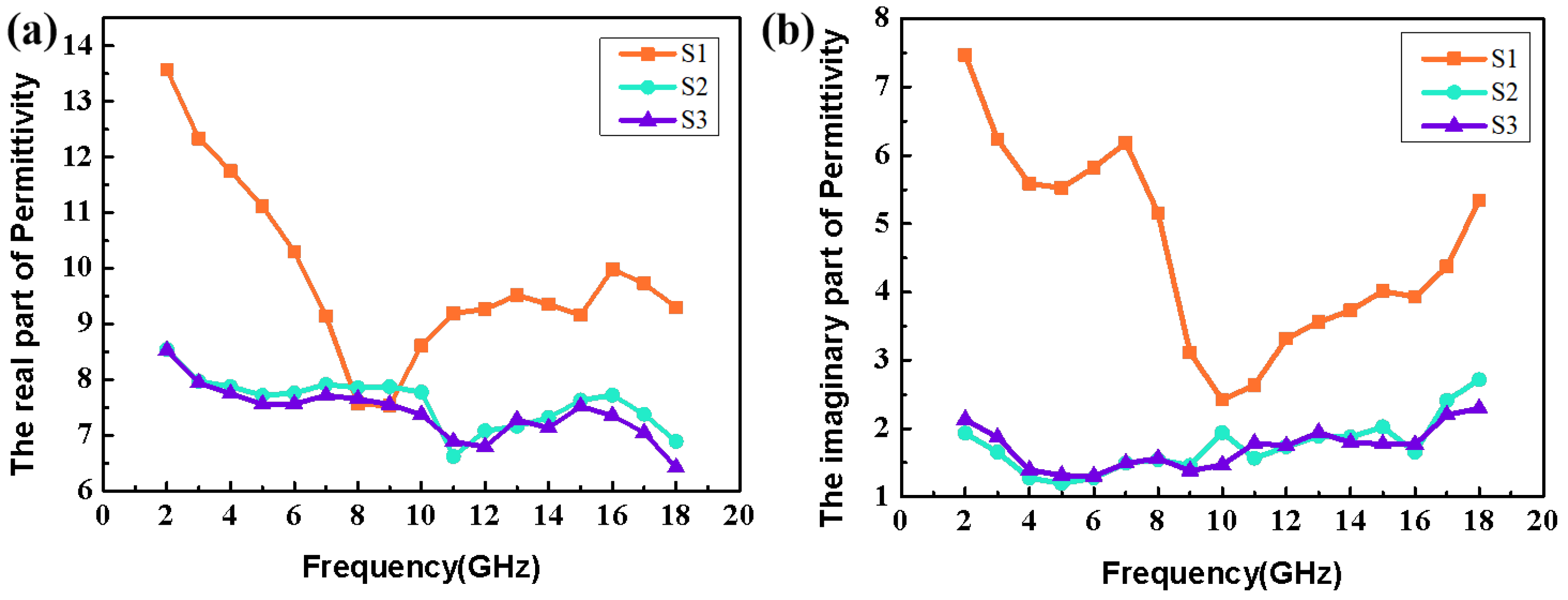
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Wang, F.; Zhang, X.; Li, W.; Guan, G. Research progress of low frequency wideband thin layer absorbing materials. J. Funct. Mater. 2019, 50, 606038–606045. [Google Scholar]
- Wu, F.; Xie, A.; Sun, M.; Wang, Y.; Wang, M. Reduced graphene oxide (RGO) modified spongelike polypyrrole (PPy) aerogel for excellent electromagnetic absorption. J. Mater. Chem. 2015, 3, 14358–14369. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, Z.; Jiang, W. Controlled synthesis of Fe3O4@SnO2/RGO nanocomposite for microwave absorption enhancement. Ceram. Int. 2016, 42, 10682–10689. [Google Scholar] [CrossRef]
- Wang, G.; Wu, Y.; Zhang, X.; Li, Y.; Guo, L.; Cao, M. Controllable synthesis of uniform ZnO nanorods and their enhanced dielectric and absorption properties. J. Mater. Chem. 2014, A2, 8644–8651. [Google Scholar] [CrossRef]
- Zhou, N.; An, D.; Xiao, Z.; Zhai, S.; Shi, Z. Solvothermal synthesis of three-dimensional, Fe2O3 NPs-embedded CNT/N-doped graphene composites with excellent microwave absorption performance. RSC Adv. 2017, 7, 45156–45169. [Google Scholar] [CrossRef] [Green Version]
- Cao, M.; Song, W.; Hou, Z.; Wen, B.; Yuan, J. The effects of temperature and frequency on the dielectric properties, electromagnetic interference shielding and microwave-absorption of short carbon fiber/silica composites. Carbon 2010, 48, 788–796. [Google Scholar] [CrossRef]
