Abstract
The control of catalytic performance using synthesis conditions is one of the main goals of catalytic research. Two series of Pt-Ti/SBA-15 catalysts with different TiO2 percentages (n = 1, 5, 10, 30 wt.%) were obtained from tetrabutylorthotitanate (TBOT) and peroxotitanate (PT), as titania precursors and Pt impregnation. The obtained catalysts were characterized using X-ray diffraction, scanning electron microscopy (SEM) and transmission electron microscopy (TEM), N2 sorption, Raman, X-ray photoelectron spectroscopy (XPS), X-ray absorption spectroscopy (XAS), hydrogen temperature-programmed reduction (H2-TPR) and H2-chemisorption measurements. Raman spectroscopy showed framework titanium species in low TiO2 loading samples. The anatase phase was evidenced for samples with higher titania loading, obtained from TBOT, and a mixture of rutile and anatase for those synthesized by PT. The rutile phase prevails in rich TiO2 catalysts obtained from PT. Variable concentrations of Pt0 as a result of the stronger interaction of PtO with anatase and the weaker interaction with rutile were depicted using XPS. TiO2 loading and precursors influenced the concentration of Pt species, while the effect on Pt nanoparticles’ size and uniform distribution on support was insignificant. The Pt/PtO ratio and their concentration on the surface were the result of strong metal–support interaction, and this influenced catalytic performance in the complete oxidation of methane at a low temperature. The highest conversion was obtained for sample prepared from PT with 30% TiO2.
1. Introduction
Titania has attracted much attention as a catalyst and/or catalyst support, with applications in catalysis and photocatalysis [1,2,3,4]. The increased interest in TiO2 supports is due to the strong metal–support interaction (SMSI) existing between noble metals and this oxide [5,6,7,8] having an important role in heterogeneous catalysis [6,7,8,9,10]. Excellent performances were obtained for platinum in catalytic reactions, such as complete or selective oxidations carried out in the gas [11,12,13,14] or liquid phase [15,16]. The catalytic performances of Pt, as well as other noble metals, are intimately related to particle size, which is a crucial parameter influencing the activity, selectivity and lifetime of a catalyst [6,17,18]. For this reason, the most commonly used strategy in the preparation of catalysts with Pt is to deposit platinum nanoparticles on large surface area supports, with the aim of improving the dispersion [19,20]. Unfortunately, titania supports have the disadvantage of a small surface area. Therefore, novel materials were obtained through the immobilization of Ti species into the framework or onto the surface of mesoporous silica, such as MCM-4 [20], HMS [21] SBA-15 [6,18,21,22,23,24], and KIT-6 [25]. Hence, the obtained mesoporous materials with large specific surface area and pore volume exhibit typical properties due to the synergistic activity of TiO2 and mesoporous silica [18,26].
Well-ordered mesoporous SBA-15 silica, with a 2D hexagonal arrangement of pores, high surface area, and stability, has been highlighted as an ideal support. The immobilization of metal oxides and metal nanoparticles onto SBA-15 silica favors their dispersion [27,28]. Thus, a stronger metal–support interaction (SMSI) was evidenced for Pt/xTiO2-SBA-15 nanocomposites than for classical Pt/TiO2 P25 catalysts [29,30]. The use of mesoporous silica-ordered channels as hosts for titanium species is a way to control and stabilize small-size particles and thus adjust interaction with Pt through the selection of the synthesis method, composition, and precursors. The most commonly used methods for titania insertion into a high surface area silica matrix are direct synthesis and impregnation in a post-synthesis method [22,25,29]. Further interaction between the inserted Ti species and the active Pt species triggers the change in the Pt’s existence status and dispersion [20]. Metal–support interaction was originally explained as an electron transfer or the formation of intermediate phases [31,32]. SMSI appears at the metal–TiO2 interface, where the oxide is reducible and is favored by dispersion on high surface area silica. It was thus shown that the crystal size of titania is an important parameter allowing us to control SMSI [29]. The effect of TiO2 content on Pt–support interaction and catalytic properties in methane combustion was correlated with the homogeneous dispersion of platinum and supported oxides, such as titania and ceria, in KIT-6 silica or SBA-15 [24,25]. However, the role of the titanium precursor and synthesis conditions on platinum interaction with titanium species immobilized on mesoporous silica is not fully known. Tuning the SMSI effect for the optimum catalytic performance of supported Pt using the synthesis method and titania loading is a less commonly approached direction in the complete oxidation of CH4 [33,34,35].
Although Pt shows lower catalytic activity in methane oxidation compared to Pd, it is more stable and resistant to sulfur poisoning [36,37]. A redox mechanism for total methane oxidation assumes an optimal metal/oxide ratio of active centers to be available in the case of catalysts based on noble metals [38]. This is a consequence of the thermodynamic equilibrium between dispersed oxide clusters and metallic surfaces. Methane conversion and sulfur tolerance were also improved by the incorporation of an optimum TiO2 mass percent in high surface area silica, with specific characteristics of acidity and morphology [21]. The promoter effect of TiO2 consists of a strong interaction with a noble metal, which influences the equilibrium between the metal and metal oxide phases. Given the fact that the sensitivity of methane oxidation to the noble metal dispersion on the silica matrix and its interaction with support has been the subject of many studies [7,25,27,31,32,39], complete oxidation of methane can be used as a test reaction in the current work.
The aim of this paper is to prepare various PtTi-SBA-15 mesoporous catalysts and to study the influence of a titanium precursor and Pt/TiO2 mass percent ratio on the SMSI effect in catalytic performances during the complete oxidation of CH4. Two series of Pt-based catalysts supported on Ti-modified SBA-15 will be prepared using two-step synthesis, in which the titanium precursor is added during the preparation of the mesoporous silica from two different precursors and Pt is added by impregnation.
2. Materials and Methods
2.1. Materials
Tetraethyl orthosilicate (TEOS), HCl (37%), triblock copolymer P123, hexachloroplatinic (IV) acid hexahydrated (H2PtCl6·6H2O) (from Sigma Aldrich, St. Louis, MO, USA) tetra butyl ortho-titanate (TBOT, 99 wt%, ACROS Organics, Waltham, MA, USA), and hydrogen peroxide (H2O2-30%, SC. Silal Trading, Bucharest, Romania) were employed as starting materials. A CH4 gas mixture (10% CH4, 5% N2, 85% Ar) from Linde Gas (Linde Gas, Bucharest, Romania) was used for catalytic tests.
2.2. Catalysts Preparation
A clear orange-colored solution of peroxotitanate was obtained, according to the procedure proposed by Sánchez et al. [40], from TBOT and H2O2 solutions in water.
The PtTi-SBA-15 mesoporous catalysts were prepared in two steps. In the first step, two series of Ti-SBA-15 supports with different TiO2 mass percents were synthesized. The first series of supports, with 1, 5, 10, and 30% titania mass percentage, was obtained from TBOT as a titanium precursor, and the second, with 5, 10, and 30% TiO2, from the obtained peroxotitanate (PT). For both syntheses, 4 g of triblock copolymer P123 was dispersed in 30 mL of bidistilled water under continuous stirring at 40 °C. After 30 min an acidic solution, a slurry was obtained by adding 110 mL of aqueous solution of HCl (2M). The slurry was continuously stirred for 2 h before a mixture of TEOS and titanium precursor was added. The obtained gels were transferred into Teflon-lined autoclaves and hydrothermally treated for 48 h at 100 °C. The solid fraction was filtered, rinsed with bidistilled water, and dried for 6 h at room temperature and overnight at 80 °C. Finally, the samples were calcined at 550 °C in the air (temperature ramp of 2 °C min−1).
The obtained supports were impregnated, in the second step, with an aqueous solution of H2PtCl6. After impregnation, the samples were dried overnight at 80 °C and cooled down at room temperature. The final nanocomposite samples with 0.25% wt. Pt calcined in air at 400 °C, were named PTnSB (samples obtained with TBOT), and PTnSP in the case of PT titania precursor (n stands for 1, 5, 10, and 30% wt. TiO2 loading).
2.3. Catalysts Characterization
The structural properties of Ti-SBA-15 supports and PtTi-SBA-15 catalysts were characterized using powder X-ray diffraction by means of a Rigaku Ultima IV diffractometer (Rigaku Corporation, Tokyo, Japan) with Cu Kα (λ = 0.15406 nm). The textural characteristics were determined using a Micromeritics ASAP 2020 instrument (Norcross, GA, USA). The morphology, sample composition, homogeneous dispersion of components, and ordered porous structure were examined using a scanning electron microscope (SEM with EDX, FEI Quanta 3D FEG scanning microscope from FEI, Brno, Czech Republic) and a transmission electron microscope (TEM, Tecnai 10 G2-F30 from Thermo Fisher Scientific, Waltham, MA, USA).
