Zinc (Zn) Doping by Hydrothermal and Alkaline Heat-Treatment Methods on Titania Nanotube Arrays for Enhanced Antibacterial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Titania Nanotube (NT) Fabrication
2.2. Zinc Doping on Ti and NT Surfaces
2.3. Material Characterization
2.4. Stability of Zn-Doped Surfaces
2.5. Mechanical Properties of the Surfaces
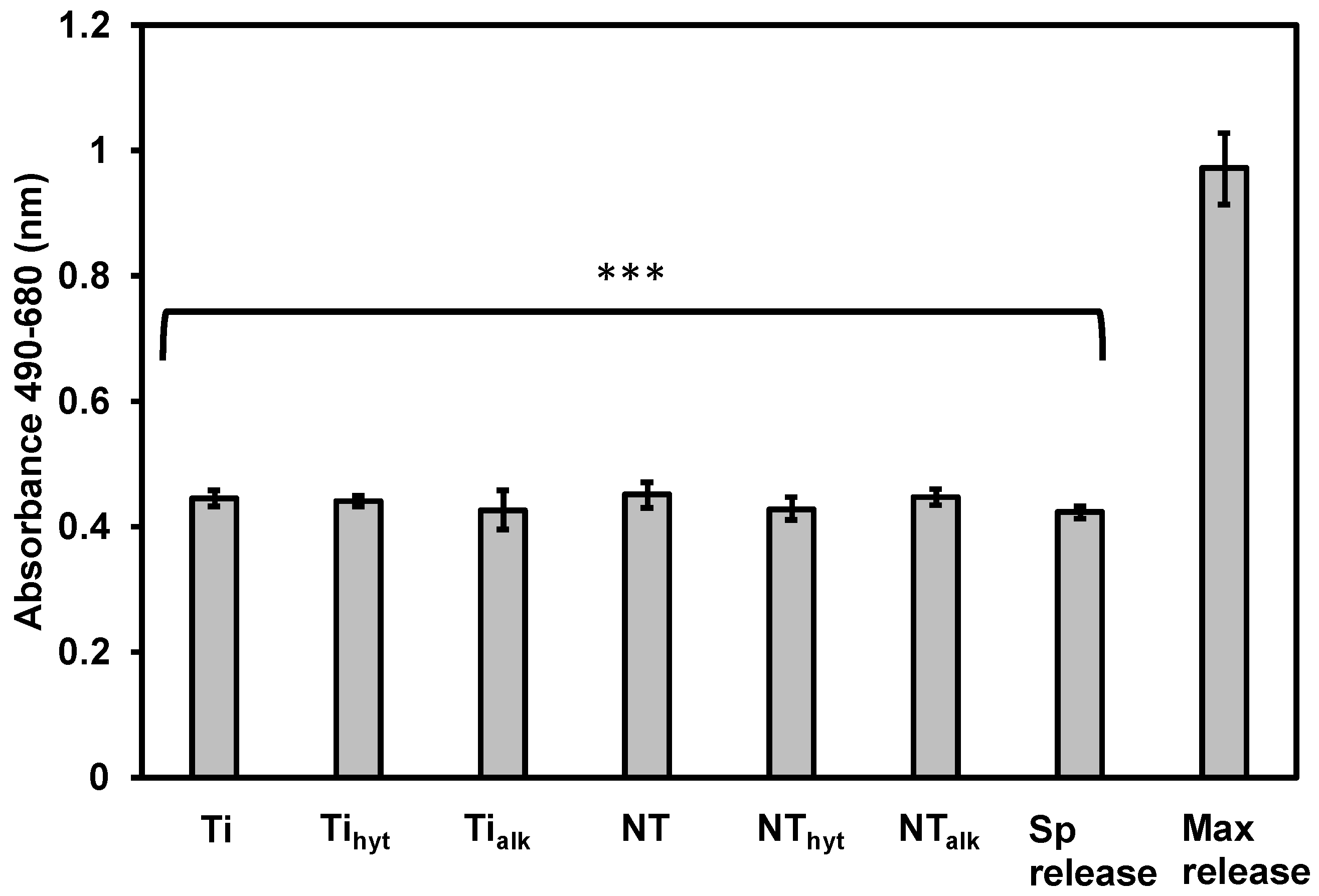
2.6. Cytotoxicity Behavior of the Surfaces
2.7. Bacteria Culture
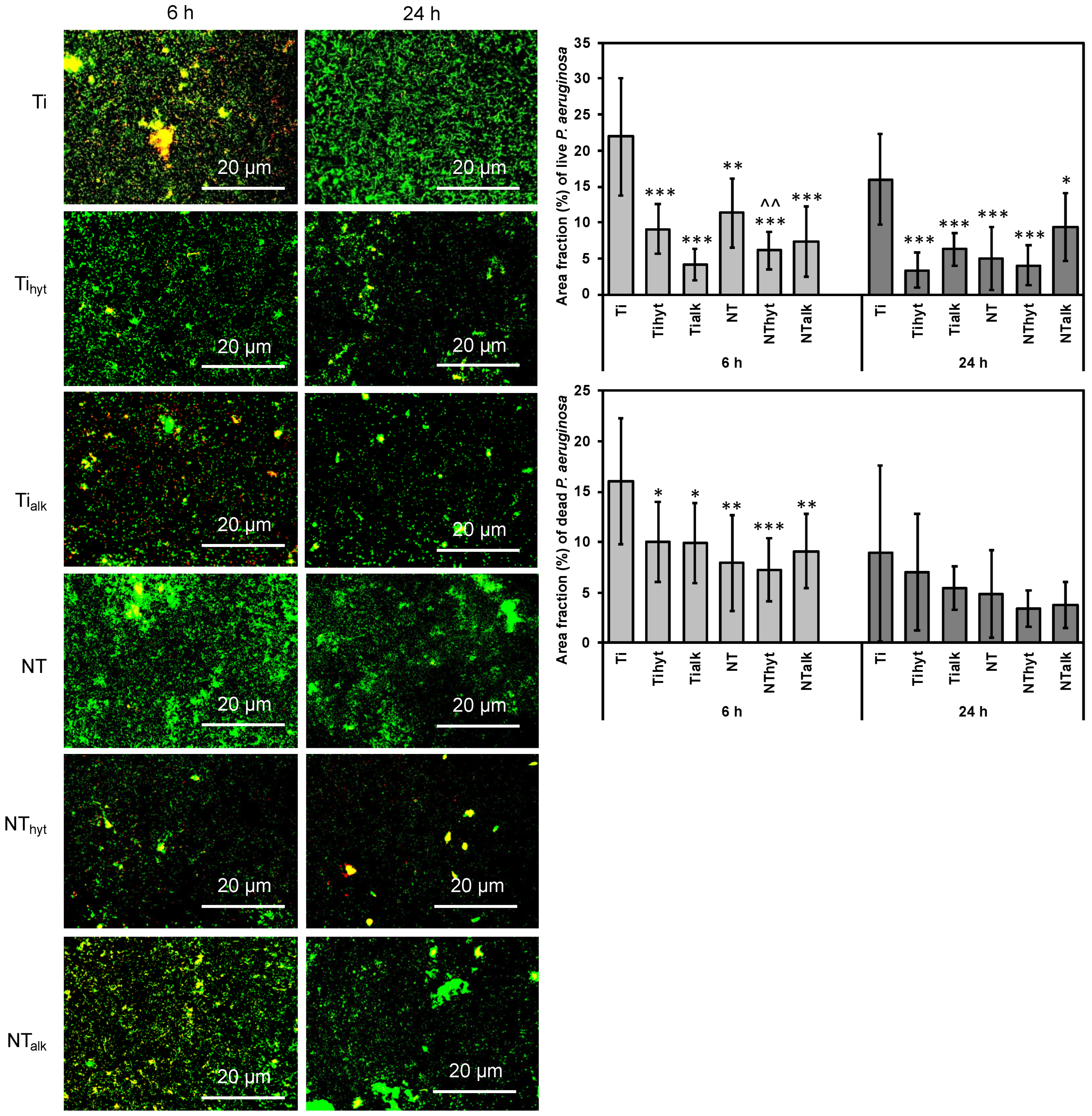
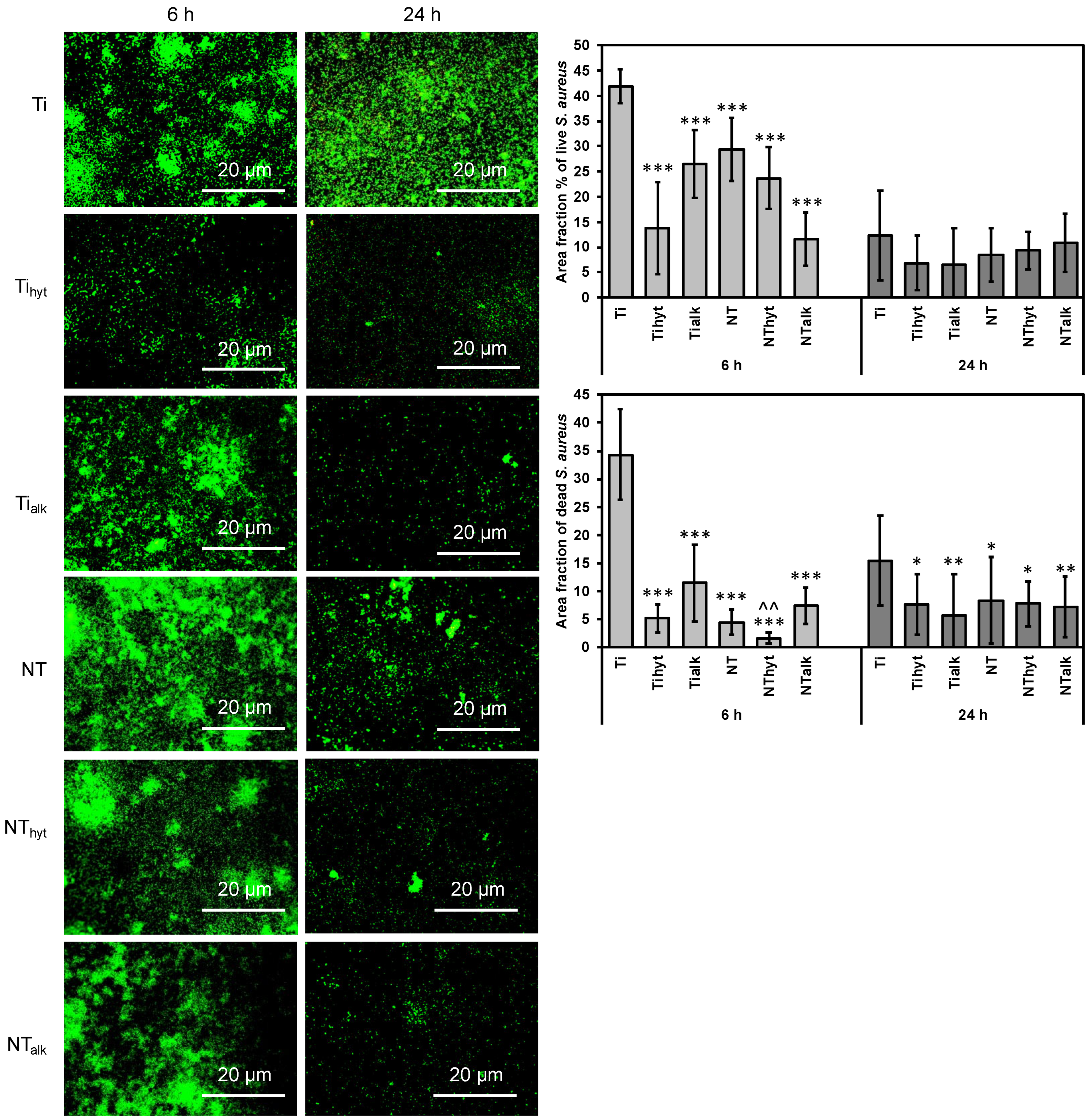
2.7.1. Bacteria Adhesion on Different Surfaces
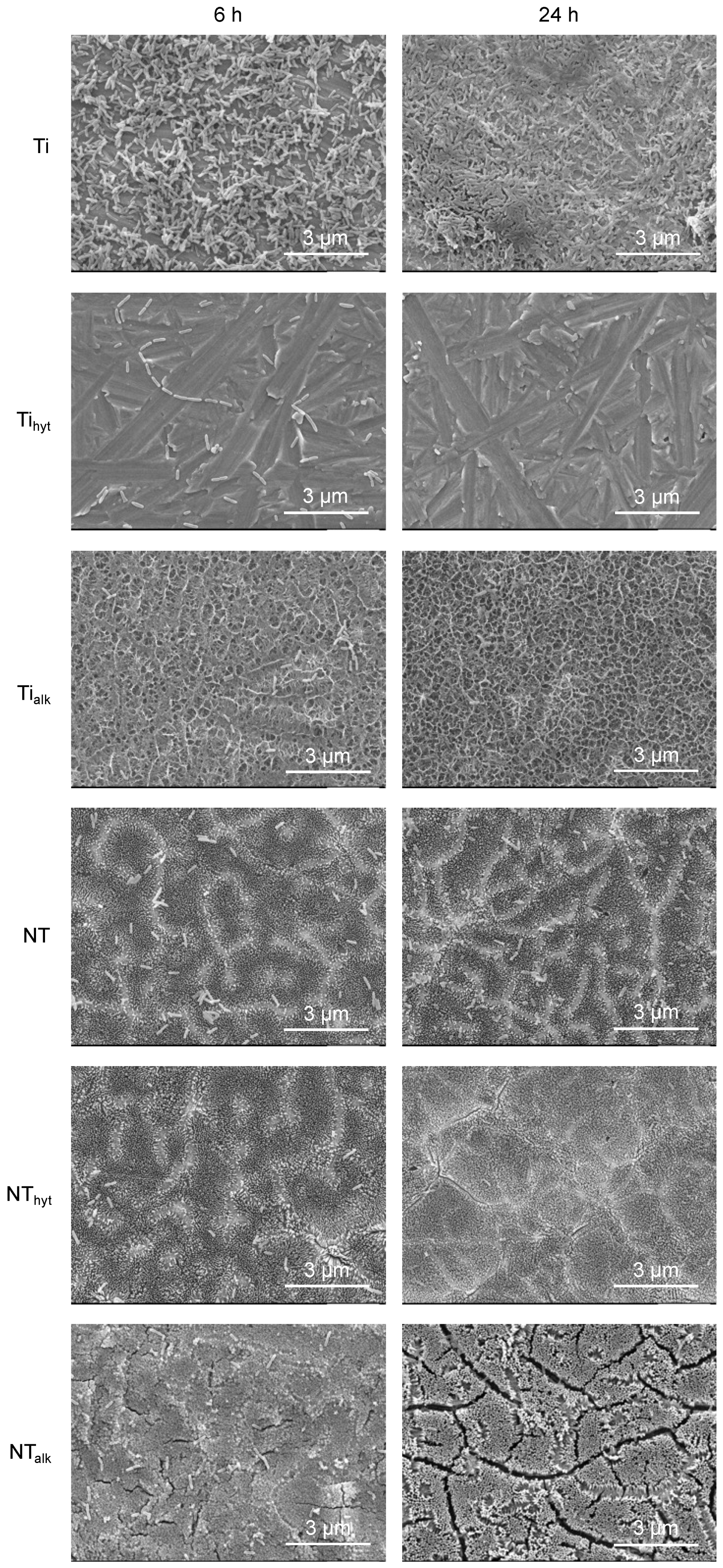
2.7.2. Bacteria Morphology on Different Surfaces
2.8. Statistical Analysis
3. Results and Discussion
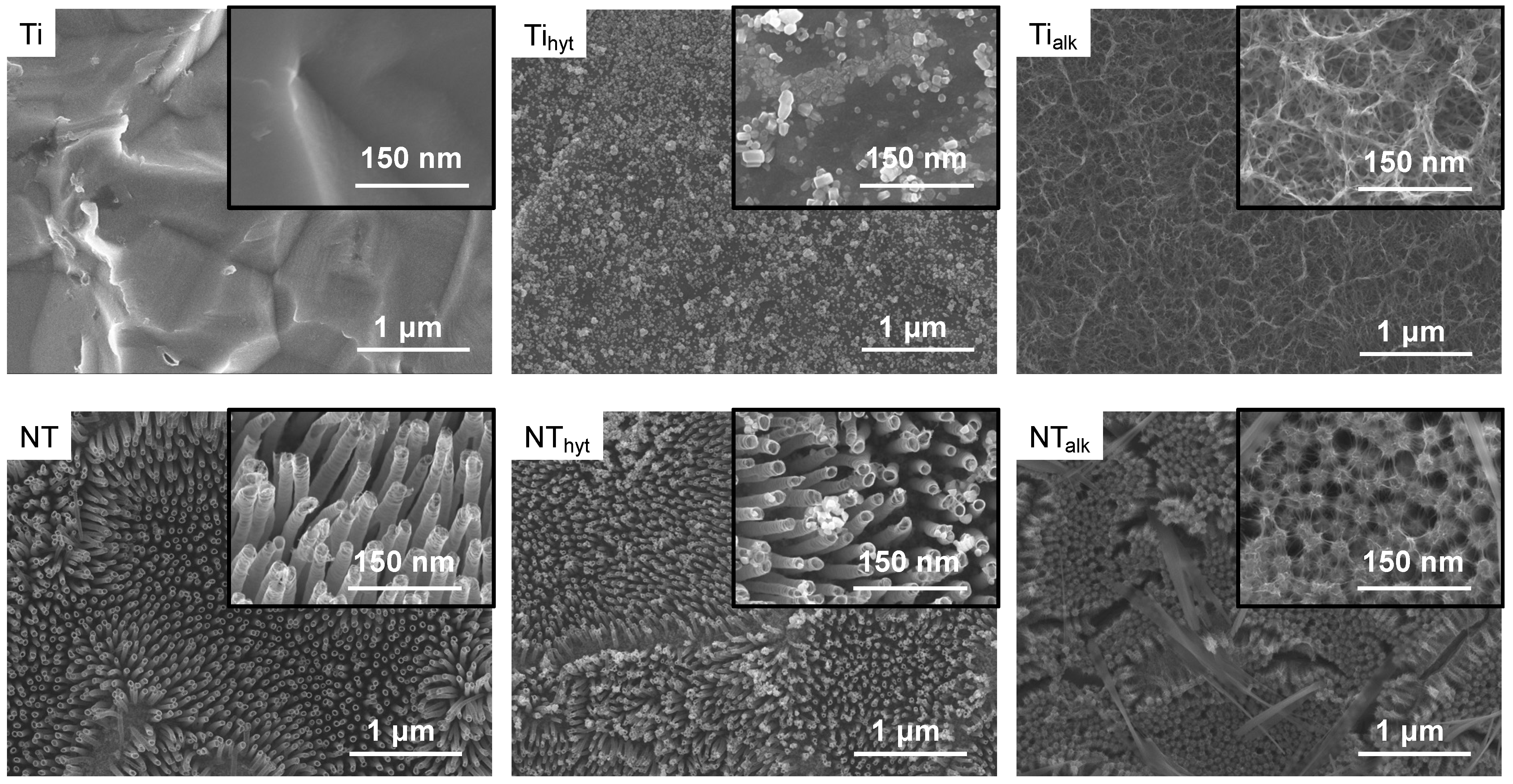
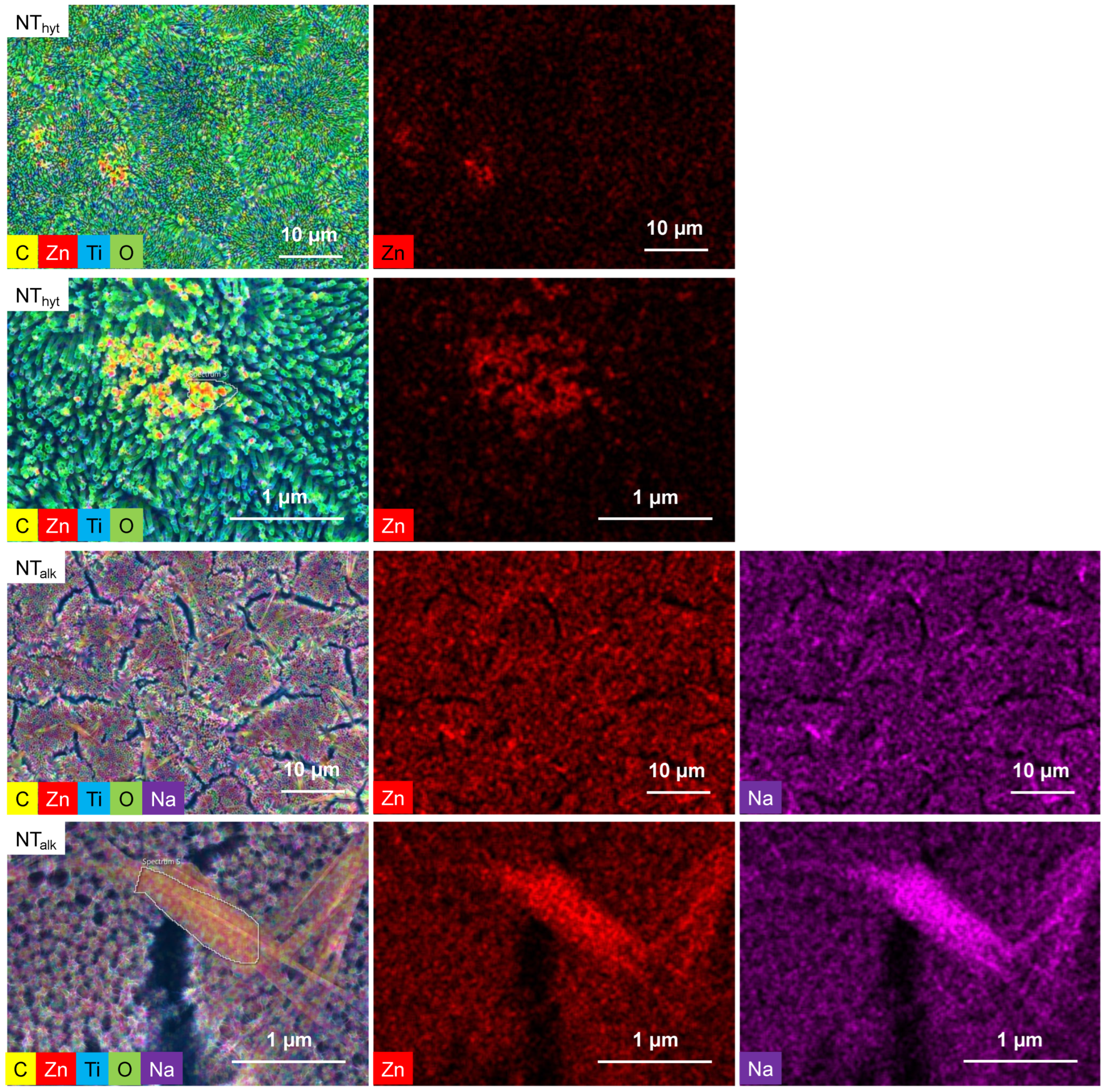
3.1. Morphology and Zn Distribution on the Surfaces
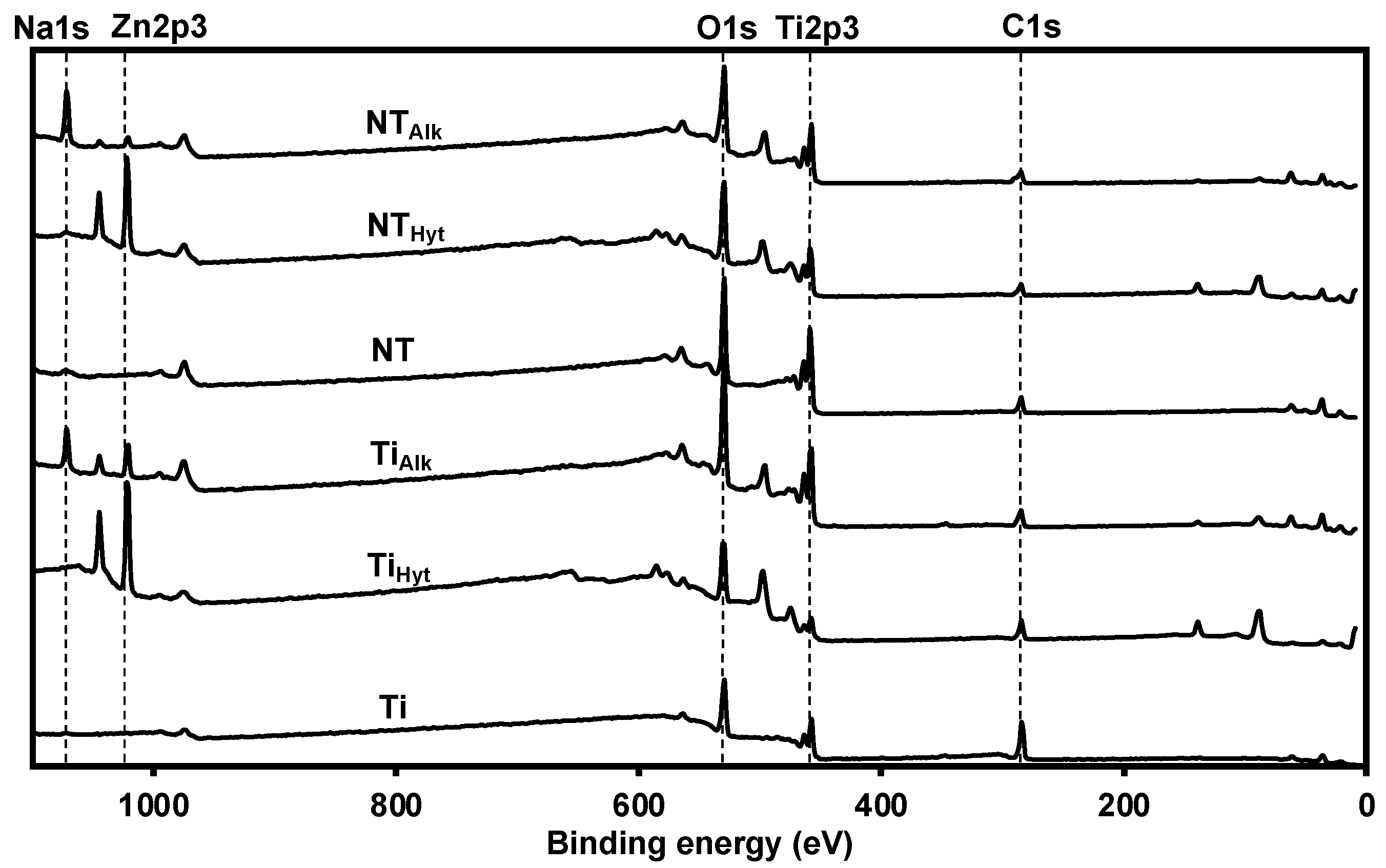
3.2. Surface Chemistry
3.3. Surface Crystallinity
3.4. Surface Stability
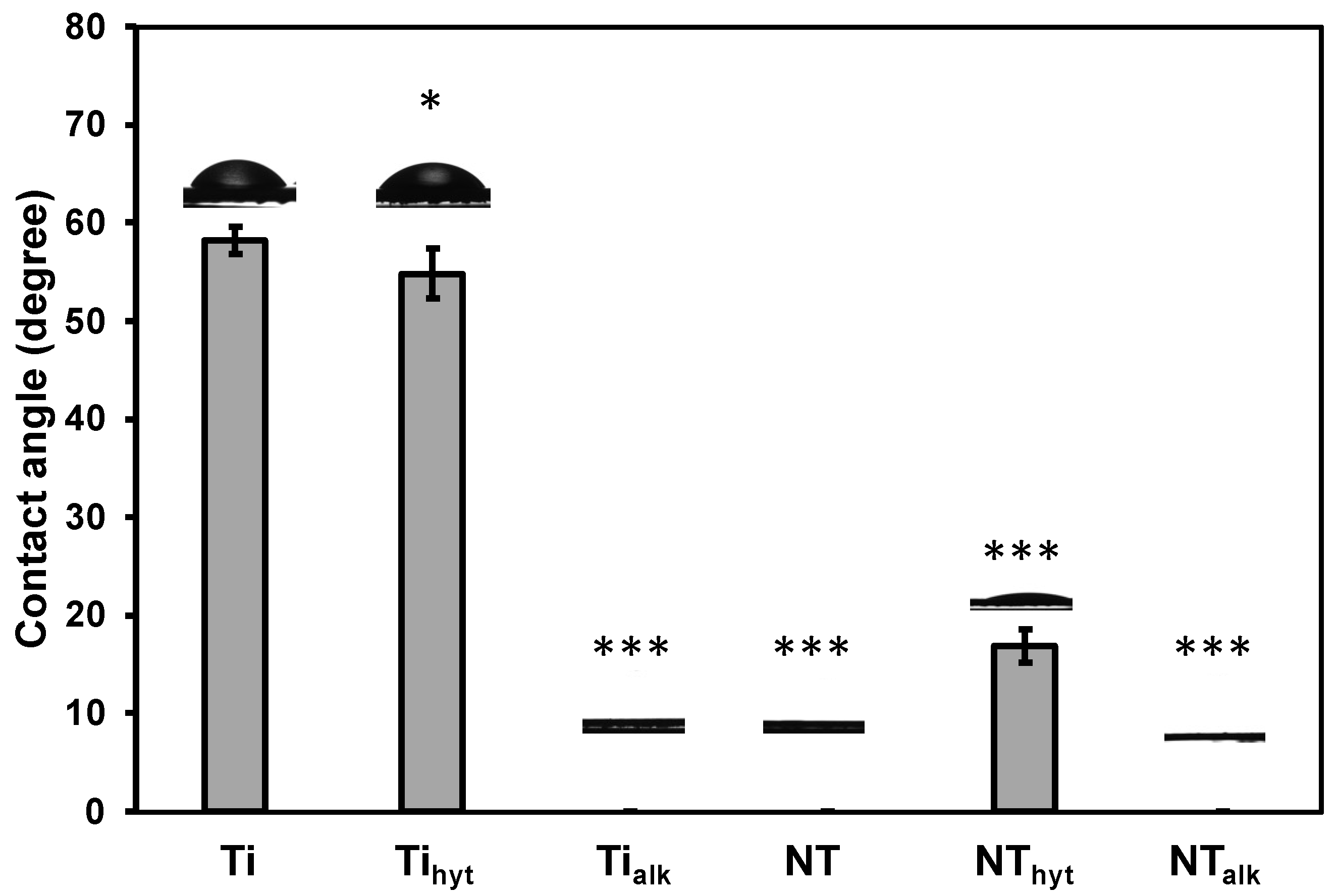
3.5. Surface Wettability
3.6. Mechanical Properties
3.7. Cytotoxicity of the Surfaces
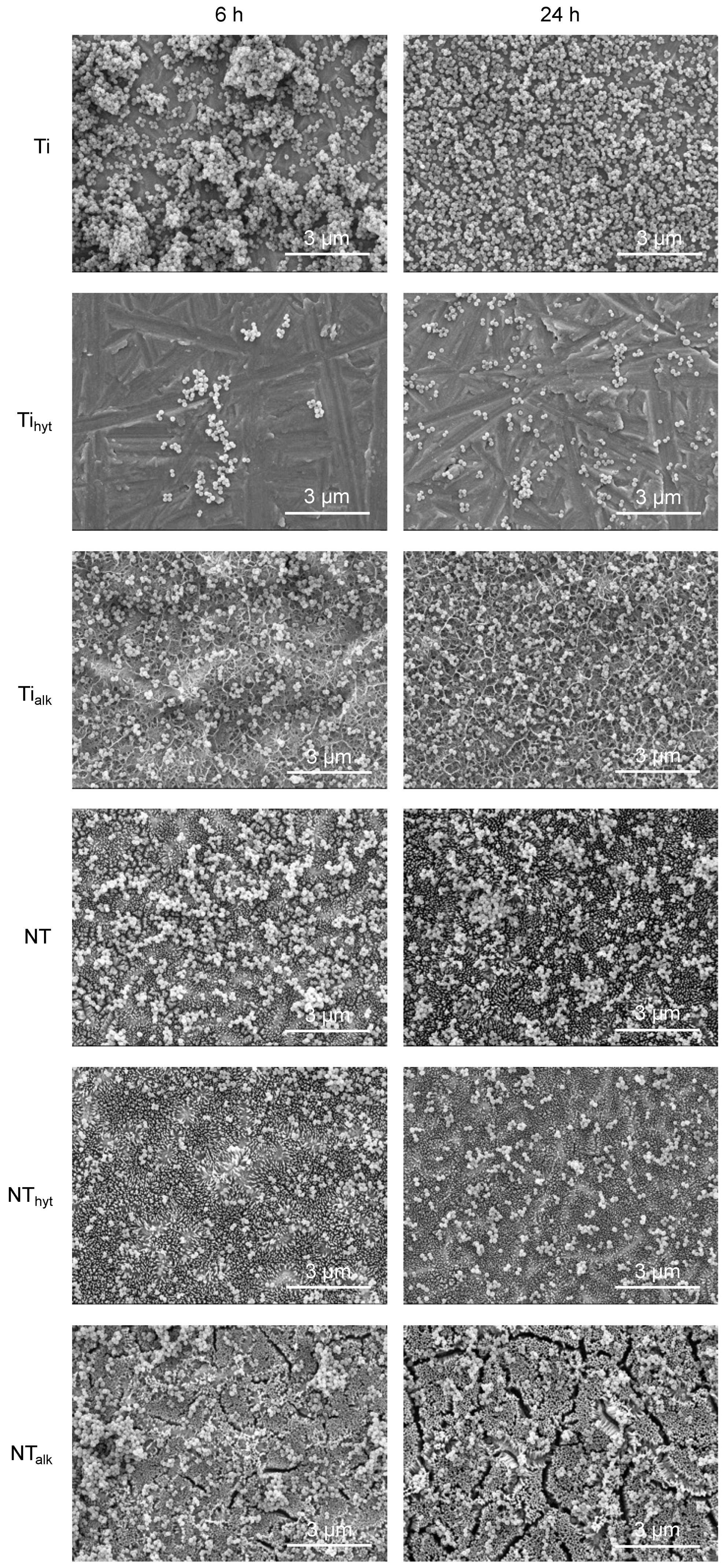
3.8. Bacteria Adhesion
3.9. Bacteria Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Effenberger, H.; Ramsauer, T.; Böhm, G.; Hilzensauer, G.; Dorn, U.; Lintner, F. Successful hip arthroplasty using cementless titanium implants in rheumatoid arthritis. Arch. Orthop. Trauma Surg. 2002, 122, 80–87. [Google Scholar] [CrossRef] [PubMed]
- John, A.A.; Jaganathan, S.K.; Supriyanto, E.; Manikandan, A. Surface modification of titanium and its alloys for the enhancement of osseointegration in orthopaedics. Curr. Sci. 2016, 111, 1003–1015. Available online: https://www.jstor.org/stable/24908502 (accessed on 22 February 2023). [CrossRef]
