Inductively Coupled Nonthermal Plasma Synthesis of Size-Controlled γ-Al2O3 Nanocrystals
Abstract
1. Introduction
2. Methods
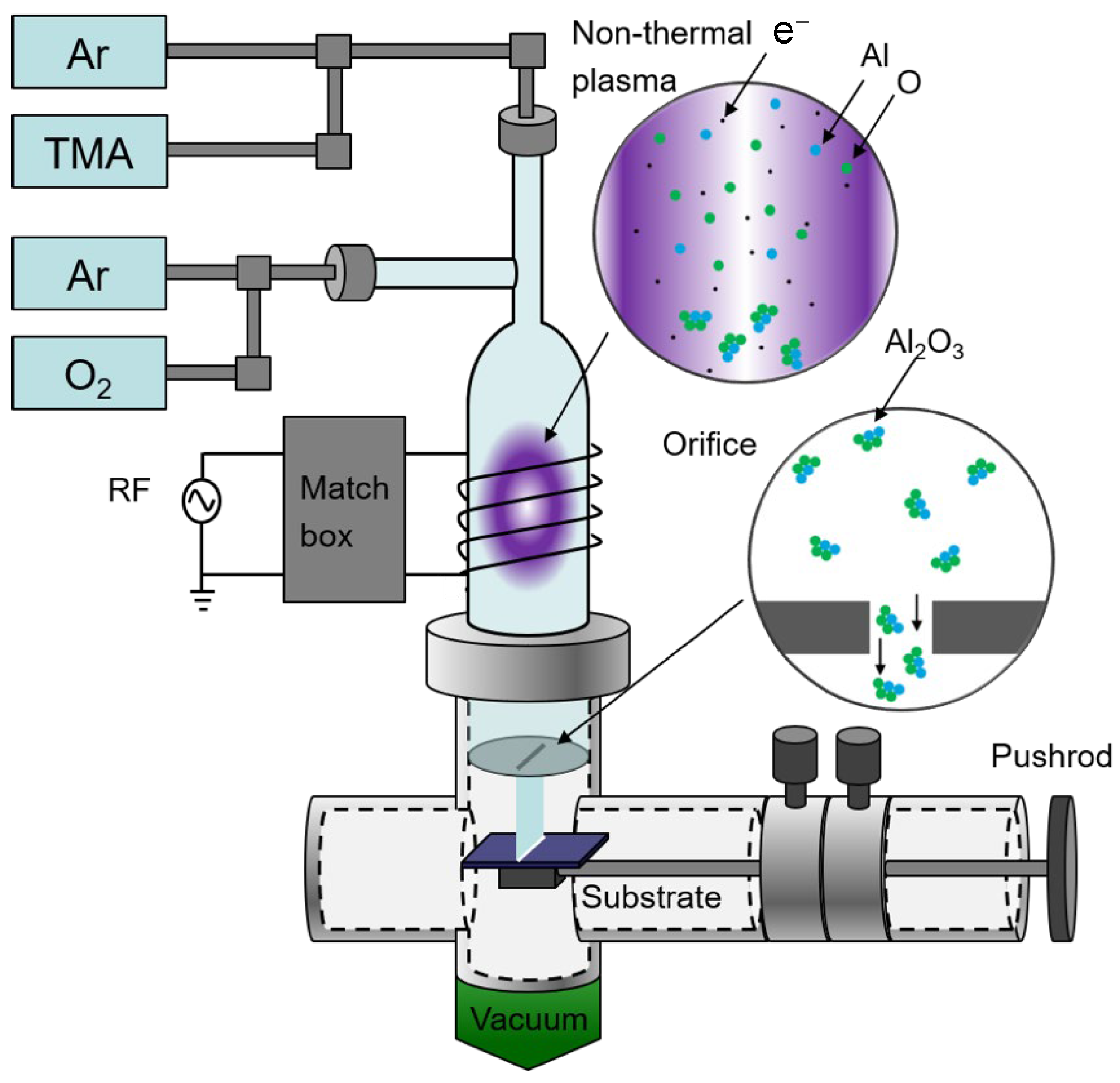
2.1. γ-Al2O3 Nanocrystal Synthesis
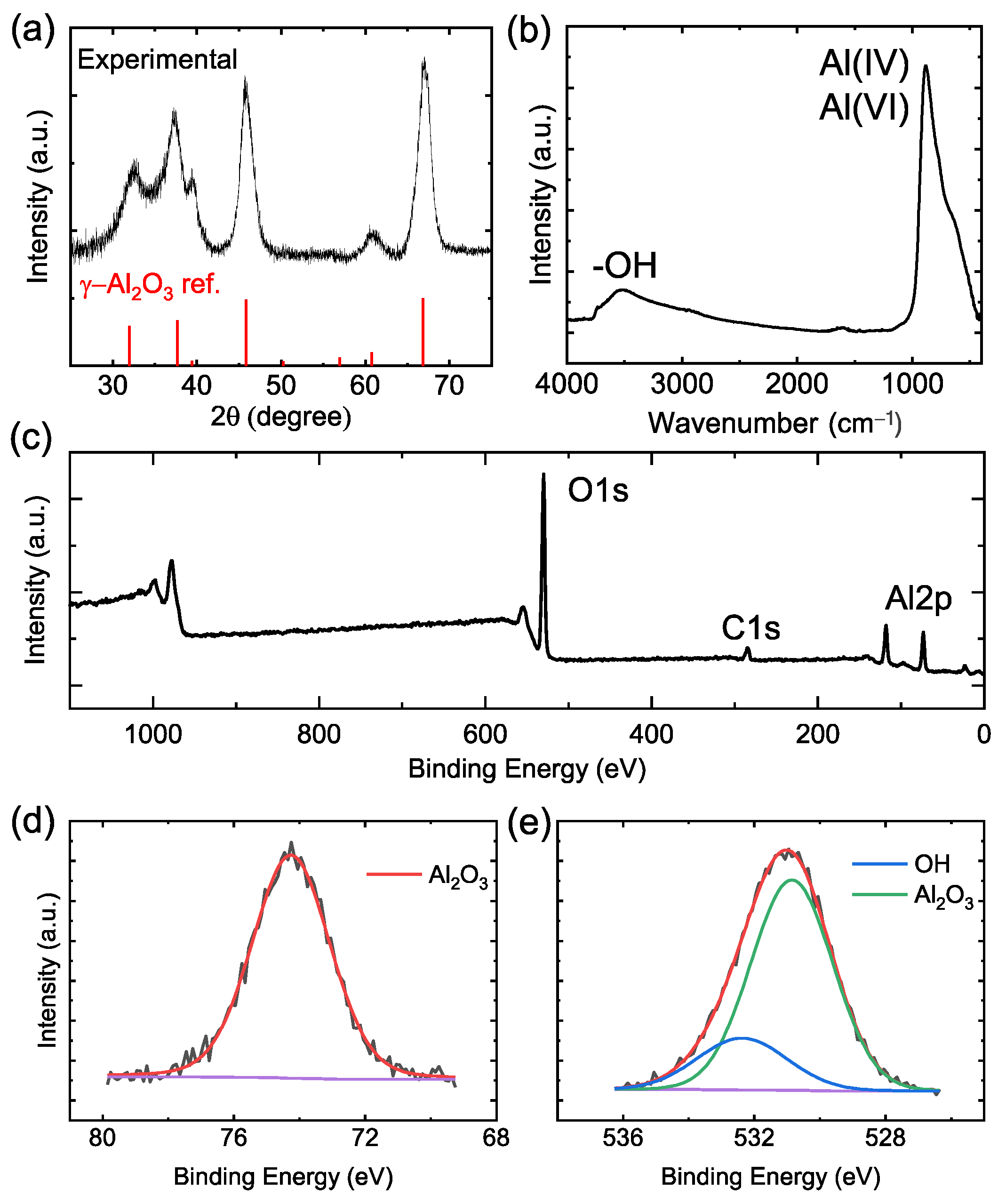
2.2. X-ray Diffraction (XRD)
2.3. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.4. X-ray Photoelectron Spectroscopy (XPS)
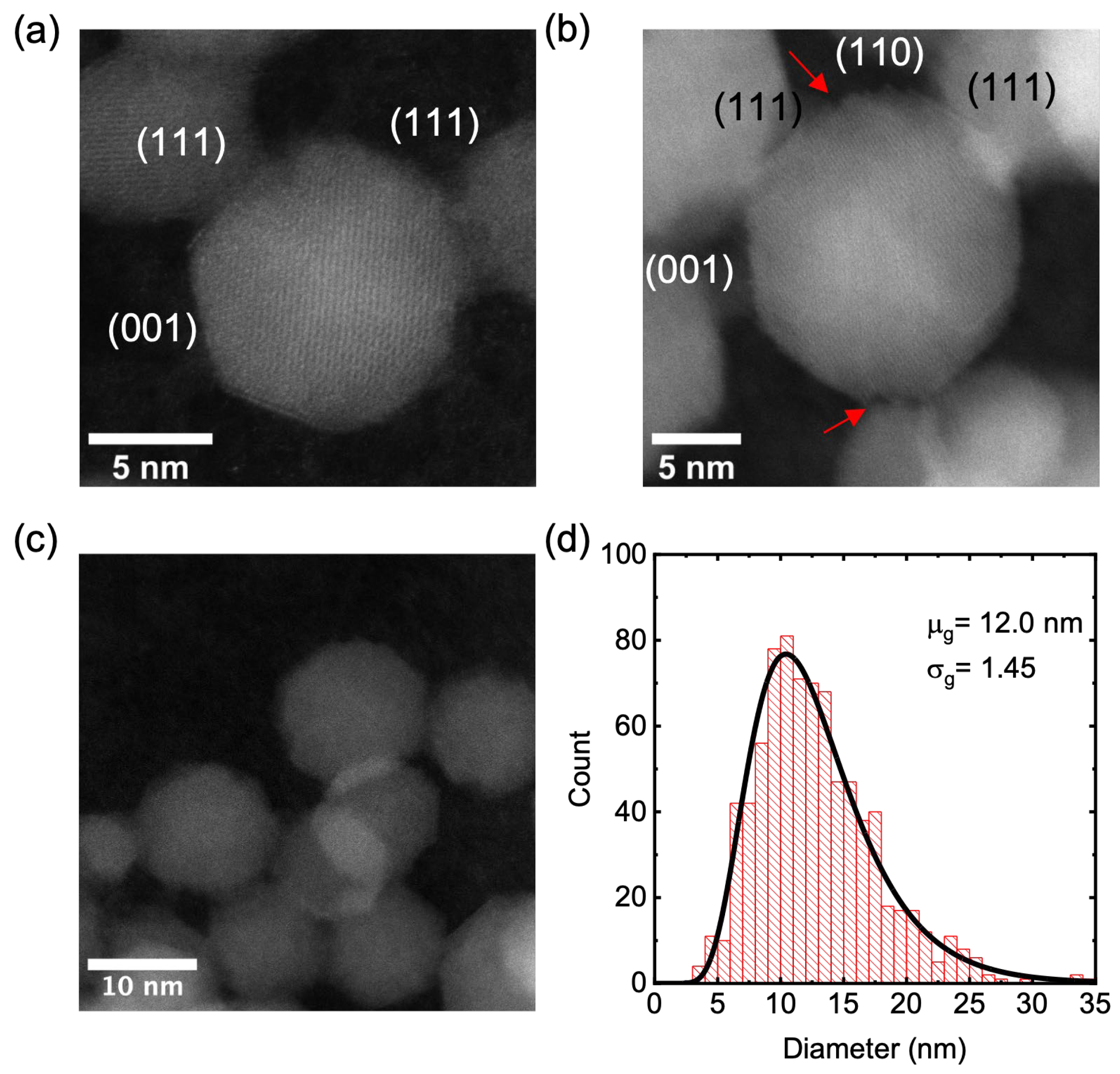
2.5. Transmission Electron Microscopy (TEM)
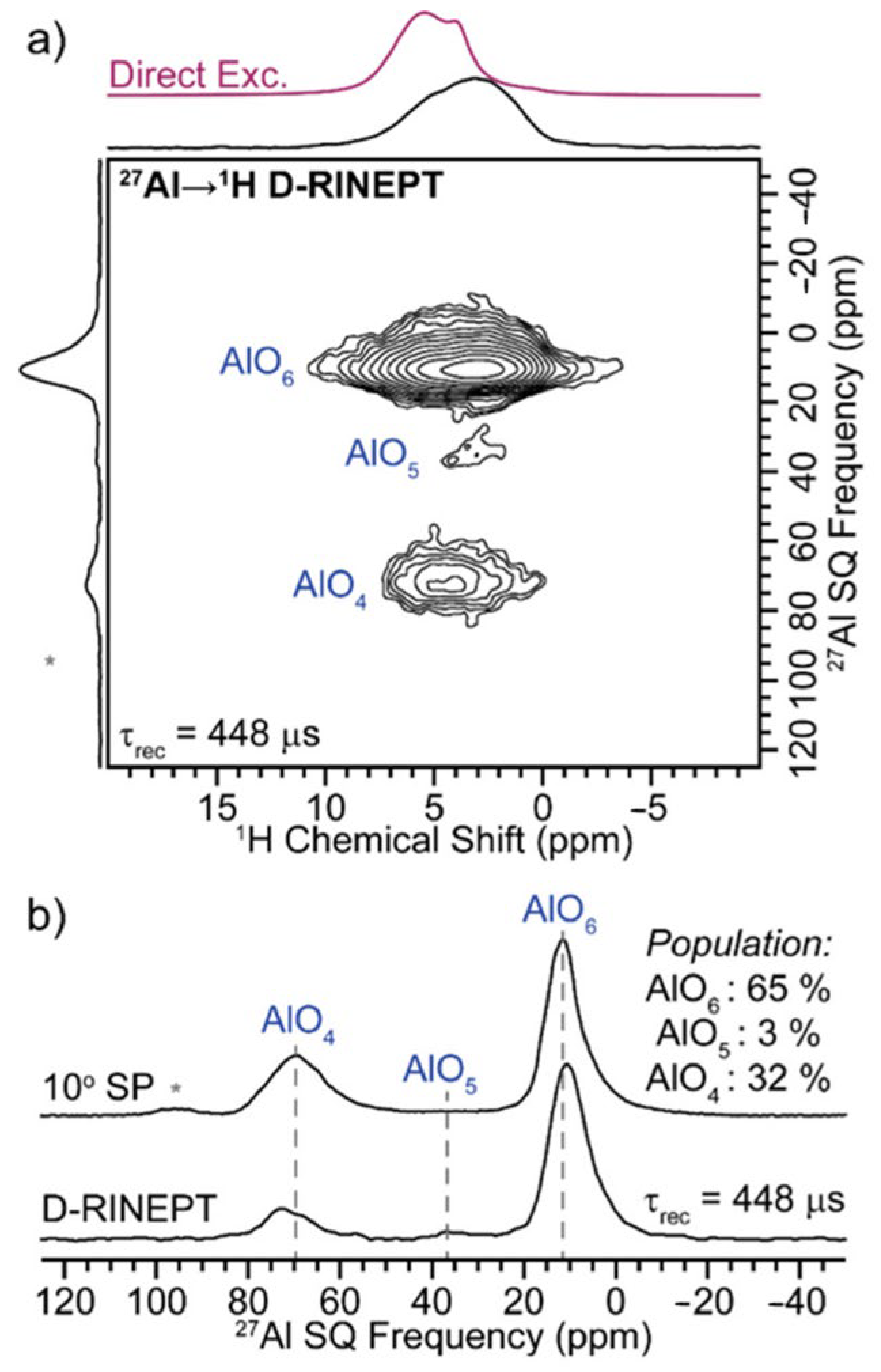
2.6. Solid-State Nuclear Magnetic Resonance (NMR) Spectroscopy
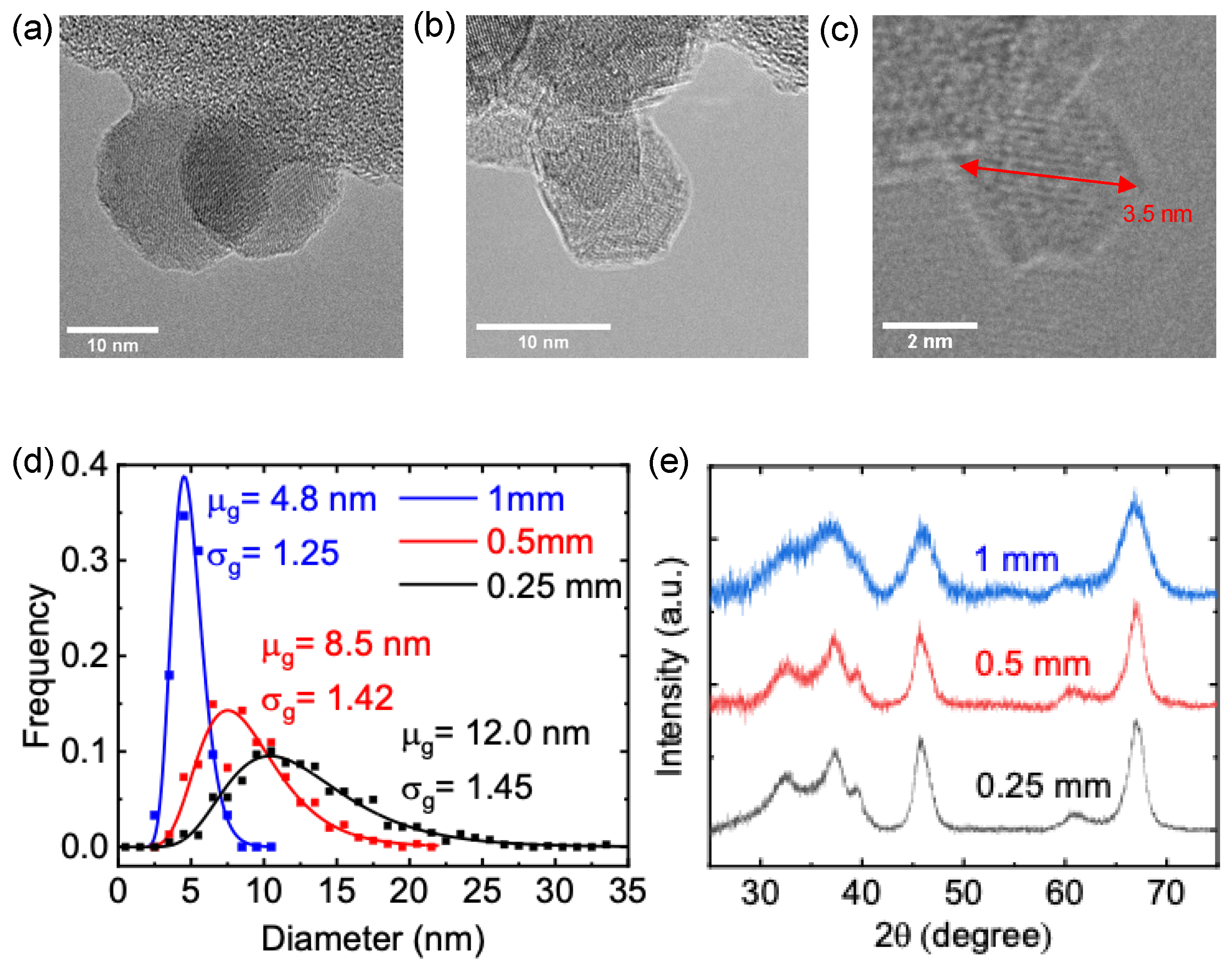
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wefers, K. Alumina Chemicals: Science and Technology Handbook; The American Ceramic Society: Westerville, OH, USA, 1990. [Google Scholar]
- Ziva, A.Z.; Suryana, Y.K.; Kurniadianti, Y.S.; Nandiyanto, A.B.D.; Kurniawan, T. Recent Progress on the Production of Aluminum Oxide (Al2O3) Nanoparticles: A Review. Mech. Eng. Soc. Ind. 2021, 1, 54–77. [Google Scholar] [CrossRef]
- Potdar, H.S.; Jun, K.-W.; Bae, J.W.; Kim, S.-M.; Lee, Y.-J. Synthesis of nano-sized porous γ-alumina powder via a precipitation/digestion route. Appl. Catal. A Gen. 2007, 321, 109–116. [Google Scholar] [CrossRef]
- Hosseini, Z.; Taghizadeh, M.; Yaripour, F. Synthesis of nanocrystalline γ-Al2O3 by sol-gel and precipitation methods for methanol dehydration to dimethyl ether. J. Nat. Gas Chem. 2011, 20, 128–134. [Google Scholar] [CrossRef]
- Baghalha, M.; Mohammadi, M.; Ghorbanpour, A. Coke deposition mechanism on the pores of a commercial Pt–Re/γ-Al2O3 naphtha reforming catalyst. Fuel Process. Technol. 2010, 91, 714–722. [Google Scholar] [CrossRef]
- Sánchez, M.; Navas, M.; Ruggera, J.F.; Casella, M.L.; Aracil, J.; Martinez, M. Biodiesel production optimization using γAl2O3 based catalysts. Energy 2014, 73, 661–669. [Google Scholar] [CrossRef]
- Rozita, Y.; Brydson, R.; Comyn, T.P.; Scott, A.J.; Hammond, C.; Brown, A.; Chauruka, S.; Hassanpour, A.; Young, N.P.; Kirkland, A.I.; et al. A Study of Commercial Nanoparticulate γ-Al2O3Catalyst Supports. Chemcatchem 2013, 5, 2695–2706. [Google Scholar] [CrossRef]
