QCM-Based MgFe2O4@CaAlg Nanocomposite as a Fast Response Nanosensor for Real-Time Detection of Methylene Blue Dye
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Clove Leaves Extract
2.3. Green Synthesis of MgFe2O4 Nanoparticles
2.4. Preparation of MgFe2O4@CaAlg Nanocomposite
2.5. Instrumentation
2.6. Establishing of QCM-Based MgFe2O4 NPs and MgFe2O4@CaAlg NCs Nanosensors
2.7. QCM-Monitoring of MB Dye
3. Results and Discussion
3.1. Characterization of Green Synthesized MgFe2O4 NPs and MgFe2O4@CaAlg NCs
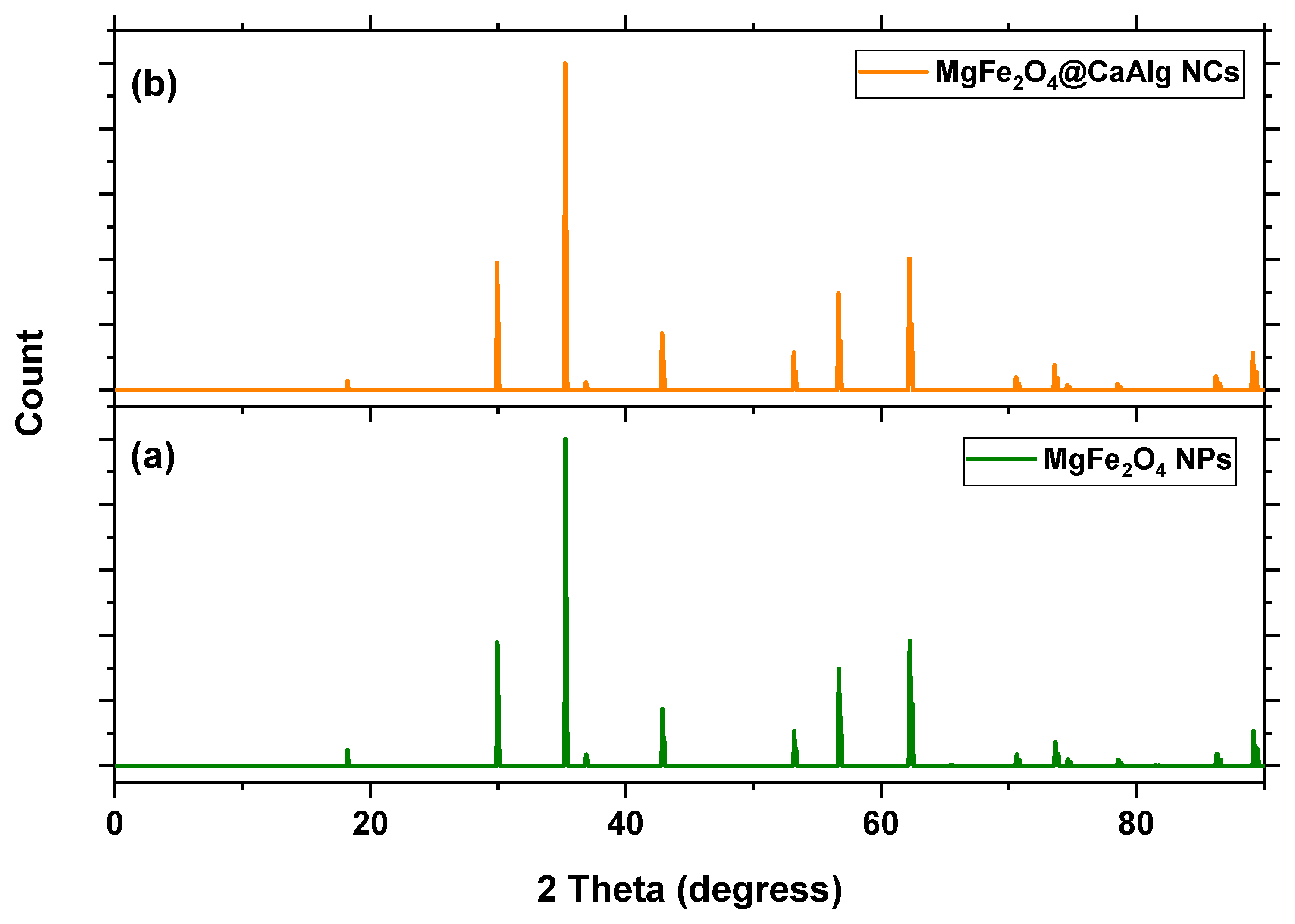
3.1.1. XRD
3.1.2. DLS and Zeta Potential
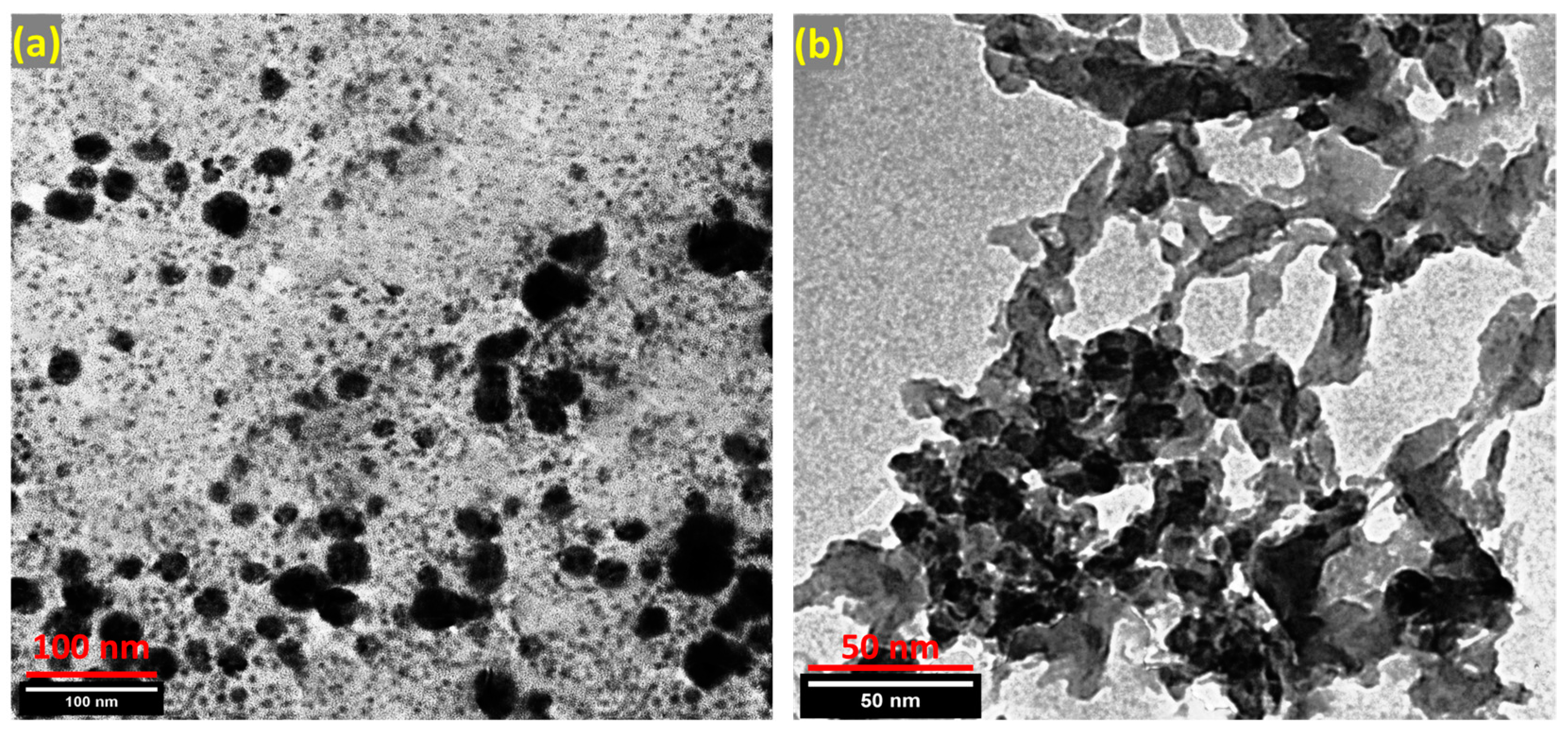
3.1.3. TEM
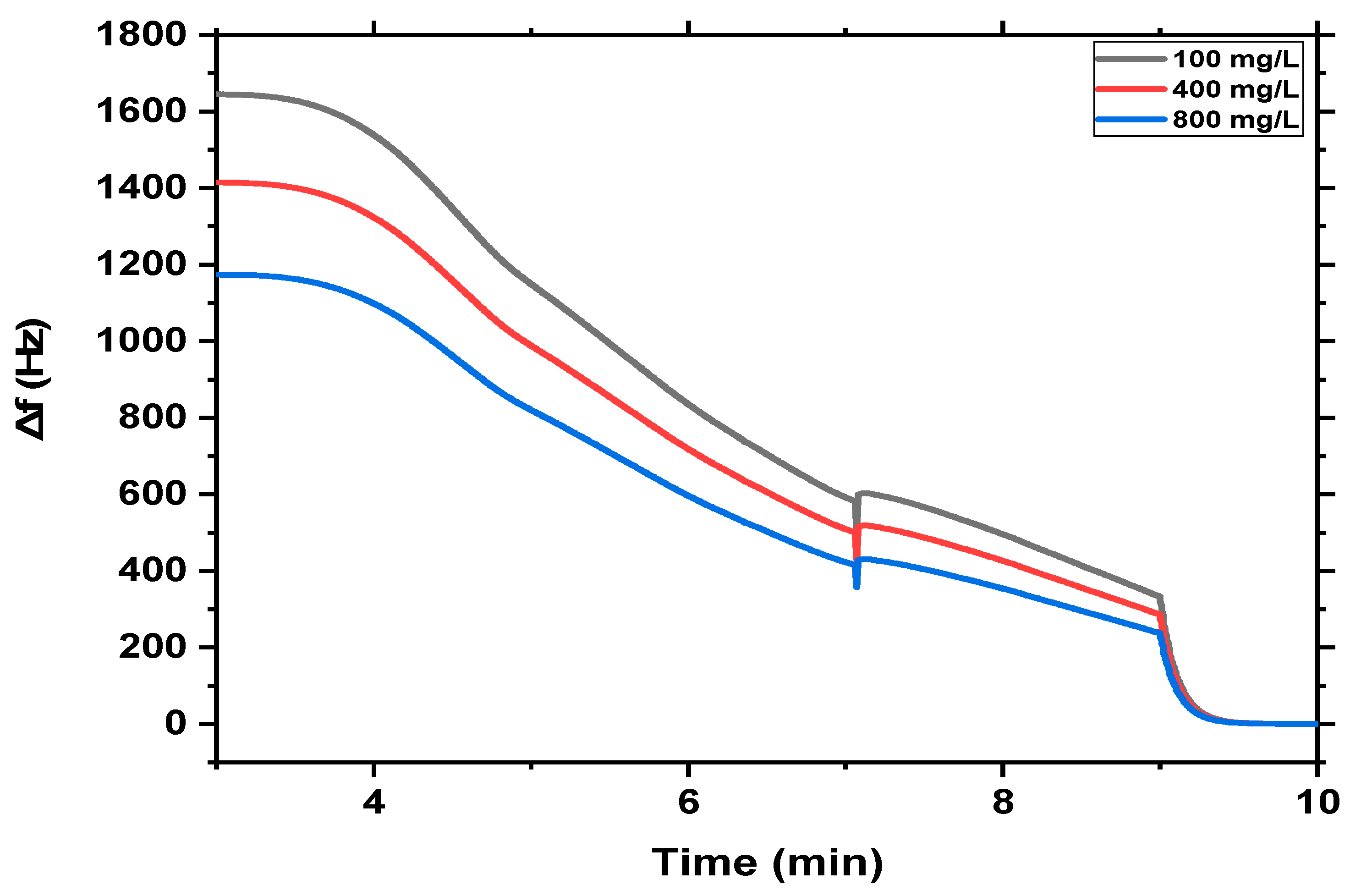
3.2. MB Monitoring Using QCM-Based MgFe2O4 NPs and MgFe2O4@CaAlg NCs Nanosensors
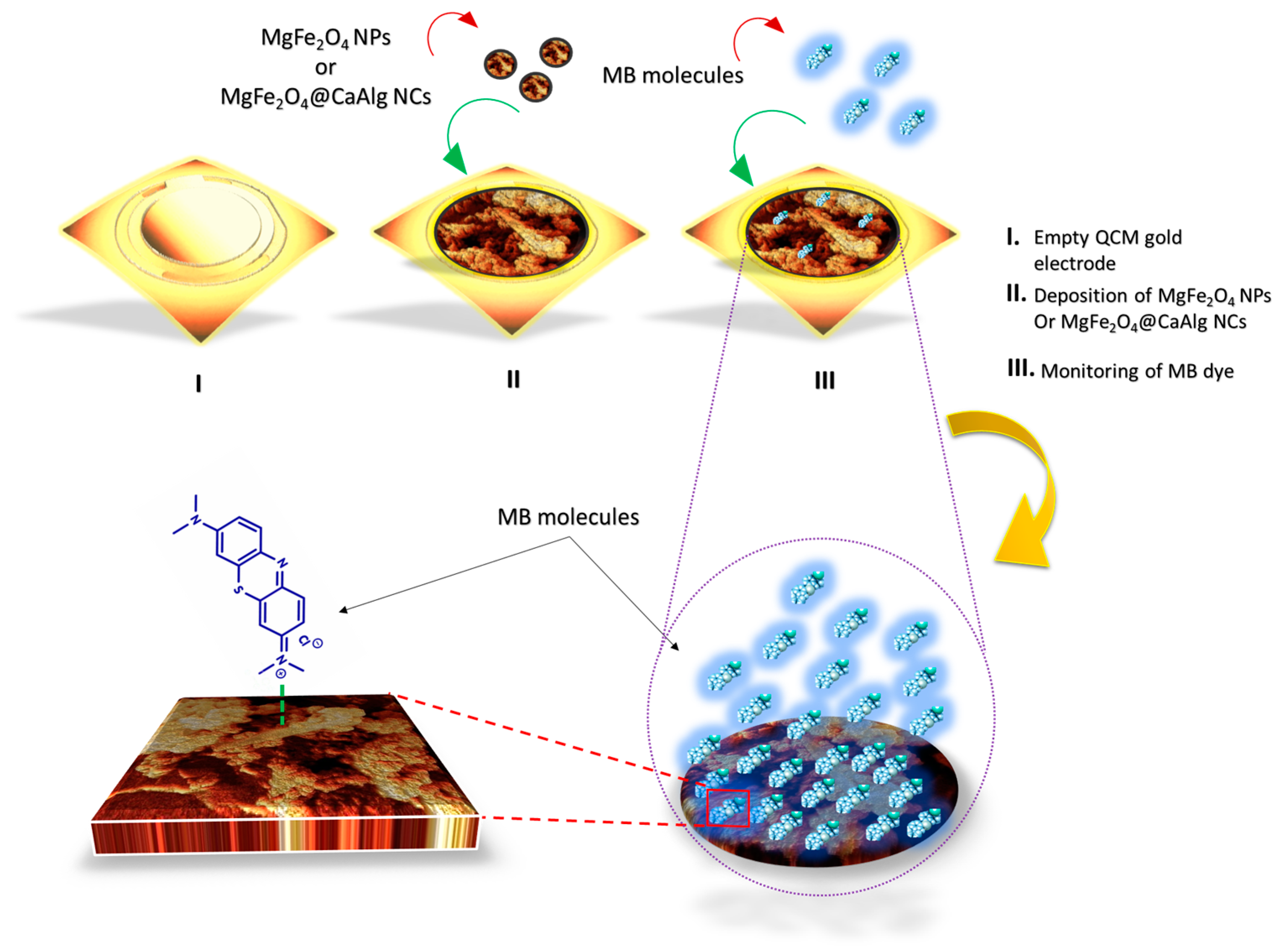
3.3. Proposed Sensing Mechanism of the QCM-Based MgFe2O4 NPs and MgFe2O4@CaAlg NCs Nanosensors
3.4. Comparison of the QCM-Based MgFe2O4 NPs and MgFe2O4@CaAlg NCs Nanosensors Method with Other Methods in the Literature
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elshayb, O.M.; Nada, A.M.; Sadek, A.H.; Ismail, S.H.; Shami, A.; Alharbi, B.M.; Alhammad, B.A.; Seleiman, M.F. The Integrative Effects of Biochar and ZnO Nanoparticles for Enhancing Rice Productivity and Water Use Efficiency under Irrigation Deficit Conditions. Plants 2022, 11, 1416. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamied, M.; Hassan, R.R.A.; Salem, M.Z.; Ashraf, T.; Mohammed, M.; Mahmoud, N.; El-din, Y.S.; Ismail, S.H. Potential effects of nano-cellulose and nano-silica/polyvinyl alcohol nanocomposites in the strengthening of dyed paper manuscripts with madder: An experimental study. Sci. Rep. 2022, 12, 19617. [Google Scholar] [CrossRef] [PubMed]
- Hassan, G.K.; Mahmoud, W.H.; Al-sayed, A.; Ismail, S.H.; El-Sherif, A.A.; Abd El Wahab, S. Multi-functional of TiO2@Ag core–shell nanostructure to prevent hydrogen sulfide formation during anaerobic digestion of sewage sludge with boosting of bio-CH4 production. Fuel 2023, 333, 126608. [Google Scholar] [CrossRef]
