A Neoteric View of sp2 Amorphous Carbon
Abstract
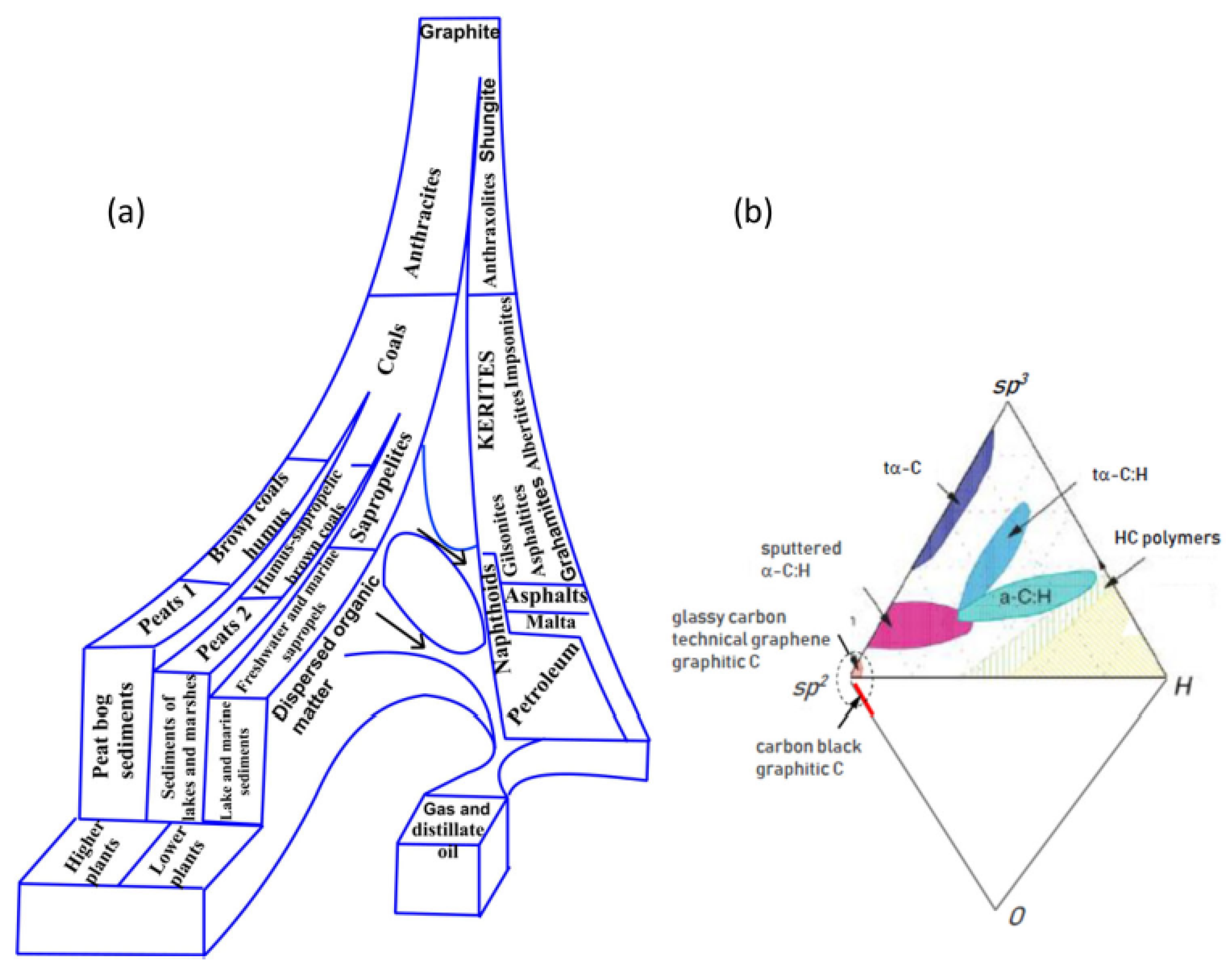
1. A Concise Historical Introduction
2. General Characteristics of the Carbon Amorphicity
3. Structure of sp2 Amorphous Carbons
3.1. Short Foreword
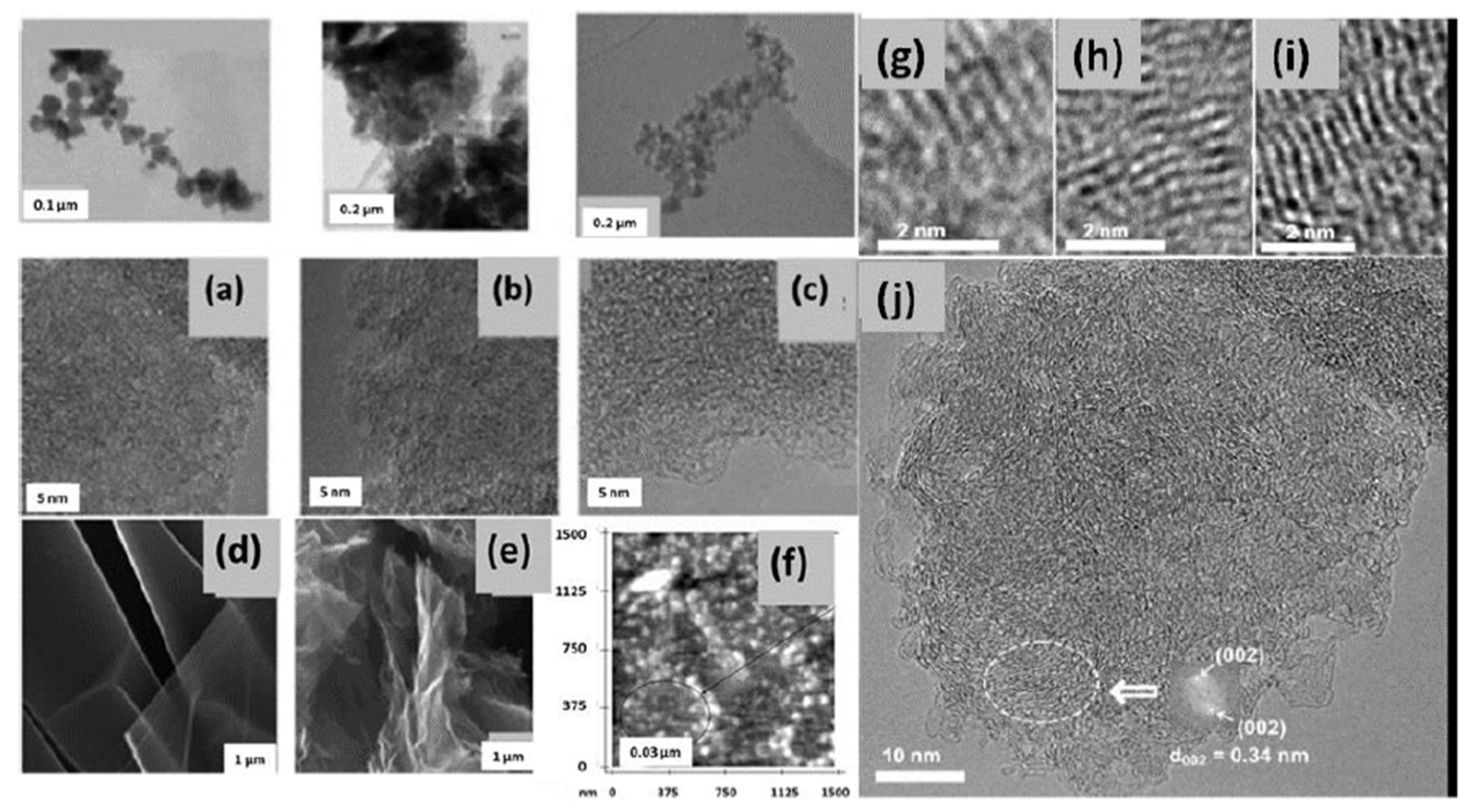
3.2. Electron Microscopy
3.3. X-ray and Neutron Powder Diffraction
4. General Atomic Content of sp2 Amorphous Carbons
4.1. Short Foreword
4.2. {CH} Analytics
4.3. {CO} Analytics
4.4. {CHO} Analytics
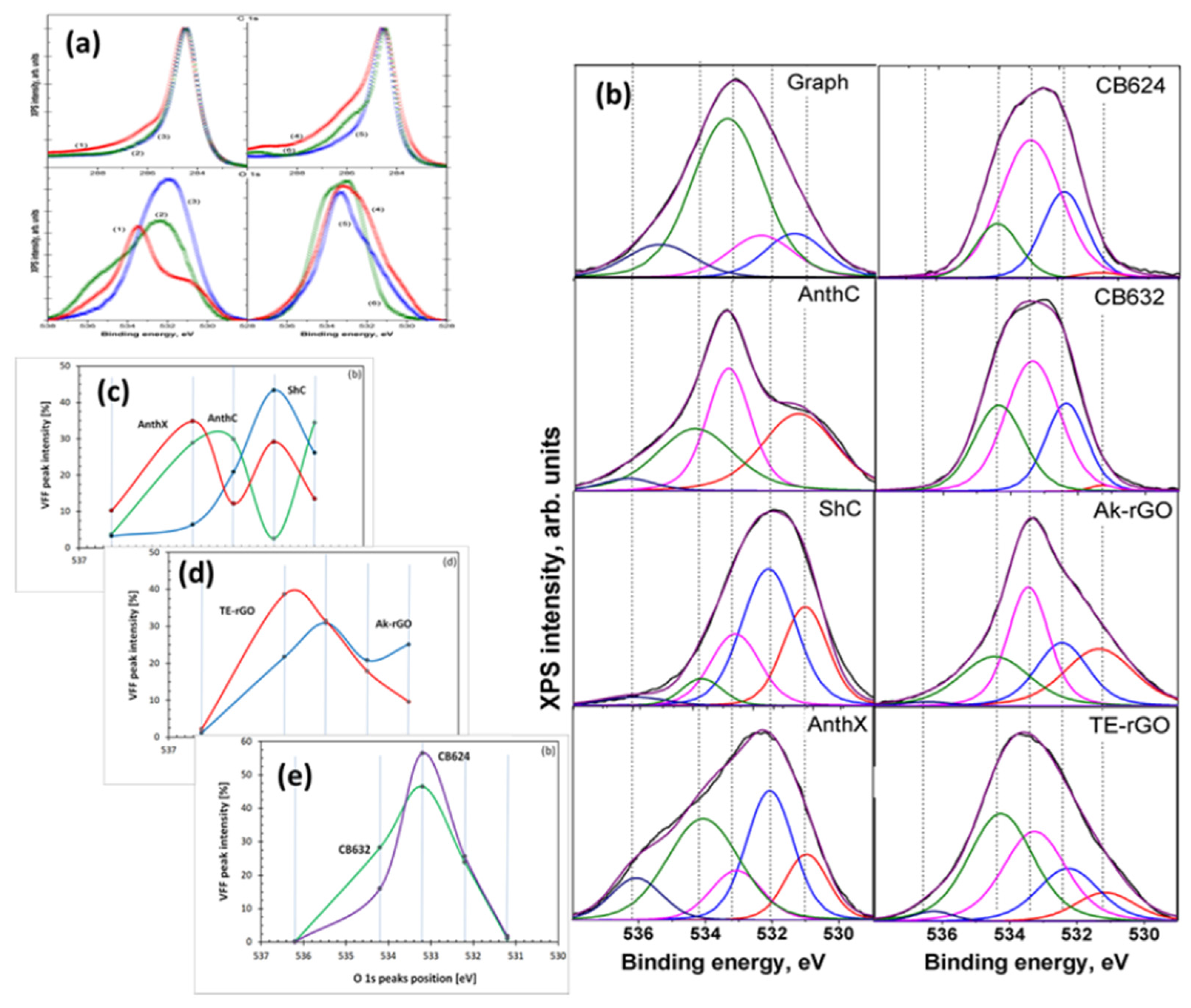
4.4.1. XPS Analysis
4.4.2. IINS Analysis
| Spectral Region, cm−1 | Group Frequencies 1 | |||
|---|---|---|---|---|
| (C, C) 2 | (C, H1) 2 | (C, CH2) 3 | (C, CH3) 4 | |
| 400–700 | 404 δ op C–C–C 606 δ ip C–C–C | - | 711 ρ CH2 | 210 r CH3 344 δ CH3 |
| 700–1200 | 707 C–C–C puckering 993 ring breathing 1010 δ C–C–C trigonal | 673 δ op in phase 846 δ op, C6 libration 967 δ op 990 δ op, trigonal 1037 δ ip 1146 δ ip, trigonal 1178 δ ip | 948 ρ CH2 | 900 ν C–CH3 1041 ρ CH3 |
| 1200–1600 | 1309 ν C–C Kekule, 1482 ν C–C 1599 ν C–C | 1350 δ ip in phase | 1409 δ internal CH2 | 1333 δ CH3 1486 δ internal CH3 |
| 2800–3200 | - | 3056 ν C–H 3057 ν trigonal C–H 3064 ν C–H 3073 ν in phase C–H | 3114 ν CH2 | 2950 ν CH3 |
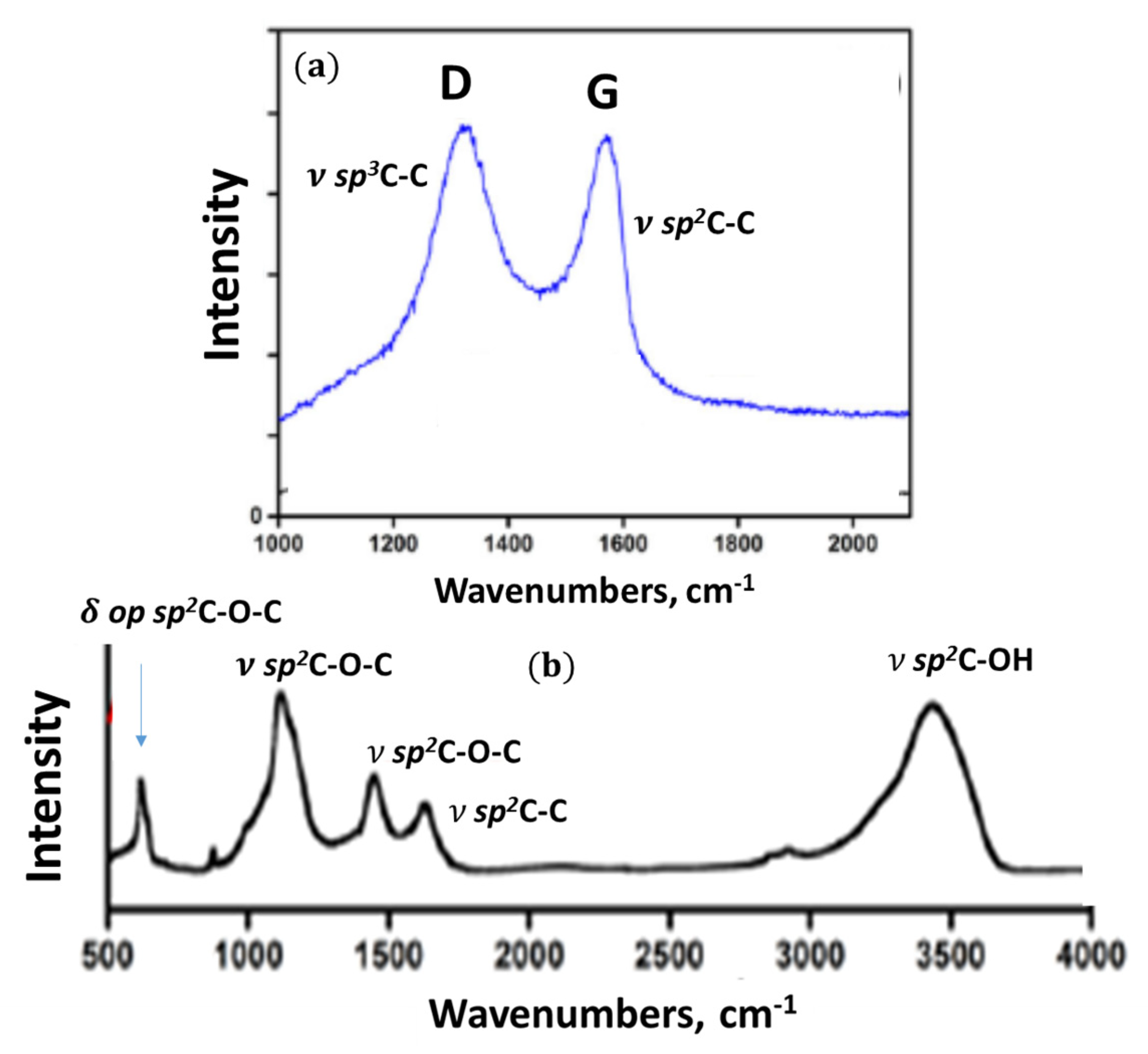
4.4.3. IR Absorption Analysis
| 300–1000 | 1000–1200 | 1200–1300 | 1300–1500 | 1500–1600 | 1600–1700 | 1800–1900 | 2600–3000 | 3000–3600 |
|---|---|---|---|---|---|---|---|---|
| δ op2, δ ip3, C–OH sp2C–O–C and sp2C–OH δ op sp2C–C–C 4 δ ip, puckering, ring breathing, δ trigonal sp2C–C–C 4, collective vibrations of graphene domain atoms 5 | ν sp2C–O–C in cyclic ether, aggregated cyclic ether and acid anhydride, | ν sp2C–OH, in lactone, hydroxyl pyran and acid anhydride | δ ip sp2C–OH, ν sp2C–O–C in cyclic ether and acid anhydride δ ip O–C=O in acid anhydride | δ ip sp2C–OH, ν sp2C–C | ν sp2C=O in acid anhydride and lactone, aggregated cyclic ether with lactone pair, pairs of lactones | ν sp2C=O in o-quinone, COOH | ν sp3C–O–H in COOH ν sp3C–H | ν sp3C–O–H |
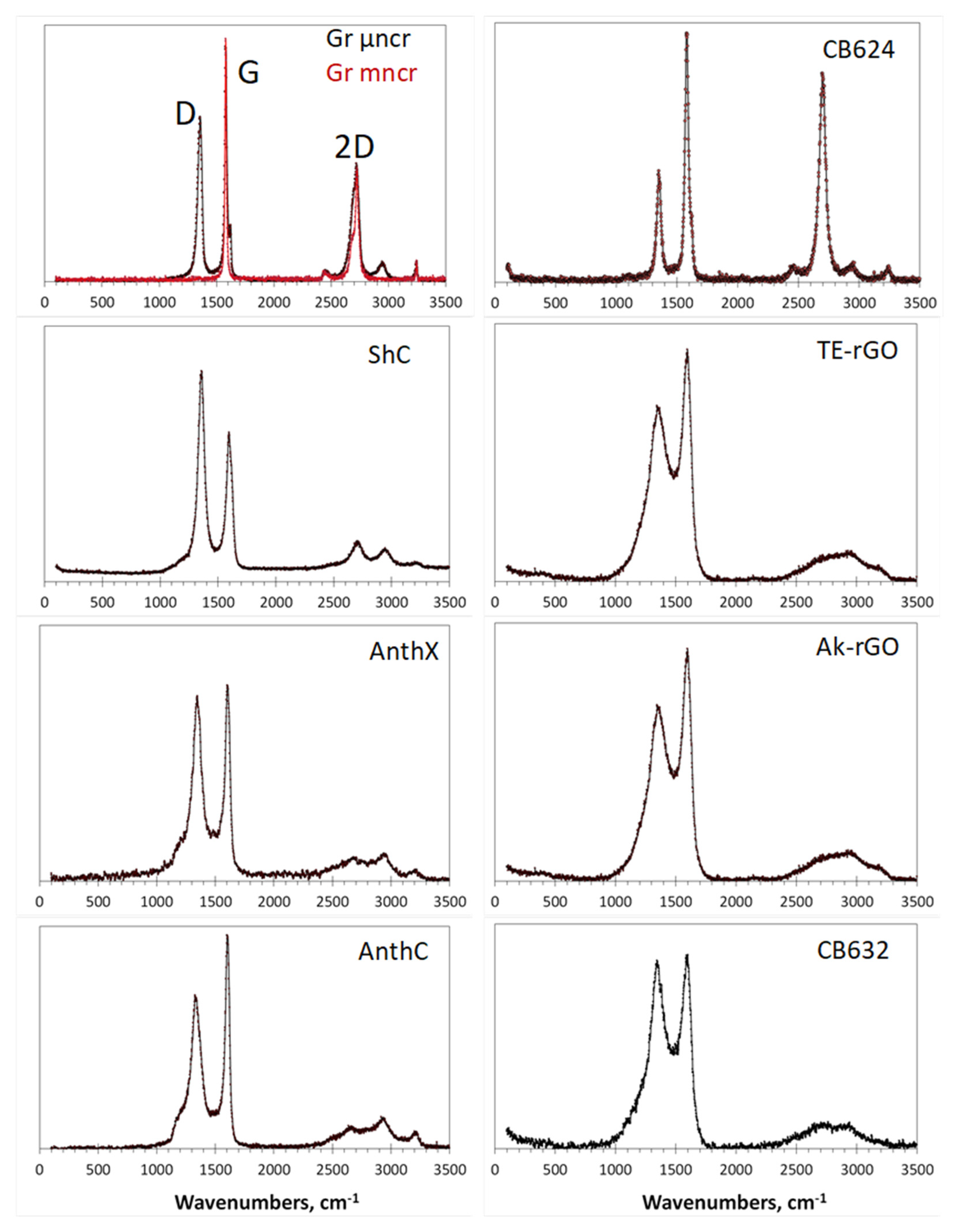
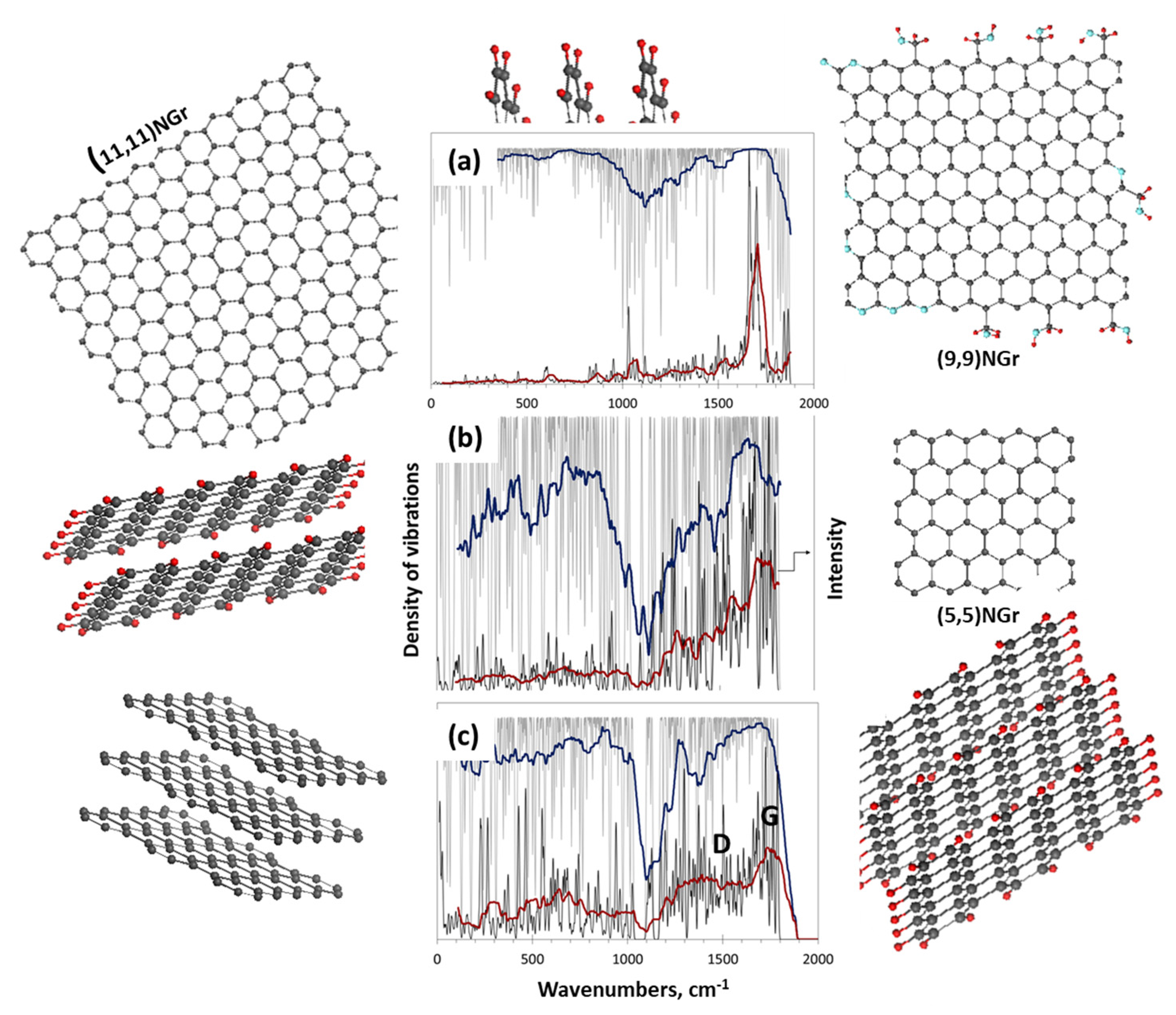
4.4.4. Raman Scattering Analysis
5. Virtual Analytics of Necklaced Graphene Molecules
5.1. Short Foreword
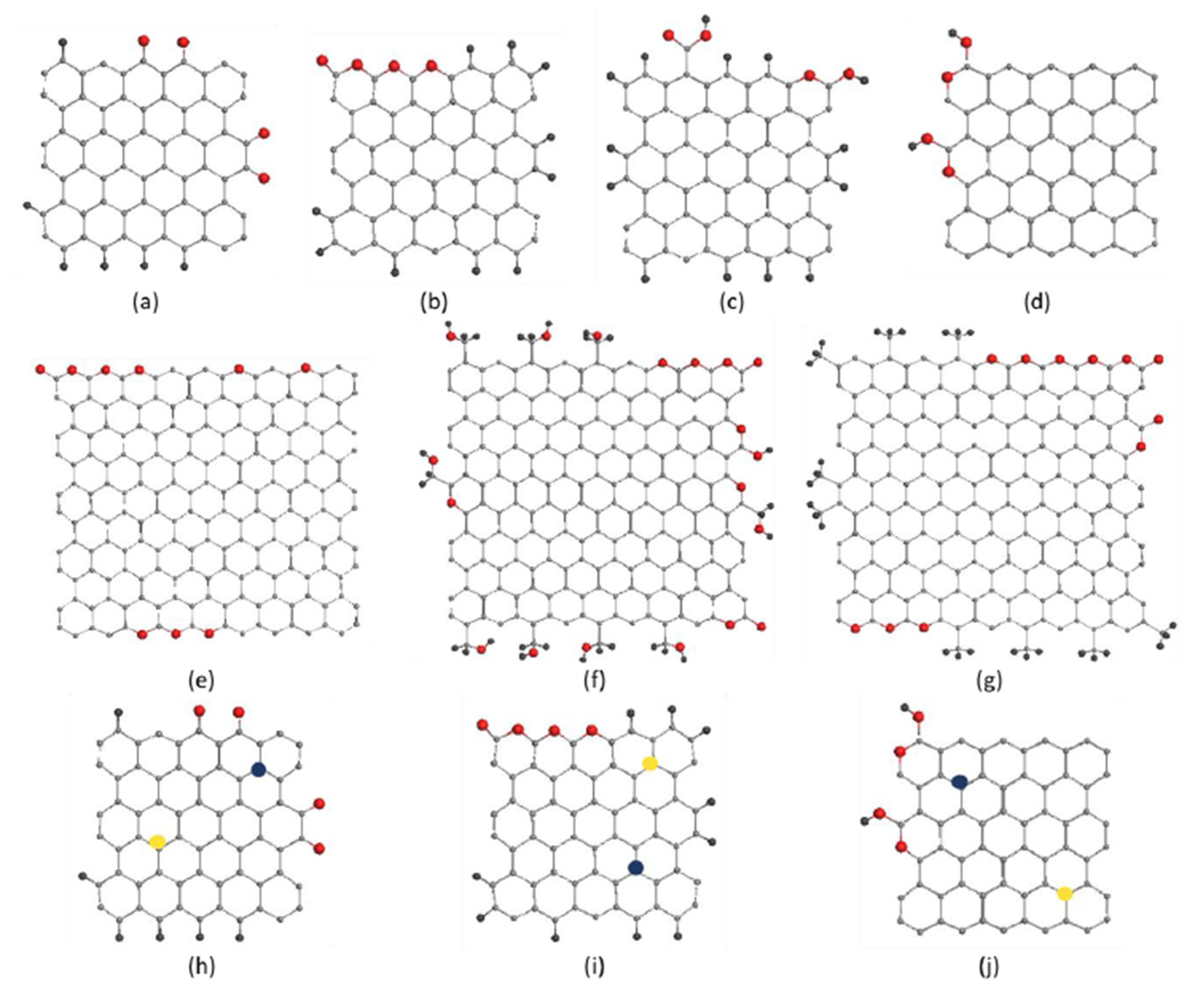
5.2. Molecular Models and Digital Twins of BSUs of sp2 Amorphous Carbons
5.3. Virtual Vibrational Analytics of sp2 Amorphous Carbon
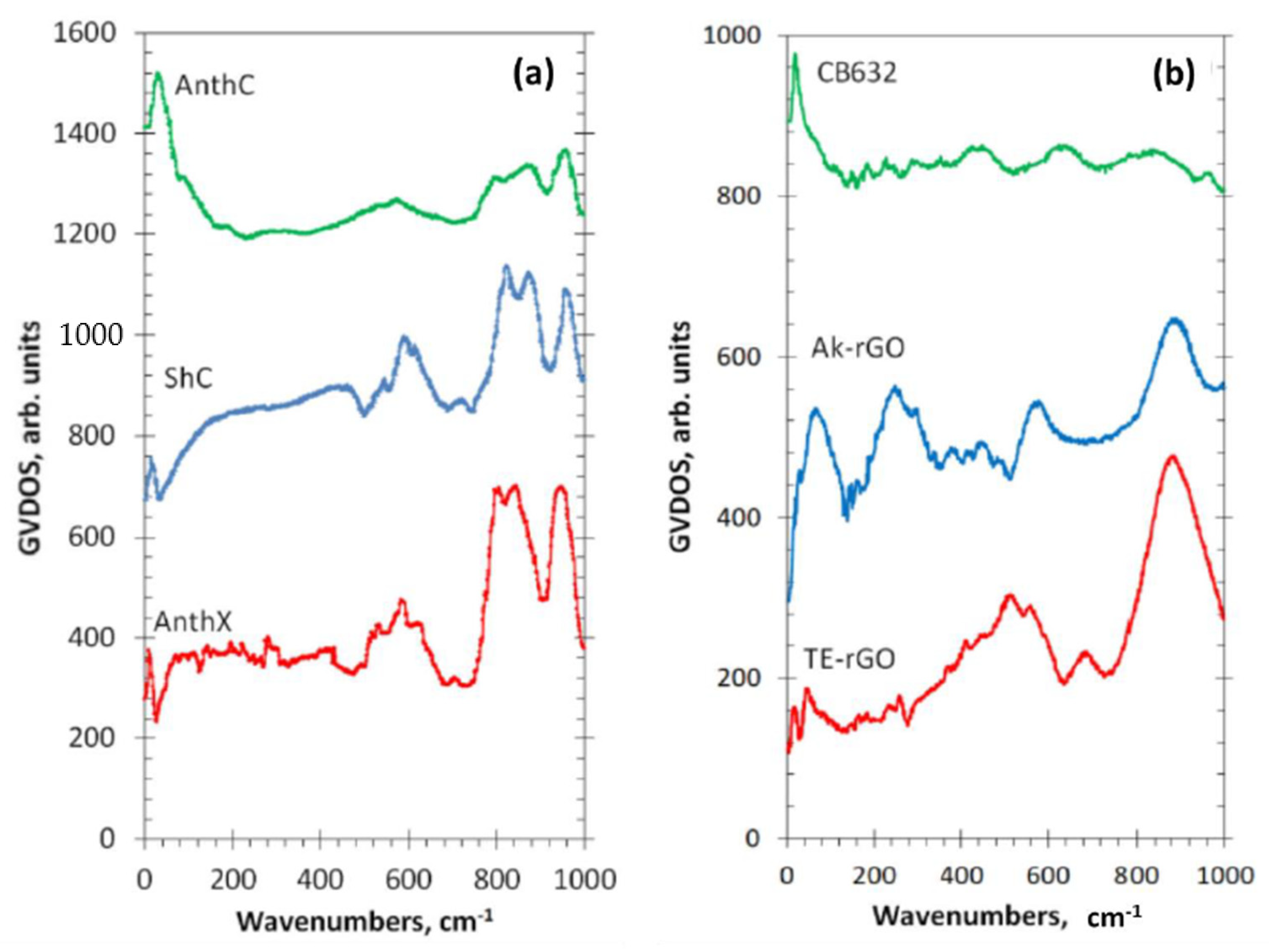
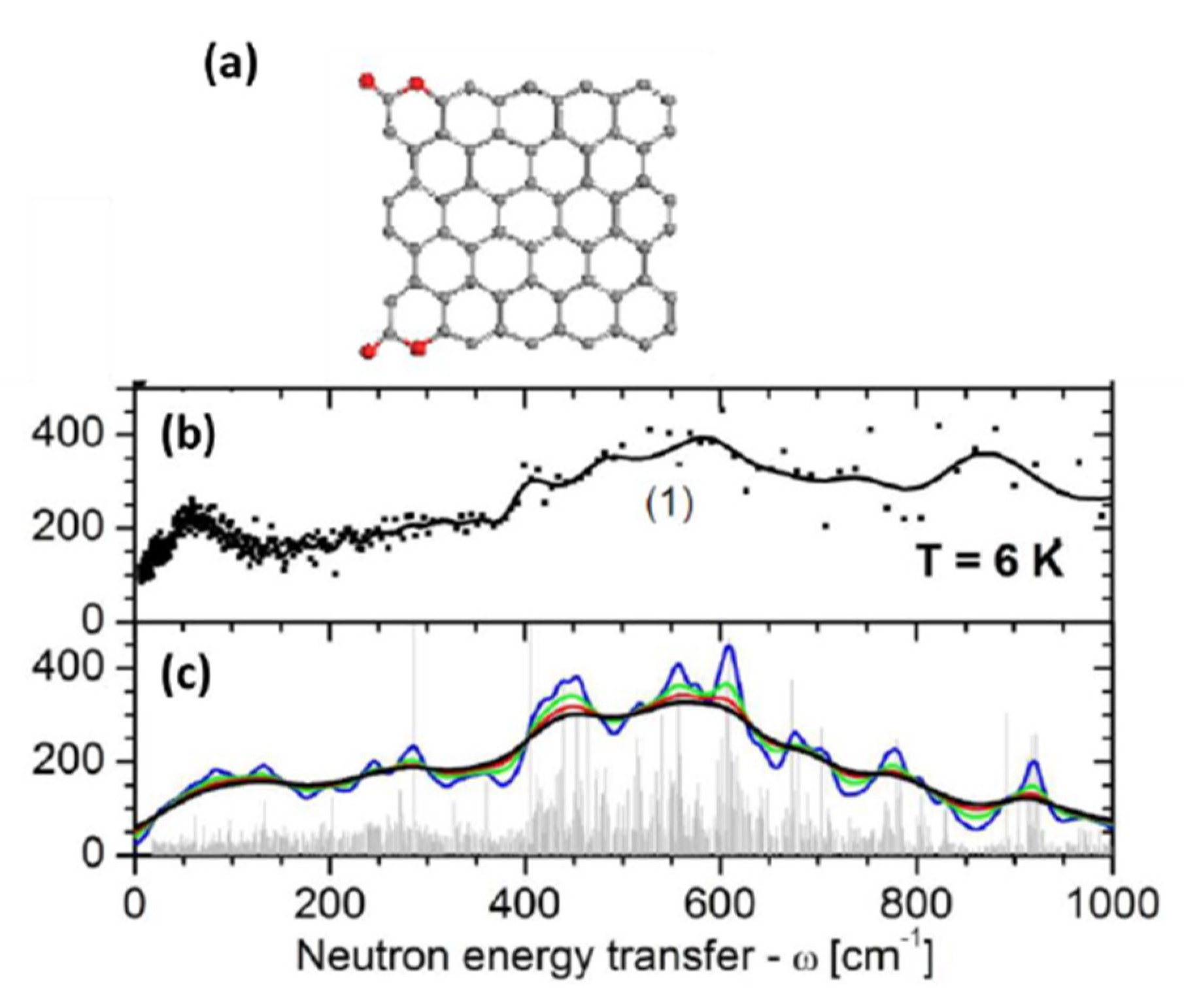
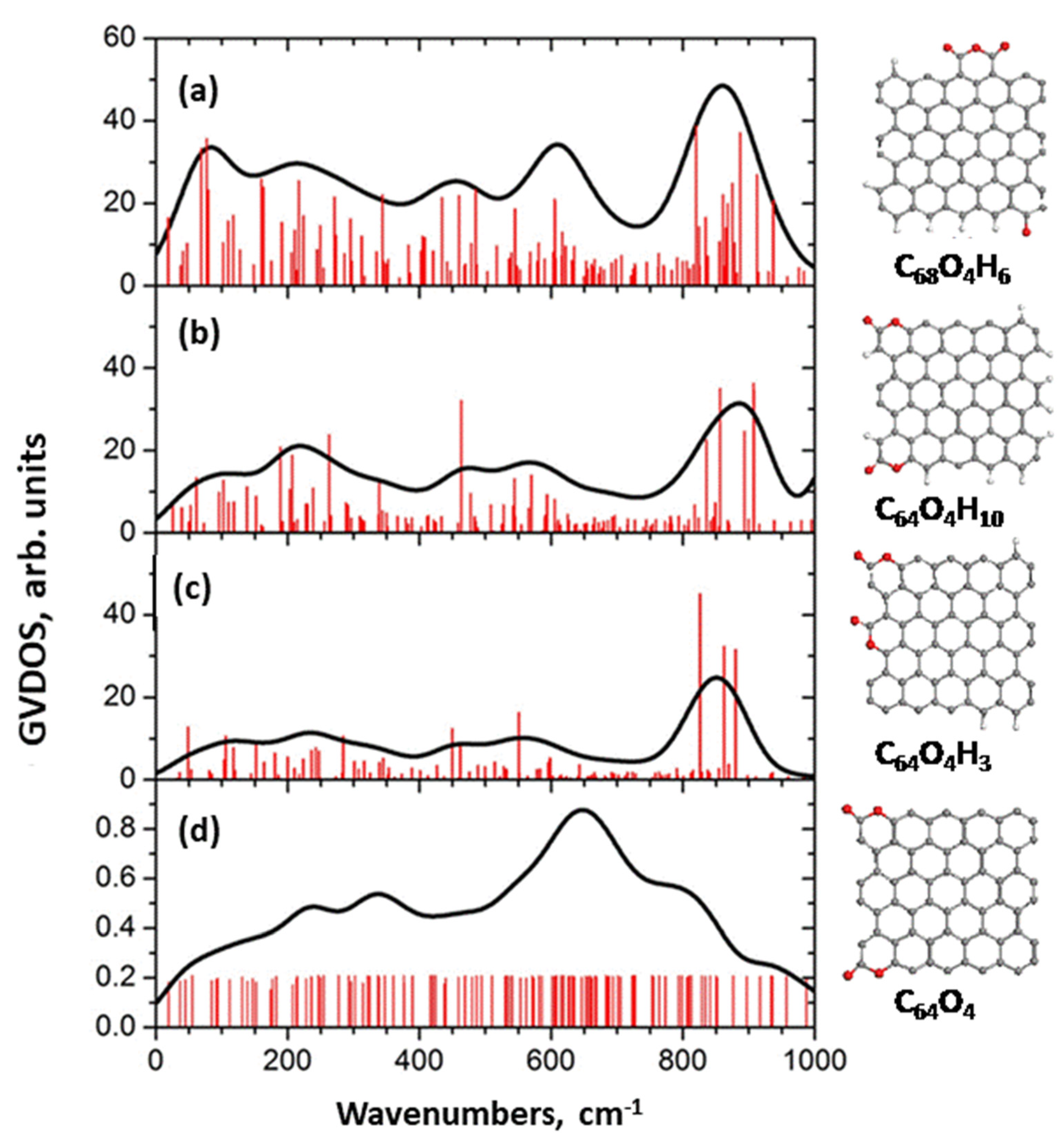
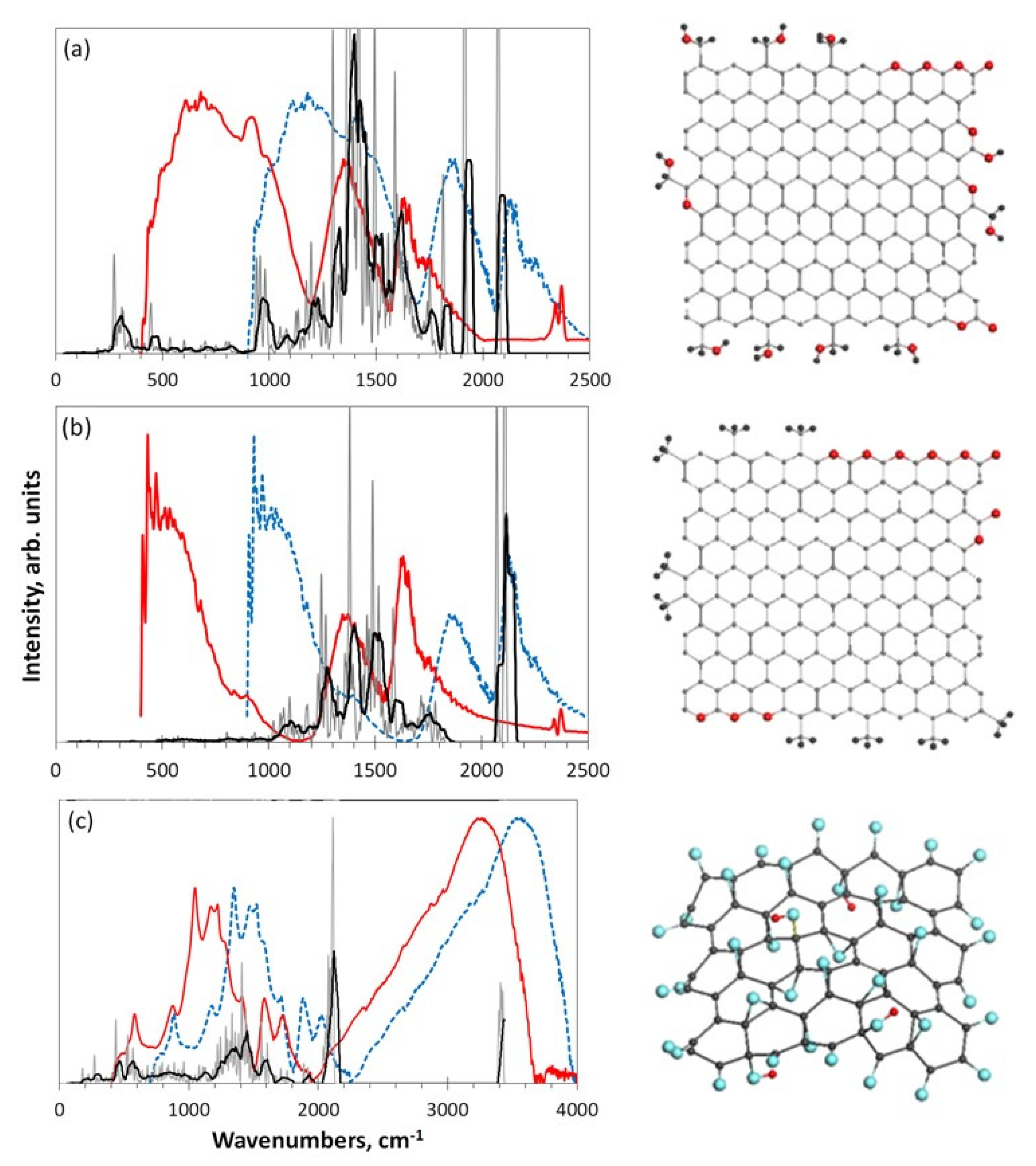
5.3.1. IINS Virtual Analytics
5.3.2. IR Absorption Virtual Analytics
5.3.3. Raman Scattering Virtual Analytics
6. Express Analysis of sp2 Amorphous Carbons Based on IR and Raman Spectra
7. Conclusive Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aC | amorphous carbon |
| AFM | atom-force microscopy |
