Gold Nanorods as Radiopharmaceutical Carriers: Preparation and Preliminary Radiobiological In Vitro Tests
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials for AuNR Synthesis and Conjugation
2.2. Characterizations
2.3. AuNR Synthesis
2.4. Preparation of Conjugate Nanorods
2.5. Cell Culture
2.6. Irradiation
2.7. Colony-Forming Assay
3. Results
3.1. AuNR Synthesis and Loading Studies
3.2. Synchrotron Radiation-Induced X-ray Photoelectron Spectral (SR-XPS) Studies
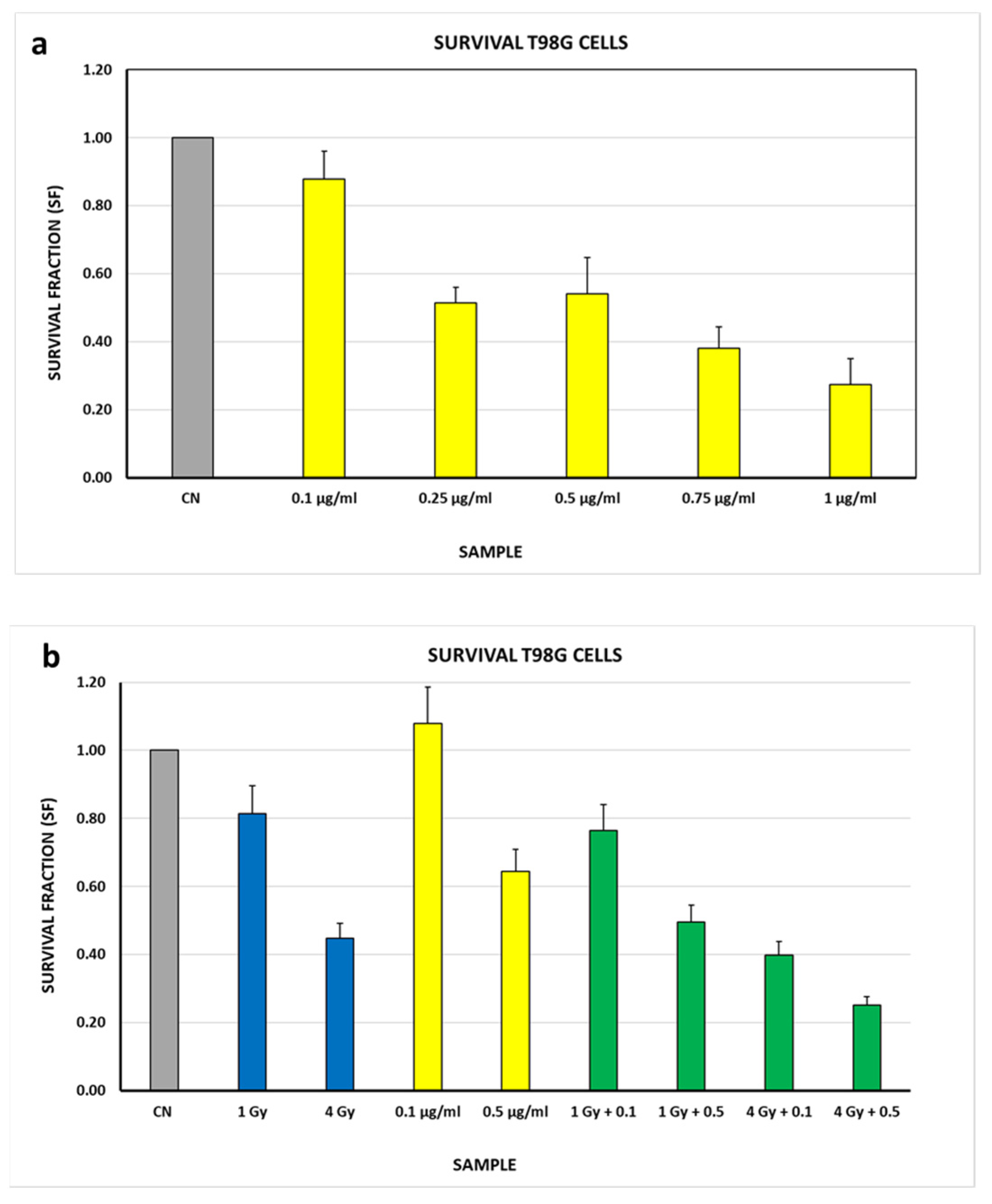
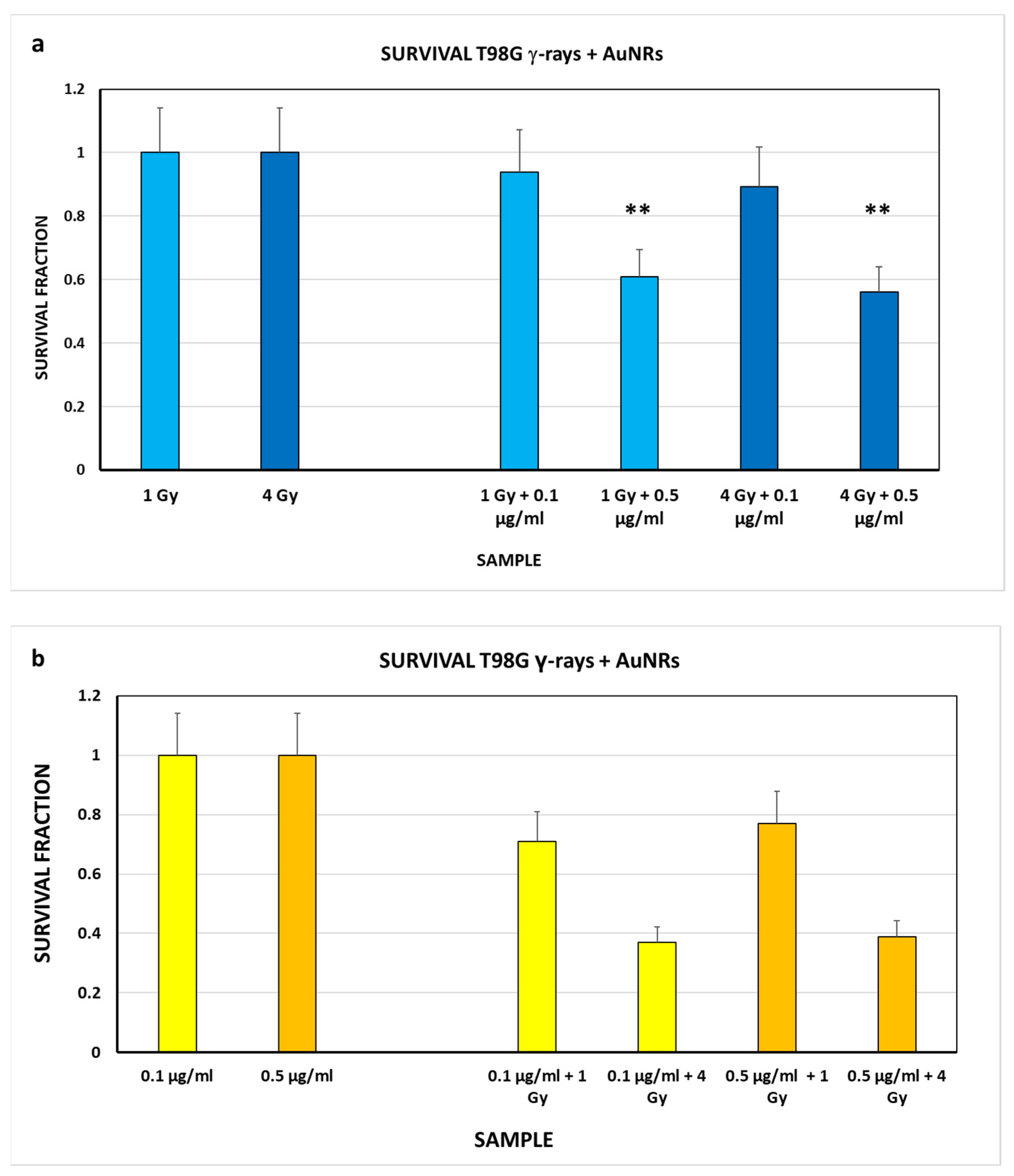
3.3. Biological Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, M.A.; Pirzada, B.M.; Price, G.; Shibiru, A.L.; Qurashi, A. Applications of nanotechnology in smart textile industry: A critical review. J. Adv. Res. 2022, 38, 55–75. [Google Scholar] [CrossRef]
- Corsi, I.; Venditti, I.; Trotta, F.; Punta, C. Environmental safety of nanotechnologies: The eco-design of manufactured nanomaterials for environmental remediation. Sci. Total Environ. 2023, 864, 161181. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A Revolution in Modern Industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef]
- Jorfi, M.; Roberts, M.N.; Foster, E.J.; Weder, C. Physiologically Responsive, Mechanically Adaptive Bio-Nanocomposites for Biomedical Applications. ACS Appl. Mater. Interfaces 2013, 5, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, M.; Das, D.B.; Salem, Z.A.; Beherei, H.H. Nanomaterials for Biomedical Applications: Production, Characterisations, Recent Trends and Difficulties. Molecules 2021, 26, 1077. [Google Scholar] [CrossRef] [PubMed]
- Venditti, I. Engineered gold-based nanomaterials: Morphologies and functionalities in biomedical applications. A mini review. Bioengineering 2019, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Kus-Liśkiewicz, M.; Fickers, P.; Tahar, I.B. Biocompatibility and Cytotoxicity of Gold Nanoparticles: Recent Advances in Methodologies and Regulations. Int. J. Mol. Sci. 2021, 22, 10952. [Google Scholar] [CrossRef] [PubMed]