- Katowah, D.F.; Alsulami, Q.A.; Alam, M.; Ismail, S.H.; Asiri, A.M.; Mohamed, G.G.; Rahman, M.M.; Hussein, M.A. The Performance of Various SWCNT Loading into CuO–PMMA Nanocomposites Towards the Detection of Mn2+ Ions. J. Inorg. Organomet. Polym. Mater. 2020, 30, 5024–5041. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, A.; Ismail, S.H.; Ebnalwaled, A.; Mohamed, G.G. Characterization of superparamagnetic/monodisperse PEG-coated magnetite nanoparticles Sonochemically prepared from the hematite ore for Cd(II) removal from aqueous solutions. J. Inorg. Organomet. Polym. Mater. 2021, 31, 397–414. [Google Scholar] [CrossRef]
- Elawwad, A.; Ragab, M.; Hamdy, A.; Husein, D.Z. Enhancing the performance of microbial desalination cells using δMnO2/graphene nanocomposite as a cathode catalyst. J. Water Reuse Desalination 2020, 10, 214–226. [Google Scholar] [CrossRef]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic nanoparticles: From design and synthesis to real world applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef]
- Hamdy, A. Experimental study of the relationship between dissolved iron, turbidity, and removal of Cu(II) ion from aqueous solutions using zero-valent iron nanoparticles. Arab. J. Sci. Eng. 2021, 46, 5543–5565. [Google Scholar] [CrossRef]
- Hamdy, A.; Mostafa, M.K.; Nasr, M. Techno-economic estimation of electroplating wastewater treatment using zero-valent iron nanoparticles: Batch optimization, continuous feed, and scaling up studies. Environ. Sci. Pollut. Res. 2019, 26, 25372–25385. [Google Scholar] [CrossRef]
- Holec, P.; Plocek, J.; Nižňanský, D.; Poltierová Vejpravová, J. Preparation of MgFe2O4 nanoparticles by microemulsion method and their characterization. J. Sol-Gel Sci. Technol. 2009, 51, 301–305. [Google Scholar] [CrossRef]
- Hossain, M.; Jamil, A.; Hossain, M.S.; Ahmed, S.; Das, H.; Rashid, R.; Hakim, M.; Khan, M. Investigation on structure, thermodynamic and multifunctional properties of Ni–Zn–Co ferrite for Gd3+ substitution. RSC Adv. 2022, 12, 4656–4671. [Google Scholar] [CrossRef] [PubMed]
- Al-Qasmi, N.; Al-Gethami, W.; Alhashmialameer, D.; Ismail, S.H.; Sadek, A.H. Evaluation of Green-Synthesized Cuprospinel Nanoparticles as a Nanosensor for Detection of Low-Concentration Cd(II) Ion in the Aqueous Solutions by the Quartz Crystal Microbalance Method. Materials 2022, 15, 6240. [Google Scholar] [CrossRef] [PubMed]
- Maensiri, S.; Sangmanee, M.; Wiengmoon, A. Magnesium ferrite (MgFe2O4) nanostructures fabricated by electrospinning. Nanoscale Res. Lett. 2009, 4, 221–228. [Google Scholar] [CrossRef]