- Yilmaz, G.; Yam, K.M.; Zhang, C.; Fan, H.J.; Ho, G.W. In situ transformation of MOFs into layered double hydroxide embedded metal sulfides for improved electrocatalytic and supercapacitive performance. Adv. Mater. 2017, 29, 1606814. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Li, T.; Hu, Y.; Huang, S. A bimetallic carbide derived from a MOF precursor for increasing electrocatalytic oxygen evolution activity. Chem. Commun. 2017, 53, 13027–13030. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Quan, B.; Sun, W.; Ji, G.; Zhang, Y.; Ma, J.; Li, D.; Zhang, B.; Du, Y. Multiple interfaces structure derived from metal–organic frameworks for excellent electromagnetic wave absorption. Part. Part. Syst. Charact. 2017, 34, 1700006. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, X.; Wei, X.; Yu, R.; Shui, J. Porous CNTs/Co composite derived from zeolitic imidazolate framework: A lightweight, ultrathin, and highly efficient electromagnetic wave absorber. ACS Appl. Mater. Interfaces 2016, 8, 34686–34698. [Google Scholar] [CrossRef]
- Liu, W.; Shao, Q.; Ji, G.; Liang, X.; Cheng, Y.; Quan, B.; Du, Y. Metal–Organic frameworks derived porous carbon-wrapped Ni composites with optimized impedance matching as excellent lightweight electromagnetic wave absorber. Chem. Eng. J. 2017, 313, 734–744. [Google Scholar] [CrossRef]
- Ma, J.; Liu, W.; Liang, X. Nonporous TiO2/C composites synthesized from directly pyrolysis of a Ti-based MOFs MIL-125(Ti) for efficient microwave absorption. J. Alloys Compd. 2017, 728, 138–144. [Google Scholar] [CrossRef]
- Shi, F.; Hu, Y.; Wang, X. Decomposition of MOFs for the preparation of nano porous Co3O4 fibers and sheets with excellent microwave absorption and photocatalytic properties. Dalton Trans. 2017, 46, 1936–1942. [Google Scholar] [CrossRef]
- Liu, W.; Tan, S.; Yang, Z.; Ji, G. Enhanced low-grequency electromagnetic properties of MOF-derived cobalt through interface design. ACS Appl. Mater. Interfaces 2018, 37, 31610–31622. [Google Scholar] [CrossRef]