| Ak-rGO | lab rGO product |
| AnthC | anthracite |
| AnthX | anthraxolite |
| BSU | basic structural unit |
| CB | carbon black |
| DFT | density functional theory |
| DRIFT | Diffused-reflection IT Fourier determined spectrometer |
| DT | digital ywin |
| DTA | differential thermal analysis |
| DSC | differential scanning calorimetry |
| EDS | energy dispersion spectroscopy |
| EA | elemental analysis |
| EM | electron microscopy |
| FTIR | Fourier determined IR spectroscopy |
| FWHM | full width at half maximum |
| GBE | group bending energy |
| GF | group frequency |
| GO | graphene oxide |
| GVDOS | generalized vibrational density of states |
| HF | Hartree-Fock approximation |
| HREELS | high-resolution electron energy loss spectroscopy |
| HRTEM | high-resolution transmission electron microscopy |
| IINS | inelastic incoherent neutron spectroscopy |
| IT | intellectual technology |
| MD | molecular dynamics |
| NGM | necklaced graphene molecule |
| NPD | neutron powder diffraction |
| PD | powder diffraction |
| rGO | reduce graphene oxide |
| SEM | scanning electron microscopy |
| ShC | shungite carbon |
| STEM | scanning transmission electron microscopy |
| TE-rGO | thermally exfoliated rGO |
| VFF | Voigt fitting function |
| XRPD | X-ray powder diffraction |
| XPS | X-ray photoelectron spectroscopy |
References
- Sheka, E.F.; Rozhkova, N.N. Shungite as the natural pantry of nanoscale reduced graphene oxide. Int. J. Smart Nano Mater. 2014, 5, 1–16. [Google Scholar] [CrossRef]
- Hoffmann, R. Small but strong lessons from chemistry for nanoscience. Angew. Chem. Int. Ed. 2013, 52, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Elliot, S.R. Physics of Amorphous Materials; Longman: London, UK; New York, NY, USA, 1983. [Google Scholar]
- Raman, C.V. Molecular structure of amorphous solids. Nature 1922, 109, 138–139. [Google Scholar] [CrossRef]
- Natkaniec, I.; Sheka, E.F.; Drużbicki, K.; Hołderna-Natkaniec, K.; Gubin, S.P.; Buslaeva, E.Y.; Tkachev, S.V. Computationally Supported Neutron Scattering Study of Parent and Chemically Reduced Graphene Oxide. J. Phys. Chem. C 2015, 119, 18650–18662. [Google Scholar] [CrossRef]
- Donet, J.-B.; Bansal, R.C.; Wang, M.-J. (Eds.) Carbon Black. Science and Technology; Marcel Dekker: New York, NY, USA; Basel, Switzerland, 1993. [Google Scholar]
- Rozhkova, N.N.; Rozhkov, S.P.; Goryunov, A.S. Natural graphene-based shungite nanocarbon. In Carbon Nanomaterials Sourcebook; Sattler, K.D., Ed.; CRC Press: Roca Raton, FL, USA; London, UK; New York, NY, USA, 2016; Volume 1, pp. 151–174. [Google Scholar]
- Filippov, M.M. Anthraxolites; VNIGRI: St. Petersburg, Russia, 2013. (In Russian) [Google Scholar]
- Skripchenko, G.B. Structure, properties and use of anthracytes of the Donetsk basin. Chem. Solid Fuel 2010, 2, 3–13. (In Russian) [Google Scholar]