Micro-Raman spectra of the Pt-containing materials were collected using a LabRam HR800 spectrometer (Horiba France SAS, Palaiseau, France) calibrated with a silicon wafer as a reference. Samples were excited with two laser lines (532 nm and 325 nm) through x50LWD/0.55 NA and x40/0.47NUV air objectives, respectively, of an Olympus microscope (Olympus Corporation, Tokyo, Japan). The Raman signal was energy-dispersed by the 600-line/mm and 2400 diffraction gratings. The spectral resolution was better than 2 cm−1.
XPS experiments were carried out on a photoelectron spectrometer (XPS-PHI Quantera equipment, Ontario, Canada). The X-ray source was Al Ka radiation (1486.6 eV, monochromatized), and the total overall energy resolution was estimated at 0.65 eV by the full width at half maximum (FWHM) of Au4f7/2 line. To consider the charging effect on the measured binding energies (BEs), the spectra were calibrated using the C1s line (BE = 284.8 eV, C-C (CH)n bonding) of the adsorbed hydrocarbon on the sample surface. A dual beam-neutralizing procedure (e− and Ar+ ion beams) was used to compensate for the charging effect in the insulating samples. The most prominent transitions of the detected elements (O1s, Pt4f, Ce3d, Ti2p, and Si2p) on the outermost surface layer (<10 nm) were recorded in a high-resolution mode, and then they were deconvoluted.
The temperature-programmed reduction (H2-TPR) and H2 chemosorption experiments were carried out in a flow system with ChemBET TPR/TPD (Quantachrome, Boynton Beach, FL, USA), instrument apparatus equipped with thermal conductivity detectors (TCD). Some 50 mg of the powder catalyst was treated in a continuous flow of 5% volume H2 in Ar (70 mL min−1). The temperature was increased to 850 °C at a heating rate of 10 °C min−1.
XAS measurements were carried out at the 1W1B beamline at the Beijing (China) Synchrotron Radiation Facilities (BSRF), operating at 30–50 mA and 2.2 GeV, at room temperature. Due to the low mass concentration of Pt, XAS measurements were performed in fluorescence mode, using X-ray energy of incident flux between 11,370 and 12,430 eV.
2.4. Catalytic Activity Measurements
Catalytic oxidation of methane was performed using the flow set-up equipment, with a gas chromatograph with a thermal conductivity detector and a flow fixed-bed reactor. The chromatographic system had two analysis channels: Porapaq QS 80/100, and carbon molecular sieve columns. About 20 mg of catalyst was loaded into the quartz reactor tube (i.d. = 6 mm), which was placed in a tubular oven equipped with a temperature controller. Measurements were carried out within a 200–500 °C temperature range, with a volume ratio air/CH4 gas mixture of 2/1. The composition of the gas mixture was 10% CH4, 5% N2, and 85% Ar. The samples were stepwise heated with steps of 50 °C and maintained for 30 min at each level temperature, as described elsewhere [25].
3. Results
3.1. Properties of Titania Modified SBA-15 Supports
The effects of the titanium precursor and its concentration on the ordered porous structure of the obtained PtTi-SBA-15 samples were investigated by small-angle X-ray diffraction and nitrogen adsorption–desorption (Figure 1 and Figure 2). XRD patterns of the PtTi-SBA-15 mesoporous silica supports exhibit five well-defined peaks in the range of 0.8 < 2θ < 3, due to (100), (110), (200), (210), and (300) reflections associated with perfect hexagonal (p6 mm) symmetry (Figure 1). The intensities of these peaks decrease with titania content and are very low for samples with 10 and 30% TiO2 content. All the samples preserved the ordered mesoporous structure of SBA-15, except for the PT30SP sample.
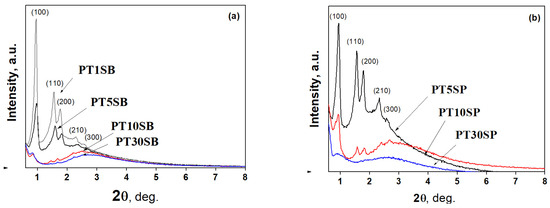
Figure 1.
The low-angle XRD patterns of PtTi-SBA-15 samples with 1%, 5%, 10% and 30% TiO2 content obtained from TBOT (a) and PT (b).
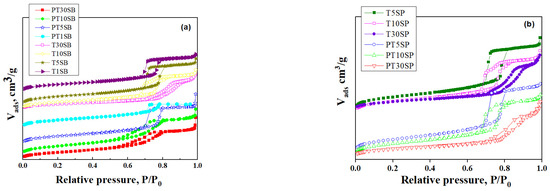
Figure 2.
N2 adsorption–desorption isotherms of Ti-SBA-15 and PtTi-SBA-15 samples with varied TiO2 content obtained from TBOT (a) and PT (b).
Nitrogen adsorption and desorption isotherms (Figure 2) can be classified as type IV, which are associated with mesoporous structures. The hysteresis loop corresponds to the H-1 type due to mesoporous SBA-15 silica. The Ti-SBA-15 supports with lower titania loading exhibit a high specific surface area (700–800 m2/g) and a large pore volume (1.1–1.4 cm3/g), with an average pore diameter between 6.4 and 7.2 nm. Conversely, richer titania content caused the specific surface area to lower to around 500 m2/g, with a pore volume of 1 cm3/g. Increasing the titania content to 10% determined a decrease in pore diameter, and for samples with 30% TiO2, a significant increase in the size of the pores was evidenced (8–9 nm). The increasing pore diameter for supports with 30% TiO2 is a result of porous structure degradation (Figure 1), which is more obvious for the sample obtained with peroxotitanate.
No significant variation in these parameters was observed after Pt immobilization. A narrow pore size distribution was obtained for the samples with lower titania content (Figure S1).
SEM and TEM images evidenced the effects of titania content and precursors on the characteristic ordering of PtTi-SBA-15 samples. A rod-like morphology is noticeable in Figure 3 for all the samples. At higher concentrations of TiO2, another phase deposited on the surface of the silica particles can be observed. At low concentrations of TiO2, the species of Ti are in the SBA-15 network or on the surface, without being able to be highlighted as a distinct phase, which is observed in the case of higher titania loading (Figure 4c,d). For the peroxotitanate-derived samples, the SEM images (Figure 4a,b) also indicate a rod-like morphology for low concentrations of TiO2. A flower-like morphology became dominant when the titania concentration increased to 30% (Figure 4c,d). SEM images recorded using a back-scattered electron (BSE) detector were presented in order to evidence Pt dispersion on the surface, using the advantage of the compositional contrast (Figure S2). It is obvious that the concentration of Pt increases with the increase in titania content. The homogeneous dispersion of Pt and Ti components in the silica network for samples with lower titania content is noticeable in the SEM elemental mapping of the microstructure (Figure S3). These images confirm the existence of Ti species and the possibility of their agglomeration on the silica surface at higher titania contents. An ordered hexagonal porous structure was highlighted in TEM images of samples obtained from TBOT (Figure 5).
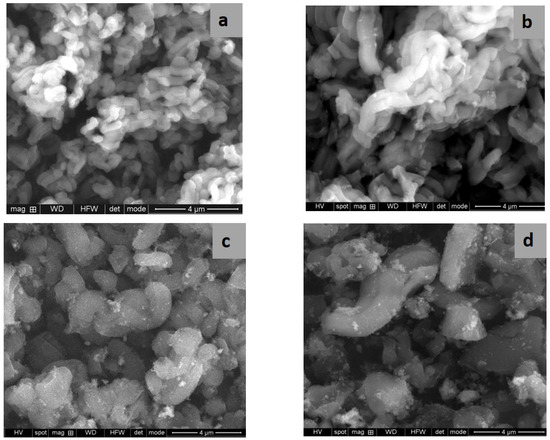
Figure 3.
SEM images of PT1SB (a), PT5SB (b), PT10SB (c) and PT30SB (d) samples.
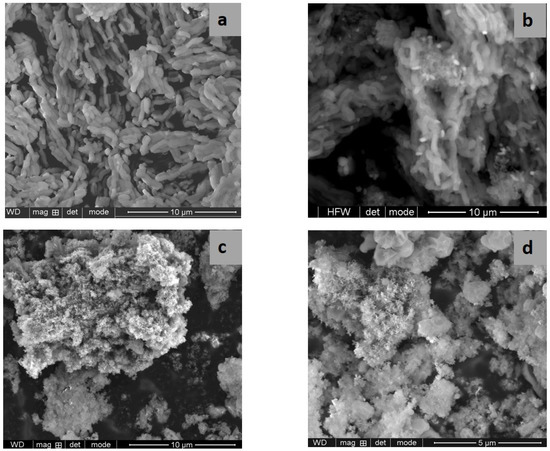
Figure 4.
