Amorphous/Nanocrystalline High-Entropy CoCrFeNiTix Thin Films with Low Thermal Coefficient of Resistivity Obtained via Magnetron Deposition
Abstract
1. Introduction
2. Materials and Methods
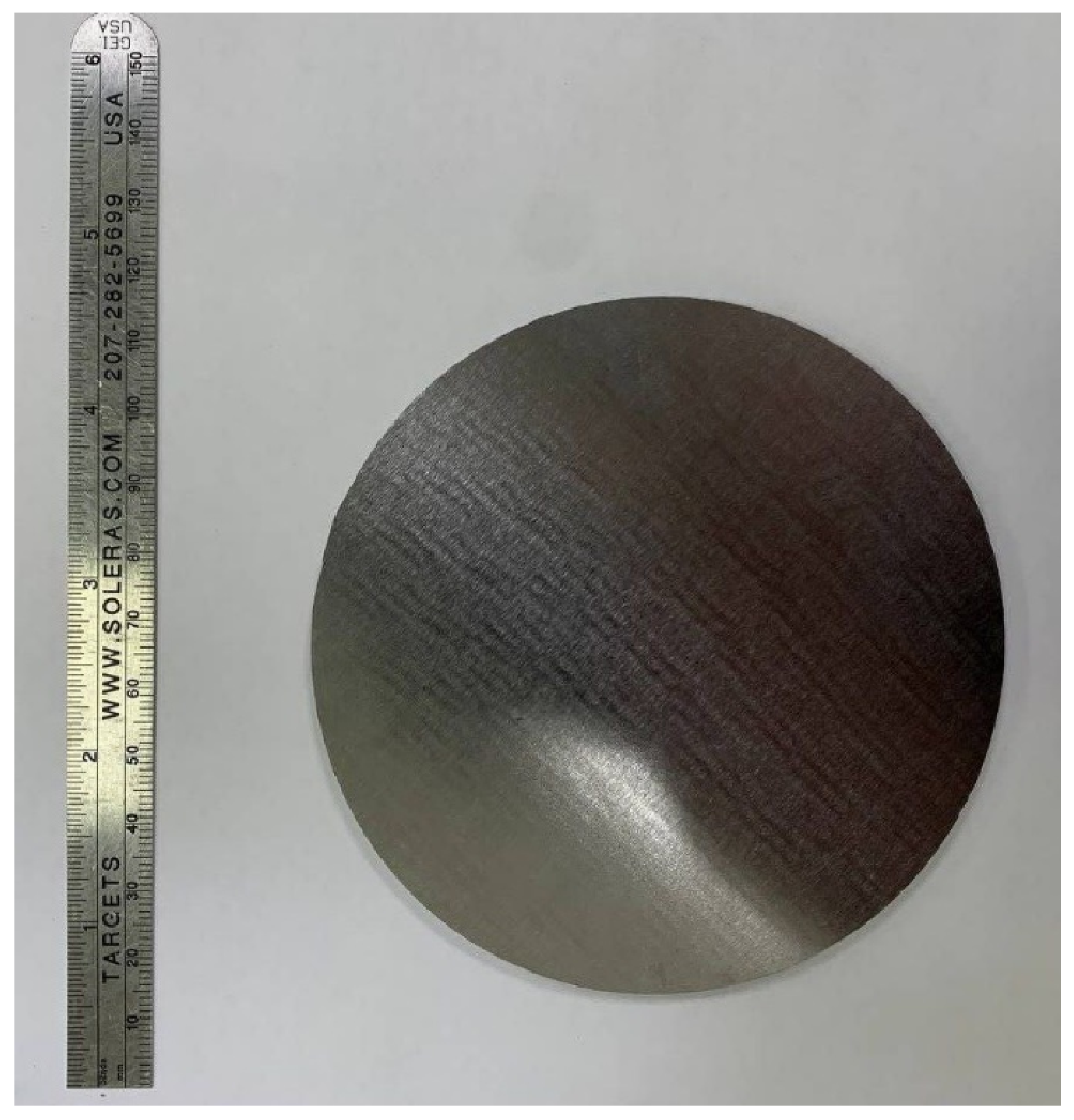
2.1. Target Synthesis
2.2. Magnetron Sputtering of the Film and Preparation of Resistive Elements
2.3. Analyses of the Films
3. Results
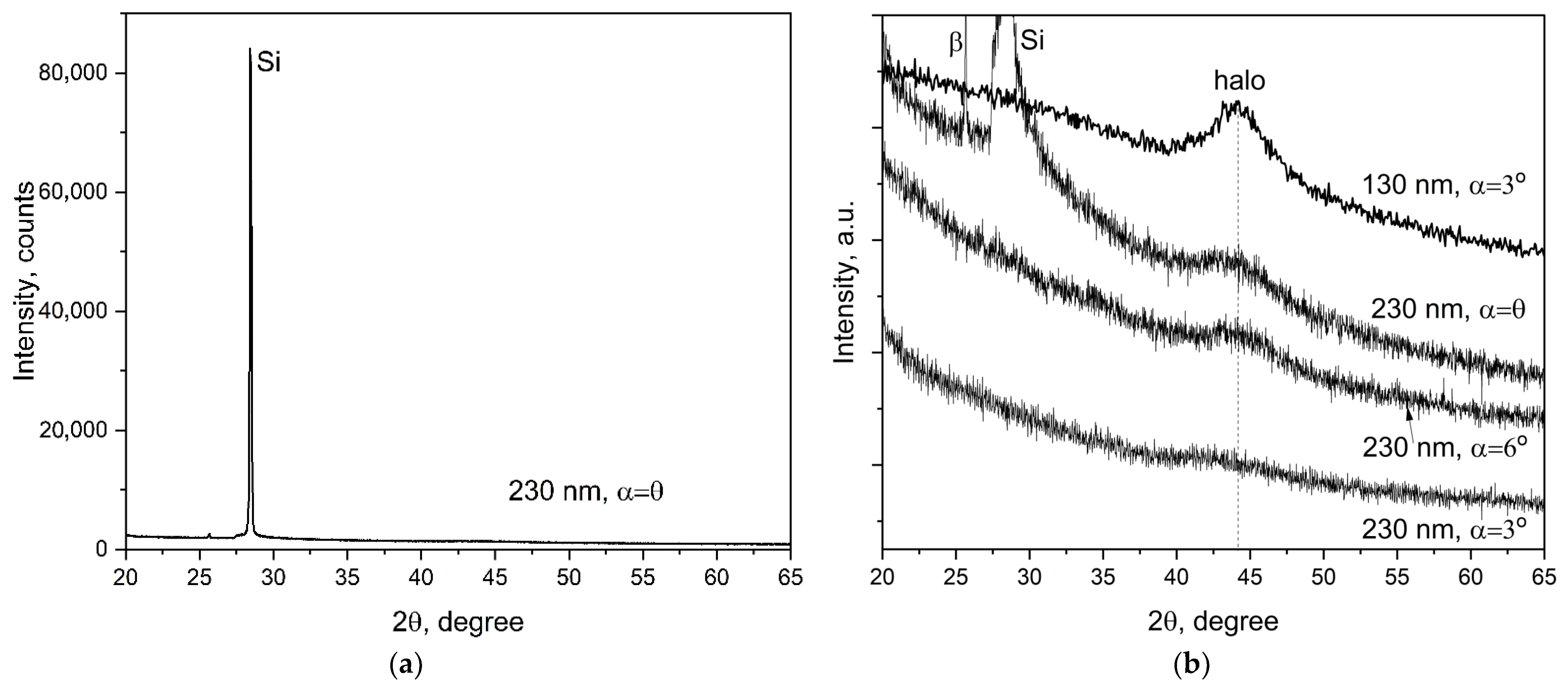
3.1. XRD Data
3.2. Electron Microscopy Data
3.3. Chemical Composition of the Film
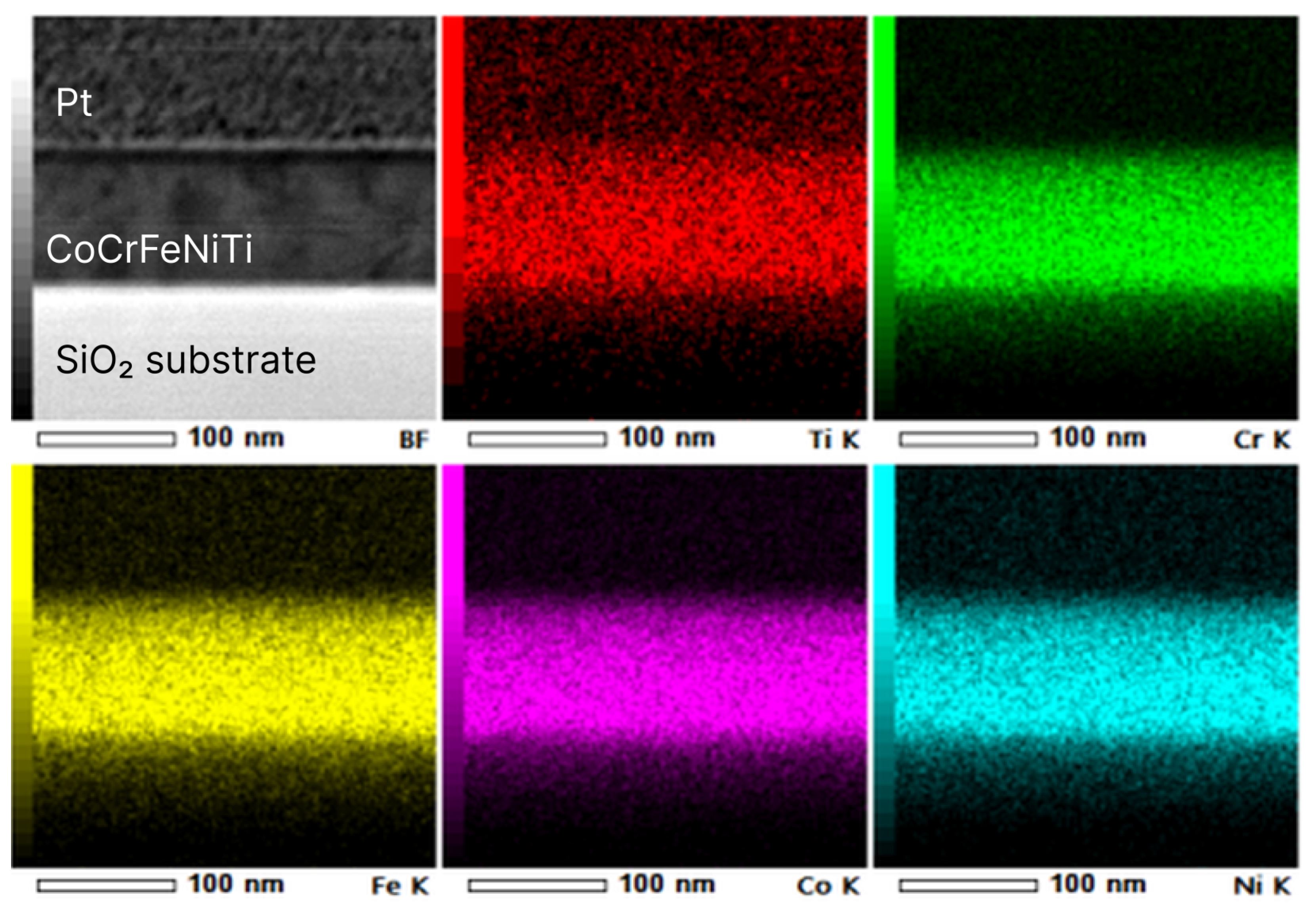
3.3.1. STEM EDX
3.3.2. Auger Electron Spectroscopy
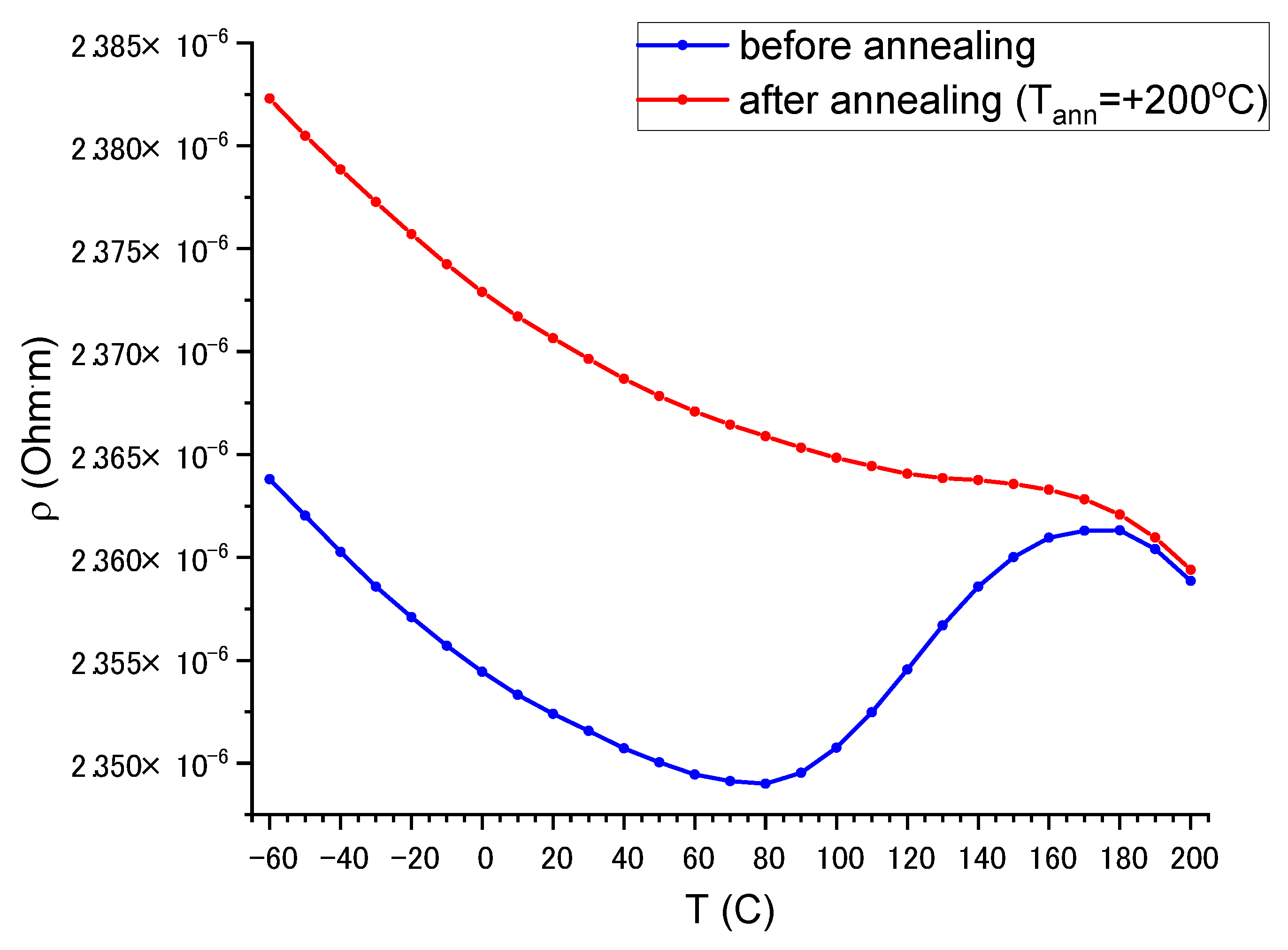
3.4. Thermal Stability of the Film
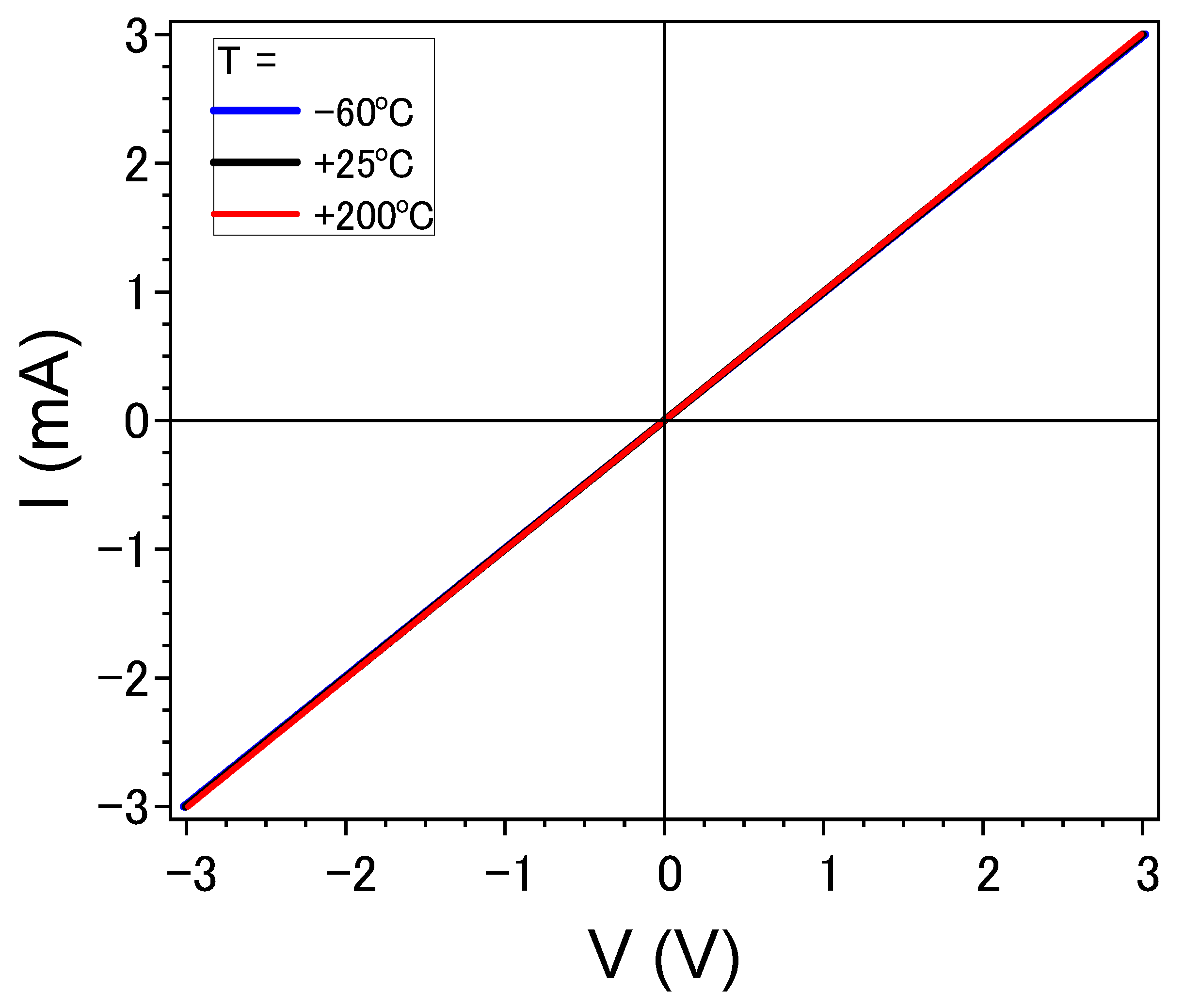
3.5. Electrical Properties of the Thin-Film Resistors
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lukose, C.C.; Zoppi, G.; Birkett, M. Thin film resistive materials: Past, present and future. IOP Conf. Ser. Mater. Sci. Eng. 2016, 104, 012003. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, Z.S.; Bai, T. Investigation on Powder Metallurgy Cr-Si-Ta-Al Alloy Target for High-Resistance Thin Film Resistors with Low Temperature Coefficient of Resistance. Mater. Des. 2010, 31, 1302–1307. [Google Scholar] [CrossRef]
- Matsuda, K.; Sato, K.; Doi, T.; Ogata, K.; Konishi, K. Super precision metal film resistors. Natl. Tech. Rep. 1980, 26, 283–288. [Google Scholar]
- Yeh, J.-W.; Chen, Y.-L.; Lin, S.-J.; Chen, S.-K. High-entropy alloys—A new era of exploration. Mater. Sci. Forum. 2007, 560, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Cantor, B. Multicomponent high-entropy Cantor alloys. Prog. Mater. Sci. 2021, 120, 100754. [Google Scholar] [CrossRef]