- Yang, R.; Yuan, J.; Yu, C.; Yan, K.; Fu, Y.; Xie, H.; Chen, J.; Chu, P.; Wu, X. Efficient electromagnetic wave absorption by SiC/Ni/NiO/C nanocomposites. J. Alloys Compd. 2020, 816, 152519. [Google Scholar] [CrossRef]
- Miao, P.; Zhou, R.; Chen, K.; Liang, J.; Ban, Q.; Kong, J. Tunable electromagnetic wave absorption of supramolecular isomer-derived nanocomposites with different morphology. Adv. Mater. Interfaces 2020, 7, 1901820. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, Y.; Guo, J.; Wu, S.; Chen, N.; Fan, H.; Gao, M.; Yang, J.; Sheng, Z.; Lang, J. One-step hydrothermal synthesis of the modified carbon cloth membrane: Towards visible light driven and self-cleaning for efficient oil-water separation. Surf. Coat. Technol. 2021, 409, 126879. [Google Scholar] [CrossRef]
- Yang, L.; Chen, W.; Sheng, C.; Wu, H.; Mao, N.; Zhang, H. Fe/N-codoped carbocatalysts loaded on carbon cloth (CC) for activating peroxymonosulfate (PMS) to degrade methyl orange dyes. Appl. Surf. Sci. 2021, 549, 149300. [Google Scholar] [CrossRef]
- Sui, S.; Sha, J.; Deng, X.; Zhu, S.; Ma, L.; He, C.; Liu, E.; He, F.; Shi, C.; Zhao, N. Boosting the charge transfer efficiency of metal oxides/carbon nanotubes composites through interfaces control. J. Power Sources 2021, 489, 229501. [Google Scholar] [CrossRef]
- Xie, L.; Chen, S.; Hu, Y.; Lan, Y.; Li, X.; Deng, Q.; Wang, J.; Zeng, Z.; Deng, S. Construction of phosphatized cobalt nickel-LDH nanosheet arrays as binder-free electrode for high-performance battery-like supercapacitor device. J. Alloys Compd. 2021, 858, 157652. [Google Scholar] [CrossRef]