- Ishaq, K.; Saka, A.A.; Kamardeen, A.O.; Abdulrahman, A.; Adekunle, I.K.; Afolabi, A.S. Application of γ alumina as catalyst support for the synthesis of CNTs in a CVD reactor. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 035012. [Google Scholar] [CrossRef]
- Trueba, M.; Trasatti, S.P. γ-Alumina as a Support for Catalysts: A Review of Fundamental Aspects. Eur. J. Inorg. Chem. 2005, 2005, 3393–3403. [Google Scholar] [CrossRef]
- Bose, S.; Das, C. Preparation, characterization, and activity of γ-alumina-supported molybdenum/cobalt catalyst for the removal of elemental sulfur. Appl. Catal. A Gen. 2016, 512, 15–26. [Google Scholar] [CrossRef]
- Zhang, X.; Huestis, P.L.; Pearce, C.I.; Hu, J.Z.; Page, K.; Anovitz, L.M.; Aleksandrov, A.B.; Prange, M.P.; Kerisit, S.; Bowden, M.E.; et al. Boehmite and Gibbsite Nanoplates for the Synthesis of Advanced Alumina Products. ACS Appl. Nano Mater. 2018, 1, 7115–7128. [Google Scholar] [CrossRef]
- Paglia, G.; Buckley, C.E.; Rohl, A.L.; Hart, R.D.; Winter, K.; Studer, A.J.; Hunter, B.A.; Hanna, J.V. Boehmite Derived γ-Alumina System. 1. Structural Evolution with Temperature, with the Identification and Structural Determination of a New Transition Phase, γ‘-Alumina. Chem. Mater. 2004, 16, 220–236. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Wang, S.; Li, Y.; Zhai, Y. Synthesis of γ-alumina via precipitation in ethanol. Mater. Lett. 2008, 62, 3552–3554. [Google Scholar] [CrossRef]
- Jbara, A.S.; Othaman, Z.; Ati, A.A.; Saeed, M.A. Characterization of γ-Al2O3 nanopowders synthesized by Co-precipitation method. Mater. Chem. Phys. 2017, 188, 24–29. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, D.; Zhou, G.; Zhang, C.; Zhang, P.; Hou, X. Synthesis of nano-sized γ-Al2O3 with controllable size by simple homogeneous precipitation method. Mater. Lett. 2020, 279, 128476. [Google Scholar] [CrossRef]
- Yi, J.-H.; Sun, Y.-Y.; Gao, J.-F.; Xu, C.-Y. Synthesis of crystalline γ-Al2O3 with high purity. Trans. Nonferrous Met. Soc. China 2009, 19, 1237–1242. [Google Scholar] [CrossRef]
- Huang, B.; Bartholomew, C.H.; Woodfield, B.F. Facile structure-controlled synthesis of mesoporous γ-alumina: Effects of alcohols in precursor formation and calcination. Microporous Mesoporous Mater. 2013, 177, 37–46. [Google Scholar] [CrossRef]