- Fratoddi, I.; Venditti, I.; Battocchio, C.; Carlini, L.; Porchia, M.; Tisato, F.; Bondino, F.; Magnano, E.; Pellei, M.; Santini, C. Highly hydrophilic gold nanoparticles as carrier for anticancer copper(I) complexes: Loading and release studies for biomedical applications. Nanomaterials 2019, 9, 772. [Google Scholar] [CrossRef]
- Ozcicek, I.; Aysit, N.; Cakici, C.; Aydeger, A. The effects of surface functionality and size of gold nanoparticles on neuronal toxicity, apoptosis, ROS production and cellular/suborgan biodistribution. Mater. Sci. Eng. C 2012, 128, 112308. [Google Scholar] [CrossRef]
- Carnovale, C.; Bryant, G.; Shukla, R.; Bansal, V. Identifying Trends in Gold Nanoparticle Toxicity and Uptake: Size, Shape, Capping Ligand, and Biological Corona. ACS Omega 2019, 4, 242. [Google Scholar] [CrossRef]
- Guo, J.; Armstrong, M.J.; O’Driscoll, C.M.; Holmes, J.D.; Rahme, K. Positively charged, surfactant-free gold nanoparticles for nucleic acid delivery. RSC Adv. 2015, 5, 17862. [Google Scholar] [CrossRef]
- Sano, K.; Ishida, Y.; Tanaka, T.; Mizukami, T.; Nagayama, T.; Haratake, Y.; Munekane, M.; Yamasaki, T.; Mukai, T. Enhanced Delivery of Thermoresponsive Polymer-Based Medicine into Tumors by Using Heat Produced from Gold Nanorods Irradiated with Near-Infrared Light. Cancers 2021, 13, 5005. [Google Scholar] [CrossRef] [PubMed]
- Maccora, D.; Dini, V.; Battocchio, C.; Fratoddi, I.; Cartoni, A.; Rotili, D.; Castagnola, M.; Faccini, R.; Bruno, I.; Scotognella, T.; et al. Gold nanoparticles and nanorods in nuclear medicine: A mini review. Appl. Sci. 2019, 9, 3232. [Google Scholar] [CrossRef]
- Jelveh, S.; Chithrani, D.B. Gold Nanostructures as a Platform for Combinational Therapy in Future Cancer Therapeutics. Cancers 2011, 3, 1081–1110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Chen, C. Gold nanorods based platforms for light-mediated theranostics. Theranostics 2013, 3, 223–238. [Google Scholar] [CrossRef]