SEM images of PT5SP (a), PT10SP (b), and P30SP (c,d) samples.
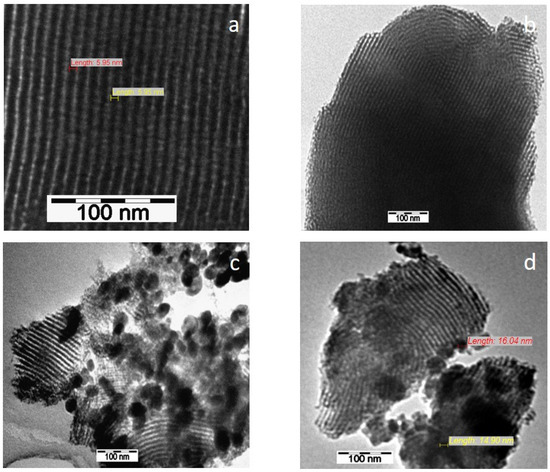
Figure 5.
TEM images of PT1SB (a), PT5SB (b), PT30SB (c) and PT10SB (d) samples.
3.2. Physicochemical Properties of the Supported Metals Species
Wide-angle XRD patterns (Figure 6) indicate distinct TiO2 crystalline peaks attributed to anatase and rutile. Unfortunately, the very low concentration of Pt (0.25% wt.) and the possibility of the Pt and TiO2 species peaks overlapping do not allow a very accurate interpretation of the results. Taking into account the concentration of TiO2 in the samples, it can be considered that most of the peaks correspond to titanium species. The intensity of the TiO2 crystalline peaks increases upon increasing its loading. This increase is more evident for the peroxotitanate-obtained samples with a richer rutile phase.
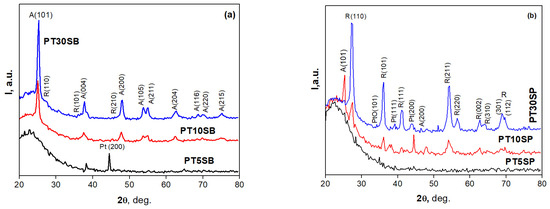
Figure 6.
The wide-angle XRD patterns of PT1nSB (a) and PTnSP (b) samples.
UV-Raman spectroscopy is a powerful technique for the surface characterization of mesoporous catalysts, while VIS-Raman spectroscopy mainly addresses the structural investigation of non-framework titania, and less so mesoporous catalyst support [41,42].
Since platinum is not Raman-active, VIS-Raman spectra of the Pt-containing materials in Figure 7 are due to TnS(B/P) materials and platinum oxides. Bands located at about 489, 604, 797, and 978 cm−1 originate from four- and three-membered SiO4 rings, and the Si-O-Si bending and Si-O stretching vibrations [43] of the SBA-15 (Figure 7a) point out that the framework titanium species (TiO4 units) [42,44] are present in the PT1SB and PT5SB samples with low TiO2 content. This finding is supported by the UV-Raman band at 1089 cm−1 (stretching vibrations of the Si-O-Ti bonds [44]) for the PT5SB sample, while the 1057 cm−1 band of the PT1SB spectrum in Figure S4 a is attributable to an asymmetric stretch of Si-O linkages [22]. Li et al. assigned the 490, 530, 960, and 1125 cm−1 bands collected with a deep UV laser (244 nm) to Ti-O-Si linkages [44]. However, the latter band is almost undetectable for the 325 nm-excited spectra [44]. This is analogous to the UV-Raman spectrum of the PT5SP catalyst (Figure S4b), wherein the 490, 530, and 960 cm−1 bands are more obvious. Moreover, Pt-O stretching modes were reported to appear at about 550 cm−1 and 532 cm−1 in the UV-Raman spectra of the platinum-rich loadings (3%), SBA-15, and platinum powder, respectively [43].
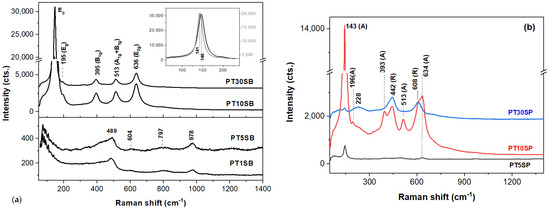
Figure 7.
VIS-Raman spectra of PTnSB (a) and PTnSP (b) samples (A and R stand for anatase and rutile).
The intensity of the two defect bands at 489 (D1) and 604 cm−1 (D2) is sensitive to the presence of HO- and the porous silica network with low TiO2 loads. The relatively intense band at ~3740 cm−1 (isolated SiO-H, H-bonded) in the UV-Raman spectra in Figure S4 confirms that OH groups are linked to the SBA surface [45]. Thus, the intensity of the D2 band decreases and vanishes when the porous structure degrades and/or collapses. Among the low TiO2 content samples, the PT5SP spectrum in Figure S4b lacks the D2 band. Since no significant modification of the surface area for the two PT5S(B/P) is recorded, the missing D2 band is a consequence of the overwhelming 634 cm−1 band of anatase [46] present in the VIS- and UV spectra (Figure 7 and Figure S4b).
Anatase vibrational modes [46] at 146 cm−1 (Eg), 195 cm−1 (Eg), 395 cm−1 (B1g), 513 cm−1 (A1g + B1g), and 639 cm−1 (Eg) are identified for higher TiO2-loaded samples, i.e., PT10SB and PT30SB, respectively (Figure 7a). The shifting of the Eg mode (146 cm−1) towards lower wavenumbers (see inset of Figure 7a) when the TiO2 content increases to 30 wt. % (PT30SB), might signal defects and/or nonstoichiometry of the anatase phase [47]. In contrast to PT5SB, the VIS-Raman spectrum of the PT5SP indicates the presence of anatase through its main bands at 143 and 636 cm−1 (Figure 7b). The relatively reduced intensity of the Eg band at 143 cm−1 is a consequence of diminished anatase content. Although obscured by strong bands of anatase, weak bands of SBA-15 at 489, ~800, and 977 cm−1 are still present in the VIS-Raman spectrum of the PT5SP.
Increasing the TiO2 content triggers the formation of the rutile [46] (143, 447, 612, and 826 cm−1 of the B1g, Eg, A1g, and B2g modes, respectively) and anatase phase. Zhang et al. [47] reported an anatase–rutile transition at lower temperatures when the initial particle size is reduced. The wide band at 228 cm−1 is due to second-order and disorder effects [48]. When TiO2 increases to 30 wt.%, the TiO2 prevailing phase is rutile. It is very likely that the anatase and rutile phases are distributed in so-called core–shell structures [49,50,51]. Hence, the non-framework titania (TiO6 clusters), namely anatase and/or rutile, is formed in the titania-rich PTnS(B/P) catalysts.
Analysis of the catalyst surface composition was carried out using XPS spectroscopy. Figure 8 displays the high-resolution XPS spectra of Ti2p and O1s, and Figure S4 shows the Si2p species detected on the surface of the PtTi-SBA-15 samples. The binding energies (Bes) of the Ti 2p doublet (2p3/2 = ~458.4 eV and 2p1/2 = 464.6 eV) reveal the presence of the Ti4+ cations in the TiO2 lattice (see Figure 8a) for the PT30SB and PT30SP samples. We assigned the higher BE (459.8 eV) in the sample PT1SB, with a small amount of Ti, to the stronger interaction of Ti with SiO2 support, as found by Lassaletta et al. [52]. Thus, the Ti oxidation state is 4+, as usual, but is shifted toward a higher BE due to the aforementioned interaction. Table 1 shows a low concentration of titanium atoms on the surface. One can see that in the sample PT1SB, Ti was not detected on the top of the surface (the first ~10 nm). In the case of titanium immobilization using direct synthesis on a mesoporous silica support, such as SBA15, the substitution of silica in the network takes place. For very low concentrations, Ti can be totally incorporated into the network [53]. This is evidenced by the high dispersion of titanium in the silica network for the samples with lower TiO2 percent, and the agglomeration of crystalline oxide on silica particles for samples with higher titania loading.