- Taddei, E.; Henriques, V.; Silva, C.; Cairo, C. Production of new titanium alloy for orthopedic implants. Mater. Sci. Eng. C 2004, 24, 683–687. [Google Scholar] [CrossRef]
- Sabino, R.M.; Mondini, G.; Kipper, M.J.; Martins, A.F.; Popat, K.C. Tanfloc/heparin polyelectrolyte multilayers improve osteogenic differentiation of adipose-derived stem cells on titania nanotube surfaces. Carbohydr. Polym. 2021, 251, 117079. [Google Scholar] [CrossRef]
- Wang, M.; Tang, T. Surface treatment strategies to combat implant-related infection from the beginning. J. Orthop. Translat. 2019, 17, 42–54. [Google Scholar] [CrossRef]
- Lucke, M.; Schmidmaier, G.; Sadoni, S.; Wildemann, B.; Schiller, R.; Haas, N.; Raschke, M. Gentamicin coating of metallic implants reduces implant-related osteomyelitis in rats. Bone 2003, 32, 521–531. [Google Scholar] [CrossRef]
- Huo, K.; Zhang, X.; Wang, H.; Zhao, L.; Liu, X.; Chu, P.K. Osteogenic activity and antibacterial effects on titanium surfaces modified with Zn-incorporated nanotube arrays. Biomaterials 2013, 34, 3467–3478. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, W.; Zhang, C.; Gao, B.; Guan, H.; Cheng, H.; Fu, J.; Li, F. Enhanced osseointegration and antibacterial action of zinc-loaded titania-nanotube-coated titanium substrates: In vitro and in vivo studies. J. Biomed. Mater. Res. Part A 2014, 102, 3939–3950. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [CrossRef]
- Parithimarkalaignan, S.; Padmanabhan, T.V. Osseointegration: An update. J. Indian Prosthodont. Soc. 2013, 13, 2–6. [Google Scholar] [CrossRef]
- Darouiche, R.O. Treatment of infections associated with surgical implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Li, R.; Tang, X.; Guo, D.; Qing, Y.; Qin, Y. Enhanced antibacterial properties of orthopedic implants by titanium nanotube surface modification: A review of current techniques. Int. J. Nanomed. 2019, 14, 7217–7236. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef]