- Ali, S.; Abbas, Y.; Zuhra, Z.; Butler, I.S. Synthesis of γ-alumina (Al2O3) nanoparticles and their potential for use as an adsorbent in the removal of methylene blue dye from industrial wastewater. Nanoscale Adv. 2019, 1, 213–218. [Google Scholar] [CrossRef]
- Mohamad, S.N.S.; Mahmed, N.; Che Halin, D.S.; Abdul Razak, K.; Norizan, M.N.; Mohamad, I.S. Synthesis of alumina nanoparticles by sol-gel method and their applications in the removal of copper ions (Cu2+) from the solution. In Proceedings of the IOP Conference Series: Materials Science and. Engineering, Wuhan, China, 10–12 October 2019; Volume 701. [Google Scholar] [CrossRef]
- Dubey, S.; Singh, A.; Nim, B.; Singh, I.B. Optimization of molar concentration of AlCl3 salt in the sol–gel synthesis of nanoparticles of gamma alumina and their application in the removal of fluoride of water. J. Sol-Gel Sci. Technol. 2017, 82, 468–477. [Google Scholar] [CrossRef]
- Kim, S.-M.; Lee, Y.-J.; Jun, K.-W.; Park, J.-Y.; Potdar, H.S. Synthesis of thermo-stable high surface area alumina powder from sol–gel derived boehmite. Mater. Chem. Phys. 2007, 104, 56–61. [Google Scholar] [CrossRef]
- Yuan, Q.; Yin, A.-X.; Luo, C.; Sun, L.-D.; Zhang, Y.-W.; Duan, W.-T.; Liu, H.-C.; Yan, C.-H. Facile Synthesis for Ordered Mesoporous γ-Aluminas with High Thermal Stability. J. Am. Chem. Soc. 2008, 130, 3465–3472. [Google Scholar] [CrossRef]
- Afruz, F.B.; Tafreshi, M.J. Synthesis of γ-Al2O3 Nano Particles by Different Combustion Modes Using Ammonium Carbonate. Indian J. Pure Appl. Phys. 2014, 52, 385–387. [Google Scholar]
- Wang, Y.; Wang, J.; Shen, M.; Wang, W. Synthesis and properties of thermostable γ-alumina prepared by hydrolysis of phosphide aluminum. J. Alloys Compd. 2009, 467, 405–412. [Google Scholar] [CrossRef]
- Ramesh, S.; Sominska, E.; Cina, B.; Chaim, R.; Gedanken, A. Nanocrystalline -Alumina Synthesized by Sonohydrolysis of Alkoxide Precursor in the Presence of Organic Acids: Structure and Morphological Properties. J. Am. Ceram. Soc. 2000, 83, 89–94. [Google Scholar] [CrossRef]