- Sasaki, T.; Ohara, S.; Naka, T.; Vejpravova, J.; Sechovsky, V.; Umetsu, M.; Takami, S.; Jeyadevan, B.; Adschiri, T. Continuous synthesis of fine MgFe2O4 nanoparticles by supercritical hydrothermal reaction. J. Supercrit. Fluids 2010, 53, 92–94. [Google Scholar] [CrossRef]
- Shahjuee, T.; Masoudpanah, S.; Mirkazemi, S. Thermal decomposition synthesis of MgFe2O4 nanoparticles for magnetic hyperthermia. J. Supercond. Nov. Magn. 2019, 32, 1347–1352. [Google Scholar] [CrossRef]
- Sadek, A.H.; Asker, M.S.; Abdelhamid, S.A. Bacteriostatic impact of nanoscale zero-valent iron against pathogenic bacteria in the municipal wastewater. Biologia 2021, 76, 2785–2809. [Google Scholar] [CrossRef]
- Materón, E.M.; Miyazaki, C.M.; Carr, O.; Joshi, N.; Picciani, P.H.; Dalmaschio, C.J.; Davis, F.; Shimizu, F.M. Magnetic nanoparticles in biomedical applications: A review. Appl. Surf. Sci. Adv. 2021, 6, 100163. [Google Scholar] [CrossRef]
- Abdelmigeed, M.O.; Sadek, A.H.; Ahmed, T.S. Novel easily separable core–shell Fe3O4/PVP/ZIF-8 nanostructure adsorbent: Optimization of phosphorus removal from Fosfomycin pharmaceutical wastewater. RSC Adv. 2022, 12, 12823–12842. [Google Scholar] [CrossRef]
- Ebnalwaled, A.; Sadek, A.H.; Ismail, S.H.; Mohamed, G.G. Structural, optical, dielectric, and surface properties of polyimide hybrid nanocomposites films embedded mesoporous silica nanoparticles synthesized from rice husk ash for optoelectronic applications. Opt. Quantum Electron. 2022, 54, 690. [Google Scholar] [CrossRef]
- Nowak-Jary, J.; Machnicka, B. Pharmacokinetics of magnetic iron oxide nanoparticles for medical applications. J. Nanobiotechnology 2022, 20, 305. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.H.; Hamdy, A.; Ismail, T.A.; Mahboub, H.H.; Mahmoud, W.H.; Daoush, W.M. Synthesis and characterization of antibacterial carbopol/ZnO hybrid nanoparticles gel. Crystals 2021, 11, 1092. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, C.-G.; Lee, E.Y. Alginate lyase: Structure, property, and application. Biotechnol. Bioprocess Eng. 2011, 16, 843–851. [Google Scholar] [CrossRef]
- Bi, D.; Yang, X.; Yao, L.; Hu, Z.; Li, H.; Xu, X.; Lu, J. Potential Food and Nutraceutical Applications of Alginate: A Review. Mar. Drugs 2022, 20, 564. [Google Scholar] [CrossRef] [PubMed]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, H.; Wu, H.; Chen, R.; Guo, S. Preparation of alginate hydrogels through solution extrusion and the release behavior of different drugs. J. Biomater. Sci. Polym. Ed. 2016, 27, 1808–1823. [Google Scholar] [CrossRef]