- Uggeri, J.; Guizzardi, S.; Scandroglio, R.; Gatti, R. Adhesion of human osteoblasts to titanium: A morpho-functional analysis with confocal microscopy. Micron 2010, 41, 210–219. [Google Scholar] [CrossRef]
- Ziebart, T.; Schnell, A.; Walter, C.; Kämmerer, P.W.; Pabst, A.; Lehmann, K.M.; Ziebart, J.; Klein, M.O.; Al-Nawas, B. Interactions between endothelial progenitor cells (EPC) and titanium implant surfaces. Clin. Oral Investig. 2013, 17, 301–309. [Google Scholar] [CrossRef]
- Stich, T.; Alagboso, F.; Křenek, T.; Kovářík, T.; Alt, V.; Docheva, D. Implant-bone-interface: Reviewing the impact of titanium surface modifications on osteogenic processes in vitro and in vivo. Bioeng. Transl. Med. 2021, 7, e10239. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, Y.; Hu, R.; Shi, B.; Zhang, L.; Huang, Q.; Yang, Y.; Tang, P.; Lin, C. Advanced surface engineering of titanium materials for biomedical applications: From static modification to dynamic responsive regulation. Bioact. Mater. 2023, 27, 15–57. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Manivasagam, V.K.; Sabino, R.M.; Kantam, P.; Popat, K.C. Surface modification strategies to improve titanium hemocompatibility: A comprehensive review. Mater. Adv. 2021, 2, 5824–5842. [Google Scholar] [CrossRef]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral Implant. Res. 2011, 22, 349–356. [Google Scholar] [CrossRef]
- Park, J.; Bauer, S.; von der Mark, K.; Schmuki, P. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 2007, 7, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bauer, S.; Schlegel, K.A.; Neukam, F.W.; von der Mark, K.; Schmuki, P. TiO2 nanotube surfaces: 15 nm—An optimal length scale of surface topography for cell adhesion and differentiation. Small 2009, 5, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Von