- Zeng, H.; Du, X.; Singh, S.C.; Kulinich, S.A.; Yang, S.; He, J.; Cai, W. Nanomaterials via Laser Ablation/Irradiation in Liquid: A Review. Adv. Funct. Mater. 2012, 22, 1333–1353. [Google Scholar] [CrossRef]
- Goddarda, Z.R.; Marín, M.J.; Russell, D.A.; Searcey, M. Active targeting of gold nanoparticles as cancer therapeutics. Chem. Soc. Rev. 2020, 49, 8774–8789. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Faure, G. Principles of Isotope Geology, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1986; pp. 15–23. [Google Scholar]
- González-Ruíz, A.; Ferro-Flores, G.; Jiménez-Mancilla, N.; Castellanos, A.E.; Ocampo-García, B.; Luna-Gutiérrez, M.; Santos-Cuevas, C.; Morales-Avila, E.; Isaac-Olivé, K. In vitro and in vivo synergistic effect of radiotherapy and plasmonic photothermal therapy on the viability of cancer cells using 177Lu–Au-NLS-RGD-Aptamer nanoparticles under laser irradiation. J. Radioanal. Nucl. Chem. 2018, 318, 1913–1921. [Google Scholar] [CrossRef]
- Pijeira, M.S.O.; Viltres, H.; Kozempel, J.; Sakmár, M.; Vlk, M.; İlem-Özdemir, D.; Ekinci, M.; Srinivasan, S.; Rajabzadeh, A.R.; Ricci-Junior, E.; et al. Radiolabeled nanomaterials for biomedical applications: Radiopharmacy in the era of nanotechnology. EJNMMI Radiopharm. Chem. 2022, 7, 8. [Google Scholar] [CrossRef]
- Saleh, T.B. Basic Sciences of Nuclear Medicine; Khalil, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Burdak-Rothkamm, S.; Prise, K.M. New molecular targets in radiotherapy: DNA damage signalling and repair in targeted and non-targeted cells. Eur. J. Pharmacol. 2009, 625, 151–155. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freudenberg, R.; Runge, R.; Maucksch, U.; Berger, V.; Kotzerke, J. On the dose calculation at the cellular level and its implications for the RBE of 99mTc and 123I. Med. Phys. 2014, 41, 062503. [Google Scholar] [CrossRef]