- Costa, T.M.H.; Gallas, M.R.; Benvenutti, E.V.; Da Jornada, J.A.H. Study of Nanocrystalline γ-Al2O3 Produced by High-Pressure Compaction. J. Phys. Chem. B 1999, 103, 4278–4284. [Google Scholar] [CrossRef]
- Paglia, G.; Buckley, C.E.; Rohl, A.L.; Hunter, B.A.; Hart, R.D.; Hanna, J.V.; Byrne, L.T. Tetragonal structure model for boehmite-derived γ-alumina. Phys. Rev. B Condens. Matter Mater Phys. 2003, 68, 144110. [Google Scholar] [CrossRef]
- Peintinger, M.F.; Kratz, M.J.; Bredow, T. Quantum-chemical study of stable, meta-stable and high-pressure alumina polymorphs and aluminum hydroxides. J. Mater. Chem. A Mater. 2014, 2, 13143–13158. [Google Scholar] [CrossRef]
- Acikgoz, M.; Harrell, J.; Pavanello, M. Seeking a Structure–Function Relationship for γ-Al2O3 Surfaces. J. Phys. Chem. C 2018, 122, 25314–25330. [Google Scholar] [CrossRef]
- Ayoola, H.O.; House, S.D.; Bonifacio, C.S.; Kisslinger, K.; Saidi, W.A.; Yang, J.C. Evaluating the accuracy of common γ-Al2O3 structure models by selected area electron diffraction from high-quality crystalline γ-Al2O3. Acta Mater. 2020, 182, 257–266. [Google Scholar] [CrossRef]
- Jefferson, D.A. The surface activity of ultrafine particles. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2000, 358, 2683–2692. [Google Scholar] [CrossRef]
- Rozita, Y.; Brydson, R.; Scott, A.J. An investigation of commercial gamma-Al2O3 nanoparticles. J. Phys. Conf. Ser. 2010, 241, 012096. [Google Scholar] [CrossRef]
- Kortshagen, U.R.; Sankaran, R.M.; Pereira, R.N.; Girshick, S.L.; Wu, J.J.; Aydil, E.S. Nonthermal Plasma Synthesis of Nanocrystals: Fundamental Principles, Materials, and Applications. Chem. Rev. 2016, 116, 11061–11127. [Google Scholar] [CrossRef] [PubMed]
- Kortshagen, U. Nonthermal Plasma Synthesis of Nanocrystals: Fundamentals, Applications, and Future Research Needs. Plasma Chem. Plasma Process. 2016, 36, 73–84. [Google Scholar] [CrossRef]
- Mangolini, L.; Thimsen, E.; Kortshagen, U. High-Yield Plasma Synthesis of Luminescent Silicon Nanocrystals. Nano Lett. 2005, 5, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Cendejas, A.J.; Sun, H.; Hayes, S.E.; Kortshagen, U.; Thimsen, E. Predicting plasma conditions necessary for synthesis of γ-Al2O3 nanocrystals. Nanoscale 2021, 13, 11387–11395. [Google Scholar] [CrossRef]