Wilmowsky, C.; Bauer, S.; Roedl, S.; Neukam, F.W.; Schmuki, P.; Schlegel, K.A. The diameter of anodic TiO2 nanotubes affects bone formation and correlates with the bone morphogenetic protein-2 expression in vivo. Clin. Oral Implants Res. 2012, 23, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Simi, V.; Rajendran, N. Influence of tunable diameter on the electrochemical behavior and antibacterial activity of titania nanotube arrays for biomedical applications. Mater. Charact. 2017, 129, 67–79. [Google Scholar] [CrossRef]
- Feng, W.; Geng, Z.; Li, Z.; Cui, Z.; Zhu, S.; Liang, Y.; Liu, Y.; Wang, R.; Yang, X. Controlled release behaviour and antibacterial effects of antibiotic-loaded titania nanotubes. Mater. Sci. Eng. C 2016, 62, 105–112. [Google Scholar] [CrossRef]
- Mei, S.; Wang, H.; Wang, W.; Tong, L.; Pan, H.; Ruan, C.; Ma, Q.; Liu, M.; Yang, H.; Zhang, L.; et al. Antibacterial effects and biocompatibility of titanium surfaces with graded silver incorporation in titania nanotubes. Biomaterials 2014, 35, 4255–4265. [Google Scholar] [CrossRef]
- Yougbaré, S.; Mutalik, C.; Okoro, G.; Lin, I.-H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Chang, C.-C.; Kuo, T.-R. Emerging trends in nanomaterials for antibacterial applications. Int. J. Nanomed. 2021, 16, 5831–5867. [Google Scholar] [CrossRef]
- Cheng, H.; Mao, L.; Wang, L.; Hu, H.; Chen, Y.; Gong, Z.; Wang, C.; Chen, J.; Li, R.; Zhu, Z. Bidirectional regulation of zinc embedded titania nanorods: Antibiosis and osteoblastic cell growth. RSC Adv. 2015, 5, 14470–14481. [Google Scholar] [CrossRef]
- Nageri, M.; Kalarivalappil, V.; Vijayan, B.K.; Kumar, V. Titania nanotube arrays surface-modified with ZnO for enhanced photocatalytic applications. Mater. Res. Bull. 2016, 77, 35–40. [Google Scholar] [CrossRef]