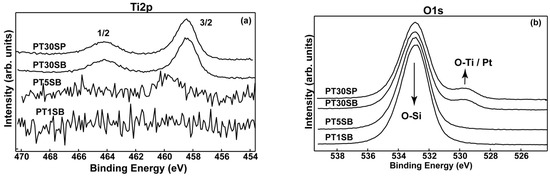
Figure 8.
The XPS photoelectron spectra: Ti2p (a) and O1s (b) superimposed spectra for PT1SB, PT5SB, PT30SB and PT30SP.

Table 1.
XPS data for the catalysts (atomic relative concentrations, and percentages of Pt chemical species).
The effect of the support and synthesis method on extra-framework titanium species has been highlighted by previous studies [24,25]. It was thus observed that the XRD results either indicated or not, for the same concentration of TiO2 (5%), the presence of its crystalline species. At the same time, the XPS spectra showed different concentrations of Ti on the surface under the conditions of the same TiO2 concentration in the sample. Thus, the different distribution of titanium in the volume compared to the surface was highlighted. Additionally, the concentration of titanium oxide and its association with other oxide species, such as those of cerium, influenced the concentration and nature of the Pt species on the surface.
The O1s spectra (Figure 8b) show a similar profile for the samples PT30SB and PT30SP with oxygen mainly bonded in SiO2 matrix (532.9 eV), as well as a small contribution assigned to oxygen bonding with titanium (530.4 eV). The Si4+ oxidation state was found in all samples and confirmed by the Si2p transition at 103.4 eV (Figure S5). Pt4f spectra exhibit a complex band-like shape, accommodating elemental Pt and Pt oxidized as Pt (OH)2 and PtO (Figure 9). Despite very low Pt concentrations, and consequently, associated noisy spectra, it is still possible to deconvolute Pt4f spectra in an attempt to determine the Pt chemical species. The deconvoluted procedure for current Pt 4f noisy spectra followed ISO-TC201 (“surface chemical analysis”) recommendations, using the constraints on the binding energies (Bes) associated with Pt0 (4f7/2 = 71.1 eV), Pt(OH)2 (4f7/2 = 72.2 eV)) and PtO (4f7/2 = 74.4 eV), the intensity ratio in the 4f7/2, 5/2 doublet, and the spin–orbit parameter. It is worth mentioning that the errors in the binding energies (Bes)’ assignments were within ±0.2 eV limits. These led to a reliable quantitative assessment, even for the spectrum with very low count rates, as a result of tiny relative concentrations (Table 1). Thus, one can see that samples PT30SB and PT30SP display about the same Pt oxide and metal concentration.
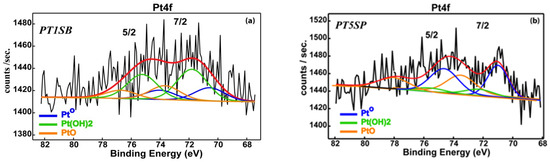
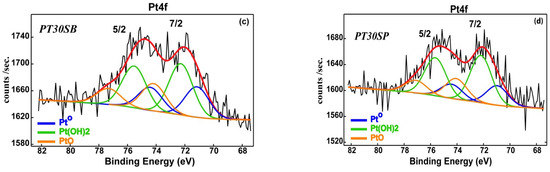
Figure 9.
XPS spectra of PtTi-SBA-15 samples with (a) 1, (b) 5, and (c,d) 30% TiO2 loading. (Red line stands for global fit).
A close examination of the spectra suggests a larger contribution of the elemental Pt to the PT5SB sample (57.4% Pt0) as compared to the PT1SB sample (22.6% Pt0).
The redox properties of the PtTi-SBA-15 catalysts, after their thermal treatment in air at 550 °C, are shown in Figure 10. High-temperature reduction peaks in the range of 300–600 °C can be observed. This is in agreement with the XPS spectra of the other Pt/TiO2 catalysts [54,55]. The low-temperature peaks were attributed to the reduction of PtOx species, whereas those appearing at high temperatures were associated with the reduction of TiO2, catalyzed by Pt through the strong metal–support interaction (SMSI). Three reduction peaks were observed for sample PT30SB, and one broad peak for all other samples. The first was attributed to the reduction of the PtOx species. The next two may be the result of two different Ti oxide species.
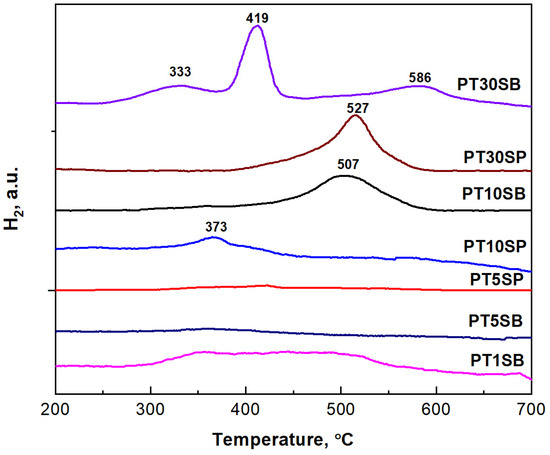
Figure 10.
H2-TPR profiles of PTnSB and PTnSP catalysts with different TiO2 loading.
The reduction of TiO2 species occurs at 419 °C, with a high interaction with Pt, and at 586 °C, titania is reduced, with a higher interaction with the silica support. The interaction of Pt with the supported TiO2 explains the hydrogen absorption at a higher temperature, e.g., 507 °C (PT10SB) and 527 °C (PT30SP). Previous studies [56,57] have also indicated that between 300 °C and 400 °C, Ti4+ to Ti3+ can be partially reduced by hydrogen spillover in the presence of Pt nanoparticles, and after 500 °C, the final reduction of the titanium species takes place.
In both cases, there is a significant effect caused by SMSI, and a spillover of dissociative hydrogen is chemisorbed [58]. The more evident spillover effect was observed for the PT1SB sample, with a large peak between 350 and 550 °C. At a low concentration of TiO2 (1%), it was proven that in the case of direct synthesis, Si-O-T-O-Si species are formed in the silica network. In this case, the probability of Pt-TiO2 interaction is negligible. In this case, the platinum is deposited on the silica. Therefore, platinum interacts with silica in the PT1SB. The broad peaks at around 350 °C were assigned to the reduction of PtO species and to the H2 spillover effect. This peak was very small for some samples, namely PT5S(B/P), with a higher Pt metal content and lower consumption of H2 (Table 2).

Table 2.
H2 chemosorption results.
The Pt particles’ nature, especially their size, and dispersion, was analyzed using the H2 chemisorption method. High dispersion of Pt, with an average size of around 1 nm, was obtained for all the samples (Table 2). The amount of hydrogen consumption needed for reduction was higher for samples with larger peaks, such as PT1SB, PT10SB, or PT10SP. All these samples have one peak of reduction as a result of both the SMSI effect and H2 spillover.
The X-ray absorption near edge spectroscopy (XANES) measurement results confirm the presence of Pt in both metallic and oxidation states. The Pt LIII-edge XANES spectra of the investigated samples together with the etalon samples are presented in Figure 11. The absorption occurring at the Pt LIII-edge corresponds to the 2p3/2→5d electronic transitions [59,60]. The energy shift observed in the Pt LIII-edge XANES spectra is not sufficiently large to distinguish between the Pt oxidation states. This is the reason that the intensity of the white line peaks was also considered. Thus, the white line intensity reflects the unoccupied Pt(5d) orbital because the absorption edge corresponds to an electron transition from the 2p3/2 to 5d orbitals in the Pt atom [61].
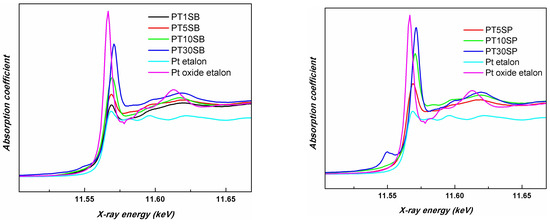
Figure 11.
Pt LIII-edge XANES spectra of investigated samples together with the etalon samples.
In order to compare the different support influences (TnSB and TnSP), the Pt LIII-edges of the samples with the same concentration of TiO2, namely PT5SB, and PT5SP, were plotted in Figure 12. The intensity of the white line is higher for the PT5SP sample. Moreover, the formation of the non-framework titania threshold is lowered to 5% in the case of the SP support, as supported by the XRD and Raman findings (Figure 6b and Figure 7b), wherein anatase was depicted.