- Rogachev, A.S. Structure, stability, and properties of high-entropy alloys. Phys. Met. Met. 2020, 121, 733–764. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.-K.; Gan, J.-Y.; Lin, S.-J.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Formation of simple crystal structures in Cu-Co-Ni-Cr-Al-Fe-Ti-V alloys with multiprincipal metallic elements. Metallurg. Mater. Trans. A 2004, 35A, 2533–2536. [Google Scholar] [CrossRef]
- Yan, X.H.; Li, J.S.; Zhang, W.R.; Zhang, Y. A brief review of high-entropy films. Mater. Chem. Phys. 2017, 210, 12–19. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Wang, X.; Yao, W.; Liang, X. Structure and Properties of High-Entropy Amorphous Thin Films: A Review. JOM 2022, 74, 794–807. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, W.; Xiao, H.; Zhou, L.; Zhu, D.; Yang, S. Fabrication and properties of nanocrystalline Co0.5FeNiCrTi0.5 high entropy alloy by MA–SPS technique. Mater. Des. 2013, 44, 535–539. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, Y.; Dong, Y.; Wang, T.; Cao, Z.; Li, T. Annealing effects on the microstructure and properties of bulk high-entropy CoCrFeNiTi0.5 alloy casting ingot. Intermetallics 2014, 44, 37–43. [Google Scholar] [CrossRef]
- Shun, T.-T.; Chang, L.-Y.; Shiu, M.-H. Microstructures and mechanical properties of multiprincipal component CoCrFeNiTix alloys. Mater. Sci. Eng. A 2012, 556, 170–174. [Google Scholar] [CrossRef]