- Alvarez, K.; Fukuda, M.; Yamamoto, O. Titanium Implants after Alkali Heating Treatment with a [Zn(OH)4]2− Complex: Analysis of Interfacial Bond Strength Using Push-Out Tests. Clin. Implant Dent. Relat. Res. 2009, 12, e114–e125. [Google Scholar] [CrossRef]
- Yusa, K.; Yamamoto, O.; Takano, H.; Fukuda, M.; Iino, M. Zinc-modified titanium surface enhances osteoblast differentiation of dental pulp stem cells in vitro. Sci. Rep. 2016, 6, 29462. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, W.; Qiao, Y.; Jiang, X.; Liu, X.; Ding, C. Antibacterial activity and increased bone marrow stem cell functions of Zn-incorporated TiO2 coatings on titanium. Acta Biomater. 2012, 8, 904–915. [Google Scholar] [CrossRef]
- Zhang, Z.; Gu, B.; Zhang, W.; Kan, G.; Sun, J. The enhanced characteristics of osteoblast adhesion to porous Zinc–TiO2 coating prepared by plasma electrolytic oxidation. Appl. Surf. Sci. 2012, 258, 6504–6511. [Google Scholar] [CrossRef]
- Le Guehennec, L.; Lopez-Heredia, M.-A.; Enkel, B.; Weiss, P.; Amouriq, Y.; Layrolle, P. Osteoblastic cell behaviour on different titanium implant surfaces. Acta Biomater. 2008, 4, 535–543. [Google Scholar] [CrossRef]
- Zareidoost, A.; Yousefpour, M.; Ghaseme, B.; Amanzadeh, A. The relationship of surface roughness and cell response of chemical surface modification of titanium. J. Mater. Sci. Mater. Med. 2012, 23, 1479–1488. [Google Scholar] [CrossRef]
- Manivasagam, V.K.; Popat, K.C. In vitro investigation of hemocompatibility of hydrothermally treated titanium and titanium alloy surfaces. ACS Omega 2020, 5, 8108–8120. [Google Scholar] [CrossRef]
- Popat, K.C.; Leoni, L.; Grimes, C.A.; Desai, T.A. Influence of engineered titania nanotubular surfaces on bone cells. Biomaterials 2007, 28, 3188–3197. [Google Scholar] [CrossRef]
- Sabino, R.M.; Kauk, K.; Movafaghi, S.; Kota, A.; Popat, K.C. Interaction of blood plasma proteins with superhemophobic titania nanotube surfaces. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102046. [Google Scholar] [CrossRef]