- El-Fayoumi, I.M.; Jones, I.R.; Turner, M.M. Hysteresis in the E- to H-mode transition in a planar coil, inductively coupled rf argon discharge. J. Phys. D Appl. Phys. 1998, 31, 3082–3094. [Google Scholar] [CrossRef]
- Lopez, T.; Mangolini, L. On the nucleation and crystallization of nanoparticles in continuous-flow nonthermal plasma reactors. J. Vac. Sci. Technol. B 2014, 32, 061802. [Google Scholar] [CrossRef]
- Kramer, N.J.; Anthony, R.J.; Mamunuru, M.; Aydil, E.S.; Kortshagen, U.R. Plasma-induced crystallization of silicon nanoparticles. J. Phys. D Appl. Phys. 2014, 47, 75202. [Google Scholar] [CrossRef]
- Li, Z.; Wray, P.R.; Su, M.P.; Tu, Q.; Andaraarachchi, H.P.; Jeong, Y.J.; Atwater, H.A.; Kortshagen, U.R. Aluminum Oxide Nanoparticle Films Deposited from a Nonthermal Plasma: Synthesis, Characterization, and Crystallization. ACS Omega 2020, 5, 24754–24761. [Google Scholar] [CrossRef]
- Holman, Z.C.; Kortshagen, U.R. A flexible method for depositing dense nanocrystal thin films: Impaction of germanium nanocrystals. Nanotechnology 2010, 21, 335302. [Google Scholar] [CrossRef]
- Harris, R.K.; Becker, E.D.; Cabral de Menezes, S.M.; Goodfellow, R.; Granger, P. NMR nomenclature. Nuclear spin properties and conventions for chemical shifts(IUPAC Recommendations 2001). Pure Appl. Chem. 2001, 73, 1795–1818. [Google Scholar] [CrossRef]
- Trébosc, J.; Hu, B.; Amoureux, J.P.; Gan, Z. Through-space R3-HETCOR experiments between spin-1/2 and half-integer quadrupolar nuclei in solid-state NMR. J. Magn. Reson. 2007, 186, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, A.; Hanrahan, M.P.; Rossini, A.J. Proton detection of MAS solid-state NMR spectra of half-integer quadrupolar nuclei. Solid State Nucl. Magn. Reson. 2017, 84, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, A.; Kentgens, A.P.M. Proton-Selective17O−1H Distance Measurements in Fast Magic-Angle-Spinning Solid-State NMR Spectroscopy for the Determination of Hydrogen Bond Lengths. J. Am. Chem. Soc. 2006, 128, 14758–14759. [Google Scholar] [CrossRef] [PubMed]