- Fujieda, T.; Shiratori, H.; Kuwabara, K.; Hirota, M.; Kato, T.; Yamanaka, K.; Koizumi, Y.; Chiba, A.; Watanabe, S. CoCrFeNiTi-based high-entropy alloy with superior tensile strength and corrosion resistance achieved by a combination of additive manufacturing using selective electron beam melting and solution treatment. Mater. Lett. 2017, 189, 148–151. [Google Scholar] [CrossRef]
- Moravcik, I.; Cizek, J.; Zapletal, J.; Kovacova, Z.; Vesely, J.; Minarik, P.; Kitzmantel, M.; Neubauer, E.; Dlouhy, I. Dlouhy, Microstructure and mechanical properties of Ni1.5Co1.5CrFeTi0.5 high entropy alloy fabricated by mechanical alloying and spark plasma sintering. Mater. Des. 2017, 119, 141–150. [Google Scholar] [CrossRef]
- He, F.; Wang, Z.; Li, Y.; Wu, Q.; Li, J.; Wang, J.; Liu, C.T. Kinetic ways of tailoring phases in high entropy alloys. Sci. Rep. 2016, 6, 34628. [Google Scholar] [CrossRef]
- Mishra, R.K.; Shahi, R. A systematic approach for enhancing magnetic properties of CoCrFeNiTi-based high entropy alloys via stoichiometric variation and annealing. J. Alloys Compd. 2019, 821, 153534. [Google Scholar] [CrossRef]
- Zhang, K.; Fu, Z. Effects of annealing treatment on properties of CoCrFeNiTiAlx multi-component alloys. Intermetallics 2012, 28, 34–39. [Google Scholar] [CrossRef]
- Mishra, R.K.; Shahi, R.R. Effect of Annealing on Phase formation and their correlation with magnetic characteristics of TiFeNiCrCo HEA. Mater. Today Proc. 2019, 18, 1422–1429. [Google Scholar] [CrossRef]