- Barthes, J.; Ciftci, S.; Ponzio, F.; Knopf-Marques, H.; Pelyhe, L.; Gudima, A.; Kientzl, I.; Bognár, E.; Weszl, M.; Kzhyshkowska, J.; et al. Review: The potential impact of surface crystalline states of titanium for biomedical applications. Crit. Rev. Biotechnol. 2018, 38, 423–437. [Google Scholar] [CrossRef]
- Wang, G.; Li, J.; Lv, K.; Zhang, W.; Ding, X.; Yang, G.; Liu, X.; Jiang, X. Surface thermal oxidation on titanium implants to enhance osteogenic activity and in vivo osseointegration. Sci. Rep. 2016, 6, 31769. [Google Scholar] [CrossRef]
- Dias-Netipanyj, M.F.; Sopchenski, L.; Gradowski, T.; Elifio-Esposito, S.; Popat, K.C.; Soares, P. Crystallinity of TiO2 nanotubes and its effects on fibroblast viability, adhesion, and proliferation. J. Mater. Sci. Mater. Med. 2020, 31, 94. [Google Scholar] [CrossRef] [PubMed]
- Del Curto, B.; Brunella, M.; Giordano, C.; Pedeferri, M.; Valtulina, V.; Visai, L.; Cigada, A. Decreased bacterial adhesion to surface-treated titanium. Int. J. Artif. Organs 2005, 28, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Ercan, B.; Taylor, E.; Alpaslan, E.; Webster, T.J. Diameter of titanium nanotubes influences anti-bacterial efficacy. Nanotechnology 2011, 22, 295102. [Google Scholar] [CrossRef] [PubMed]
- Puckett, S.D.; Taylor, E.; Raimondo, T.; Webster, T.J. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials 2010, 31, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, C.; Nygren, H.; Ohlson, K. Implantation of hydrophilic and hydrophobic titanium discs in rat tibia: Cellular reactions on the surfaces during the first 3 weeks in bone. Biomaterials 2004, 25, 4759–4766. [Google Scholar] [CrossRef]