- Schnell, I.; Lupulescu, A.; Hafner, S.; Demco, D.E.; Spiess, H.W. Resolution Enhancement in Multiple-Quantum MAS NMR Spectroscopy. J. Magn. Reson. 1998, 133, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Feike, M.; Demco, D.E.; Graf, R.; Gottwald, J.; Hafner, S.; Spiess, H. Broadband Multiple-Quantum NMR Spectroscopy. J. Magn. Reson. A 1996, 122, 214–221. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, B.; Lafon, O.; Trébosc, J.; Deng, F.; Amoureux, J. Double-quantum homonuclear NMR correlation spectroscopy of quadrupolar nuclei subjected to magic-angle spinning and high magnetic field. J. Magn. Reson. 2009, 200, 251–260. [Google Scholar] [CrossRef]
- Mali, G.; Fink, G.; Taulelle, F. Double-quantum homonuclear correlation magic angle sample spinning nuclear magnetic resonance spectroscopy of dipolar-coupled quadrupolar nuclei. J. Chem. Phys. 2004, 120, 2835. [Google Scholar] [CrossRef]
- Kwak, H.-T.; Prasad, S.; Clark, T.; Grandinetti, P.J. Enhancing sensitivity of quadrupolar nuclei in solid-state NMR with multiple rotor assisted population transfers. Solid State Nucl. Magn. Reson. 2003, 24, 71–77. [Google Scholar] [CrossRef]
- Yao, Z.; Kwak, H.-T.; Sakellariou, D.; Emsley, L.; Grandinetti, P.J. Sensitivity enhancement of the central transition NMR signal of quadrupolar nuclei under magic-angle spinning. Chem. Phys. Lett. 2000, 327, 85–90. [Google Scholar] [CrossRef]
- Fung, B.M.; Khitrin, A.K.; Ermolaev, K. An Improved Broadband Decoupling Sequence for Liquid Crystals and Solids. J. Magn. Reson. 2000, 142, 97–101. [Google Scholar] [CrossRef]
- Szymanski, S.F.; Seman, M.T.; Wolden, C.A. Plasma and gas-phase characterization of a pulsed plasma-enhanced chemical vapor deposition system engineered for self-limiting growth of aluminum oxide thin films. Surf. Coat. Technol. 2007, 201, 8991–8997. [Google Scholar] [CrossRef]
- Nguyen, H.M.T.; Tang, H.-Y.; Huang, W.-F.; Lin, M.C. Mechanisms for reactions of trimethylaluminum with molecular oxygen and water. Comput. Theor. Chem. 2014, 1035, 39–43. [Google Scholar] [CrossRef]
- Belenguer, P.; Blondeau, J.P.; Boufendi, L.; Toogood, M.; Plain, A.; Bouchoule, A.; Laure, C.; Boeuf, J.P. Numerical and experimental diagnostics of rf discharges in pure and dusty argon. Phys. Rev. A 1992, 46, 7923–7933. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Cheng, C.-F.; Heine, V.; Klinowski, J. Distribution of tetrahedral and octahedral A1 sites in gamma alumina. Chem. Phys. Lett. 1997, 265, 673–676. [Google Scholar] [CrossRef]