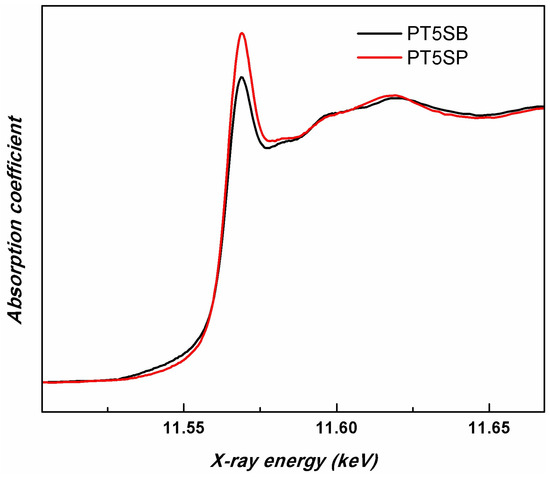
Figure 12.
Pt LIII-edge XANES spectra of PT5SB and PT5SP samples.
3.3. Catalytic Properties of PtTi-SBA-15 Materials in CH4 Oxidation
The methane oxidation occurring in Ti-SBA-15 supports and Pt-based catalysts was monitored as a function of temperature (Figure 13 and Figure 14). All the samples are active in CH4 oxidation reactions, and a higher selectivity to CO2 was obtained for samples immobilized with Pt (>90%).
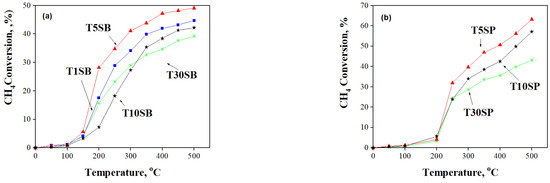
Figure 13.
CH4 conversion as a function of temperature for (a) TnSB and (b) TnSP supports with different titania loadings.
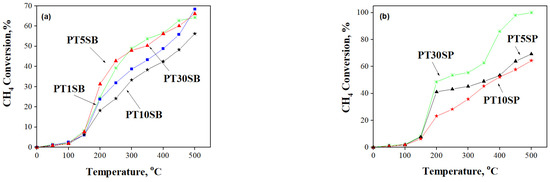
Figure 14.
CH4 conversion and its variation with temperature for (a) PTnSB and (b) PTnSP samples after Pt immobilization.
All TnSB and TnSP supports with 1, 5, 10, and 30% TiO2 content are active in methane oxidation (Figure 13). High activity was obtained for TnSB samples after Pt immobilization (Figure 14). The highest conversion of methane was obtained above 400 °C for the sample prepared from peroxotitanate with 30% TiO2.
4. Discussion
Two different Ti-SBA-15 supports were prepared using the direct synthesis method. One was obtained from tetra butyl ortho-titanate (TBOT) as a titanium dioxide precursor [22,24], and the other from peroxotitanate synthesized from TBOT and H2O2. The nature and concentration of the titanium precursors influenced the sol–gel process in the presence of surfactant, and thus the formation of a silica network and titania crystalline species. The addition of TBOT in combination with TEOS to the aqueous solution of surfactant reduced the rate of titanium alkoxide hydrolysis in order to obtain its advanced dispersion within the silica species. The increasing TBOT concentration led to the growth of the hydrolyzed alkoxide species, and their agglomeration thus blocked the polycondensation of the silica. Thus, the TBOT precursor influenced the morphology and porous structure of the SBA-15 silica. After hydrothermal treatment and calcination, the presence of non-framework TiO2 crystals on the silica surface was detected for the titania-rich samples (Figure 6).
In the second synthesis method, the precursor of titania was peroxotitanate. The peroxo groups (O-O) were found either to accelerate photocatalytic activity through superoxide generation or to shift the catalytic activity to visible light due to oxygen vacancies [62]. The peroxotitanate route used in the synthesis of the silica–titania composite, using the sol–gel method with small TiO2 loadings, led to interesting properties; this is due to the formation of Si-O-Ti linkages and small crystalline anatase particles because silica suppresses the growth and aggregation of TiO2 crystals. A well-crystallized rutile phase was obtained using the sol–gel method and hydrothermal peroxo route at high TiO2 loadings. Thus, the Ti-hydroperoxy (OH)4-n-Ti(OOH)n species formed during preparation from TBOT and H2O2 in an aqueous solution further dissociated into protonated Ti-peroxo species (OH)4-n-Ti(OO-)n. Chung et al. [63] reported lower free energies for the anatase overall reaction than in the rutile reaction in TiO2 catalysts. The influence of these anions on the formation of the silica network is smaller in an acidic solution of surfactant and TEOS silica precursor. However, they can be adsorbed on the surface of the silica at small concentrations and may form aggregates of rutile when the concentration of titania is higher. The formation of rutile nanocrystals was considered the result of hydrothermal treatment and of the TBOT/H2O2 molar ratio.
The presence of different titania polymorphs and Pt species, the change in the morphology (Figure 4c,d and Figure 5c,d), and the diminishing of the silica-ordered structure was evidenced for the samples with 10 and 30% TiO2 (Figure 6c,d). XRD patterns (Figure 1), SEM (Figure 3 and Figure 5), and TEM (Figure 5) images show the significant effect of the titanium precursor and its concentration on the morphology and porous structure of PtTi-SBA-15 samples, which is more significant in the case of SBA-15 compared to KIT-6 [25]. Although TBOT was the titanium precursor for both synthesis methods, the type of the non-framework titanium dioxide polymorph was determined by the SBA-15 and KIT-6 supports as well as the TBOT and PT precursors. Thus, direct synthesis of Ti-SBA-15 favored the attainment of anatase and rutile phases. The concentration of these species increased significantly in the case of peroxo-synthesized catalysts (PTnSP materials). According to Raman data in Figure 7, the titania-rich sample PT30SP contains mostly rutile phase in comparison with the PT30SB counterpart in which the anatase phase prevails. Hence, when using the peroxo route, the non-framework rutile phase of titania formed at high titania loadings. Moreover, the TiO2 content threshold is lowered to 5% in the case of the PTnSP, in which the non-framework anatase prevailed.
XPS results showed the presence of Pt0 species on the surface. Their content decreased for the samples with higher titania loadings. A very low effect of the titania precursor was evidenced for the superficial Pt species. Comparatively, with PtTi-KIT-6 samples, XPS of PtTi-SBA-15 samples evidenced a lower percentage of Pt on the surface under conditions of samples with the same composition [25]. These differences are caused by changes in morphologies, porous structures, the dispersion of titanium species, and their interaction with the supports.
H2-TPR results evidenced the interaction of Pt species with supports of different compositions that modified PtOx’s reducibility to Pt0 [64,65]. Different degrees of the metal–support interaction was also observable in the TiO2 reduction pattern above 400 °C [66]. The strong interaction of Pt with the TiO2 substrate is attributable to an electron transfer from the substrate to Pt atoms. However, with the increase in TiO2 concentration, this transference is reduced, and for the sample with higher TiO2 content, the Pt oxide concentration increases. This phenomenon is a result of TiO2 crystalline phase agglomeration with the increase in its concentration, which causes a decrease in Pt-TiO2 surface interaction (Figure 4 and Figure S2). The small differences between XPS and XAS results concerning the relative concentration of Pt species can be explained by considering that XPS provides surface information, while XAS is a volume technique. Therefore, the presence of Pt0 in all the PTnS samples sustains the metal–support interaction. However, the interaction between Pt and titania depends on titania’s dispersion on a mesoporous silica support, its particle size, and its crystalline structure.
The best conversion was obtained for samples with the highest dispersion and an insignificant crystalline phase of titania (see Figure 2 and Figure S2). A higher CH4 conversion was obtained for PTnSB samples with 5% and 30% TiO2 concentrations, respectively. This result was explained by the SMSI effect evidenced both in the reduction of PtO to Pt0, at a higher percentage for sample PT5SB and at a higher Pt percentage on the surface of the PT30SB sample, as determined by XPS (Table 1). The Pt/PtO ratio of the atomic species (Table 1) is 1.35 for the PT5SB sample and 0.43 for the PT30SP sample. Parmon et al. [67] showed using XPS measurements that the catalyst activation in methane oxidation may be associated with the predominant formation of the metallic platinum, whereas the oxidation of the platinum surface is responsible for the less active state. This effect of the metal oxidation state (Pd2+/Pd0 ratio) on activity in the total oxidation reaction of methane was evidenced for Pd-Pt/Al2O3 catalysts in situ XPS experiments [68]. The presence of Pt on the surface influenced the Pd2+/Pd0 ratio and hence the activity. The literature data indicate that only the coexistence of Pd0 and Pd2+ determines the high values of the activity in the total oxidation of methane or propane [69]. In this study, the conversion obtained for the PT30SB sample was considered the result of SMSI, in which the effect of the Pt/PtOx value is offset by increasing the Ti and Pt species on the surface. Thus, catalysts with 5% TiO2 may be considered the best catalysts. The catalyst PT30SP exhibited more effective conversion, reaching the maximum value (100%) at 500 °C, while SMSI had a lesser effect on the samples, with a maximum percentage of rutile on a silica support. The published studies [66] have shown that the crystalline composition of anatase and rutile TiO2 can affect the interaction with the support, the dispersion, and the oxidation state of the metal oxide support, i.e., PtO. For the PT30SP sample, rutile provides large amounts of oxygen atoms for reaction with methane. In the presence of higher Pt atoms on the surface, a great conversion of methane was achieved. Increased conversion at higher temperatures was supported by the catalytic test results and TPR data. Thus, the H2-TPR results showed the possibility of reducing oxide metal species at higher temperatures. In this way, taking methane as a reducing agent, the increase in conversion with temperature can be explained.