- Fujieda, T.; Chen, M.; Shiratori, H.; Kuwabara, K.; Yamanaka, K.; Koizumi, Y.; Chiba, A. Watanabe, Mechanical and corrosion properties of CoCrFeNiTi-based high-entropy alloy additive manufactured using selective laser melting. Addit. Manuf. 2019, 25, 412–420. [Google Scholar] [CrossRef]
- Ikeda, T.; Yonehara, M.; Ikeshoji, T.-T.; Nobuki, T.; Hatate, M.; Kuwabara, K.; Otsubo, Y.; Kyogoku, H. Influences of Process Parameters on the Microstructure and Mechanical Properties of CoCrFeNiTi Based High-Entropy Alloy in a Laser Powder Bed Fusion Process. Crystals 2021, 11, 549. [Google Scholar] [CrossRef]
- Liu, H.; Gao, W.; Liu, J.; Du, X.; Li, X.; Yang, H. Microstructure and Properties of CoCrFeNiTi High-Entropy Alloy Coating Fabricated by Laser Cladding. J. Mater. Eng. Perform. 2020, 29, 7170–7178. [Google Scholar] [CrossRef]
- Zhang, S.; Han, B.; Li, M.; Zhang, Q.; Hu, C.; Niu, S.; Li, Z.; Wang, Y. Investigation on solid particles erosion resistance of laser cladded CoCrFeNiTi high entropy alloy coating. Intermetallics 2021, 131, 107111. [Google Scholar] [CrossRef]
- Phuong, N.M.; Kim, D.-J.; Kang, B.-D.; Kim, C.S.; Yoon, S.-G. Effect of Chromium Concentration on the Electrical Properties of NiCr Thin Films Resistor Deposited at Room Temperature by Magnetron Cosputtering Technique. J. Electro Chem. Soc. 2006, 153, G27. [Google Scholar] [CrossRef]
- Lai, L.; Fu, X.; Sun, R.; Du, R. Comparison of Microstructure and Electrical Properties of NiCr Alloy Thin Film Deposited on Different Substrates. Surf. Coat. Technol. 2013, 235, 552–560. [Google Scholar] [CrossRef]