- Qin, Y.; Jiang, J.; Zhao, L.; Zhang, J.; Wang, F. Applications of Alginate as a Functional Food Ingredient. In Biopolymers for Food Design; Elsevier: Amsterdam, The Netherlands, 2018; pp. 409–429. [Google Scholar]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 1–22. [Google Scholar]
- Darwish, M.S.; Kim, H.; Lee, H.; Ryu, C.; Lee, J.Y.; Yoon, J. Synthesis of magnetic ferrite nanoparticles with high hyperthermia performance via a controlled co-precipitation method. Nanomaterials 2019, 9, 1176. [Google Scholar] [CrossRef]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green synthesis of metallic nanoparticles via biological entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef]
- Farag, R.; El-Shafei, M.M.; Mahmoud, A.S.; Mostafa, M.; Peters, R. Green synthesis of nano iron carbide: Preparation, characterization and application for removal of phosphate from aqueous solutions. In Proceedings of the 2018 AIChE Annual Meeting, Pittsburgh, PA, USA, 27–30 October 2018. [Google Scholar]
- Irshad, S.; Siddiqui, B.u.; Rehman, A.; Farooq, R.K.; Ahmed, N. Recent trends and development in targeted delivery of therapeutics through enzyme responsive intelligent nanoplatform. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 403–413. [Google Scholar] [CrossRef]
- Bao, Y.; He, J.; Song, K.; Guo, J.; Zhou, X.; Liu, S. Plant-extract-mediated synthesis of metal nanoparticles. J. Chem. 2021, 2021, 6562687. [Google Scholar] [CrossRef]
- Bloch, K.; Pardesi, K.; Satriano, C.; Ghosh, S. Bacteriogenic platinum nanoparticles for application in nanomedicine. Front. Chem. 2021, 9, 624344. [Google Scholar] [CrossRef] [PubMed]
- Al-Gethami, W.; Alhashmialameer, D.; Al-Qasmi, N.; Ismail, S.H.; Sadek, A.H. Design of a Novel Nanosensors Based on Green Synthesized CoFe2O4/Ca-Alginate Nanocomposite-Coated QCM for Rapid Detection of Pb(II) Ions. Nanomaterials 2022, 12, 3620. [Google Scholar] [CrossRef] [PubMed]