5. Conclusions
Two series of catalysts with highly dispersed Pt nanoparticles were prepared on Ti-modified SBA-15 mesoporous silica. SEM, XPS, XAS, TPR, and H2 chemisorption measurements revealed changes in Pt-TiO2 interaction with titanium species’ dispersion on the silica support and its crystalline structure. The properties of the PTnS(B/P) are a consequence of the synthesis conditions and titania loading. The catalysts exhibited a different concentration of Pt0 nanoparticles resulting from the stronger interaction of PtO with anatase and the weak interaction of PtO with rutile TiO2. Titania loading and precursors influenced the anatase and rutile concentration, the dispersion of titania species, and the Pt-TiO2-supported interaction. The results of the catalytic tests highlighted the synergistic effect of Pt and TiO2 species. The Pt/PtO ratio and their concentration on the catalyst surface, trigged by the SMSI effect, also influenced methane conversion. The increase in TiO2 concentration led to an increase in Pt species on the surface, which may explain the high conversion value of the PT30SB and PT30SP samples. In the case of the PT5SB sample, the high conversion value may be due to the higher percentage of metallic Pt dispersed on the surface. The best conversion (100%) was obtained for the PT30SP sample at 500 °C, and this may be the result of the Pt’s interaction with more crystalline TiO2 and a richer rutile phase. Although the impact of the TiO2 crystalline phase on catalytic performance was less known, here, its beneficial effect on methane oxidation has been evidenced.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano13101647/s1. Figure S1: Pore size distribution of PtTi-SBA-15 samples, before and after Pt immobilization on samples obtained with tetrabutylorthotitanate; Figure S2: Pore size distribution of PtTi-SBA-15 samples, before and after Pt immobilization on samples obtained with peroxotitanate; Figure S3: Elemental mapping images obtained by SEM; Figure S4: UV-Raman spectra of the PT(1/5)S(B/P) catalysts. Figure S5: The XPS photoelectron spectra of Si2p, superimposed spectra for PT1SB, PT5SB, PT30SB and PT30SP.
Author Contributions
Conceptualization, V.P.; methodology, M.F. and V.P.; software, V.R.; validation, E.M.A., V.P., N.A. and J.Z.; formal analysis, M.F., E.M.A., N.A. and J.Z.; investigation, M.F., E.M.A., V.R., F.P., S.S. and V.P.; data curation, M.F., E.M.A., V.R., F.P., S.S. and C.M.; writing—original draft preparation, M.F.; writing—review and editing, E.M.A., V.R., S.S. and V.P.; visualization, E.M.A. and V.P.; supervision, V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank M. Enachescu for access to the Raman facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Oseghe, E.O.; Maddila, S.; Ndungu, P.G.; Jonnalagadda, S.B. Effect of surfactant concentration on active species generation and photocatalytic properties of TiO2. Appl. Catal. B 2015, 176–177, 288–297. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, Q.; Liu, X.; Li, J.; Xu, H.; Ding, H.; Li, G. Facile Assembly of InVO4/TiO2 Heterojunction for Enhanced Photo-Oxidation of Benzyl Alcohol. Nanomaterials 2022, 12, 1544. [Google Scholar] [CrossRef] [PubMed]
- Gowrisankaran, S.; Thirunavukkarasu, G.K.; Makarov, T.; Roch, T.; Plesch, G.; Motola, M.; Mailhot, G.; Brigante, M.; Monfort, O. New insights into the mechanism of coupled photocatalysis and Fenton-based processes using Fe surface-modified TiO2 nanotube layers: The case study of caffeine degradation. Catal. Today 2023, 413–415, 114027. [Google Scholar] [CrossRef]
- Rui, Z.; Wu, S.; Peng, C.; Ji, H. Comparison of TiO2 Degussa P25 with anatase and rutile crystalline phases for methane combustion. Chem. Eng. J. 2014, 243, 254. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Magdziarz, A.; Aramendia, M.A.; Marinas, A.; Marinas, J.M.; Urbano, F.J.; Navio, J.A. Influence of the strong metal support interaction effect (SMSI) of Pt/TiO2 and Pd/TiO2 systems in the photocatalytic biohydrogen production from glucose solution. Catal. Commun. 2011, 16, 1–6. [Google Scholar] [CrossRef]
- Li, X.; Zheng, W.; Pan, H.; Yu, Y.; Chen, L.; Wu, P. Pt nanoparticles supported on highly dispersed TiO2 coated on SBA-15 as an efficient and recyclable catalyst for liquid-phase hydrogenation. J. Catal. 2013, 300, 9–19. [Google Scholar] [CrossRef]
- Bertella, F.; Concepción Heydorn, P.; Martinez Feliu, A. The impact of support surface area on the SMSI decoration effect and catalytic performance for Fischer-Tropsch synthesis of Co-Ru/TiO2-anatase catalysts. Catal. Today 2017, 296, 170–180. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, X.; Liu, J.; Xiao, J.; Hu, Z. Si doping influence on the catalytic performance of Pt/TiO2 mesoporous film catalyst for low-temperature methanol combustion. Appl. Surf. Sci. 2014, 309, 144–152. [Google Scholar] [CrossRef]
- Alonso, F.; Riente, P.; Rodríguez-Reinoso, F.; Ruiz-Martínez, J.; Sepúlveda-Escribano, A.; Yus, M. Platinum nanoparticles supported on titania as an efficient hydrogen-transfer catalyst. J. Catal. 2008, 260, 113–118. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Liu, Y.-Y.; Wang, A.; Sheng, Q.; Zhang, C.-X. Insight into metal-support interactions from the hydrodesulfurization of dibenzothiophene over Pd catalysts supported on UiO-66 and its aminofunctionalized analogues. J. Catal. 2022, 407, 333–341. [Google Scholar] [CrossRef]
- Hazlett, M.J.; Moses-Debusk, M.; Parks, J.E.; Allard, L.F.; Epling, W.S. Kinetic and mechanistic study of bimetallic Pt-Pd/Al2O3 catalysts for CO and C3H6 oxidation. Appl. Catal. B 2017, 202, 404–417. [Google Scholar] [CrossRef]
- Daneshvar, K.; Dadi, R.K.; Luss, D.; Balakotaiah, V.; Kang, S.B.; Kalamaras, C.M.; Epling, W.S. Experimental and modeling study of CO and hydrocarbons light-off on various Pt-Pd/γ-Al2O3 diesel oxidation catalysts. Chem. Eng. J. 2017, 323, 347–360. [Google Scholar] [CrossRef]
- Peng, R.; Zhang, H.; Guo, Y.; Huang, W.; Zhang, Y.; Wu, J.; Fu, M.; Yu, G.; Ye, D. The lanthanide doping effect on toluene catalytic oxidation over Pt/CeO2 catalyst. J. Colloid Interface Sci. 2022, 614, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Lunagómez Rocha, M.A.; Del Ángel, G.; Torres-Torres, G.; Cervantes, A.; Vázquez, A.; Arrieta, A.; Beltramini, J.N. Effect of the Pt oxidation state and Ce3+/Ce4+ ratio on the Pt/TiO2-CeO2 catalysts in the phenol degradation by catalytic wet air oxidation (CWAO). Catal. Today 2015, 250, 145–154. [Google Scholar] [CrossRef]
- Yurdakal, S.; Tek, B.S.; Değirmenci, Ç.; Palmisano, G. Selective photocatalytic oxidation of aromatic alcohols in solar-irradiated aqueous suspensions of Pt, Au, Pd and Ag loaded TiO2 catalysts. Catal. Today 2017, 281, 53–59. [Google Scholar] [CrossRef]