- Nie, Z.; Zhang, H.; Lu, Y.; Han, C.; Du, Y.; Sun, Z.; Yan, Y.; Yu, H.; Zhang, X.; Zhu, J. Manipulation of porosity and electrochemical artificial separator interphase for durable lithium-sulfur batteries. Chem. Eng. J. 2021, 409, 128137. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, D.; Zhu, X.; Wang, S.; Chen, E.; Yang, Y.; Liao, Q. A self-pumping microfluidic fuel cell powered by formatted with Pd coated carbon cloth electrodes. J. Power Sources 2021, 490, 229553. [Google Scholar] [CrossRef]
- Dai, K.; Zhang, N.; Zhang, L.; Yin, L.; Zhao, Y.; Zhang, B. Self-supported Co/CoO anchored on N-doped carbon composite as bifunctional electrocatalyst for efficient overall water splitting. Chem. Eng. J. 2021, 414, 128804. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, J.; Ci, C.; Bai, R.; Shi, D.; Xia, K.; Wu, D. Preparation of NiSe2/CC composite and its electrocatalytic performance for hydrogen evolution. Micro/Nano. Electron. Technol. 2021, 58, 207–213. [Google Scholar]
- Zhan, J.; Cao, X.; Zhou, J.; Xu, G.; Lei, B.; Wu, M. Porous array with CoP nanoparticle modification derived from MOF grown on carbon cloth for effective alkaline hydrogen evolution. Chem. Eng. J. 2021, 416, 128943. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Li, Q.; Yu, X.; Zhao, Y.; Zhang, J.; Wang, M.; Che, R. Oriented polarization tuning broadband absorption from flexible hierarchical ZnO arrays vertically supported on carbon cloth. Small 2019, 15, 1900900. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, C.; Gao, S.; Guan, C.; Huang, Y.; He, W. N-doped porous carbon nanoplates embedded with CoS2 vertically anchored on carbon cloths for flexible and ultrahigh microwave absorption. Carbon 2020, 163, 348–359. [Google Scholar] [CrossRef]