- Lee, D.W.; Kim, Y.N.; Cho, M.Y.; Ko, P.J.; Lee, D.; Koo, S.M.; Moon, K.S.; Oh, J.M. Reliability and Characteristics of Magnetron Sputter Deposited Tantalum Nitride for Thin Film Resistors. Thin Solid Film. 2018, 660, 688–694. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Ing, N.G.; Gopalakrishnan, R. Reactive sputter deposition and characterization of tantalum nitride thin films. Mater. Sci. Eng. B 1999, 57, 224–227. [Google Scholar] [CrossRef]
- Wang, K.Y.; Chang, T.C.; Chen, W.C.; Zhang, Y.C.; Tseng, Y.T.; Yang, C.C.; Lin, C.C.; Wu, P.Y.; Tan, Y.F.; Tsai, T.M. Investigations on TaHf Alloys for Thin Film Resistor Applications. Mater. Chem. Phys. 2022, 285, 126027. [Google Scholar] [CrossRef]
- Lin, C.H.; Lee, H.Y.; Tseng, Y.T.; Lee, Y.C. A Study on the NiCrMnZr Thin Film Resistors Prepared Using the Magnetron Sputtering Technique. Thin Solid Film. 2018, 660, 695–704. [Google Scholar] [CrossRef]
- Lin, R.C.; Lee, T.K.; Wu, D.H.; Lee, Y.C. A Study of Thin Film Resistors Prepared Using Ni-Cr-Si-Al-Ta High Entropy Alloy. Adv. Mater. Sci. Eng. 2015, 2015, 847191. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Li, Z.; Wang, Q.; Zheng, Y.; Ma, Y.; Bi, L.; Zhang, Y.; Yuan, X.; Zhang, X.; et al. The Resistivity–Temperature Behavior of AlxCoCrFeNi High-Entropy Alloy Films. Thin Solid Film. 2020, 700, 137895. [Google Scholar] [CrossRef]
- Kim, H.; Nam, S.; Roh, A.; Son, M.; Ham, M.H.; Kim, J.H.; Choi, H. Mechanical and Electrical Properties of NbMoTaW Refractory High-Entropy Alloy Thin Films. Int. J. Refract. Met. Hard Mater. 2019, 80, 286–291. [Google Scholar] [CrossRef]
