Lead Monoxide Nanostructures for Nanophotonics: A Review
Abstract
1. Introduction
2. Synthesis of PbO Nanostructures
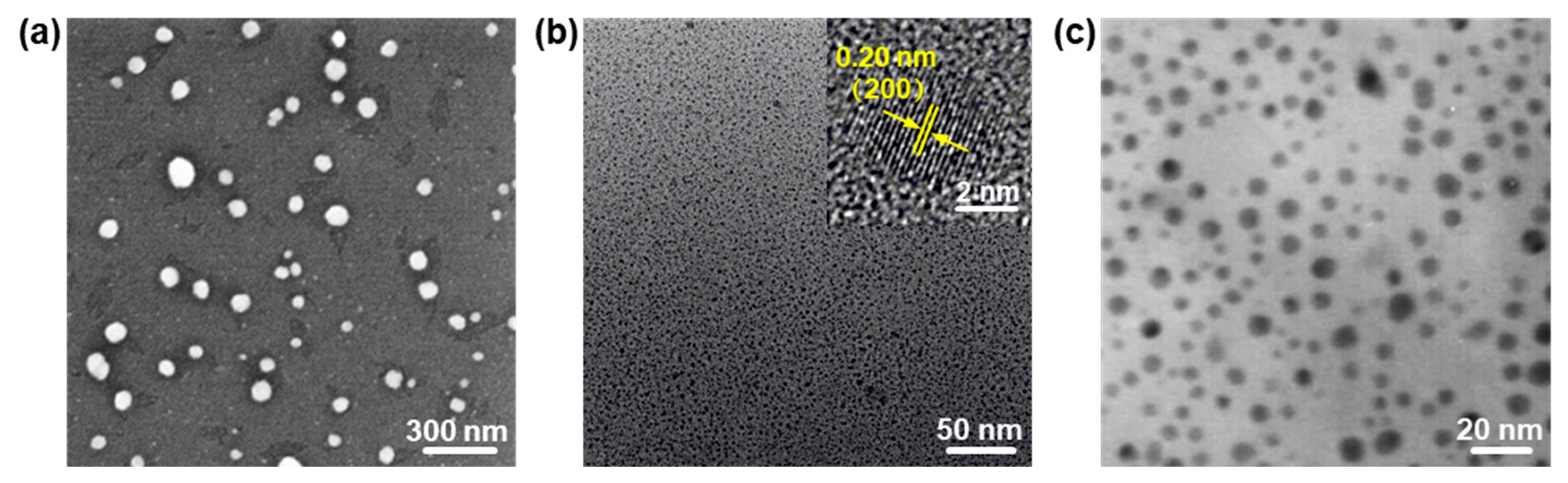
2.1. Zero-Dimensional PbO NPs
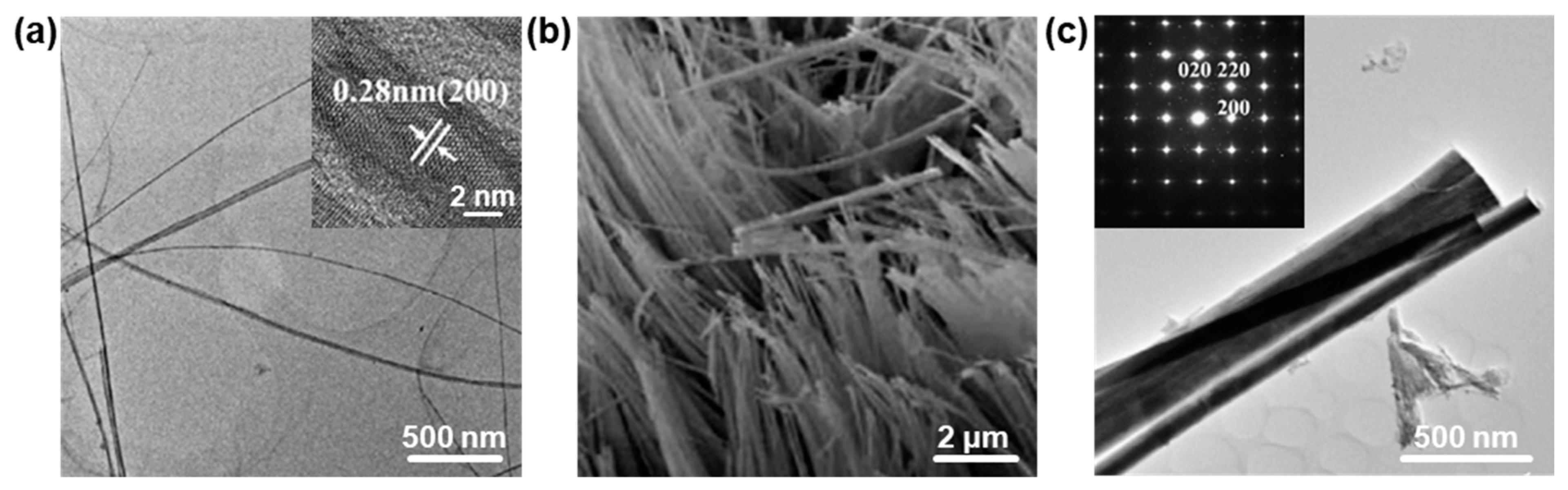
2.2. One-Dimensional PbO Nanostructures
2.3. Two-Dimensional PbO NSs
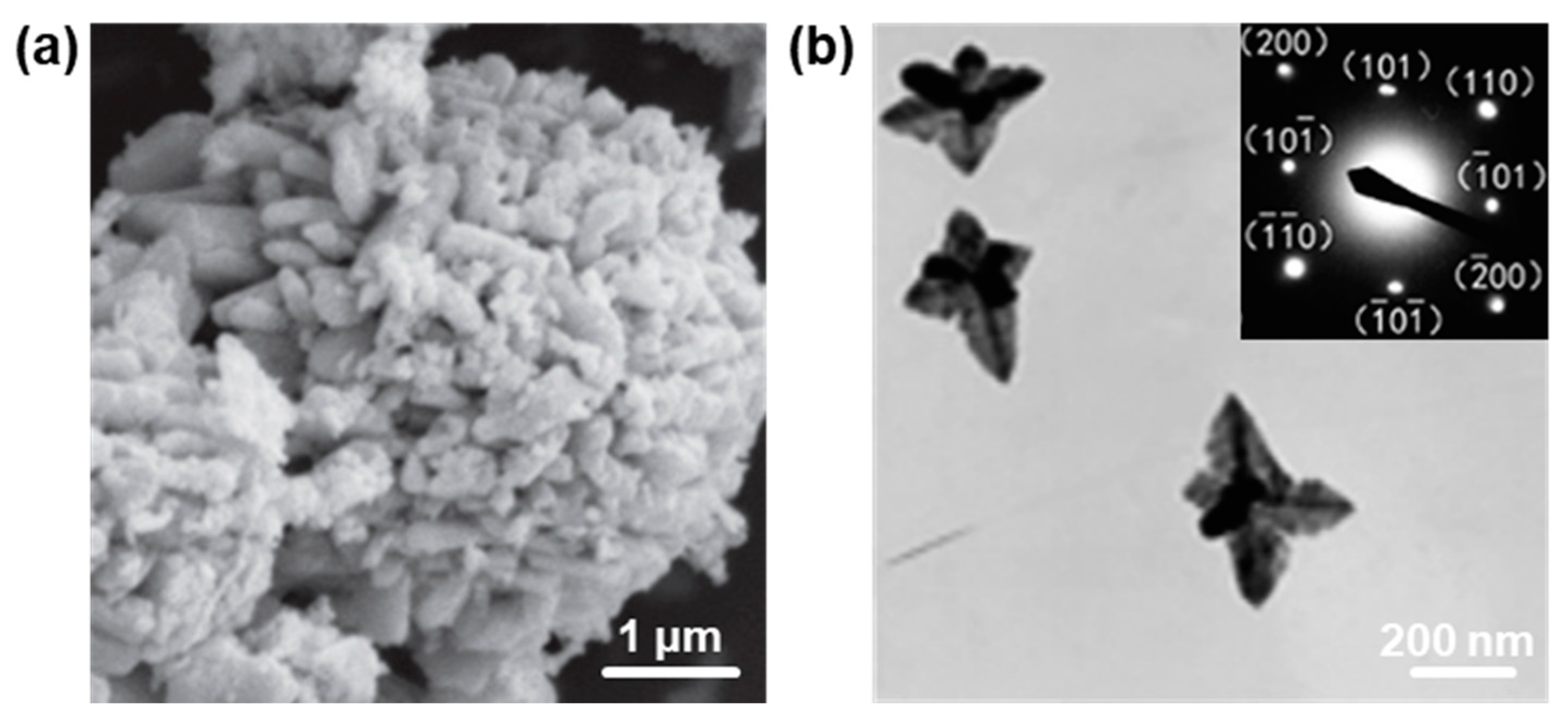
2.4. Three-Dimensional PbO Hierarchical Heterostructures
3. Properties and Applications in Nanophotonics
3.1. Optical Properties
3.2. Bandgap Properties
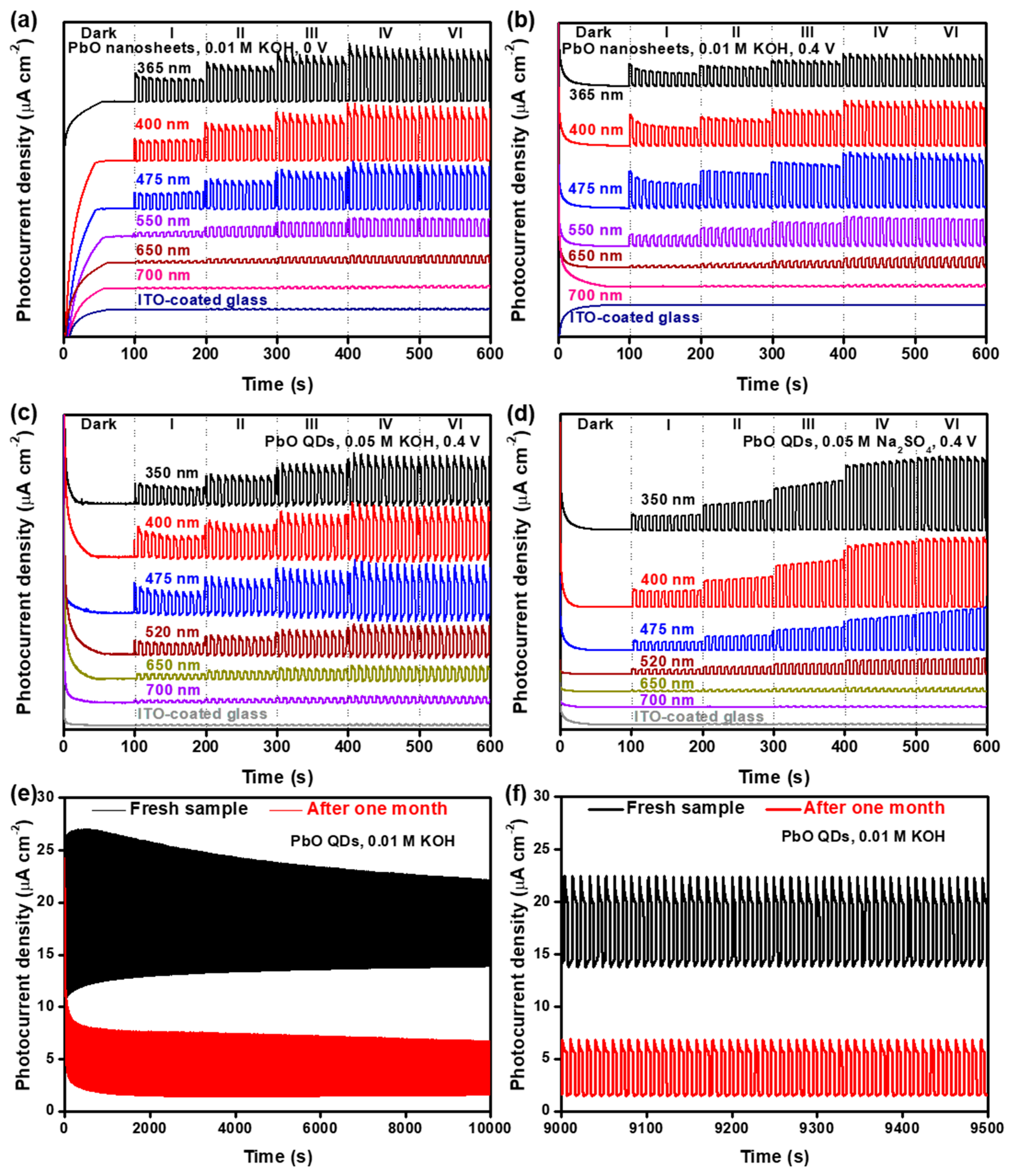
3.3. Photodetection Performance
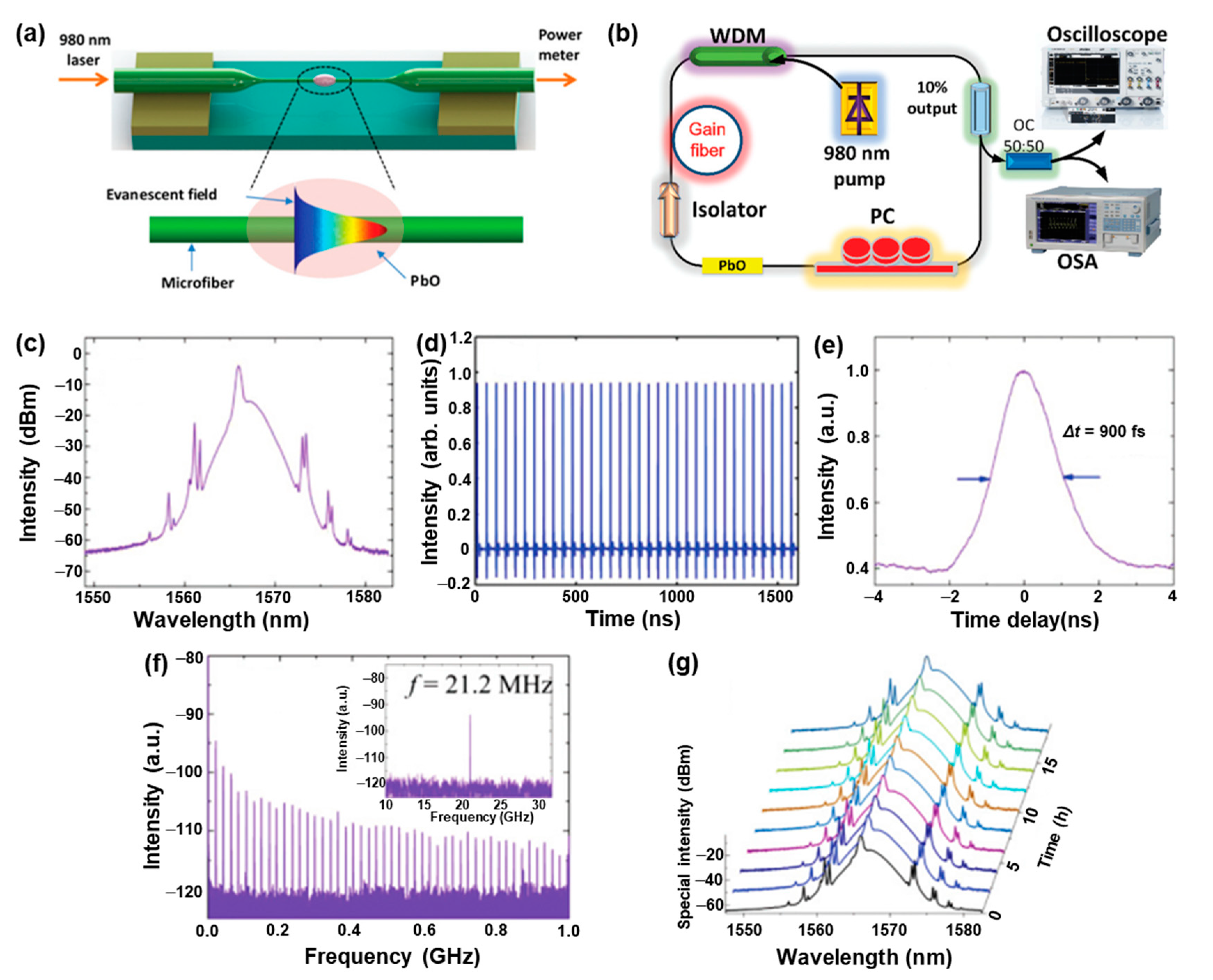
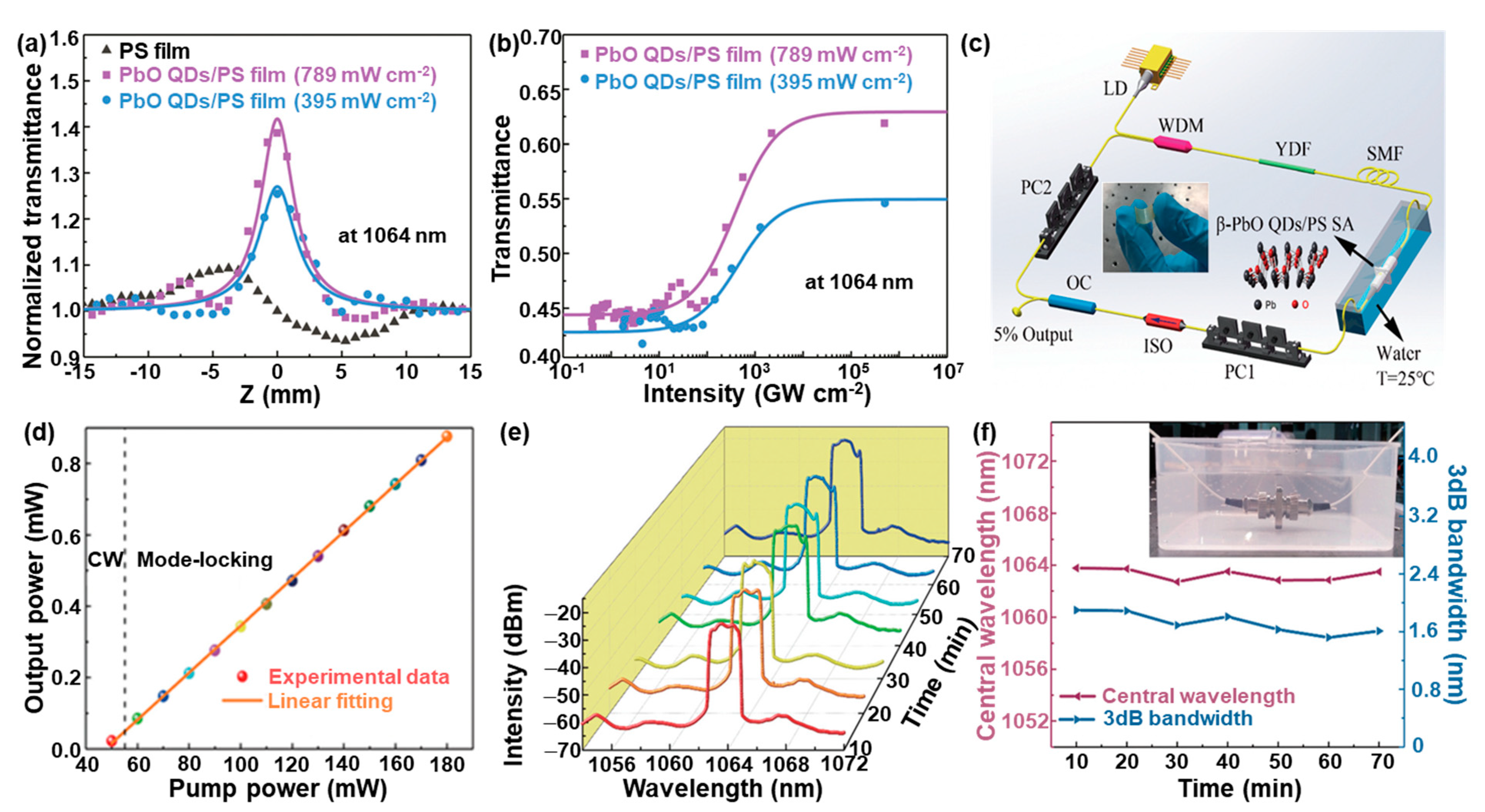
3.4. Nonlinear Photonics
4. Conclusions and Outlook
Funding
Data Availability Statement
Conflicts of Interest
References
- Pan, H.; Chu, H.; Li, Y.; Pan, Z.; Zhao, J.; Zhao, S.; Huang, W.; Li, D. Bismuthene Quantum Dots Integrated D-Shaped Fiber as Saturable Absorber for Multi-Type Soliton Fiber Lasers. J. Mater. 2023, 9, 183–190. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, H.; Zi, Y.; Wang, M.; Huang, W. Size-Tunable Bismuth Quantum Dots for Self-Powered Photodetectors under Ambient Conditions. Nanotechnology 2023, 34, 025202. [Google Scholar] [CrossRef] [PubMed]
- Zi, Y.; Hu, Y.; Pu, J.; Wang, M.; Huang, W. Recent Progress in Interface Engineering of Nanostructures for Photoelectrochemical Energy Harvesting Applications. Small 2023, 19, 2208274. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhu, J.; Wang, M.; Hu, L.; Tang, Y.; Shu, Y.; Xie, Z.; Zhang, H. Emerging Mono-Elemental Bismuth Nanostructures: Controlled Synthesis and Their Versatile Applications. Adv. Funct. Mater. 2020, 31, 2007584. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, W.; Wang, R.; Li, H.; Xia, X.; Zhao, X.; Hu, L.; Chen, T.; Tang, Y.; Zhang, H. Photocarrier Relaxation Pathways in Selenium Quantum Dots and Their Application in UV-Vis Photodetection. Nanoscale 2020, 12, 11232–11241. [Google Scholar] [CrossRef]
- Huang, W.; Wang, M.; Hu, L.; Wang, C.; Xie, Z.; Zhang, H. Recent Advances in Semiconducting Monoelemental Selenium Nanostructures for Device Applications. Adv. Funct. Mater. 2020, 30, 2003301. [Google Scholar] [CrossRef]
- Huang, W.; Li, C.; Gao, L.; Zhang, Y.; Wang, Y.; Huang, Z.N.; Chen, T.; Hu, L.; Zhang, H. Emerging Black Phosphorus Analogue Nanomaterials for High-Performance Device Applications. J. Mater. Chem. C 2020, 8, 1172–1197. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Q.; Mo, F.; Li, N.; Liang, G.; Li, X.; Huang, Z.; Wang, D.; Huang, W.; Fan, J.; et al. Aqueous Zinc-Tellurium Batteries with Ultraflat Discharge Plateau and High Volumetric Capacity. Adv. Mater. 2020, 32, 2001469. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, W.; Wang, C.; Guo, J.; Zhang, F.; Song, Y.; Ge, Y.; Wu, L.; Liu, J.; Li, J.; et al. An All-Optical, Actively Q-Switched Fiber Laser by an Antimonene-Based Optical Modulator. Laser Photonics Rev. 2019, 13, 1800313. [Google Scholar] [CrossRef]
- Wang, M.; Hu, Y.; Zi, Y.; Huang, W. Functionalized Hybridization of Bismuth Nanostructures for Highly Improved Nanophotonics. APL Mater. 2022, 10, 050901. [Google Scholar] [CrossRef]
- Pumera, M.; Sofer, Z. 2D Monoelemental Arsenene, Antimonene, and Bismuthene: Beyond Black Phosphorus. Adv. Mater. 2017, 29, 1605299. [Google Scholar] [CrossRef] [PubMed]