- Tkachev, S.V.; Buslaeva, E.Y.; Naumkin, A.V.; Kotova, S.L.; Laure, S.V.; Gubin, S.P. Reduced graphene oxide. Inorg. Mater. 2012, 48, 796–802. [Google Scholar] [CrossRef]
- Sheka, E.F.; Natkaniec, I.; Mel’nikov, V.; Druzbicki, K. Neutron scattering from graphene oxide paper and thermally exfoliated reduced graphene oxide. Nanosyst. Phys. Chem. Math. 2015, 6, 378–393. [Google Scholar] [CrossRef]
- SIGMA-ALDRICH. Synthesis of mesoporous materials. Mater. Matters 2008, 3, 17–18. [Google Scholar]
- Sheka, E.F.; Golubev, E.A. Technical graphene (reduced graphene oxide) and its natural analog (schungite). Tech. Phys. 2016, 61, 1032–1038. [Google Scholar] [CrossRef]
- Sheka, E.F. Digital Twins in the graphene technology. arXiv 2022, arXiv:2208.14926. [Google Scholar] [CrossRef]
- Radovic, L. (Ed.) Chemistry and Physics of Carbon; Marcel Dekker: New York, NY, USA, 2001. [Google Scholar]
- Setton, R.; Bernier, P.; Lefrant, S. (Eds.) Carbon Molecules and Materials; Taylor and Francis: London, UK; New York, NY, USA, 2002. [Google Scholar]
- Filippov, M.M. Shungite-Bearing Rocks of the Onega Structure; Karelian Science Centre of RAS: Petrozavodsk, Russia, 2002. (In Russian) [Google Scholar]
- Silva, R.; Silva, S.R.P. (Eds.) Properties of Amorphous Carbon; INSPEC: London, UK, 2003. [Google Scholar]
- Marsh, H.; Rodriguez-Reinoso, F. (Eds.) Activated Carbon; Elsevier Science & Technology Books: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Gogotsi, Y. (Ed.) Carbon Nanomaterials; Taylor and Francis Group, LLC: Boca Raton, FL, USA, 2006. [Google Scholar]
- Radovic, L. (Ed.) Chemistry and Physics of Carbon; Taylor and Francis Group, CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Serp, P.; Figueiredo, J.L. (Eds.) Carbon Materials for Catalysis; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Colombo, L.; Fasolino, A. (Eds.) Computer-Based Modeling of Novel Carbon Systems and Their Properties, Carbon Materials: Chemistry and Physics 3; Springer Science + Business Media B.V.: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Oberlin, A.; Bonnamy, S. A realistic approach to disordered carbons. In Chemistry and Physics of Carbon; Radovic, L., Ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Serp, P.; Machado, B. Nanostructured Carbon Materials for Catalysis; RSC: Croydon, UK, 2015. [Google Scholar]
- Putz, M.V.; Ori, O. (Eds.) Exotic Properties of Carbon Nanomatter. Advances in Physics and Chemistry. Carbon Materials: Chemistry and Physics; Springer Science + Business Media: Dordtrecht, The Netherlands, 2015. [Google Scholar]
- Sattler, K.D. (Ed.) Carbon Nanomaterials Sourcebook; CRS Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2016. [Google Scholar]
- Sheka, E. Spin Chemical Physics of Graphene; Pan Stanford: Singapore, 2018. [Google Scholar]
- Kharisov, B.I.; Kharissova, O.V. Carbon Allotropes: Metal-Complex Chemistry, Properties and Applications; Springer Nature: Cham, Switzerland, 2019. [Google Scholar]