- Yao, X.; Peng, R.; Ding, J. Cell-material interactions revealed via material techniques of surface patterning. Adv. Mater. 2013, 25, 5257–5286. [Google Scholar] [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y.-L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef]
- Sorkin, J.A.; Hughes, S.; Soares, P.; Popat, K.C. Titania nanotube arrays as interfaces for neural prostheses. Mater. Sci. Eng. C 2015, 49, 735–745. [Google Scholar] [CrossRef]
- Sun, Q.; Hou, Y.; Chu, Z.; Wei, Q. Soft overcomes the hard: Flexible materials adapt to cell adhesion to promote cell mechanotransduction. Bioact. Mater. 2022, 10, 397–404. [Google Scholar] [CrossRef]
- Arciola, C.; An, Y.; Campoccia, D.; Donati, M.; Montanaro, L. Etiology of Implant Orthopedic Infections: A Survey on 1027 Clinical Isolates. Int. J. Artif. Organs 2005, 28, 1091–1100. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef]
- Schierholz, J.; Beuth, J. Implant infections: A haven for opportunistic bacteria. J. Hosp. Infect. 2001, 49, 87–93. [Google Scholar] [CrossRef]
- Wilson, W.; Wade, M.M.; Holman, S.C.; Champlin, F.R. Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J. Microbiol. Methods 2001, 43, 153–164. [Google Scholar] [CrossRef]
- Diggle, S.P.; Whiteley, M. Microbe Profile: Pseudomonas aeruginosa: Opportunistic pathogen and lab rat. Microbiology 2020, 166, 30–33. [Google Scholar] [CrossRef]
- Harris, L.; Foster, S.; Richards, R. An introduction to Staphylococcus aureus, and techniques for identifying and quantifying S. aureus adhesins in relation to adhesion to biomaterials: Review. Eur. Cells Mater. 2002, 4, 39–60. [Google Scholar] [CrossRef]

| % C | % O | % Ti | % Zn | % Na | |
|---|---|---|---|---|---|
| Ti | 55.6 | 34.7 | 9.7 | 0.0 | 0.0 |
| Tihyt | 29.7 | 42.8 | 5.9 | 21.6 | 0.0 |
| Tialk | 19.2 | 51.7 | 15.2 | 4.1 | 9.8 |
| NT | 21.3 | 57.3 | 21.4 | 0.0 | 0.0 |
| NThyt | 17.7 | 53.6 | 11.7 | 16.9 | 0.0 |
| NTalk | 19.5 | 49.0 | 12.0 | 1.3 | 18.1 |
| % Zn | % Na | |||||