- Huo, W.; Liu, X.; Tan, S.; Fang, F.; Xie, Z.; Shang, J.; Jiang, J. Ultrahigh Hardness and High Electrical Resistivity in Nano-Twinned, Nanocrystalline High-Entropy Alloy Films. Appl. Surf. Sci. 2018, 439, 222–225. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Zhao, Y.L.; Kai, J.J.; Liu, C.T.; Hsueh, C.H. Nanotwinned CoCrFeMnNi High Entropy Alloy Films for Flexible Electronic Device Applications. Vacuum 2021, 189, 110249. [Google Scholar] [CrossRef]
- Rogachev, A.; Vadchenko, S.; Kovalev, D.; Kochetov, N.; Zhukovskyi, M.; Orlova, T.; Mukasyan, A. Long term stability of a high-entropy CoCrFeNiTi alloy fabricated by mechanical alloying. J. Alloys Compd. 2023, 931, 167470. [Google Scholar] [CrossRef]
- Briggs, D.; Seah, M.P. Practical Surface Analysis by Auger and X-Ray Photoelectron Spectroscopy; Practical Surface Analysis; Wiley: Hoboken, NJ, USA, 1983; ISBN 047126279X. [Google Scholar]
- Monakhova, Y.B.; Myshtakova, S.P. Mathematical treatment of spectra in analysis of mixtures by independent components method: Identification and quantitative analysis. J. Anal. Chem. 2012, 67, 1044–1051. (In Russian) [Google Scholar]





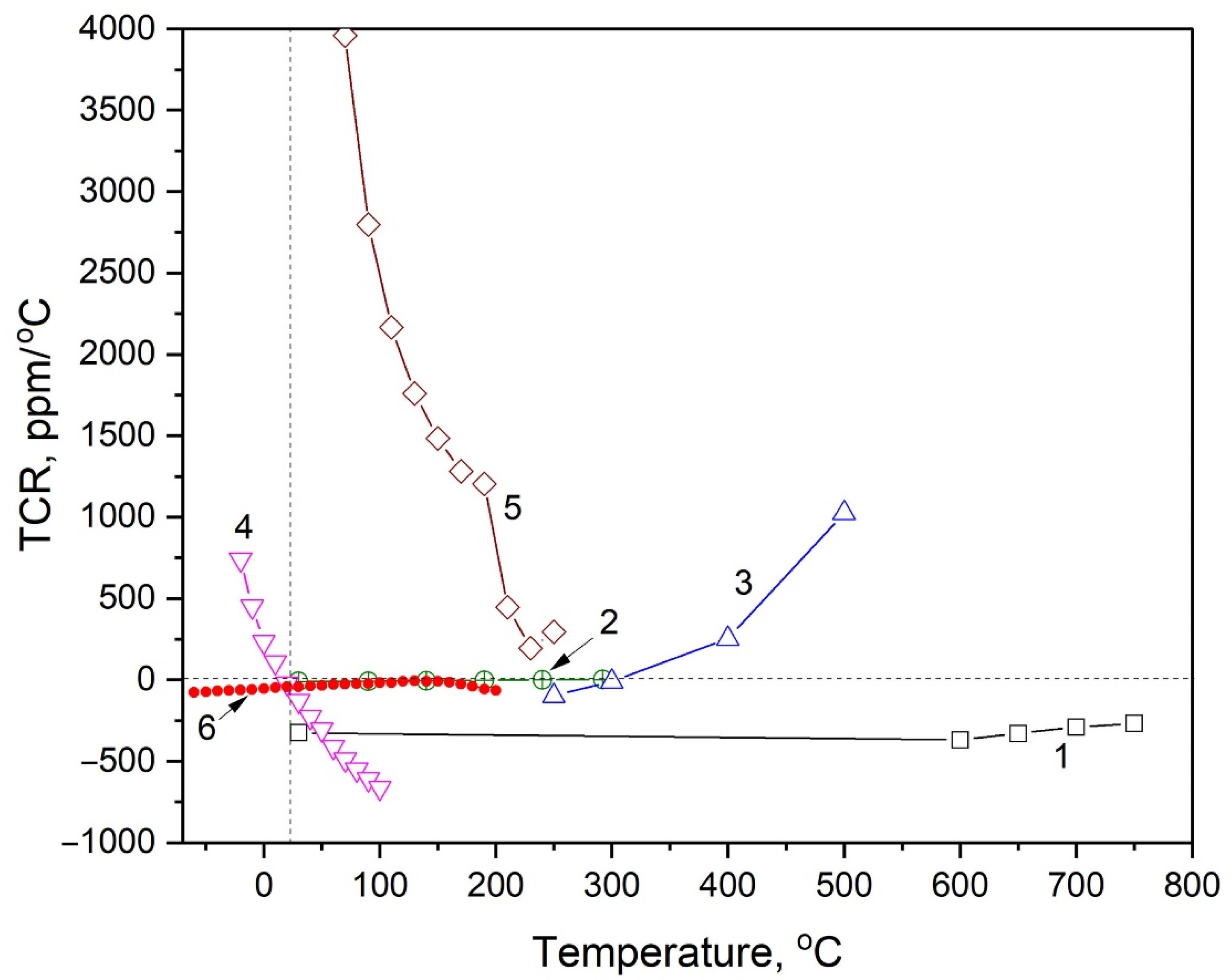
| Alloy | Power, W (DC) | Working Pressure, Pa | Microstructure | Annealing, °C | Material of Substrate | Ω, 10−6 Ohm·m | TCR, ppm/°C | Thikness, nm | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| NiCr | 40/70 * | 0.67 | amorphous | No | SiO2 (600 nm)/Si | 2.4 | ±10 | 100 | [26] |
| NiCr | 405 | 0.85 | amorphous with Ni (111) | 350 | Si | 0.74 | ~6000 (RT) | 86 | [27] |
| TaN | 700 | 0.13 | amorphous | 300 | SiO2 (50 nm)/Si (100) | 13,860 | −67 (RT) | 198 | [28] |