- Ray, S.C. Magnetism and Spintronics in Carbon and Carbon Nanostructured Materials; Elsevier: Dodrecht, The Netherlands, 2020. [Google Scholar]
- Borghi, F.; Soavi, F.; Milani, P. (Eds.) Nanoporous Carbons for Soft and Flexible Energy Devices. Carbon Materials: Chemistry and Physics; Springer Nature: Cham, Switzerland, 2022. [Google Scholar]
- Uspensky, V.A.; Radchenko, O.A.; Glebovskaya, E.A. Basics of Genetic Classification of Bitumen; Nedra: Leningrad, Russia, 1964. (In Russian) [Google Scholar]
- Cornelius, C.D. Classification of natural bitumen: A physical and chemical approach. In Exploration for Heavy Crude Oil Fnd Natural Bitumen; Meyer, R.F., Ed.; AAPG Studies in Geology: Tulsa, OK, USA, 1987; Volume 25, pp. 165–174. [Google Scholar]
- Jakob, H. Nomenclature, classification, characterization and genesis of natural solid bitumen (Migrabitumen). In Bitumen in Ore Deposits; Parnell, J., Kucha, H., Landais, P., Eds.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 11–27. [Google Scholar]
- Mossman, D.J.; Nagy, B. Solid bitumens: An assessment of their characteristic, genesis, and role in geological processes. Terra Nova 1996, 8, 114–128. [Google Scholar] [CrossRef]
- Sheka, E.F.; Golubev, Y.A.; Popova, N.A. Amorphous state of sp2 solid carbon. FNCN 2021, 29, 107–113. [Google Scholar] [CrossRef]
- Shumilova, T.G. Mineralogy of Native Carbon; Ural Dept RAS: Yekaterinburg, Russia, 2003. (In Russian) [Google Scholar]
- Shumilova, T.G.; Isaenko, S.I.; Divaev, F.K. Mineralogical features of diamond, amorphous diamond like carbon and graphite from Chagatay carbonatites (Uzbekistan). Miner. J. 2013, 35, 81–89. [Google Scholar]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R Rep. 2002, 37, 129–281. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, X.; Lin, Y.; Wang, F. A ternary phase diagram for amorphous carbon. Carbon 2015, 94, 202–213. [Google Scholar] [CrossRef]
- Harris, P.J.F. New perspectives on the structure of graphitic carbons. Crit. Rev. Solid State Mater. Sci. 2005, 30, 235–253. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Krishnan, D.; Kim, F.; Luo, J.; Cruz-Silva, R.; Cote, L.J.; Jang, H.D.; Huang, J. Energetic graphene oxide: Challenges and opportunities. Nano Today 2012, 7, 137–152. [Google Scholar] [CrossRef]
- Chyan, Y.; Ye, R.; Li, Y.; Singh, S.P.; Arnusch, C.J.; Tour, J.M. Laser-induced graphene by multiple lasing: Toward electronics on cloth, paper, and food. ACS Nano 2018, 12, 2176–2183. [Google Scholar] [CrossRef]
- Luong, D.X.; Bets, K.V.; Algozeeb, W.A.; Stanford, M.G.; Kittrell, C.; Chen, W.; Salvatierra, R.V.; Ren, M.; McHugh, E.A.; Advincula, P.A.; et al. Gram-scale bottom-up flash graphene synthesis. Nature 2020, 577, 647–651. [Google Scholar] [CrossRef]
- Seal, M. The effect of surface orientation on the graphitization of diamond. Phys. Status Solidi 1963, 3, 658. [Google Scholar] [CrossRef]