- Ares, P.; Palacios, J.J.; Abellán, G.; Gómez-Herrero, J.; Zamora, F. Recent Progress on Antimonene: A New Bidimensional Material. Adv. Mater. 2018, 30, 1703771. [Google Scholar] [CrossRef]
- Wu, W.; Qiu, G.; Wang, Y.; Wang, R.; Ye, P. Tellurene: Its Physical Properties, Scalable Nanomanufacturing, and Device Applications. Chem. Soc. Rev. 2018, 47, 7203–7212. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yang, Y.; Liu, J.-W.; Yu, S.-H. Emerging Tellurium Nanostructures: Controllable Synthesis and Their Applications. Chem. Soc. Rev. 2017, 46, 2732–2753. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, B.; Gnanakumar, G.; Therasa Alphonsa, A. Future is on Cheap Metal Oxide-A Review. In Nano Metal Oxides; Springer: Singapore, 2023. [Google Scholar]
- Geldasa, F.T.; Kebede, M.A.; Shura, M.W.; Andoshe, D.M.; Tegegne, N.A.; Hone, F.G. Facile Synthesis of Different Metals Doped α-PbO Nanoparticles for Photocatalytic Degradation of Methylene Blue Dye. Phys. Scr. 2023, 98, 065701. [Google Scholar] [CrossRef]
- Ge, Y.; Huang, W.; Yang, F.; Liu, J.; Wang, C.; Wang, Y.; Guo, J.; Zhang, F.; Song, Y.; Xu, S.; et al. Beta-Lead Oxide Quantum Dot (β-PbO QD)/Polystyrene (PS) Composite Films and Their Applications in Ultrafast Photonics. Nanoscale 2019, 11, 6828–6837. [Google Scholar] [CrossRef]
- Song, Y.; You, K.; Chen, Y.; Zhao, J.; Jiang, X.; Ge, Y.; Wang, Y.; Zheng, J.; Xing, C.; Zhang, H. Lead Monoxide: A Promising Two-Dimensional Layered Material for Applications in Nonlinear Photonics in the Infrared Band. Nanoscale 2019, 11, 12595–12602. [Google Scholar] [CrossRef]
- Xing, C.; Chen, X.; Huang, W.; Song, Y.; Li, J.; Chen, S.; Zhou, Y.; Dong, B.; Fan, D.; Zhu, X.; et al. Two-Dimensional Lead Monoxide: Facile Liquid Phase Exfoliation, Excellent Photoresponse Performance, and Theoretical Investigation. ACS Photonics 2018, 5, 5055–5067. [Google Scholar] [CrossRef]
- Huang, W.; Jiang, X.; Wang, Y.; Zhang, F.; Ge, Y.; Zhang, Y.; Wu, L.; Ma, D.; Li, Z.; Wang, R.; et al. Two-Dimensional Beta-Lead Oxide Quantum Dots. Nanoscale 2018, 10, 20540–20547. [Google Scholar] [CrossRef]
- Terpstra, H.J.; de Groot, R.A.; Haas, C. Electronic Structure of the Lead Monoxides: Band-Structure Calculations and Photoelectron Spectra. Phys. Rev. B 1995, 52, 11690–11697. [Google Scholar] [CrossRef]
- Ferreira, A.F.; Falcão, E.H.L.; de Azevedo, W.M. New Mechanistic Insight on the Synthesis of Metal Carbonates by Laser Ablation in Ethanol. Mater. Lett. 2023, 344, 134365. [Google Scholar] [CrossRef]
- Panturotai, K.; Krataithong, C.; Pluengphon, P.; Wongrat, E.; Tubtimtae, A.; Inceesungvorn, B. Structural and Optical Properties of Undoped and Sb-Doped Lead Oxide Thin Films Synthesized via the Chemical Bath Deposition Method. Opt. Mater. 2022, 126, 112179. [Google Scholar] [CrossRef]
- Kumar, P.; Liu, J.; Ranjan, P.; Hu, Y.; Yamijala, S.S.; Pati, S.K.; Irudayaraj, J.; Cheng, J.G. Alpha Lead Oxide (α-PbO): A New 2D Material with Visible Light Sensitivity. Small 2018, 14, 1703346. [Google Scholar] [CrossRef]
- Jin, J.; Chen, S.; Wang, J.; Chen, C.; Peng, T. One-Pot Hydrothermal Preparation of PbO-Decorated Brookite/Anatase TiO2 Composites with Remarkably Enhanced CO2 Photoreduction Activity. Appl. Catal. B Environ. 2020, 263, 118353. [Google Scholar] [CrossRef]