- Choi, P.G.; Ohno, T.; Masui, T.; Imanaka, N. Catalytic liquid-phase oxidation of acetaldehyde to acetic acid over a Pt/CeO2–ZrO2–SnO2/γ-alumina catalyst. J. Environ. Sci. 2015, 36, 63–66. [Google Scholar] [CrossRef]
- Kumar, S.M.; Chen, D.; Walmsley, J.C.; Holmen, A. Dehydrogenation of propane over Pt-SBA-15: Effect of Pt particle size. Catal. Commun. 2008, 9, 747–750. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, T.; Xu, X.; Xiao, P.; Li, J. Pt nanoparticles supported on SBA-15: Synthesis, characterization and applications in heterogeneous catalysis. Appl. Catal. B 2013, 130–131, 197–217. [Google Scholar] [CrossRef]
- Usón, L.; Colmenares, M.G.; Hueso, J.L.; Sebastián, V.; Balas, F.; Arruebo, M.; Santamaría, J. VOCs abatement using thick eggshell Pt/SBA-15 pellets with hierarchical porosity. Catal. Today 2014, 227, 179–186. [Google Scholar] [CrossRef]
- Li, L.; Wua, P.; Yu, Q.; Wu, G.; Guan, N. Low temperature H2-SCR over platinum catalysts supported on Ti-containing MCM-41. Appl. Catal. B 2010, 94, 254–262. [Google Scholar] [CrossRef]
- Venezia, A.M.; Di Carlo, G.; Liotta, L.F.; Pantaleo, G.; Kantcheva, M. Effect of Ti(IV) loading on CH4 oxidation activity and SO2 tolerance of Pd catalysts supported on silica SBA-15 and HMS. Appl. Catal. B 2011, 106, 529–539. [Google Scholar] [CrossRef]
- Filip, M.; Petcu, G.; Anghel, E.; Petrescu, S.; Trica, B.; Osiceanu, P.; Stanica, N.; Atkinson, I.; Munteanu, C.; Mureseanu, M.; et al. FeTi- SBA-15 magnetic nanocomposites with photocatalytic properties. Catal. Today 2021, 366, 10–19. [Google Scholar] [CrossRef]
- Ganiyu, S.A.; Alhooshani, K.; Ali, S.A. Single-pot synthesis of Ti-SBA-15-NiMo hydrodesulfurization catalysts: Role of calcination temperature on dispersion and activity. Appl. Catal. B 2017, 203, 428–441. [Google Scholar] [CrossRef]
- Ciobanu, M.; Petcu, G.; Anghel, E.M.; Papa, F.; Apostol, N.G.; Culita, D.C.; Atkinson, I.; Todorova, S.; Shopska, M.; Naydenov, A.; et al. Influence of Ce addition and Pt loading upon the catalytic properties of modified mesoporous PtTi-SBA-15 in total oxidation reactions. Appl. Catal. A 2021, 619, 118123. [Google Scholar] [CrossRef]
- Filip, M.; Todorova, S.; Shopska, M.; Ciobanu, M.; Papa, F.; Somacescu, S.; Munteanu, C.; Parvulescu, V. Effects of Ti loading on activity and redox behavior of metals in PtCeTi/KIT-6 catalysts for CH4 and CO oxidation. Catal. Today 2018, 306, 138–144. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Lin, S.D.; Wu, J.C.S. Synergetic photo-epoxidation of propylene over VTi/MCM-41 mesoporous photocatalysts. J. Catal. 2015, 331, 21–227. [Google Scholar] [CrossRef]
- Shanmugam, V.; Zapf, R.; Neuberg, S.; Hessel, V.; Kolb, G. Effect of ceria and zirconia promotors on Ni/SBA-15 catalysts for coking and sintering resistant steam reforming of propylene glycol in microreactors. Appl. Catal. B 2017, 203, 859–869. [Google Scholar] [CrossRef]
- Li, D.; Zeng, L.; Li, X.; Wang, X.; Ma, H.; Assabumrungrat, S.; Gong, J. Ceria-promoted Ni/SBA-15 catalysts for ethanol steam reforming with enhanced activity and resistance to deactivation. Appl. Catal. B 2015, 176–177, 532–541. [Google Scholar] [CrossRef]
- Bonne, M.; Samoila, P.; Ekou, T.; Especel, C.; Epron, F.; Marecot, P.; Royer, S.; Duprez, D. Control of titania nanodomain size as a route to modulate SMSI effect in Pt/TiO2 catalysts. Catal. Commun. 2010, 12, 86–91. [Google Scholar] [CrossRef]
- Benz, D.; Van Bui, H.; Hintzen, H.T.; Kreutzer, M.T.; Ruud van Ommen, J. Synthesis of a Rationally Designed Multi-Component Photocatalyst Pt:SiO2:TiO2(P25) with Improved Activity for Dye Degradation by Atomic Layer Deposition. Nanomaterials 2020, 10, 1496. [Google Scholar] [CrossRef]
- Valdés-Martínez, O.U.; Suárez-Toriello, V.A.; de los Reyes, J.A.; Pawelec, B.; Fierro, J.L.G. Support effect and metals interactions for NiRu/Al2O3, TiO2 and ZrO2 catalysts in the hydrodeoxygenation of phenol. Catal. Today 2017, 296, 219–227. [Google Scholar] [CrossRef]
- Bailón-García, E.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J. Influence of the pretreatment conditions on the development and performance of active sites of Pt/TiO2 catalysts used for the selective citral hydrogenation. J. Catal. 2015, 327, 86–95. [Google Scholar] [CrossRef]
- Shah, M.S.A.S.; Oh, C.; Park, H.; Hwang, Y.J.; Ma, M.; Park, J.H. Catalytic Oxidation of Methane to Oxygenated Products: Recent Advancements and Prospects for Electrocatalytic and Photocatalytic Conversion at Low Temperatures. Adv. Sci. 2020, 7, 2001946. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Lin, H.; Song, H.; Yang, L.; Tang, D.; Deng, F.; Ye, J. Efficient and selective photocatalytic CH4 conversion to CH3OH with O2 by controlling overoxidation on TiO2. Nat. Commun. 2021, 12, 4652. [Google Scholar] [CrossRef]
- Stakheev, A.Y.; Gololobov, A.M.; Beck, I.E.; Bragina, G.O.; Zaikovsky, V.I.; Ayupov, A.B.; Telegina, N.S.; Bukhtiyarov, V.I. Effect of Pt nanoparticle size on the specific catalytic activity of Pt/SiO2 and Pt/TiO2 in the total oxidation of methane and n-butane. Russ. Chem. Bull. 2010, 59, 1713–1719. [Google Scholar] [CrossRef]
- Wilburn, M.S.; Epling, W.S. Sulfur deactivation and regeneration of mono- and bimetallic Pd-Pt methane oxidation catalysts. Appl. Catal. B 2017, 206, 589–598. [Google Scholar] [CrossRef]
- Yashnik, S.A.; Chesalov, Y.A.; Ishchenko, A.V.; Kaichev, V.V.; Ismagilov, Z.R. Effect of Pt addition on sulfur dioxide and water vapor tolerance of Pd-Mn-hexaaluminate catalysts for high-temperature oxidation of methane. Appl. Catal. B 2017, 204, 89–106. [Google Scholar] [CrossRef]
- Alegre, V.V.; da Silva, M.A.P.; Schmal, M. Catalytic combustion of methane over palladium alumina modified by niobia. Catal. Commun. 2006, 7, 314–322. [Google Scholar] [CrossRef]
- Damyanova, S.; Bueno, J.M.C. Effect of CeO2 loading on the surface and catalytic behaviors of CeO2-Al2O3-supported Pt catalysts. Appl. Catal. A 2003, 253, 135–150. [Google Scholar] [CrossRef]
- de Lucas, A.; Rodrίguez, L.; Sánchez, P. Synthesis of TS-2 in the system SiO2–TiO2–H2O2–TBAOH. Influence of the synthesis variables. Appl. Catal. A 1999, 180, 375–383. [Google Scholar] [CrossRef]
- Hess, C. New advances in using Raman spectroscopy for the characterization of catalysts and catalytic reactions. Chem. Soc. Rev. 2021, 50, 3519. [Google Scholar] [CrossRef]
- Xiong, G.; Cao, Y.; Guo, Z.; Jia, Q.; Tiana, F.; Liu, L. The roles of different titanium species in TS-1 zeolite in propylene epoxidation studied by in situ UV Raman spectroscopy. Phys. Chem. Chem. Phys. 2016, 18, 190. [Google Scholar] [CrossRef] [PubMed]