- Marx, K.A. Quartz crystal microbalance: A useful tool for studying thin polymer films and complex biomolecular systems at the solution−surface interface. Biomacromolecules 2003, 4, 1099–1120. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, A.; Mustafa, G.; Dickert, F.L. Label-free bioanalyte detection from nanometer to micrometer dimensions—Molecular imprinting and QCMs. Biosensors 2018, 8, 52. [Google Scholar] [CrossRef]
- Hu, Y.; Xing, H.; Li, G.; Wu, M. Magnetic Imprinted Polymer-Based Quartz Crystal Microbalance Sensor for Sensitive Label-Free Detection of Methylene Blue in Groundwater. Sensors 2020, 20, 5506. [Google Scholar] [CrossRef]
- Na Songkhla, S.; Nakamoto, T. Overview of quartz crystal microbalance behavior analysis and measurement. Chemosensors 2021, 9, 350. [Google Scholar] [CrossRef]
- Chandanshive, V.; Kadam, S.; Rane, N.; Jeon, B.-H.; Jadhav, J.; Govindwar, S. In situ textile wastewater treatment in high rate transpiration system furrows planted with aquatic macrophytes and floating phytobeds. Chemosphere 2020, 252, 126513. [Google Scholar] [CrossRef]
- Alhadhrami, A.; Mohamed, G.G.; Sadek, A.H.; Ismail, S.H.; Ebnalwaled, A.A.; Almalki, A.S.A. Behavior of silica nanoparticles synthesized from rice husk ash by the sol–gel method as a photocatalytic and antibacterial agent. Materials 2022, 15, 8211. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, A.; Mostafa, M.K.; Nasr, M. Zero-valent iron nanoparticles for methylene blue removal from aqueous solutions and textile wastewater treatment, with cost estimation. Water Sci. Technol. 2018, 78, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Din, M.I.; Khalid, R.; Najeeb, J.; Hussain, Z. Fundamentals and photocatalysis of methylene blue dye using various nanocatalytic assemblies-a critical review. J. Clean. Prod. 2021, 298, 126567. [Google Scholar] [CrossRef]
- Hamdy, A.; Mostafa, M.; Nasr, M. Regression analysis and artificial intelligence for removal of methylene blue from aqueous solutions using nanoscale zero-valent iron. Int. J. Environ. Sci. Technol. 2019, 16, 357–372. [Google Scholar] [CrossRef]
- Jung, K.-W.; Lee, S.; Lee, Y.J. Synthesis of novel magnesium ferrite (MgFe2O4)/biochar magnetic composites and its adsorption behavior for phosphate in aqueous solutions. Bioresour. Technol. 2017, 245, 751–759. [Google Scholar] [CrossRef]
- Al-Qasmi, N. Facial eco-friendly synthesis of copper oxide nanoparticles using chia seeds extract and evaluation of its electrochemical activity. Processes 2021, 9, 2027. [Google Scholar] [CrossRef]