| TaN | 250 | 0.93 | TaN | 250 | GaAs | 4.8 | −200 (RT) | 600–750 | [29] |
| TaHf | 100 ** | 0.5 | Hf6O17Ta2 (200) | No | Si | 5.7 | −330 (RT) | 120 | [30] |
| NiCrSiAlTa | 100 | 0.57 | amorphous | 300 | Al2O3 | 22.15 | −10 (300 °C) | 80 | [32] |
| Al0.7CoCrFeNi | 100 *** | 1.4 | FCC and BCC | No | Si (100) | 5.36 | −5 (RT to 292 °C) | 570 | [33] |
| NiCrMnZr | 50/30 **** | 0.4 | amorphous | 300 | Al2O3 | 5.1 | 53 (from 25 to 125 °C) | 80 | [31] |
| NbMoTaW | 50 | 0.67 | BCC | No | Si (100) | 1.68 | None | 350 | [34] |
| Elements | ρ, g/cm3 | at.% | AEM | g/mol | wt.% |
|---|---|---|---|---|---|
| Co | 8.9 | 22 | 58.93 | 12.9646 | 23.3 |
| Cr | 7.19 | 23 | 51.996 | 11.95908 | 21.5 |
| Fe | 7.874 | 29 | 55.845 | 16.19505 | 29.1 |
| Ni | 8.904 | 20 | 58.6934 | 11.73868 | 21.1 |
| Ti | 4.54 | 6 | 47.867 | 2.87202 | 5.2 |
| Name | Power, W | Working Pressure, Pa | Duration, s | Working Gas | Thikness, nm |
|---|---|---|---|---|---|
| Specimen 1 | 500 | 0.1 | 75 | Ar | 130 |
| Specimen 2 | 500 | 0.1 | 180 | Ar | 230 |
| Name | Incident Angle, ° | Registration Interval, ° | Scan Step, ° | Time at Point, Seconds |
|---|---|---|---|---|
| Specimen 1 | 3 | 10–90 | 0.07 | 1 |
| Specimen 2 | 3 and 6 | 25–100 | 0.02 | 3 |
| Atomic Concentrations of the Elements, at.% | ||||
|---|---|---|---|---|
| Co | Cr | Fe | Ni | Ti |
| 25.2 | 24.9 | 25.5 | 19.8 | 4.6 |
| Atomic Concentrations of the Elements, at.% | |||||
|---|---|---|---|---|---|
| Co | Cr | Fe | Ni | Ti | O |