- Fu, H.; Liu, G.; Bao, H.; Zhou, L.; Zhang, H.; Zhao, Q.; Li, Y.; Cai, W. Ultrathin Hexagonal PbO Nanosheets Induced by Laser Ablation in Water for Chemically Trapping Surface-Enhanced Raman Spectroscopy Chips and Detection of Trace Gaseous H2S. ACS Appl. Mater. Interfaces 2020, 12, 23330–23339. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Pan, J.; Gong, S.; Sun, Y. A Green Preparation Method of Battery Grade α-PbO based on Pb-O2 Fuel Cell. J. Power Sources 2017, 360, 324–327. [Google Scholar] [CrossRef]
- Sivaram, H.; Selvakumar, D.; Alsalme, A.; Alswieleh, A.; Jayavel, R. Enhanced Performance of PbO Nanoparticles and PbO-CdO and PbO-ZnO Nanocomposites for Supercapacitor Application. J. Alloys Compd. 2018, 731, 55–63. [Google Scholar] [CrossRef]
- Majima, E.; Kozuka, Y.; Uchida, M.; Nakamura, M.; Kawasaki, M. Band Alignment and Photovoltaic Effect of Epitaxial α-PbO Thin Films. Appl. Phys. Express 2015, 8, 074001. [Google Scholar] [CrossRef]
- Zi, Y.; Zhu, J.; Wang, M.; Hu, L.; Hu, Y.; Wageh, S.; Al-Hartomy, O.A.; Al-Ghamdi, A.; Huang, W.; Zhang, H. CdS@CdSe Core/Shell Quantum Dots for Highly Improved Self-Powered Photodetection Performance. Inorg. Chem. 2021, 60, 18608–18613. [Google Scholar] [CrossRef]
- Huang, W.; Hu, L.; Tang, Y.; Xie, Z.; Zhang, H. Recent Advances in Functional 2D MXene-Based Nanostructures for Next-Generation Devices. Adv. Funct. Mater. 2020, 30, 2005223. [Google Scholar] [CrossRef]
- Pan, H.; Huang, W.; Chu, H.; Li, Y.; Zhao, S.; Li, G.; Zhang, H.; Li, D. Bismuthene Quantum Dots Based Optical Modulator for MIR Lasers at 2 μm. Opt. Mater. 2020, 102, 109830. [Google Scholar] [CrossRef]
- Dong, L.; Huang, W.; Chu, H.; Li, Y.; Wang, Y.; Zhao, S.; Li, G.; Zhang, H.; Li, D. Passively Q-Switched Near Infrared Lasers with Bismuthene Quantum Dots as the Saturable Absorber. Opt. Laser Technol. 2020, 128, 106219. [Google Scholar] [CrossRef]
- Xing, C.; Huang, W.; Xie, Z.; Zhao, J.; Ma, D.; Fan, T.; Liang, W.; Ge, Y.; Dong, B.; Li, J.; et al. Ultrasmall Bismuth Quantum Dots: Facile Liquid-Phase Exfoliation, Characterization, and Application in High-Performance UV-Vis Photodetector. ACS Photonics 2018, 5, 621–629. [Google Scholar] [CrossRef]
- Liang, X.-L.; Bao, N.; Luo, X.; Ding, S.-N. CdZnTeS Quantum Dots Based Electrochemiluminescent Image Immunoanalysis. Biosens. Bioelectron. 2018, 117, 145–152. [Google Scholar] [CrossRef]
- Gao, W.; Wang, W.; Yao, S.; Wu, S.; Zhang, H.; Zhang, J.; Jing, F.; Mao, H.; Jin, Q.; Cong, H.; et al. Highly Sensitive Detection of Multiple Tumor Markers for Lung Cancer Using Gold Nanoparticle Probes and Microarrays. Anal. Chim. Acta 2017, 958, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, T.; Cai, Y.; Chen, X.; Shang, S.; Li, J. Highly Fluorescent N,S-Co-Doped Carbon Dots: Synthesis and Multiple Applications. New J. Chem. 2017, 41, 11125–11137. [Google Scholar] [CrossRef]
- Shen, J.; Shang, S.; Chen, X.; Wang, D.; Cai, Y. Highly Fluorescent N, S-Co-Doped Carbon Dots and Their Potential Applications as Antioxidants and Sensitive Probes for Cr (VI) Detection. Sens. Actuators B-Chem. 2017, 248, 92–100. [Google Scholar] [CrossRef]