- Tavares, A.A.; Tavares, J.M. (99m)Tc Auger electrons for targeted tumourtherapy: A review. Int. J. Radiat. Biol. 2010, 86, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Ku, A.; Facca, V.J.; Cai, Z.; Reilly, R.M. Auger electrons for cancer therapy—A review. EJNMMI Radiopharm. Chem. 2019, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Choppin, G.R.; Liljenzin, J.-O.; Rydberg, J. Absorption of Nuclear Radiation. Radiochem. Nucl. Chem. 2002, 1, 123–165. [Google Scholar]
- Puck, T.T.; Marcus, P.I. Action of X-rays on mammalian cells. J. Exp. Med. 1956, 103, 653–666. [Google Scholar] [CrossRef]
- Moreau, L.; Jones, M.; Roth, E.; Wu, J.; Kewalramani, S.; O’Brien, M.; Chen, B.-R.; Mirkin, C.A.; Bedzyk, M. The role of trace Ag in the synthesis of Au nanorods. Nanoscale 2019, 11, 11744–11754. [Google Scholar] [CrossRef]
- Calzolai, L.; Gilliland, D.; Rossi, F. Measuring nanoparticles size distribution in food and consumer products: A review. Food Addit. Contam. 2012, 29, 1183–1193. [Google Scholar] [CrossRef]
- Venditti, I.; Iucci, G.; Fratoddi, I.; Cipolletti, M.; Montalesi, E.; Marino, M.; Secchi, V.; Battocchio, C. Directly Resveratrol immobilization on hydrophilic charged gold nanoparticles: Structural investigations and cytotoxic studies. Nanomaterials 2020, 10, 1898. [Google Scholar] [CrossRef]
- Venditti, I.; Cartoni, A.; Fontana, L.; Testa, G.; Scaramuzzo, F.A.; Faccini, R.; Terracciano, C.M.; Camillocci, E.S.; Morganti, S.; Giordano, A.; et al. Y3+ embedded in polymeric nanoparticles: Morphology, dimension and stability of composite colloidal system. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 125–131. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database; Version 4.1; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012. Available online: http://srdata.nist.gov/xps/ (accessed on 1 April 2023).