- Zhou, R.-S.; Snyder, R.L. Structures and transformation mechanisms of the η, γ and θ transition aluminas. Acta Crystallogr. Sect. B 1991, 47, 617–630. [Google Scholar] [CrossRef]
- Bradley, S.M.; Hanna, J.V. 27Al and 23Na MAS NMR and Powder X-ray Diffraction Studies of Sodium Aluminate Speciation and the Mechanistics of Aluminum Hydroxide Precipitation upon Acid Hydrolysis. J. Am. Chem. Soc. 1994, 116, 7771–7783. [Google Scholar] [CrossRef]
- Boumaza, A.; Favaro, L.; Lédion, J.; Sattonnay, G.; Brubach, J.B.; Berthet, P.; Huntz, A.M.; Roy, P.; Tétot, R. Transition alumina phases induced by heat treatment of boehmite: An X-ray diffraction and infrared spectroscopy study. J. Solid State Chem. 2009, 182, 1171–1176. [Google Scholar] [CrossRef]
- Zagrajczuk, B.; Dziadek, M.; Olejniczak, Z.; Sulikowski, B.; Cholewa-Kowalska, K.; Laczka, M. Structural investigation of gel-derived materials from the SiO2Al2O3 system. J. Mol. Struct. 2018, 1167, 23–32. [Google Scholar] [CrossRef]
- Kuech, T.F.; Veuhoff, E.; Kuan, T.S.; Deline, V.; Potemski, R. The influence of growth chemistry on the MOVPE growth of GaAs and AlxGa1−xAs layers and heterostructures. J. Cryst. Growth 1986, 77, 257–271. [Google Scholar] [CrossRef]
- Kobayashi, N.; Makimoto, T. Reduced Carbon Contamination in OMVPE Grown GaAs and AlGaAs. Jpn. J. Appl. Phys. 1985, 24, L824. [Google Scholar] [CrossRef]
- Paparazzo, E. XPS analysis of iron aluminum oxide systems. Appl. Surf. Sci. 1986, 25, 1–12. [Google Scholar] [CrossRef]
- van den Brand, J.; Snijders, P.C.; Sloof, W.G.; Terryn, H.; De Wit, J.H.W. Acid−Base Characterization of Aluminum Oxide Surfaces with XPS. J. Phys. Chem. B 2004, 108, 6017–6024. [Google Scholar] [CrossRef]
- McCafferty, E.; Wightman, J.P. Determination of the Concentration of Surface Hydroxyl Groups on Metal Oxide Films by a Quantitative XPS Method. Surf. Interface Anal. 1998, 26, 549–564. [Google Scholar] [CrossRef]