- Hao, C.; Shen, Y.; Wang, Z.; Wang, X.; Feng, F.; Ge, C.; Zhao, Y.; Wang, K. Preparation and Characterization of Fe2O3 Nanoparticles by Solid-Phase Method and Its Hydrogen Peroxide Sensing Properties. ACS Sustain. Chem. Eng. 2016, 4, 1069–1077. [Google Scholar] [CrossRef]
- Qian, K.; Hao, F.; Wei, S.; Wang, Y.; Ge, C.; Chen, P.; Zhang, Y. Synthesis of Well-Dispersed Pt-Pd Nanoparticles Stabilized by Silsesquioxanes with Enhanced Catalytic Activity for Formic Acid Electrooxidation. J. Solid State Electrochem. 2016, 21, 297–304. [Google Scholar] [CrossRef]
- Ding, S.-N.; Jin, Y.; Chen, X.; Bao, N. Tunable Electrochemiluminescence of CdSe@ZnSe Quantum Dots by Adjusting ZnSe Shell Thickness. Electrochem. Commun. 2015, 55, 30–33. [Google Scholar] [CrossRef]
- Shur, V.Y.; Gunina, E.V.; Esin, A.A.; Shishkina, E.V.; Kuznetsov, D.K.; Linker, E.A.; Greshnyakov, E.D.; Pryakhina, V.I. Influence of Hot Water Treatment During Laser Ablation in Liquid on the Shape of PbO Nanoparticles. Appl. Surf. Sci. 2019, 483, 835–839. [Google Scholar] [CrossRef]
- Elawam, S.A.; Morsi, W.M.; Abou-Shady, H.M.; Guirguis, O.W. Characterizations of Beta-Lead Oxide “Massicot” Nano-particles. Braz. J. Aquat. Sci. Technol. 2016, 17, 28143. [Google Scholar] [CrossRef]
- Chen, K.-C.; Wang, C.-W.; Lee, Y.-I.; Liu, H.-G. Nanoplates and Nanostars of β-PbO Formed at the Air/Water Interface. Colloids Surf. A 2011, 373, 124–129. [Google Scholar] [CrossRef]
- Fan, K.; Jia, Y.; Ji, Y.; Kuang, P.; Zhu, B.; Liu, X.; Yu, J. Curved Surface Boosts Electrochemical CO2 Reduction to Formate via Bismuth Nanotubes in a Wide Potential Window. ACS Catal. 2020, 10, 358–364. [Google Scholar] [CrossRef]
- Yang, C.; Gu, B.; Zhang, D.; Ge, C.; Tao, H. Coaxial Carbon Fiber/ZnO Nanorods as Electrodes for the Electrochemical Determination of Dopamine. Anal. Methods 2016, 8, 650–655. [Google Scholar] [CrossRef]
- Yin, H.; Yu, K.; Hu, J.; Song, C.; Guo, B.; Wang, Z.; Zhu, Z. Novel Photoluminescence Properties and Enhanced Photocatalytic Activities for V2O5-Loaded ZnO Nanorods. Dalton Trans. 2015, 44, 4671–4678. [Google Scholar] [CrossRef]
- Yan, X.; Chen, Y.; Deng, S.; Yang, Y.; Huang, Z.; Ge, C.; Xu, L.; Sun, D.; Fu, G.; Tang, Y. In Situ Integration of Ultrathin PtCu Nanowires with Reduced Graphene Oxide Nanosheets for Efficient Electrocatalytic Oxygen Reduction. Chem. Eur. J. 2017, 23, 16871–16876. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Huang, M.; Hao, S.; He, C.; Li, X.; Fan, L.; Li, Y. One-Pot and High-Yield Preparation of Ultrathin β-PbO Nanowires and Nanosheets for High-Capacity Positive Electrodes in Lead-Acid Batteries. J. Alloys Compd. 2020, 831, 154845. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, X.; Luo, S.; Sun, L.; Wu, X.; Cao, M.; Hu, C. Controllable Synthesis of PbO Nano/Microstructures Using a Porous Alumina Template. Cryst. Growth Des. 2007, 7, 2665–2669. [Google Scholar] [CrossRef]
- Jia, B.; Gao, L. Synthesis and Characterization of Single Crystalline PbO Nanorods via a Facile Hydrothermal Method. Mater. Chem. Phys. 2006, 100, 351–354. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, M.; Hu, L.; Hu, Y.; Guo, J.; Xie, Z.; Wei, S.; Wang, Y.; Zi, Y.; Zhang, H.; et al. Recent Advances in Two-Dimensional Graphdiyne for Nanophotonic Applications. Chem. Eng. J. 2022, 450, 138228. [Google Scholar] [CrossRef]