- Venditti, I.; Cartoni, A.; Cerra, S.; Fioravanti, R.; Salamone, T.A.; Sciubba, F.; Tabocchini, M.A.; Dini, V.; Battocchio, C.; Iucci, G.; et al. Hydrophilic Gold Nanoparticles as anti-PD-L1 Antibody carriers: Synthesis and Interface Properties. Part. Part. Syst. Charact. 2022, 39, 2100282. [Google Scholar] [CrossRef]
- Stumpf, T.; Foerstendorf, H.; Bok, F.; Richter, A. Annual Report 2018; Institute of Resource Ecology: Dresden, Germany, 2019. [Google Scholar]
- Vales, G.; Suhonen, S.; Siivola, K.M.; Savolainen, K.M.; Catalán, J.; Norppa, H. Genotoxicity and Cytotoxicity of Gold Nanoparticles In Vitro: Role of Surface Functionalization and Particle Size. Nanomaterials 2020, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Abu-Dahab, R.; Mahmoud, N.N.; Abdallah, M.; Hamadneh, L.; Hikmat, S.; Zaza, R.; Abuarqoub, D.; Khalil, E.A. Cytotoxicity and Cellular Death Modality of Surface-Decorated Gold Nanorods against a Panel of Breast Cancer Cell Lines. ACS Omega 2021, 6, 15903. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhang, X.; Chu, C.; Dong, Y.; Zhang, T.; Li, X.; Liu, G.; Cai, W.; Han, S. Preparation, toxicity reduction and radiation therapy application of gold nanorods. J. Nanobiotechnol. 2021, 19, 454. [Google Scholar] [CrossRef]
- Xu, X.; Ding, Y.; Hadianamrei, R.; Lv, S.; You, R.; Pan, F.; Zhang, P.; Wang, N.; Zhao, X. Antimicrobial peptide functionalized gold nanorods combining near-infrared photothermal therapy for effective wound healing. Colloids Surf. B Biointerfaces 2022, 220, 112887. [Google Scholar] [CrossRef] [PubMed]