- Sheka, E.F.; Hołderna-Natkaniec, K.; Natkaniec, I.; Krawczyk, J.X.; Golubev, Y.A.; Rozhkova, N.N.; Kim, V.V.; Popova, N.A.; Popova, V.A. Computationally supported neutron scattering study of natural and synthetic amorphous carbons. J. Phys. Chem. C 2019, 123, 15841–15850. [Google Scholar] [CrossRef]
- Golubev, Y.A.; Rozhkova, N.N.; Kabachkov, E.N.; Shul’ga, Y.M.; Natkaniec-Hołderna, K.; Natkaniec, I.; Antonets, I.V.; Makeev, B.A.; Popova, N.A.; Popova, V.A.; et al. sp2 Amorphous carbons in view of multianalytical consideration: Normal, expected and new. J. Non Cryst. Solids 2019, 524, 119608. [Google Scholar] [CrossRef]
- Sheka, E.F.; Natkaniec, I.; Ipatova, E.U.; Golubev, Y.A.; Kabachkov, E.N.; Popova, V.A. Heteroatom necklaces of sp2 amorphous carbons. XPS supported INS and DRIFT spectroscopy. FNCN 2020, 28, 1010–1029. [Google Scholar] [CrossRef]
- Sheka, E.F.; Golubev, Y.A.; Popova, N.A. Graphene domain signature of Raman spectra of sp2 amorphous carbons. Nanomaterials 2020, 10, 2021. [Google Scholar] [CrossRef]
- Razbirin, B.S.; Rozhkova, N.N.; Sheka, E.F.; Nelson, D.K.; Starukhin, A.N. Fractals of graphene quantum dots in photoluminescence of shungite. J. Exp. Theor. Phys. 2014, 5, 838–850. [Google Scholar]
- Avdeev, M.V.; Tropin, T.V.; Aksenov, V.L.; Rosta, L.; Garamus, V.M.; Rozhkova, N.N. Pore structures in shungites as revealed by small-angle neutron scattering. Carbon 2006, 44, 954–961. [Google Scholar] [CrossRef]
- Broude, V.L.; Klimusheva, G.V.; Prikhotjko, A.F.; Sheka, E.F.; Yatcenko, L.P. Absorption Spectra of Molecular Crystals; Polysubstituted Benzene Compounds; Naukova Dumka: Kiev, Russia, 1972. (In Russian) [Google Scholar]
- Sheka, E.F. Spectroscopy of amorphous substances with molecular structure. Sov. Phys. Usp. 1990, 33, 147–166. [Google Scholar] [CrossRef]
- Golubev, Y.A.; Ulyashev, V.V.; Veligzhanin, A.A. Porosity and structural parameters of Karelian shungites according to the data of small-angle synchrotron radiation scattering and microscopy. Crystallgr. Rep. 2016, 61, 66–77. [Google Scholar] [CrossRef]
- Rozhkova, N.N. Aggregation and stabilization of shungite carbon nanoparticles. Ecol. Chem. 2012, 4, 240–251. [Google Scholar] [CrossRef]
- Duan, X.; Ao, Z.; Zhang, H.; Saunders, M.; Sun, H.; Shao, Z.; Wang, S. Nanodiamonds in sp2/sp3 configuration for radical to nonradical oxidation: Core-shell layer dependence. Appl. Cat. B Environ. 2018, 222, 176–181. [Google Scholar] [CrossRef]
- Sadovnichii, R.V.; Rozhkov, S.S.; Rozhkova, N.N. The use of shungite processing products in nanotechnology: Geological and Mineralogical Justification. Smart Nanocomp. 2016, 7, 111–119. [Google Scholar]
- Garvie, L.A.J.; Buseck, P.R. Carbonaceous materials in the acid residue from the Orgueil carbonaceous chondrite meteorite. Meteorit. Planet. Sci. 2006, 41, 633–642. [Google Scholar] [CrossRef]