- Borodko, Y.; Ager, J.W.; Marti, G.E.; Song, H.; Niesz, K.; Somorjai, G.A. Structure Sensitivity of Vibrational Spectra of Mesoporous Silica SBA-15 and Pt/SBA-15. J. Phys. Chem. B 2005, 109, 17386–17390. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xiong, G.; Liu, J.; Ying, P.; Xin, A.Q.; Feng, Z. Identifying Framework Titanium in TS-1 Zeolite by UV Resonance Raman Spectroscopy. J. Phys. Chem. B 2001, 105, 2993–2997. [Google Scholar] [CrossRef]
- Malfait, B.; Moréac, A.; Jani, A.; Lefort, R.; Huber, P.; Fröba, M.; Morineau, D. Structure of Water at Hydrophilic and Hydrophobic Interfaces: Raman Spectroscopy of Water Confined in Periodic Mesoporous (Organo) Silicas. J. Phys. Chem. C 2022, 126, 3520–3531. [Google Scholar] [CrossRef]
- Araújo, M.M.; Silva, L.K.R.; Sczancosk, J.C.; Orlandi, M.O.; Longo, E.; Santos, A.G.D.; Sá, J.L.S.; Santos, R.S.; Luz, G.E., Jr.; Cavalcante, L.S. Anatase TiO2 nanocrystals anchored at inside of SBA-15 mesopores and their optical behavior. Appl. Surf. Sci. 2016, 389, 1137–1147. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Q.; Li, M.; Feng, Z.; Li, C. UV Raman Spectroscopic Study on TiO2. II. Effect of Nanoparticle Size on the Outer/Inner Phase Transformations. J. Phys. Chem. C 2009, 113, 1698–1704. [Google Scholar] [CrossRef]
- Gotic, M.; Ivanda, M.; Popovic, S.; Music, S.; Sekulic, A.; Turkovic, A.; Furic, K. Raman investigation of nanosized TiO2. J. Ram. Spectrosc. 1997, 228, 555–558. [Google Scholar] [CrossRef]
- Wang, Q.; Fan, J.; Zhang, S.; Yun, Y.; Zhang, J.; Zhang, P.; Hu, J.; Wang, L.; Shao, G. In situ coupling of Ti2O with rutile TiO2 as a core–shell structure and its photocatalysis performance. RSC Adv. 2017, 7, 54662. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, M.; Qin, X. Photocatalytic Activity of TiO2 Nanofibers: The Surface Crystalline Phase Matters. Nanomaterials 2019, 9, 535. [Google Scholar] [CrossRef]
- Su, W.; Zhang, J.; Feng, Z.; Chen, T.; Ying, P.; Li, C. Surface Phases of TiO2 Nanoparticles Studied by UV Raman Spectroscopy and FT-IR Spectroscopy. J. Phys. Chem. C 2008, 112, 7710–7716. [Google Scholar] [CrossRef]
- Lassaletta, G.; Caballero, A.; Wu, S.; Gonzalez-Elipe, A.R.; Fernandez, A. Photoelectron spectroscopy of metal oxide particles: Size and support effects. Vacuum 1994, 45, 1085–1086. [Google Scholar] [CrossRef]
- Das, S.K.; Bhunia, M.K.; Bhaumik, A. Highly ordered Ti-SBA-15: Efficient H2 adsorbent and photocatalyst for eco-toxic dye degradation. J. Solid State Chem. 2010, 183, 1326–1333. [Google Scholar] [CrossRef]
- Li, N.; Chen, Q.Y.; Luo, L.F.; Huang, W.X.; Luo, M.F.; Hu, G.S.; Lu, J.Q. Kinetic study and the effect of particle size on low temperature CO oxidation over Pt/TiO2 catalysts. Appl. Catal. B 2013, 142–143, 523–532. [Google Scholar] [CrossRef]
- Aramendía, M.A.; Colmenares, J.C.; Marinas, A.; Marinas, J.M.; Moreno, J.M.; Navío, J.A.; Urbano, F.J. Effect of the redox treatment of Pt/TiO2 system on its photocatalytic behaviour in the gas phase selective photooxidation of propan-2-ol. Catal. Today 2007, 128, 235–244. [Google Scholar] [CrossRef]
- Saputera, W.H.; Scott, J.A.; Friedmann, D.; Amal, R. Revealing the key oxidative species generated by Pt-loaded metal oxides under dark and light conditions. Appl. Catal. B 2018, 223, 216–227. [Google Scholar] [CrossRef]
- Vasile, A.; Papa, F.; Bratan, V.; Munteanu, C.; Teodorescu, M.; Atkinson, I.; Anastasescu, M.; Kawamoto, D.; Negrila, C.; Ene, C.D.; et al. Water denitration over titania-supported Pt and Cu by combined photocatalytic and catalytic processes: Implications for hydrogen generation properties in a photocatalytic system. J. Environ. Chem. Eng. 2022, 10, 107129. [Google Scholar] [CrossRef]
- Petallidou, K.C.; Polychronopoulou, K.; Fierro, J.L.G.; Efstathiou, A.M. Low-temperature water-gas shift on Pt/Ce0.8La0.2O2−δ–CNT: The effect of Ce0.8La0.2O2−δ/CNT ratio. Appl. Catal. A 2015, 504, 585–598. [Google Scholar] [CrossRef]
- Chen, A.; Guo, H.; Song, Y.; Chen, P.; Lou, H. Recyclable CeO2–ZrO2 and CeO2–TiO2 mixed oxides based Pt catalyst for aqueous-phase reforming of the low-boiling fraction of bio-oil. Int. J. Hydrogen Energy 2017, 42, 9577–9588. [Google Scholar] [CrossRef]
- Kim, M.S.; Chung, S.H.; Yoo, C.J.; Lee, M.S.; Cho, I.H.; Lee, D.W.; Lee, K.Y. Catalytic Reduction of Nitrate in Water over Pd-Cu/TiO2 Catalyst: Effect of the Strong Metal-Support Interaction (SMSI) on the Catalytic Activity. Appl. Catal. B 2013, 142–143, 354–361. [Google Scholar] [CrossRef]
- Kim, S.S.; Lee, H.H.; Hong, S.C. A study on the effect of support’s reducibility on the reverse water-gas shift reaction over Pt catalysts. Appl. Catal. A 2012, 423–424, 100–107. [Google Scholar] [CrossRef]
- Lee, J.W.; Jeong, R.H.; Kim, D.I.; Yu, J.-H.; Nam, S.-H.; Boo, J.-H. Facile synthesis of amorphous Ti-peroxo complex for photocatalytic activity under visible-light irradiation. J. Clean. Prod. 2019, 239, 118013. [Google Scholar] [CrossRef]
- Chung, S.-H.; Park, G.H.; Schukkink, N.; Lee, H.; Shiju, N.R. Structure-sensitive epoxidation of dicyclopentadiene over TiO2 catalysts. Chem. Commun. 2023, 59, 756. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Choi, S.M.; Green, S.; Tompsett, G.A.; Seo, M.H.; Lee, S.H.; Cho, J.; Huber, G.W.; Kim, W.B. Highly active and stable PtRuSn/C catalyst for electrooxidations of ethylene glycol and glycerol. Appl. Catal. B 2011, 101, 366–375. [Google Scholar] [CrossRef]
- Tanaka, K.; Watanabe, N. Study on the Coordination Structure of Pt Sorbed on Bacterial Cells Using X-ray Absorption Fine Structure Spectroscopy. PLoS ONE 2015, 10, e0127417. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.Y.; Yun, H.J.; Yu, S.; Kim, W.; Kim, N.D.; Yi, J. Effect of TiO2 crystalline phase on CO oxidation over CuO catalysts supported on TiO2. J. Mol. Catal. A Chem. 2013, 368–369, 72–77. [Google Scholar] [CrossRef]
- Pakharukov, I.Y.; Prosvirin, I.P.; Chetyrin, I.A.; Bukhtiyarov, V.I.; Parmon, V.N. In situ XPS studies of kinetic hysteresis in methane oxidation over Pt/γ-Al2O3 catalysts. Catal. Today 2016, 278, 135–139. [Google Scholar] [CrossRef]
- Chetyrin, I.A.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Khudorozhkov, A.K.; Bukhtiyarov, V.I. In Situ XPS and MS Study of Methane Oxidation on the Pd–Pt/Al2O3 Catalysts. Top. Catal. 2020, 63, 66–74. [Google Scholar] [CrossRef]
- Khudorozhkov, A.K.; Chetyrin, I.A.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Bukhtiyarov, V.I. Propane Oxidation Over Pd/Al2O3: Kinetic and In Situ XPS Study. Top. Catal. 2017, 60, 190–197. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).