- Boglaienko, D.; Soltis, J.; Kukkadapu, R.; Du, Y.; Sweet, L.; Holfeltz, V.; Hall, G.; Buck, E.; Segre, C.; Emerson, H.; et al. Spontaneous redox continuum reveals sequestered technetium clusters and retarded mineral transformation of iron. Commun. Chem. 2020, 3, 87. [Google Scholar] [CrossRef]
- Chew, M.T.; Bradley, D.A.; Suzuki, M.; Matsufuji, N.; Murakami, T.; Jones, B.; Nisbet, A. The radiobiological effects of He, C and Ne ions as a function of LET on various glioblastoma cell lines. J. Radiat. Res. 2019, 60, 178–188. [Google Scholar] [CrossRef]
- Burdak-Rothkamm, S.; Smith, A.; Lobachevsky, P.; Martin, R.; Prise, K.M. Radioprotection of targeted and bystander cells by methylproamine. Strahlenther. Onkol. 2015, 191, 248–255. [Google Scholar] [CrossRef]
- Short, S.; Mayes, C.; Woodcock, M.; Johns, H.; Joiner, M.C. Low dose hypersensitivity in the T98G human glioblastoma cell line. Int. J. Radiat. Biol. 1999, 75, 847–855. [Google Scholar] [CrossRef]

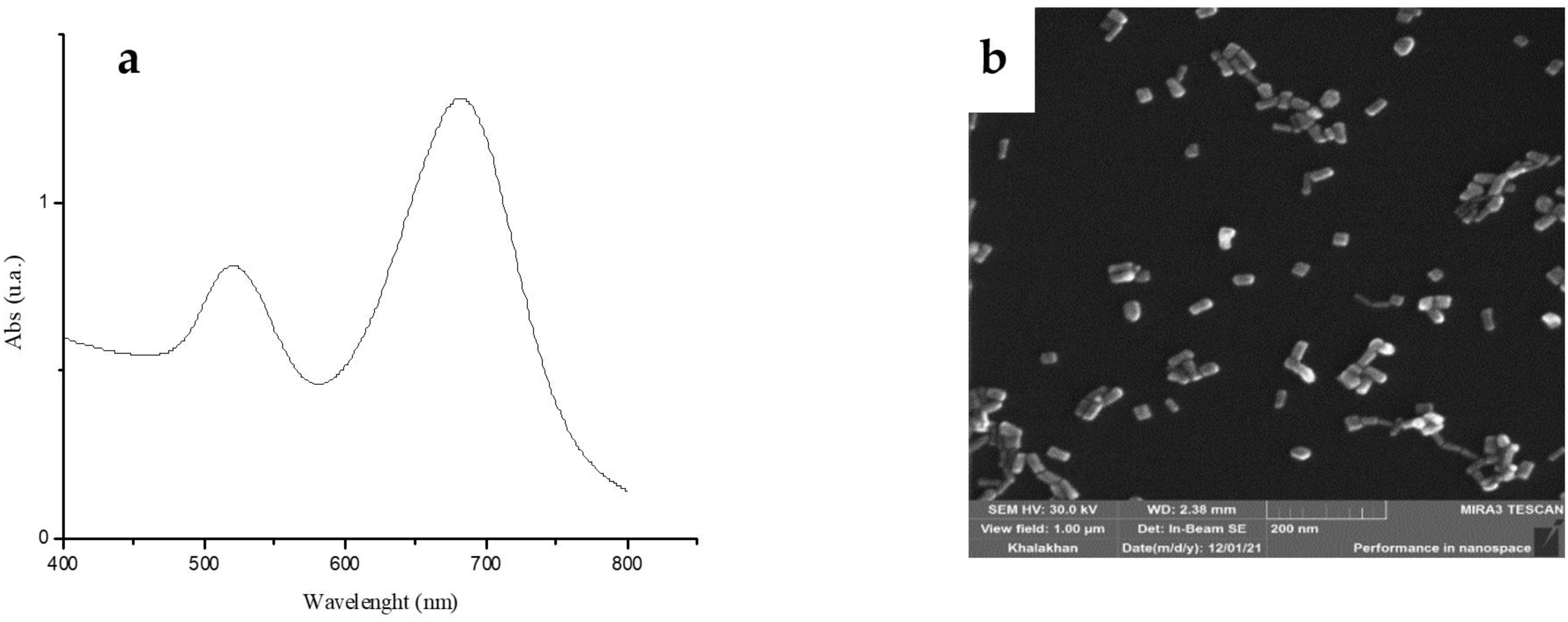
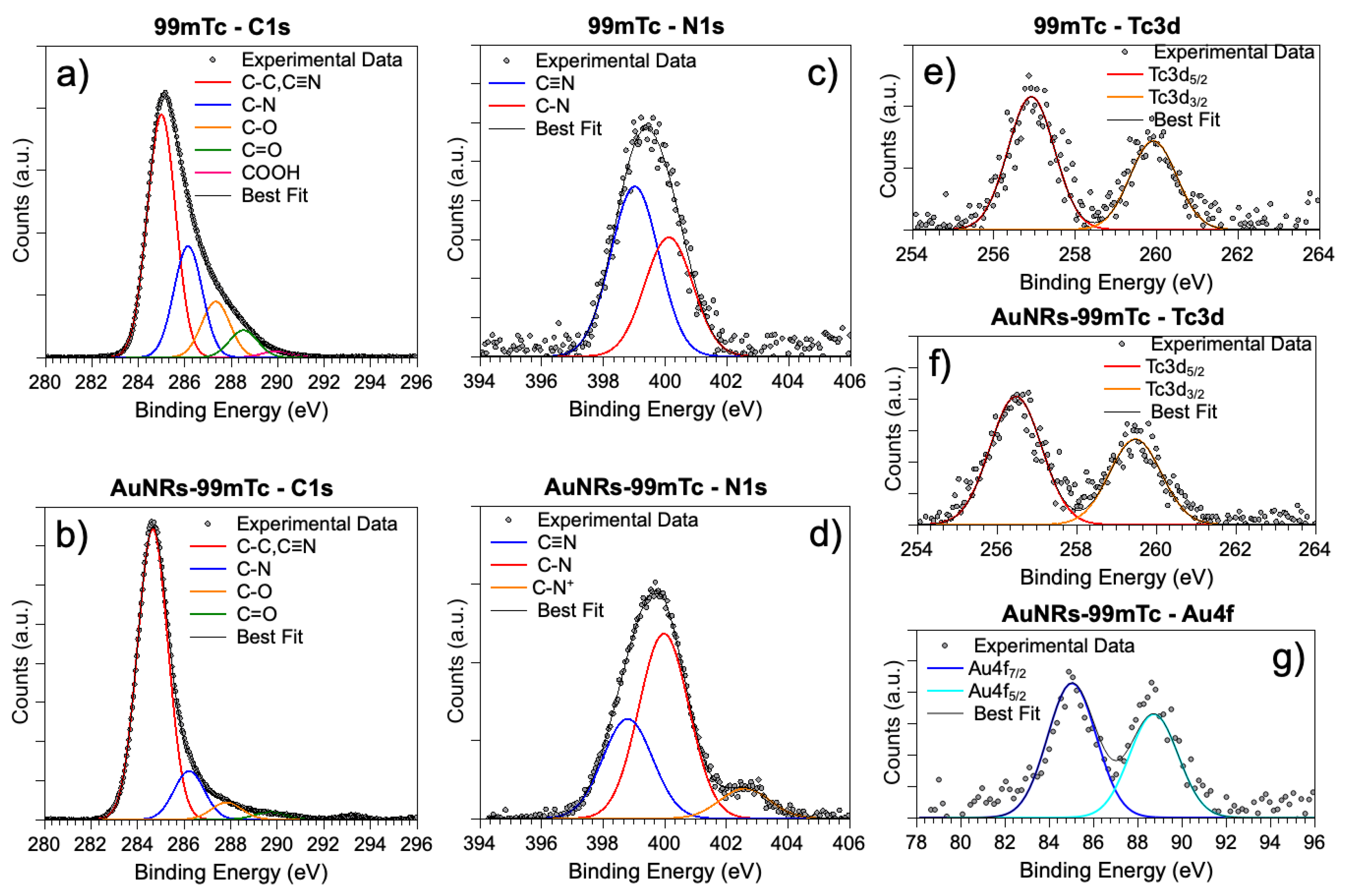
| CTAB mL (M) | HAuCl4 mL (M) | AgNO3 μL (M) | AA μL (M) | Seed Solution, μL |
|---|---|---|---|---|
| 10 (0.2) | 10 (0.001) | 400 (0.004) | 70 (0.078) | 24 |
| Sample | Dose (Gy) | AuNRs (μg/mL) | Cells Plated |
|---|---|---|---|
| CN | - | - | 400 |
| 0.1 μg/mL | - | 0.1 | 400 |
| 0.5 μg/mL | - | 0.5 | 400 |
| 1 Gy | 1 | - | 400 |
| 4 Gy | 4 | - | 1000 |
| 1a | 1 | 0.1 | 900 |
| 2a | 1 | 0.5 | 2000 |
| 3a | 4 | 0.1 | 3000 |
| 4a | 4 | 0.5 | 4000 |
| Sample | SF (see Figure 3b) | Sample | SF (see Figure 4b) | Effect of Auger Electrons |
|---|---|---|---|---|
| CN | 1 ± 0.14 | 0.1 μg/mL 0.5 μg/mL | 1 ± 0.14 1 ± 0.14 | - - |