- Isawi, H. Using zeolite/polyvinyl alcohol/sodium alginate nanocomposite beads for removal of some heavy metals from wastewater. Arab. J. Chem. 2020, 13, 5691–5716. [Google Scholar] [CrossRef]
- Kumar, S.; Sreenivas, K. Effects of dl-alanine fuel and annealing on auto-combustion derived MgFe2O4 powder with low carbon content and improved magnetic properties. Appl. Phys. A 2021, 127, 165. [Google Scholar] [CrossRef]
- Doan, M.Q.; Anh, N.H.; Quang, N.X.; Dinh, N.X.; Tri, D.Q.; Huy, T.Q.; Le, A.-T. Ultrasensitive Detection of Methylene Blue Using an Electrochemically Synthesized SERS Sensor Based on Gold and Silver Nanoparticles: Roles of Composition and Purity on Sensing Performance and Reliability. J. Electron. Mater. 2022, 51, 150–162. [Google Scholar] [CrossRef]
- Sadrolhosseini, A.R.; Ghasemi, E.; Pirkarimi, A.; Hamidi, S.M.; Ghahrizjani, R.T. Highly sensitive surface plasmon resonance sensor for detection of Methylene Blue and Methylene Orange dyes using NiCo-Layered Double Hydroxide. Opt. Commun. 2023, 529, 129057. [Google Scholar] [CrossRef]
- Pradel, J.S.; Tong, W.G. Determination of malachite green, crystal violet, brilliant green and methylene blue by micro-cloud-point extraction and nonlinear laser wave-mixing detection interfaced to micellar capillary electrophoresis. Anal. Methods 2017, 9, 6411–6419. [Google Scholar] [CrossRef]
- Borwitzky, H.; Haefeli, W.E.; Burhenne, J. Analysis of methylene blue in human urine by capillary electrophoresis. J. Chromatogr. B 2005, 826, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Roybal, J.E.; Munns, R.K.; Hurlbut, J.A.; Shimoda, W. High-performance liquid chromatography of Gentian violet, its demethylated metabolites, leucogentian violet and methylene blue with electrochemical detection. J. Chromatogr. A 1989, 467, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Khan, M.A.; Alothman, Z.A.; Alsohaimi, I.H.; Naushad, M.; Al-Shaalan, N.H. Quantitative determination of methylene blue in environmental samples by solid-phase extraction and ultra-performance liquid chromatography-tandem mass spectrometry: A green approach. RSC Adv. 2014, 4, 34037–34044. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, X.; Wei, C.; Qu, Y.; Xiao, X.; Cheng, H. One-step synthesis of red-emitting carbon dots via a solvothermal method and its application in the detection of methylene blue. RSC Adv. 2019, 9, 29533–29540. [Google Scholar] [CrossRef]