|---|---|---|---|---|---|---|
| Day 0 | Day 28 | % Change | Day 0 | Day 28 | % Change | |
| Ti | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Tihyt | 21.6 | 8.9 | 58.8 | 0.0 | 0.0 | 0.0 |
| Tialk | 4.1 | 5.2 | −26.8 | 9.8 | 4.4 | 55.1 |
| NT | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| NThyt | 16.9 | 9.2 | 45.6 | 0.0 | 0.0 | 0.0 |
| NTalk | 1.3 | 2.6 | −100.0 | 18.1 | 0.0 | 100.0 |
| Indentation Hardness (GPa) | Elastic Modulus (GPa) | |
|---|---|---|
| Ti | 2.30 ± 0.4 | 138 ± 20 |
| Tihyt | 1.55 ± 0.08 | 94 ± 5 |
| Tialk | 2.40 ± 0.2 | 91 ± 20 |
| NT | 0.32 ± 0.02 | 23 ± 1 |
| NThyt | 0.26 ± 0.02 | 45 ± 4 |
| NTalk | 1.00 ± 0.4 | 76 ± 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattacharjee, A.; Goodall, E.; Pereira, B.L.; Soares, P.; Popat, K.C. Zinc (Zn) Doping by Hydrothermal and Alkaline Heat-Treatment Methods on Titania Nanotube Arrays for Enhanced Antibacterial Activity. Nanomaterials 2023, 13, 1606. https://doi.org/10.3390/nano13101606
Bhattacharjee A, Goodall E, Pereira BL, Soares P, Popat KC. Zinc (Zn) Doping by Hydrothermal and Alkaline Heat-Treatment Methods on Titania Nanotube Arrays for Enhanced Antibacterial Activity. Nanomaterials. 2023; 13(10):1606. https://doi.org/10.3390/nano13101606
Chicago/Turabian StyleBhattacharjee, Abhishek, Emma Goodall, Bruno Leandro Pereira, Paulo Soares, and Ketul C. Popat. 2023. "Zinc (Zn) Doping by Hydrothermal and Alkaline Heat-Treatment Methods on Titania Nanotube Arrays for Enhanced Antibacterial Activity" Nanomaterials 13, no. 10: 1606. https://doi.org/10.3390/nano13101606
APA StyleBhattacharjee, A., Goodall, E., Pereira, B. L., Soares, P., & Popat, K. C. (2023). Zinc (Zn) Doping by Hydrothermal and Alkaline Heat-Treatment Methods on Titania Nanotube Arrays for Enhanced Antibacterial Activity. Nanomaterials, 13(10), 1606. https://doi.org/10.3390/nano13101606