| 1 Gy | 0.81 ± 0.14 | 0.1 μg/mL + 1 Gy 0.5 μg/mL + 1 Gy | 0.71 ± 0.10 0.77 ± 0.11 | 0.11 ± 0.13 0.05 ± 0.14 |
| 4 Gy | 0.45 ± 0.14 | 0.1 μg/mL + 4 Gy 0.5 μg/mL + 4 Gy | 0.37 ± 0.05 0.39 ± 0.05 | 0.08 ± 0.07 0.06 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binelli, L.; Dini, V.; Amatori, S.; Scotognella, T.; Giordano, A.; De Berardis, B.; Bertelà, F.; Battocchio, C.; Iucci, G.; Fratoddi, I.; et al. Gold Nanorods as Radiopharmaceutical Carriers: Preparation and Preliminary Radiobiological In Vitro Tests. Nanomaterials 2023, 13, 1898. https://doi.org/10.3390/nano13131898
Binelli L, Dini V, Amatori S, Scotognella T, Giordano A, De Berardis B, Bertelà F, Battocchio C, Iucci G, Fratoddi I, et al. Gold Nanorods as Radiopharmaceutical Carriers: Preparation and Preliminary Radiobiological In Vitro Tests. Nanomaterials. 2023; 13(13):1898. https://doi.org/10.3390/nano13131898
Chicago/Turabian StyleBinelli, Ludovica, Valentina Dini, Simone Amatori, Teresa Scotognella, Alessandro Giordano, Barbara De Berardis, Federica Bertelà, Chiara Battocchio, Giovanna Iucci, Ilaria Fratoddi, and et al. 2023. "Gold Nanorods as Radiopharmaceutical Carriers: Preparation and Preliminary Radiobiological In Vitro Tests" Nanomaterials 13, no. 13: 1898. https://doi.org/10.3390/nano13131898
APA StyleBinelli, L., Dini, V., Amatori, S., Scotognella, T., Giordano, A., De Berardis, B., Bertelà, F., Battocchio, C., Iucci, G., Fratoddi, I., Cartoni, A., & Venditti, I. (2023). Gold Nanorods as Radiopharmaceutical Carriers: Preparation and Preliminary Radiobiological In Vitro Tests. Nanomaterials, 13(13), 1898. https://doi.org/10.3390/nano13131898