- Zi, Y.; Zhu, J.; Hu, L.; Wang, M.; Huang, W. Nanoengineering of Tin Monosulfide (SnS)-Based Structures for Emerging Applications. Small Sci. 2022, 2, 2100098. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, Z.; Pu, J.; Hu, L.; Zi, Y.; Wang, M.; Feng, X.; Huang, W. 2D MXene Ti(3)C(2)T(x) Nanosheets in the Development of a Mechanically Enhanced and Efficient Antibacterial Dental Resin Composite. Front. Chem. 2022, 10, 1090905. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, M.; Huang, W. Two-Dimensional Selenium Nanosheet-Based Sponges with Superior Hydrophobicity and Excellent Photothermal Performance. Nanomaterials 2022, 12, 3756. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, J.; Zi, Y.; Huang, W. 3D MXene Sponge: Facile Synthesis, Excellent Hydrophobicity, and High Photothermal Efficiency for Waste Oil Collection and Purification. ACS Appl. Mater. Interfaces 2021, 13, 47302–47312. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, J.; Wang, Y.; Song, Y.; Guo, J.; Huang, W.; Ge, Y.; Hu, L.; Liu, J.; Zhang, H. MXene (Ti2NTx): Synthesis, Characteristics and Application as a Thermo-Optical Switcher for All-Optical Wavelength Tuning Laser. Sci. China Mater. 2020, 64, 259–265. [Google Scholar] [CrossRef]
- Huang, W.; Ma, C.; Li, C.; Zhang, Y.; Hu, L.; Chen, T.; Tang, Y.; Ju, J.; Zhang, H. Highly Stable MXene (V2CTx)-Based Harmonic Pulse Generation. Nanophotonics 2020, 9, 2577–2585. [Google Scholar] [CrossRef]
- Xu, H.; Yang, S.; Li, B. Ultrathin Bismuth Nanosheets as an Efficient Polysulfide Catalyst for High Performance Lithium-Sulfur Batteries. J. Mater. Chem. A 2020, 8, 149–157. [Google Scholar] [CrossRef]
- Shen, C.; Cheng, T.; Liu, C.; Huang, L.; Cao, M.; Song, G.; Wang, D.; Lu, B.; Wang, J.; Qin, C.; et al. Bismuthene from Sonoelectrochemistry as a Superior Anode for Potassium-Ion Batteries. J. Mater. Chem. A 2020, 8, 453–460. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, X.; Qiao, X.; Zhang, N.; Zhang, C.; Ma, Z.; Wang, H. Lithium-Ion-Engineered Interlayers of V2C MXene as Advanced Host for Flexible Sulfur Cathode with Enhanced Rate Performance. J. Phys. Chem. Lett. 2020, 11, 885–890. [Google Scholar] [CrossRef]
- Xiong, J.; Song, P.; Di, J.; Li, H.; Liu, Z. Freestanding Ultrathin Bismuth-Based Materials for Diversified Photocatalytic Applications. J. Mater. Chem. A 2019, 7, 25203–25226. [Google Scholar] [CrossRef]
- Hu, S.; Zhu, M. Ultrathin Two-Dimensional Semiconductors for Photocatalysis in Energy and Environment Applications. ChemCatChem 2019, 11, 6147–6165. [Google Scholar] [CrossRef]
- Xiong, J.; Di, J.; Xia, J.; Zhu, W.; Li, H. Surface Defect Engineering in 2D Nanomaterials for Photocatalysis. Adv. Funct. Mater. 2018, 28, 1801983. [Google Scholar] [CrossRef]
- Xue, T.; Bongu, S.R.; Huang, H.; Liang, W.; Wang, Y.; Zhang, F.; Liu, Z.; Zhang, Y.; Zhang, H.; Cui, X. Ultrasensitive Detection of MicroRNA Using a Bismuthene-Enabled Fluorescence Quenching Biosensor. Chem. Commun. 2020, 56, 7041–7044. [Google Scholar] [CrossRef]