| 25.17 | 26.73 | 21.8 | 18.95 | 3.42 | 3.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poliakov, M.; Kovalev, D.; Vadchenko, S.; Moskovskikh, D.; Kiryukhantsev-Korneev, P.; Volkova, L.; Dudin, A.; Orlov, A.; Goryachev, A.; Rogachev, A. Amorphous/Nanocrystalline High-Entropy CoCrFeNiTix Thin Films with Low Thermal Coefficient of Resistivity Obtained via Magnetron Deposition. Nanomaterials 2023, 13, 2004. https://doi.org/10.3390/nano13132004
Poliakov M, Kovalev D, Vadchenko S, Moskovskikh D, Kiryukhantsev-Korneev P, Volkova L, Dudin A, Orlov A, Goryachev A, Rogachev A. Amorphous/Nanocrystalline High-Entropy CoCrFeNiTix Thin Films with Low Thermal Coefficient of Resistivity Obtained via Magnetron Deposition. Nanomaterials. 2023; 13(13):2004. https://doi.org/10.3390/nano13132004
Chicago/Turabian StylePoliakov, Maksim, Dmitry Kovalev, Sergei Vadchenko, Dmitry Moskovskikh, Philipp Kiryukhantsev-Korneev, Lidiya Volkova, Alexander Dudin, Andrey Orlov, Andrey Goryachev, and Alexander Rogachev. 2023. "Amorphous/Nanocrystalline High-Entropy CoCrFeNiTix Thin Films with Low Thermal Coefficient of Resistivity Obtained via Magnetron Deposition" Nanomaterials 13, no. 13: 2004. https://doi.org/10.3390/nano13132004
APA StylePoliakov, M., Kovalev, D., Vadchenko, S., Moskovskikh, D., Kiryukhantsev-Korneev, P., Volkova, L., Dudin, A., Orlov, A., Goryachev, A., & Rogachev, A. (2023). Amorphous/Nanocrystalline High-Entropy CoCrFeNiTix Thin Films with Low Thermal Coefficient of Resistivity Obtained via Magnetron Deposition. Nanomaterials, 13(13), 2004. https://doi.org/10.3390/nano13132004