- Chen, N.; Chen, D.; Wei, F.; Zhao, S.; Luo, Y. Effect of structures on the adsorption performance of cobalt metal organic framework obtained by microwave-assisted ball milling. Chem. Phys. Lett. 2018, 705, 23–30. [Google Scholar] [CrossRef]
- Davydovskaya, P.; Pentyala, V.; Yurchenko, O.; Hussein, L.; Pohle, R.; Urban, G. Work function based sensing of alkanes and alcohols with benzene tricarboxylate linked metal organic frameworks. Sen. Actuat. B Chem. 2014, 193, 911–917. [Google Scholar] [CrossRef]
- Wan, G.; Wang, G.; Huang, X.; Zhao, H.; Li, X.; Wang, K.; Yu, L.; Peng, X.; Qin, Y. Uniform Fe3O4 coating on flower-like ZnO nanostructures by atomic layer deposition for electromagnetic wave absorption. Dalton Trans. 2015, 44, 18804–18809. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Z.; Long, Y. Multishelled NixCo3-xO4 hollow microspheres derived from bimetal-organic frameworks as anode materials for high-performance lithium-ion batteries. Small 2017, 13, 1604270. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yu, T.; Sow, C.; Liu, Y.; Wee, A.; Xu, X.; Lim, C.; Thong, J. Efficient field emission from α-Fe2O3 nanoflakes on an atomic force microscope tip. Appl. Phys. Lett. 2005, 87, 023103. [Google Scholar] [CrossRef]
- Feng, W.; Wang, Y.; Chen, J.; Wang, L.; Guo, L.; Yang, J.; Jia, D.; Zhou, Y. Reduced graphene oxide decorated with in-situ growing ZnO nanocrystals: Facile synthesis and enhanced microwave absorption properties. Carbon 2016, 108, 52–60. [Google Scholar] [CrossRef]