- Xue, T.; Liang, W.; Li, Y.; Sun, Y.; Xiang, Y.; Zhang, Y.; Dai, Z.; Duo, Y.; Wu, L.; Qi, K.; et al. Ultrasensitive Detection of MiRNA with an Antimonene-Based Surface Plasmon Resonance Sensor. Nat. Commun. 2019, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Mayorga-Martinez, C.C.; Gusmão, R.; Sofer, Z.; Pumera, M. Pnictogen-Based Enzymatic Phenol Biosensors: Phosphorene, Arsenene, Antimonene, and Bismuthene. Angew. Chem. Int. Ed. 2019, 58, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Han, F.; Li, W.; Bao, N.; Yu, C.; Gu, H. Sensitive Determination of Sialic Acid Expression on Living Cells by Using an ITO Electrode Modified with Graphene, Gold Nanoparticles and Thionine for Triple Signal Amplification. Microchim. Acta 2017, 184, 3841–3850. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.; You, Q.; Huang, P.; Wang, Y.; Huang, Z.N.; Ge, Y.; Wu, L.; Dong, Z.; Dai, X.; et al. Enhanced Photodetection Properties of Tellurium@Selenium Roll-to-Roll Nanotube Heterojunctions. Small 2019, 15, 1900902. [Google Scholar] [CrossRef]
- Ma, C.; Huang, W.; Wang, Y.; Adams, J.; Wang, Z.; Liu, J.; Song, Y.; Ge, Y.; Guo, Z.; Hu, L.; et al. MXene Saturable Absorber Enabled Hybrid Mode-Locking Technology: A New Routine of Advancing Femtosecond Fiber Lasers Performance. Nanophotonics 2020, 9, 2451–2458. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Y.; Huang, W.; Wang, C.; Zheng, Z.; Zhang, M.; Zhang, H. MXene-Based High-Performance All-Optical Modulators for Actively Q-switched Pulse Generation. Photonics Res. 2020, 8, 1140–1147. [Google Scholar] [CrossRef]
- Wu, L.; Huang, W.; Wang, Y.; Zhao, J.; Ma, D.; Xiang, Y.; Li, J.; Ponraj, J.S.; Dhanabalan, S.C.; Zhang, H. 2D Tellurium Based High-Performance All-Optical Nonlinear Photonic Devices. Adv. Funct. Mater. 2019, 29, 1806346. [Google Scholar] [CrossRef]
- Qiu, M.; Ren, W.X.; Jeong, T.; Won, M.; Park, G.Y.; Sang, D.K.; Liu, L.-P.; Zhang, H.; Kim, J.S. Omnipotent Phosphorene: A Next-Generation, Two-Dimensional Nanoplatform for Multidisciplinary Biomedical Applications. Chem. Soc. Rev. 2018, 47, 5588–5601. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Wang, D.; Liang, W.; Liu, L.; Zhang, Y.; Chen, X.; Sang, D.K.; Xing, C.; Li, Z.; Dong, B.; et al. Novel Concept of the Smart NIR-Light-Controlled Drug Release of Black Phosphorus Nanostructure for Cancer Therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 501–506. [Google Scholar] [CrossRef]
- Qiu, M.; Sun, Z.T.; Sang, D.K.; Han, X.G.; Zhang, H.; Niu, C.M. Current Progress in Black Phosphorus Materials and Their Applications in Electrochemical Energy Storage. Nanoscale 2017, 9, 13384–13403. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lv, X.; Zhu, X.; Du, J.; Lu, S.; Alshehri, A.A.; Alzahrani, K.A.; Zheng, B.; Sun, X. Bi Nanodendrites for Efficient Electrocatalytic N2 Fixation to NH3 under Ambient Conditions. Chem. Commun. 2020, 56, 2107–2110. [Google Scholar] [CrossRef]
- Wang, M.; Hu, X.; Zhan, Z.; Sun, T.; Tang, Y. Facile Fabrication of CeVO4 Hierarchical Hollow Microspheres with Enhanced Photocatalytic Activity. Mater. Lett. 2019, 253, 259–262. [Google Scholar] [CrossRef]
- Qiu, Y.; Du, J.; Dai, C.; Dong, W.; Tao, C. Bismuth Nano-Flowers as a Highly Selective Catalyst for Electrochemical Reduction of CO2 to Formate. J. Electrochem. Soc. 2018, 165, H594–H600. [Google Scholar] [CrossRef]