- Taskaev, S.; Skokov, K.; Khovaylo, V.; Donner, W.; Faske, T.; Dudorov, A.; Gorkavyi, N.; Muratov, D.S.; Savosteenko, G.; Dyakonov, A.; et al. Exotic carbon microcrystals in meteoritic dust of the Chelyabinsk superbolide: Experimental investigations and theoretical scenarios of their formation. Eur. Phys. J. Plus 2022, 137, 562. [Google Scholar] [CrossRef]
- Povarennykh, M.Y.; Matvienko, E.N.; Knot’ko, A.V.; Shatalova, T.B. Characteristic of natural carbon nanominerals and their aggregates from the Dzhaharduk area (Uzbekistan). Mineral 2018, 4, 85–97. [Google Scholar]
- Allen, K.D.; Wegener, G.; Matthew Sublett, D., Jr.; Bodnar, R.J.; Feng, X.; Wendt, J.; White, R.H. Biogenic formation of amorphous carbon by anaerobic methanotrophs and select methanogens. Sci. Adv. 2021, 7, eabg9739. [Google Scholar] [CrossRef]
- Melezhik, V.A.; Filippov, M.M.; Romashkin, A.E. A giant Palaeoproterozoic deposit of shungite in NW Russia: Genesis and practical applications. Ore Geol. Rev. 2004, 24, 135–154. [Google Scholar] [CrossRef]
- Sheka, E.F.; Popova, N.A. Virtual vibrational analytics of reduced graphene oxide. Int. J. Mol. Sci. 2022, 23, 6978. [Google Scholar] [CrossRef]
- Sheka, E.F. sp2 Carbon stable radicals. C 2021, 7, 31. [Google Scholar] [CrossRef]
- Deng, D.; Yu, L.; Pan, X.; Wang, S.; Chen, X.; Hu, P.; Sunb, L.; Bao, X. Size effect of graphene on electrocatalytic activation of oxygen. Chem. Commun. 2011, 47, 10016–10018. [Google Scholar] [CrossRef]
- Sheka, E.F. Graphene oxyhydride catalysts in view of spin radical chemistry. Materials 2020, 13, 565. [Google Scholar] [CrossRef] [PubMed]
- Jurkiewicz, K.; Pawlyta, M.; Burian, A. Structure of carbon materials explored by local transmission electron microscopy and global powder diffraction probes. C 2018, 4, 68. [Google Scholar] [CrossRef]
- Warren, B.E. X-ray diffraction in random layer lattices. Phys. Rev. 1941, 59, 693–698. [Google Scholar] [CrossRef]
- Biscoe, J.; Warren, B.E. An X-ray study of carbon black. J. Appl. Phys. 1942, 13, 364–371. [Google Scholar] [CrossRef]
- Busek, P.R.; Galdobina, I.P.; Kovalevski, V.V.; Rozhkova, N.N.; Valley, J.W.; Zaidenberg, A.Z. Shungites: The C-rich rocks of Karelia, Russia. Can. Mineralog. 1997, 35, 1363–1378. [Google Scholar]
- Kovalevskii, V.V. The Structure of Carbon Matter and the Genesis of Shungite Rocks. Dr. Sci. Dissertation, Geology and Mineralogy, Petrozavodsk, Russia, 2007. [Google Scholar]
- Golubev, E.A. Globular structure of higher anthraxolites according to scanning probe microscopy. Dokl. Ak. Nauk. 2009, 425, 519–521. (In Russian) [Google Scholar] [CrossRef]
- Golubev, E.A. Electrophysical properties and structural features of shungite. Phys. Solid State 2013, 55, 1078. [Google Scholar] [CrossRef]
- ImageJ. Available online: http//rsb.info.nih.gov/ij (accessed on 1 January 2023).