- Yan, J.; Huang, Y.; Zhang, Z.; Liu, X. Novel 3D micro sheets contain cobalt particles and numerous interlaced carbon nanotubes for high-performance electromagnetic wave absorption. J. Alloys Compd. 2019, 785, 1206–1214. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, X.; Liu, W.; Tang, D.; Zhang, B.; Ji, G. A simple hydrothermal process to grow MoS2 nanosheets with excellent dielectric loss and microwave absorption performance. J. Mater. Chem. C 2016, 4, 6816–6821. [Google Scholar] [CrossRef]
- Cui, X.; Liang, X.; Liu, W.; Gu, W.; Ji, G.; Du, Y. Stable microwave absorber derived from 1D customized heterogeneous structures of Fe3N@C. Chem. Eng. J. 2020, 381, 122589. [Google Scholar] [CrossRef]
- Liang, X.; Quan, B.; Ji, G.; Liu, W.; Cheng, Y.; Zhang, B.; Du, Y. Novel nanoporous carbon derived from metal-organic frameworks with tunable electromagnetic wave absorption capabilities. Inorg. Chem. Front. 2016, 3, 1516–1526. [Google Scholar] [CrossRef]
- Cao, M.; Qin, R.; Qiu, C.; Zhu, J. Matching design and mismatching analysis towards radar absorbing coatings based on conducting plate. Mater. Eng. 2003, 24, 391–396. [Google Scholar] [CrossRef]
- Song, W.; Cao, M.; Hou, Z.; Yuan, J. High-temperature microwave absorption and evolutionary behavior of multiwalled carbon nanotube nanocomposite. Scr. Mater. 2009, 61, 201–204. [Google Scholar] [CrossRef]
- Wang, L.; Jia, X.; Li, Y.; Yang, F.; Zhang, L.; Liu, L.; Ren, X.; Yang, H. Synthesis and microwave absorption property of flexible magnetic film based on graphene oxide/carbon nanotubes and Fe3O4 nanoparticles. J. Mater. Chem. A 2014, 2, 14940–14946. [Google Scholar] [CrossRef]