- Hayat, M.; Shah, A.; Nisar, J.; Shah, I.; Haleem, A.; Ashiq, M.N. A Novel Electrochemical Sensing Platform for the Sensitive Detection and Degradation Monitoring of Methylene Blue. Catalysts 2022, 12, 306. [Google Scholar] [CrossRef]
- Nekouei, F.; Kargarzadeh, H.; Nekouei, S.; Keshtpour, F.; Makhlouf, A.S.H. Efficient method for determination of methylene blue dye in water samples based on a combined dispersive solid phase and cloud point extraction using Cu(OH)2 nanoflakes: Central composite design optimization. Anal. Bioanal. Chem. 2017, 409, 1079–1092. [Google Scholar] [CrossRef]
- Wang, R.; Guo, M.; Hu, Y.; Zhou, J.; Wu, R.; Yang, X. A molecularly imprinted fluorescence sensor based on the ZnO quantum dot core–shell structure for high selectivity and photolysis function of methylene blue. ACS Omega 2020, 5, 20664–20673. [Google Scholar] [CrossRef]
- Vu, X.H.; Dien, N.D.; Pham, T.T.H.; Trang, T.T.; Ca, N.; Tho, P.; Vinh, N.D.; Van Do, P. The sensitive detection of methylene blue using silver nanodecahedra prepared through a photochemical route. RSC Adv. 2020, 10, 38974–38988. [Google Scholar] [CrossRef]
- Yang, S.; Zeng, T.; Li, Y.; Liu, J.; Chen, Q.; Zhou, J.; Ye, Y.; Tang, B. Preparation of graphene oxide decorated Fe3O4@SiO2 nanocomposites with superior adsorption capacity and SERS detection for organic dyes. J. Nanomater. 2015, 2015, 337. [Google Scholar] [CrossRef]




| Method | LOD | Reference |
|---|---|---|
| Electrochemically synthesized SERS sensor based on gold and silver nanoparticles | 9 × 10−11 M for e-AuNPs 5 × 10−12 M for e-AgNPs | [51] |
| SPR sensor using NiCo-Layered Double Hydroxide (NiCo-LDH) | 0.005 mg/L | [52] |
| Micro-cloud point extraction and nonlinear laser wave-mixing detection interfaced with micellar capillary electrophoresis | 81.6 pg/mL | [53] |
| Capillary electrophoresis | 1.0 μg/mL | [54] |
| High-performance liquid chromatography | 3 pmol | [55] |
| Solid-phase extraction (SPE) and ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) | 0.1 ng/mL | [56] |
| Fluorescence red-emitting CDs (CD-tetra) | 10 nM | [57] |
| Electrochemical sensor consisting of amino-group-functionalized, multi-walled carbon nanotubes (NH2-fMWCNTs) immobilized on a glassy carbon electrode (GCE) | 0.21 nM | [58] |