- Osváth, Z.; Deák, A.; Kertész, K.; Molnár, G.; Vértesy, G.; Zámbó, D.; Hwang, C.; Biró, L.P. The structure and properties of graphene on gold nanoparticles. Nanoscale 2015, 7, 5503–5509. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials, 2nd ed.; John Wiley: New York, NY, USA, 1974. [Google Scholar]
- Ingham, B. X-ray scattering characterization of nanoparticles. Cryst. Rev. 2015, 21, 229–303. [Google Scholar] [CrossRef]
- Kisi, E.H.; Howard, C.J. Applications of Neutron Powder Diffraction; Oxford University Press: New York, NY, USA, 2008; 384p. [Google Scholar]
- Sheka, E.F.; Rozhkova, N.N.; Holderna-Natkaniec, K.; Natkaniec, I. Nanoscale reduced-graphene-oxide origin of shungite in light of neutron scattering. Nanosyst. Phys. Chem. Math. 2014, 5, 659–676. [Google Scholar]
- Lobzova, R.V. Graphite and Alkaline Rocks of the Botogol’sk Massif; Nauka: Moscow, Russia, 1975. (In Russian) [Google Scholar]
- Langford, J.I.; Wilson, A.J.C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Cryst. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Vorokh, A.S. Scherrer formula: Estimation of error in determining small nanoparticle size. Nanosyst. Phys. Chem. Mat. 2018, 9, 364–369. [Google Scholar] [CrossRef]
- Sofer, Z.; Šimek, P.; Jankovsky, O.; Sedmidubsky, D.; Beran, P.; Pumera, M. Neutron diffraction as a precise and reliable method for obtaining structural properties of bulk quantities of graphene. Nanoscale 2014, 6, 13082–13089. [Google Scholar] [CrossRef] [PubMed]
- Opalev, S.V.; Belenkov, E.A. Experimental study of graphites structure change under mechanical grinding. Bull. Chelyabinsk. Sci. Cent. 2004, 3, 27–30. (In Russian) [Google Scholar]
- Klancnik, G.; Medved, J.; Mrvar, P. Differential thermal analysis (DTA) and differential scanning calorimetry (DSC) as a method of material investigation. RMZ Mat. Geoenv. 2010, 57, 127–142. [Google Scholar]
- Mitchel, P.C.H.; Parker, S.F.; Ramirez-Cuesta, A.J.; Tomkinson, J. Vibrational Spectroscopy with Neutrons. In With Application in Chemistry, Biology, Materials Science and Catalysis; World Scientific: Singapore, 2005. [Google Scholar]
- Huang, H.; Sun, G.; Hu, J.; Jiao, T. Low temperature synthesis of MnO2/graphene nanocomposites for supercapacitors. J. Chem. 2015, 2015, 629362. [Google Scholar] [CrossRef]
- Goldstein, J.; Newbury, D.E.; Echlin, P.; Joy, D.C.; Romig, A.D.; Lyman, C.E. Scanning Electron Microscopy and X-Ray Microanalysis. In A Text for Biologists, Materials Scientists, and Geologists, 2nd ed.; Plenum Press: New York, NY, USA, 1992. [Google Scholar]
- Casa Software Ltd. CasaXPS User’s Manual; Casa Software Ltd.: San Diego, CA, USA, 2001. [Google Scholar]
- Plyusnina, L.P.; Likhoidov, G.G.; Kuzmina, T.V. Graphitization and naphthide-ore genesis. Litosphere 2011, 5, 111–116. (In Russian) [Google Scholar]
- Shumilova, T.G.; Shevchuk, S.S.; Isayenko, S.I. Metal concentrations and carbonaceous matter in the black shale type rocks of the Urals. Dokl. Earth Sci. 2016, 469, 695–698. [Google Scholar] [CrossRef]
- Moldoveanu, S.C. Pyrolysis of Organic Molecules with Application to Health and Enviromental Issues, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Darmstadt, H.; Roy, C.; Kaliaguine, S. ESCA characterization of commercial carbon blacks and of carbon blacks from vacuum pyrolysis of used tires. Carbon 1994, 32, 1399–1406. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Lehmann, J.; Thies, J.E.; Burton, S.D.; Engelhard, M.H. Oxidation of black carbon by biotic and abiotic processes. Org. Geochem. 2006, 37, 1477–1488. [Google Scholar] [CrossRef]
- Shao, Y.; Yin, G.; Zhang, J.; Gao, Y. Comparative investigation of the resistance to electrochemical oxidation of carbon black and carbon nanotubes in aqueous sulfuric acid solution. Electrochim. Acta 2006, 51, 5853–5857. [Google Scholar] [CrossRef]
- Nikitin, A.; Li, X.; Zhang, Z.; Ogasawara, H.; Dai, H.; Nilsson, A. Hydrogen storage in carbon nanotubes through the formation of stable C−H bonds. Nano Lett. 2008, 8, 162–167. [Google Scholar] [CrossRef]
- Yan, J.; Wei, T.; Shao, B.; Ma, F.; Fan, Z.; Zhang, M.; Zheng, C.; Shang, Y.; Qian, W.; Wei, F. Electrochemical properties of graphene nanosheet/carbon black composites as electrodes for supercapacitors. Carbon 2010, 48, 1731–1737. [Google Scholar] [CrossRef]
- Schuster, M.E.; Havecker, M.; Arrigo, R.; Blume, R.; Knauer, M.; Ivleva, N.P.; Su, D.S.; Niessner, R.; Schlogl, R. Surface sensitive study to determine the reactivity of soot with the focus on the European emission standards IV and VI. J. Phys. Chem. A 2011, 115, 2568–2580. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhu, Z.; Li, S.; Chen, C.; Yan, L. Efficient preparation of highly hydrogenated graphene and its application as a high-performance anode material for lithium ion batteries. Nanoscale 2012, 4, 2124–2129. [Google Scholar] [CrossRef]
- Kalazhokov, Z.K.; Naumkin, A.V.; Zhansitov, A.; Khashirova, S.Y.; Kalazhokov, K.H.; Karamurzov, B.S. Investigation of carbon fiber/polyphenylenesulfone composites by method of X-ray photoelectron spectroscopy. Key Eng. Mat. 2019, 816, 37–42. [Google Scholar] [CrossRef]
- Komarova, N.S.; Krivenko, A.G.; Ryabenko, A.G.; Naumkin, A.V. Active forms of oxygen as agents for electrochemical functionalization of SWCNTs. Carbon 2013, 53, 188–196. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Chen, B. Adsorption of polycyclic aromatic hydrocarbons by graphene and graphene oxide nanosheets. Environ. Sci. Technol. 2014, 48, 4817–4825. [Google Scholar] [CrossRef]
- Gusmjo, R.; Sofer, Z.; Bouša, D.; Pumera, M. Synergetic metals on carbocatalyst shungite. Chem. Eur. J. 2017, 23, 18232–18238. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers. In The Scienta ESCA300 Database; Wiley: Chichester, UK, 1992. [Google Scholar]
- Acik, M.; Lee, G.; Mattevi, C.; Chhowalla, M.; Cho, K.; Chabal, Y.J. Unusual infrared-absorption mechanism in thermally reduced graphene oxide. Nat. Mater. 2010, 9, 840–845. [Google Scholar] [CrossRef]
- Acik, M.; Lee, G.; Mattevi, C.; Pirkle, A.; Wallace, R.M.; Chhowalla, M.; Cho, K.; Chabal, Y. The role of oxygen during thermal reduction of graphene oxide studied by infrared absorption spectroscopy. J. Phys. Chem. C 2011, 115, 19761–19781. [Google Scholar] [CrossRef]
- Kim, S.; Zhou, S.; Hu, Y.; Acik, M.; Chabal, Y.J.; Berger, C.; De Heer, W.; Bongiorno, A.; Riedo, E. Room-temperature metastability of multilayer graphene oxide films. Nat. Mater. 2012, 11, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Radovich, L.R.; Bockrath, B. On the chemical nature of graphene edges: Origin of stability and potential for magnetism in carbon materials. JACS 2005, 127, 5917–5927. [Google Scholar] [CrossRef]
- Serp, P.; Machado, B. Doped Nanostructured carbon materials as catalysts. In Nanostructured Carbon Materials for Catalysis; Royal Society of Chemistry: Croydon, UK, 2015; pp. 223–267. [Google Scholar]
- Villa, A.; Dimitratos, N. (Eds.) Metal-Free Functionalized Carbons in Catalysis: Synthesis, Characterization and Applications; Royal Society of Chemistry: Croydon, UK, 2018. [Google Scholar]
- Larciprete, R.; Lacovig, P.; Gardonio, S.; Baraldi, A.; Lizzit, S. Atomic oxygen on graphite: Chemical characterization and thermal reduction. J. Phys. Chem. C 2012, 116, 9900–9908. [Google Scholar] [CrossRef]
- Yamada, Y.; Yasuda, H.; Murota, K.; Nakamura, M.; Sodesawa, T.; Sato, S. Analysis of heat-treated graphite oxide by X-ray photoelectron spectroscopy. J. Mater. Sci. 2013, 48, 8171–8198. [Google Scholar] [CrossRef]
- Alternative Asymmetric Line-Shapes. Available online: http://www.casaxps.com/help_manual/line_shapes.htm (accessed on 1 January 2023).
- Coates, J. Encyclopedia of Analytical Chemistry. In Application, Theory and Instrumentation, 2nd ed.; Meyers, R.A., Ed.; Wiley: Chichester, UK, 2006; pp. 1–23. [Google Scholar]
- Sheka, E.; Natkaniec, I.; Rozhkova, N.; Buslaeva, E.; Tkachev, S.; Gubin, S.; Mel’Nikov, V. Parent and reduced graphene oxide of different origin in light of neutron scattering. Nanosyst. Phys. Chem. Math. 2016, 7, 71–80. [Google Scholar] [CrossRef]
- Fillaux, F.; Papoular, R.; Lautié, A.; Tomkinson, J. Inelastic neutron-scattering study of the proton dynamics in coals. Fuel 1995, 74, 865–873. [Google Scholar] [CrossRef]
- Varsanyi, G. Assignments for Vibrational Spectra of Seven Hundred Benzene Derivatives; Hilger, A., Ed.; Wiley: London, UK, 1974. [Google Scholar]
- Baskir, E.G.; Maltsev, A.K.; Korolev, V.A.; Khabashesku, V.N.; Nefedov, O.M. Generation and IR spectroscopic study of benzyl radical. Russ. Chem. Bull. 1993, 42, 1438–1440. [Google Scholar] [CrossRef]
- Crespo-Otero, R.; Bravo-Rodriguez, K.; Roy, S.; Benighaus, T.; Thiel, W.; Sander, W.; Sánchez-García, E. Interactions of aromatic radicals with water. Chem. Phys. Chem. 2013, 14, 805–811. [Google Scholar] [CrossRef]
- Wilmshurst, J.K.; Bernstein, H.J. The infrared and Raman spectra of toluene, toluene –alfa-d4, m-xylene, and m-xylene = alfaalfa’-d6. Can. J. Chem. 1957, 35, 911–925. [Google Scholar] [CrossRef]
- Gardner, A.M.; Green, A.M.; Tamé-Reyes, V.M.; Wilton, V.H.K.; Wright, T.G. Vibrations of the low energy states of toluene (1A1 and 1B2) and the toluene cation (2B1). J. Chem. Phys. 2013, 138, 134303. [Google Scholar] [CrossRef] [PubMed]
- Fuente, E.; Menndez, J.A.; Diez, M.A.; Montes-Moran, M.A. Infrared spectroscopy of carbon materials: A quantum chemical study of model compounds. J. Phys. Chem. B 2003, 107, 6350–6359. [Google Scholar] [CrossRef]
- Sheka, E.F. Digital Twins solve the mystery of Raman spectra of parental and reduced graphene oxides. Nanomaterials 2022, 12, 4209. [Google Scholar] [CrossRef] [PubMed]
- Sheka, E.F. Virtual vibrational spectrometry of stable radicals—Necklaced graphene molecules. Nanomaterials 2022, 12, 597. [Google Scholar] [CrossRef]
- Singh, S.K.; Peeters, F.M. Vibrational properties of nanographene. NanoMMTA 2013, 2, 10–29. [Google Scholar] [CrossRef]