- Shi, X.; Wang, H.; Xie, X.; Xue, Q.; Zhang, J.; Kang, S.; Wang, C.; Liang, J.; Chen, Y. Bioinspired Ultrasensitive and Stretchable MXene-Based Strain Sensor via Nacre-Mimetic Microscale “Brick-and-Mortar” Architecture. ACS Nano 2019, 13, 649–659. [Google Scholar] [CrossRef]
- Das, A.; Sangaranarayanan, M.V. Shape-Controlled Synthesis of Three-Dimensional Triangular Bismuth Microstructures and Sensing of H2O2. CrystEngComm 2016, 18, 1147–1155. [Google Scholar] [CrossRef]
- Lin, J.; Yu, Y.; Zhang, Z.; Gao, F.; Liu, S.; Wang, W.; Li, G. A Novel Approach for Achieving High-Efficiency Photoelectrochemical Water Oxidation in InGaN Nanorods Grown on Si System: MXene Nanosheets as Multifunctional Interfacial Modifier. Adv. Funct. Mater. 2020, 30, 1910479. [Google Scholar] [CrossRef]
- Yan, D.; Fu, X.; Shang, Z.; Liu, J.; Luo, H. A BiVO4 Film Photoanode with Re-Annealing Treatment and 2D Thin Ti3C2Tx Flakes Decoration for Enhanced Photoelectrochemical Water Oxidation. Chem. Eng. J. 2019, 361, 853–861. [Google Scholar] [CrossRef]
- Behnoudnia, F.; Dehghani, H. Synthesis and Characterization of Novel Three-Dimensional-Cauliflower-Like Nanostructure of Lead(II) Oxalate and its Thermal Decomposition for Preparation of PbO. Inorg. Chem. Commun. 2012, 24, 32–39. [Google Scholar] [CrossRef]
- Kwon, Y.; Lee, H.; Lee, J. Autonomous Interfacial Creation of Nanostructured Lead Oxide. Nanoscale 2011, 3, 4984–4988. [Google Scholar] [CrossRef]
- Yutomo, E.B.; Noor, F.A.; Winata, T. The Effect of Vacancies on the Magnetic and Optical Properties of Monolayer Alpha Lead Oxide (α-PbO): A Density Functional Theory Study. Micro Nanostruct. 2022, 163, 107125. [Google Scholar] [CrossRef]
- Geldasa, F.T.; Kebede, M.A.; Shura, M.W.; Hone, F.G. Density Functional Theory Study of Different Metal Dopants Influence on the Structural and Electronic Properties of a Tetragonal α-PbO. AIP Adv. 2022, 12, 115302. [Google Scholar] [CrossRef]
- Yin, Q.; Kang, S.; Wang, X.; Li, S.; He, D.; Hu, L. Effect of PbO on the Spectral and Thermo-Optical Properties of Nd3+-Doped Phosphate Laser Glass. Opt. Mater. 2017, 66, 23–28. [Google Scholar] [CrossRef]
- Cattley, C.A.; Stavrinadis, A.; Beal, R.; Moghal, J.; Cook, A.G.; Grant, P.S.; Smith, J.M.; Assender, H.; Watt, A.A.R. Colloidal Synthesis of Lead Oxide Nanocrystals for Photovoltaics. Chem. Commun. 2010, 46, 2802–2804. [Google Scholar] [CrossRef]
- Suryawanshi, V.N.; Varpe, A.S.; Deshpande, M.D. Band Gap Engineering in PbO Nanostructured Thin Films by Mn Doping. Thin Solid Film. 2018, 645, 87–92. [Google Scholar] [CrossRef]
- Ren, X.; Li, Z.; Huang, Z.; Sang, D.; Qiao, H.; Qi, X.; Li, J.; Zhong, J.; Zhang, H. Environmentally Robust Black Phosphorus Nanosheets in Solution: Application for Self-Powered Photodetector. Adv. Funct. Mater. 2017, 27, 1606834. [Google Scholar] [CrossRef]
- Huang, W.; Xie, Z.; Fan, T.; Li, J.; Wang, Y.; Wu, L.; Ma, D.; Li, Z.; Ge, Y.; Huang, Z.N.; et al. Black-Phosphorus-Analogue Tin Monosulfide: An Emerging Optoelectronic Two-Dimensional Material for High-Performance Photodetection with Improved Stability under Ambient/Harsh Conditions. J. Mater. Chem. C 2018, 6, 9582–9593. [Google Scholar] [CrossRef]
- Kobtsev, S.M. Artificial Saturable Absorbers for Ultrafast Fibre Lasers. Opt. Fiber Technol. 2022, 68, 102764. [Google Scholar] [CrossRef]