- van den Brand, J.; Sloof, W.G.; Terryn, H.; De Wit, J.H.W. Correlation between hydroxyl fraction and O/Al atomic ratio as determined from XPS spectra of aluminium oxide layers. Surf. Interface Anal. 2004, 36, 81–88. [Google Scholar] [CrossRef]
- Pinto, H.P.; Nieminen, R.M.; Elliott, S.D. Ab Initio study of γ−Al2O3 surfaces. Phys. Rev. B 2004, 70, 125402. [Google Scholar] [CrossRef]
- Gresback, R.; Holman, Z.; Kortshagen, U. Nonthermal plasma synthesis of size-controlled, monodisperse, freestanding germanium nanocrystals. Appl. Phys. Lett. 2007, 91, 093119. [Google Scholar] [CrossRef]
- Xiong, Z.; Lanham, S.; Husmann, E.; Nelson, G.; Eslamisaray, M.A.; Polito, J.; Liu, Y.; Goree, J.; Thimsen, E.; Kushner, M.J.; et al. Particle trapping, size-filtering, and focusing in the nonthermal plasma synthesis of sub-10 nanometer particles. J. Phys. D Appl. Phys. 2022, 55, 235202. [Google Scholar] [CrossRef]
- Tavakoli, A.H.; Maram, P.S.; Widgeon, S.J.; Rufner, J.; van Benthem, K.; Ushakov, S.; Sen, S.; Navrotsky, A. Amorphous Alumina Nanoparticles: Structure, Surface Energy, and Thermodynamic Phase Stability. J. Phys. Chem. C 2013, 117, 17123–17130. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Z.; Andaraarachchi, H.P.; Held, J.T.; Dorn, R.W.; Jeong, Y.-J.; Rossini, A.; Kortshagen, U.R. Inductively Coupled Nonthermal Plasma Synthesis of Size-Controlled γ-Al2O3 Nanocrystals. Nanomaterials 2023, 13, 1627. https://doi.org/10.3390/nano13101627
Xiong Z, Andaraarachchi HP, Held JT, Dorn RW, Jeong Y-J, Rossini A, Kortshagen UR. Inductively Coupled Nonthermal Plasma Synthesis of Size-Controlled γ-Al2O3 Nanocrystals. Nanomaterials. 2023; 13(10):1627. https://doi.org/10.3390/nano13101627
Chicago/Turabian StyleXiong, Zichang, Himashi P. Andaraarachchi, Jacob T. Held, Rick W. Dorn, Yong-Jin Jeong, Aaron Rossini, and Uwe R. Kortshagen. 2023. "Inductively Coupled Nonthermal Plasma Synthesis of Size-Controlled γ-Al2O3 Nanocrystals" Nanomaterials 13, no. 10: 1627. https://doi.org/10.3390/nano13101627
APA StyleXiong, Z., Andaraarachchi, H. P., Held, J. T., Dorn, R. W., Jeong, Y.-J., Rossini, A., & Kortshagen, U. R. (2023). Inductively Coupled Nonthermal Plasma Synthesis of Size-Controlled γ-Al2O3 Nanocrystals. Nanomaterials, 13(10), 1627. https://doi.org/10.3390/nano13101627