- Lv, H.; Ji, G.; Liang, X.; Zhang, H.; Hai, Q.; Du, Y. A novel rod-like MnO2@Fe loading on graphene giving excellent electromagnetic absorption properties. J. Mater. Chem. C 2015, 3, 5056–5064. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, G.; Liu, W.; Quan, B.; Liang, X.; Shang, C.; Cheng, Y.; Du, Y. Thermal conversion of an Fe3O4@metal-organic framework: A new method for an efficient Fe-Co/nanoporous carbon microwave absorbing material. Nanoscale 2015, 7, 12932–12942. [Google Scholar] [CrossRef]

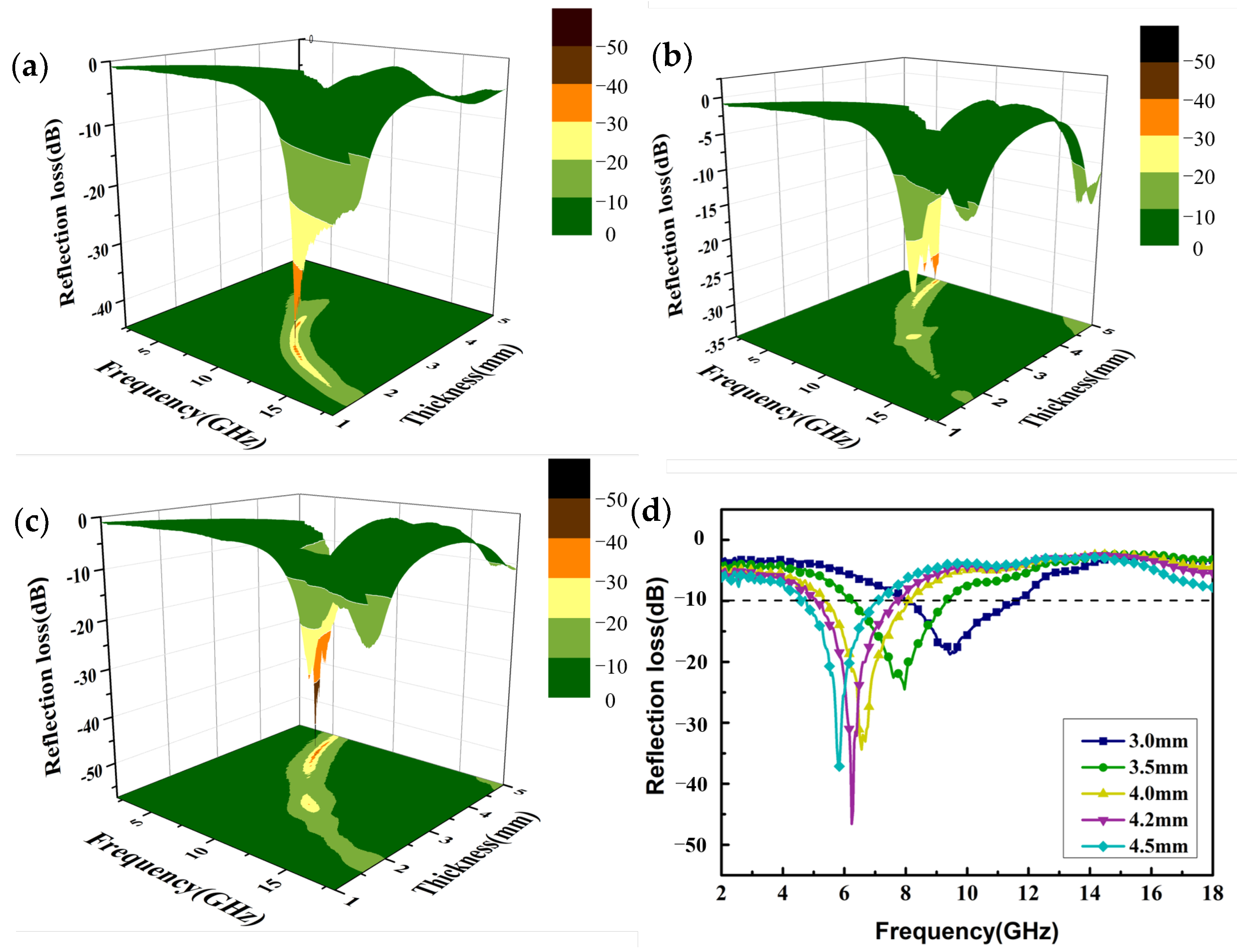
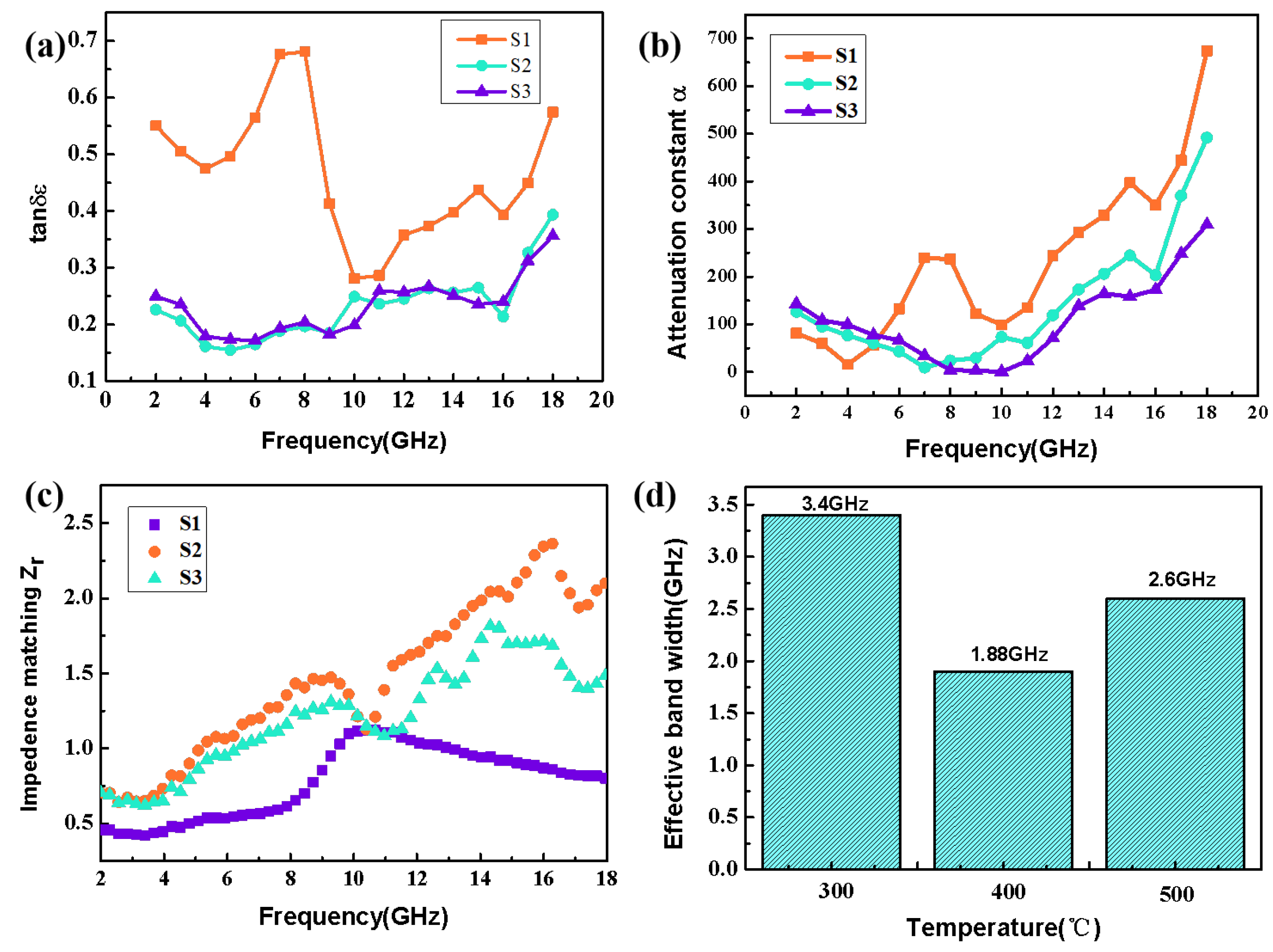

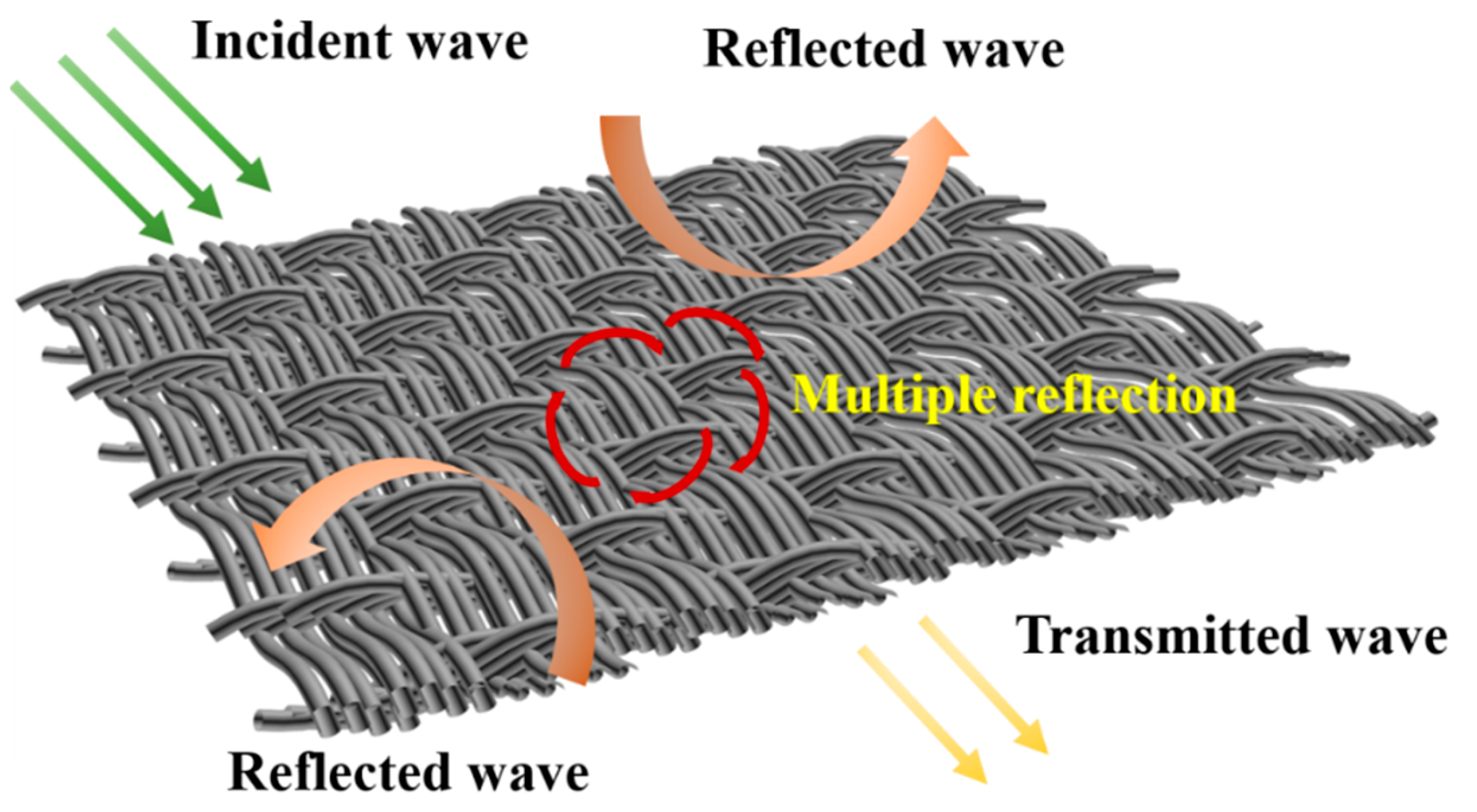
| Filler | RL (dB) | Thickness (mm) | Effective Bandwidth (GHz) | Ref. |
|---|---|---|---|---|
| (Zn0.8Mn0.2)2Y and Co2Z department hexagonal ferrite | −15 | >5 | 3.2 | [40] |
| NiZn Spinel type ferrite | −10 | 12 | 0.7 | [41] |
| Magnesium doped barium ferrite | −10 | 6.0 | 3 | [13] |
| Mg doped lithium zinc ferrite | −39 | 5.5 | 6.5 | [13] |
| Co3O4/CC | −46.59 | 4.2 | 3.04 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, B.; Wu, M.; Yang, X.; Man, Z.; Feng, C.; Liang, X. Lightweight Co3O4/CC Composites with High Microwave Absorption Performance. Nanomaterials 2023, 13, 1903. https://doi.org/10.3390/nano13131903
An B, Wu M, Yang X, Man Z, Feng C, Liang X. Lightweight Co3O4/CC Composites with High Microwave Absorption Performance. Nanomaterials. 2023; 13(13):1903. https://doi.org/10.3390/nano13131903
Chicago/Turabian StyleAn, Bing, Mei Wu, Xinhuang Yang, Zengming Man, Chunyang Feng, and Xiaohui Liang. 2023. "Lightweight Co3O4/CC Composites with High Microwave Absorption Performance" Nanomaterials 13, no. 13: 1903. https://doi.org/10.3390/nano13131903
APA StyleAn, B., Wu, M., Yang, X., Man, Z., Feng, C., & Liang, X. (2023). Lightweight Co3O4/CC Composites with High Microwave Absorption Performance. Nanomaterials, 13(13), 1903. https://doi.org/10.3390/nano13131903