- Hu, H.; Shi, Z.; Khan, K.; Cao, R.; Liang, W.; Tareen, A.K.; Zhang, Y.; Huang, W.; Guo, Z.; Luo, X.; et al. Recent Advances in Doping Engineering of Black Phosphorus. J. Mater. Chem. A 2020, 8, 5421–5441. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, D.; Fan, T.; Xing, C.; Li, Z.; Tao, W.; Liu, L.; Bao, S.; Fan, D.; Zhang, H. Black Phosphorus Analogue Tin Sulfide Nanosheets: Synthesis and Application as Near-Infrared Photothermal Agents and Drug Delivery Platforms for Cancer Therapy. J. Mater. Chem. B 2018, 6, 4747–4755. [Google Scholar] [CrossRef] [PubMed]
- Britnell, L.; Ribeiro, R.M.; Eckmann, A.; Jalil, R.; Belle, B.D.; Mishchenko, A.; Kim, Y.-J.; Gorbachev, R.V.; Georgiou, T.; Morozov, S.V.; et al. Strong Light-Matter Interactions in Heterostructures of Atomically Thin Films. Science 2013, 340, 1311–1314. [Google Scholar] [CrossRef]
- Suresh, B.; Dineshbabu, N.; Shanmugam, G.; Dash, C.K.; Sundararajan, M.; Ramachandran, S. Influence of Eu3+ Dopant on the Third Order Nonlinear Optical Properties of PbO/PMMA Nanocomposites. Mater. Res. Innov. 2023. [Google Scholar] [CrossRef]
- Du, J.; Wang, Q.; Jiang, G.; Xu, C.; Zhao, C.; Xiang, Y.; Chen, Y.; Wen, S.; Zhang, H. Ytterbium-Doped Fiber Laser Passively Mode Locked by Few-Layer Molybdenum Disulfide (MoS2) Saturable Absorber Functioned with Evanescent Field Interaction. Sci. Rep. 2014, 4, 6346. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, S.; Liang, W.; Luo, S.; He, Z.; Ge, Y.; Wang, H.; Cao, R.; Zhang, F.; Wen, Q.; et al. Broadband Nonlinear Photonics in Few-Layer MXene Ti3C2Tx (T = F, O, or OH). Laser Photonics Rev. 2018, 12, 1700229. [Google Scholar] [CrossRef]
- Li, P.; Chen, Y.; Yang, T.; Wang, Z.; Lin, H.; Xu, Y.; Li, L.; Mu, H.; Shivananju, B.N.; Zhang, Y.; et al. Two-Dimensional CH3NH3PbI3 Perovskite Nanosheets for Ultrafast Pulsed Fiber Lasers. ACS Appl. Mater. Interfaces 2017, 9, 12759–12765. [Google Scholar] [CrossRef]
- Chai, T.; Li, X.; Feng, T.; Guo, P.; Song, Y.; Chen, Y.; Zhang, H. Few-Layer Bismuthene for Ultrashort Pulse Generation in a Dissipative System Based on an Evanescent Field. Nanoscale 2018, 10, 17617–17622. [Google Scholar] [CrossRef]
- Chintapalli, M.; Le, T.N.P.; Venkatesan, N.R.; Mackay, N.G.; Rojas, A.A.; Thelen, J.L.; Chen, X.C.; Devaux, D.; Balsara, N.P. Structure and Ionic Conductivity of Polystyrene-block-Poly(ethylene oxide) Electrolytes in the High Salt Concentration Limit. Macromolecules 2016, 49, 1770–1780. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Wang, M.; Huang, W. Lead Monoxide Nanostructures for Nanophotonics: A Review. Nanomaterials 2023, 13, 1842. https://doi.org/10.3390/nano13121842
Chen H, Wang M, Huang W. Lead Monoxide Nanostructures for Nanophotonics: A Review. Nanomaterials. 2023; 13(12):1842. https://doi.org/10.3390/nano13121842
Chicago/Turabian StyleChen, Hongyan, Mengke Wang, and Weichun Huang. 2023. "Lead Monoxide Nanostructures for Nanophotonics: A Review" Nanomaterials 13, no. 12: 1842. https://doi.org/10.3390/nano13121842
APA StyleChen, H., Wang, M., & Huang, W. (2023). Lead Monoxide Nanostructures for Nanophotonics: A Review. Nanomaterials, 13(12), 1842. https://doi.org/10.3390/nano13121842