- Jorio, A.; Dresselhaus, M.S.; Saito, R.; Dresselhaus, G. Raman Spectroscopy in Graphene Related Systems; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Merlen, A.; Buijnsters, J.G.; Pardanaud, C. A guide to and review of the use of multiwavelength Raman spectroscopy for characterizing defective aromatic carbon solids: From graphene to amorphous carbons. Coatings 2017, 7, 153. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef]
- Venezuela, P.; Lazzeri, M.; Mauri, F. Theory of double-resonant spectra in graphene: Intensity and line shape of defect-induced and two-phonon bands. Phys. Rev. B. 2011, 84, 035433. [Google Scholar] [CrossRef]
- Ramos, S.L.; Pimenta, M.A.; Champi, A. Multiple-excitation study of the double-resonance Raman bands in rhombohedral graphite. Carbon 2021, 179, 683–691. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Raman spectroscopy of amorphous, nanostructured, diamond–like carbon, and nanodiamond. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2004, 362, 2477–2512. [Google Scholar] [CrossRef] [PubMed]
- Mallet-Ladeira, P.; Puech, P.; Toulouse, C.; Cazayous, M.; Ratel-Ramond, N.; Weisbecker, P.; Monthioux, M. A Raman study to obtain crystallite size of carbon materials: A better alternative to the Tuinstra and Koenig law. Carbon 2017, 80, 629–639. [Google Scholar] [CrossRef]
- Mapelli, C.; Castiglioni, C.; Meroni, E.; Zerbi, G. Graphite and graphitic compounds: Vibrational spectra from oligomers to real materials. J. Mol. Struct. 1999, 480–481, 615–620. [Google Scholar] [CrossRef]
- Mapelli, C.; Castiglioni, C.; Zerbi, G.; Müllen, K. Common force field for graphite and polycyclic aromatic hydrocarbons. Phys. Rev. B 1999, 60, 12710–12725. [Google Scholar] [CrossRef]
- Castiglioni, C.; Mapelli, C.; Negri, F.; Zerbi, G. Origin of the D line in the Raman spectrum of graphite: A study based on Raman frequencies and intensities of polycyclic aromatic hydrocarbon molecules. J. Chem. Phys. 2001, 114, 963–974. [Google Scholar] [CrossRef]
- Negri, F.; Castiglioni, C.; Tommasini, M.; Zerbi, G. A computational study of the Raman spectra of large polycyclic aromatic hydrocarbons: Toward molecularly defined subunits of graphite. J. Phys. Chem. A 2002, 106, 3306–3317. [Google Scholar] [CrossRef]
- Di Donato, E.; Tommasini, M.; Fustella, G.; Brambilla, L.; Castiglioni, C.; Zerbi, G.; Simpson, C.D.; Müllen, K.; Negri, F. Wavelength-dependent Raman activity of D2h symmetry polycyclic aromatic hydrocarbons in the D-band and acoustic phonon regions. Chem. Phys. 2004, 301, 81–93. [Google Scholar] [CrossRef]
- Negri, F.; di Donato, E.; Tommasini, M.; Castiglioni, C.; Zerbi, G.; Müllen, K. Resonance Raman contribution to the D band of carbon materials: Modeling defects with quantum chemistry. J. Chem. Phys. 2004, 120, 11889–11900. [Google Scholar] [CrossRef]
- Tommasini, M.; Castiglioni, C.; Zerbi, G. Raman scattering of molecular graphenes. Phys. Chem. Chem. Phys. 2009, 11, 10185–10194. [Google Scholar] [CrossRef]
- Golubev, Y.A.; Sheka, E.F. Peculiarities of the molecular character of the vibrational spectra of amorphous sp2 carbon: IR absorption and Raman scattering. In Proceedings of the 14th International Conference Carbon: Fundamental Problems of Material Science and Technology, Moscow, Russia, 7–9 June 2022; pp. 59–60. (In Russian). [Google Scholar]
- Yang, X.; Han, D.; Fan, H.; Wang, M.; Du, M.; Wang, X. First-principles calculations of phonon behaviors in graphether: A comparative study with graphene. Phys. Chem. Chem. Phys. 2020, 23, 123–130. [Google Scholar] [CrossRef]
- Savosteenko, G.; Taskaev, S.; Avramov, P. Structure and Raman spectra of exotic carbon microcrystals from meteoritic dust of Chelyabinsk superbolide. Nanomaterials 2023, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Sheka, E.F. Molecular theory of graphene chemical modification. In Graphene Science Handbook: Mechanical and Chemical Properties; Aliofkhazraei, M., Ali, N., Miln, W.I., Ozkan, C.S., Mitura, S., Gervasoni, J., Eds.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: Abingdon, UK, 2016; pp. 312–338. [Google Scholar]
- Rasheed, A.; San, O.; Kvamsdal, T. Digital twins: Values, challenges and enablers from a modeling perspective. IEEE Access 2020, 8, 21980–22012. [Google Scholar] [CrossRef]
- Belousova, I.M.; Videnichev, D.A.; Kislyakov, I.M.; Krisko, T.K.; Rozhkova, N.N.; Rozhkov, S.S. Comparative studies of optical limiting in fullerene and shungite nanocarbon aqueous dispersions. Opt. Mater. Express 2015, 5, 169–175. [Google Scholar] [CrossRef]
- Skrypnik, L.; Babich, O.; Sukhikh, S.; Shishko, O.; Ivanova, S.; Mozhei, O.; Kochish, I.; Nikonov, I. A Study of the Antioxidant, Cytotoxic Activity and Adsorption Properties of Karelian Shungite by Physicochemical Methods. Antioxidants 2021, 10, 1121. [Google Scholar] [CrossRef]
- Sheka, E.F.; Popova, N.A.; Popova, V.A. Virtual spectrometer for sp2 carbon clusters. 1. Polycyclic benzenoid-fused hydrocarbons. FNCN 2021, 29, 703–715. [Google Scholar] [CrossRef]
- Bloino, J. A VPT2 route to near-infrared spectroscopy: The role of mechanical and electrical anharmonicity. J. Phys. Chem. A 2015, 119, 5269–5287. [Google Scholar] [CrossRef]
- Dewar, M.J.S.; Ford, G.P.; McKee, M.-L.; Rzepa, H.S.; Thiel, W.; Yamaguchi, Y. Semiempirical calculations of molecular vibrational frequencies: The MNDO method. J. Mol. Struct. 1978, 43, 135–138. [Google Scholar] [CrossRef]
- Dolganov, V.K.; Kroo, N.; Rosta, L.; Sheka, E.F.; Szabort, J. Multimode polymorphism of solid MBBA. Mol. Cryst. Liq. Cryst. 1985, 127, 187–194. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, V.; Jain, Y.; Kumari, M.; Gupta, R.; Sharma, S.K.; Sachdev, K. Synthesis and characterization of graphene oxide (GO) and reduced graphene oxide (rGO) for gas sensing application. Macromol. Symp. 2017, 376, 1700006. [Google Scholar] [CrossRef]







| No | Samples | Abbreviation | Origin | References |
|---|---|---|---|---|
| 1 | Shungite carbon | ShC | Shun’ga deposit of Karelia, Russia | [7] |
| 2 | Anthraxolite | AnthX | Pavlovsk deposit of Novaya Zemlya, Russia | [8] |
| 3 | Anthracite | AnthC | Donetsk deposit, Russia | [9] |
| 4 | rGo | Ak–rGO | Institute of the Inorganic Chemistry, RAS, Moscow, Russia | [10] |
| 5 | rGO | TE–rGO | Institute of the Chemical Physics, RAS, Moscow, Russia | [11] |
| 6 | Black carbon | CB632 | Sigma-Aldririch-Merk company, USA | [12] |
| 7 | Black carbon | CB624 | Sigma-Aldririch-Merk company, USA | [12] |
| Samples | (Å) | , nm | Number of BSU Layers | , nm | Ref |
|---|---|---|---|---|---|
| ShC | 3.47(n); 3.48(X) | 2.5(n); 2.0(X) | 7(n); 5–6(X) | 2.1(X) | [48] |
| AnthX | 3.47(n); 3.47(X) | 2.5(n); 1.9(X) | 7(n); 5–6(X) | 1.6(X) | [48] |
| AnthC (Donetsk) | 3.50(X) | 2.2(X) | 5–6(X) | 2.1(X) | [49] |
| Ak-rGO | 3.50(n) | 2.4 | 7(n) | >20 | [5] |
| TE-rGO | 3.36(n) | 2.9 | 8(n) | >20 | [12] |
| CB632 | 3.57(n); 3.58(X) | 2.2(n); 1.6(X) | 6(n); 4–5(X) | 1.4(X) | [48] |
| CB624 | 3.40(n); 3.45(X) | 7.8(n); 4.1(X) | 23(n); 12(X) | 2.5(X) | [48] |
| μncr Gr | 3.35 | >202 | ~100 | >20 | [48] |
| Samples | Elemental Analysis, wt% | XPS Analysis, wt% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | S | Ref. | C | O | Minor Impurities | Ref. | |
| ShC | 94.44 | 0.63 | 0.88 | 4.28 | 1.11 | [48] | 88.5 | 8.6 | 2.9 | [48] |
| AnthX | 94.01 | 1.11 | 0.86 | 2.66 | 1.36 | [48] | 89.5 | 7.7 | 2.8 | [48] |
| AnthC | 90.53 | 1.43 | 0.74 | 6.44 | 0.89 | [49] | 89.6 | 8.1 | 2.3 | [49] |
| TE-rGO | 84.51 | 1.0 | 0.01 | 13.5 | 1.0 | [49] | 82.3 | 14.8 | 2.9 | [49] |
| AK-rGO | 89.67 | 0.96 | 0.01 | 8.98 | 0.39 | [49] | 89.5 | 7.6 | 2.9 | [49] |
| CB624 | 99.67 | 0.18 | 0 | 0.15 | - | [48] | 93.1 | 5.9 | 1.0 | [48] |
| CB632 | 97.94 | 0.32 | 0.04 | 1.66 | 0.68 | [48] | 90.7 | 7.8 | 1.5 | [48] |
| GBEs | BE, eV | Assignments |
|---|---|---|
| 1 | 531.2 | C=O, O=C–O–C=O, O=C–O–C (lactones and pairs of lactones) |
| 2 | 532.2 | O=C–O–C (lactones); O=C–C=O (o-quinones); O=C–OH; C=O in aggregated cyclic ethers with lactone |
| 3 | 533.2 | sp2C–OH; C–O–C in cyclic ethers; C–O–C–OH (hydroxypyrans: singles and pairs); O=C–O–C (lactones and pairs of lactones); O=C–OH; C–O–C in aggregated cyclic ethers with lactones |
| 4 | 534.2 | C–O–C in aggregated cyclic ethers; C–O–C–OH (hydroxypyran: singles and pairs); C–O–C in aggregated cyclic ethers with lactones |
| 5 | 536.2 | O=C–O–C–O–C–O–C–O–C=O in aggregated cyclic ethers with lactones |
| ShC | carbonyls sp2C=O; acid anhydride O=C–O–C=O; o-quinone O=sp2C–sp2C=O, carboxyls sp2C=OOH. |
| AntX | hydroxyls sp2–OH; C–O–C–OH (hydroxypyran-HP) and pairs of HPs; C=OOC(lactone) and pairs of lactones; aggregated cyclic ethers with lactones. |
| AntC | carboxyls sp2C–COOH; cyclic ethers; aggregated cyclic ethers; pyran and hydroxypyran. |
| Ak-rGO | aggregated cyclic ethers and aggregated cyclic ethers with lactones; lactones and pairs of lactones. |
| TR-rGO | aggregated cyclic ethers and aggregated cyclic ethers with lactones; hydroxypyrans and lactones, both singles and pairs. |
| CB632 | C–O–C in cyclic ether and aggregated cyclic ether; C–O–C of pairs of cyclic ether and aggregated cyclic ether with lactone. |
| CB624 | C–O–C in cyclic ether, aggregated cyclic ether and aggregated cyclic ether with lactone |
| No | Samples | Chemical Formula |
|---|---|---|
| 1. | ShC | C66H6O4 |
| 2. | AnthX | C66H10O4 |
| 3. | AnthC | C66H14O4 |
| 4. | Ak-rGO | C181H27O11 |
| 5. | TE-rGO | C185H28O19 |
| 6. | CB632 | C66H2O4 |
| 7. | CB624 | C181O9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheka, E.F. A Neoteric View of sp2 Amorphous Carbon. Nanomaterials 2023, 13, 1648. https://doi.org/10.3390/nano13101648
Sheka EF. A Neoteric View of sp2 Amorphous Carbon. Nanomaterials. 2023; 13(10):1648. https://doi.org/10.3390/nano13101648
Chicago/Turabian StyleSheka, Elena F. 2023. "A Neoteric View of sp2 Amorphous Carbon" Nanomaterials 13, no. 10: 1648. https://doi.org/10.3390/nano13101648
APA StyleSheka, E. F. (2023). A Neoteric View of sp2 Amorphous Carbon. Nanomaterials, 13(10), 1648. https://doi.org/10.3390/nano13101648