| UV–Vis spectrophotometry | 0.65 μg/L | [59] |
| QCM-based MgFe2O4 NPs and MgFe2O4@CaAlg NCs nanosensors | 400 mg/L (1.25× 10−3 M) | The current work |
| Sensor | MB Concentration | Sensor Material Concentration | Operating Temperature (°C) | Response Time | Reference |
|---|---|---|---|---|---|
| Molecularly imprinted polymer-based QCM sensor (MIPs) | 1–150 μg/L | 1.5 mg | RT * | 6000 s | [38] |
| Surface plasmon resonance sensor using NiCo-layered double hydroxide (SPR-glass/Au/NiCo-LDH) | 0.005 mg/L | 27.6 nm thick layer | RT | 268 s | [52] |
| Electrochemical sensor consisting of amino-group-functionalized, multi-walled carbon nanotubes (NH2-fMWCNTs) immobilized on a glassy carbon electrode (GCE) | 10 µM | 0.89 mg/mL | RT | 30 min | [58] |
| Molecularly imprinted fluorescence sensor based on the ZnO quantum dot core−shell structure (ZCF@MB-MIP) | 0 to 100 μmol/L | 37 mg/L | RT | 15 min | [60] |
| Silver nanodecahedra (AgND) | 10−8 to 10−4 M | 0.5 mM | RT | 15–50 min | [61] |
| Fe3O4@SiO2-GO microspheres based on SERS | 1 × 10−5, to 1× 10−7 M | 5 mg | RT | 40 min | [62] |
| QCM-based MgFe2O4 NPs and MgFe2O4@CaAlg NCs nanosensors | 400 mg/L (1.25× 10−3 M) | 5 g/L | RT | 5 min | The current work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Gethami, W.; Al-Qasmi, N.; Ismail, S.H.; Sadek, A.H. QCM-Based MgFe2O4@CaAlg Nanocomposite as a Fast Response Nanosensor for Real-Time Detection of Methylene Blue Dye. Nanomaterials 2023, 13, 97. https://doi.org/10.3390/nano13010097
Al-Gethami W, Al-Qasmi N, Ismail SH, Sadek AH. QCM-Based MgFe2O4@CaAlg Nanocomposite as a Fast Response Nanosensor for Real-Time Detection of Methylene Blue Dye. Nanomaterials. 2023; 13(1):97. https://doi.org/10.3390/nano13010097
Chicago/Turabian StyleAl-Gethami, Wafa, Noha Al-Qasmi, Sameh H. Ismail, and Ahmed H. Sadek. 2023. "QCM-Based MgFe2O4@CaAlg Nanocomposite as a Fast Response Nanosensor for Real-Time Detection of Methylene Blue Dye" Nanomaterials 13, no. 1: 97. https://doi.org/10.3390/nano13010097
APA StyleAl-Gethami, W., Al-Qasmi, N., Ismail, S. H., & Sadek, A. H. (2023). QCM-Based MgFe2O4@CaAlg Nanocomposite as a Fast Response Nanosensor for Real-Time Detection of Methylene Blue Dye. Nanomaterials, 13(1), 97. https://doi.org/10.3390/nano13010097








