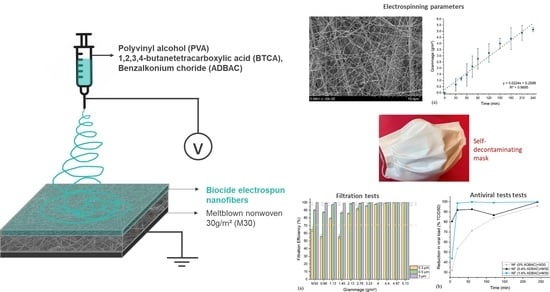
Electrospun Filtering Membrane Designed as Component of Self-Decontaminating Protective Masks
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning
2.3. Characterization Techniques
2.3.1. Electrospun Solution Viscosities
2.3.2. Scanning Electron Microscopy
2.3.3. Thermal Analysis
2.3.4. Air Filtration Performance
2.3.5. Antibacterial Tests
2.3.6. Antiviral Tests
3. Results and Discussion
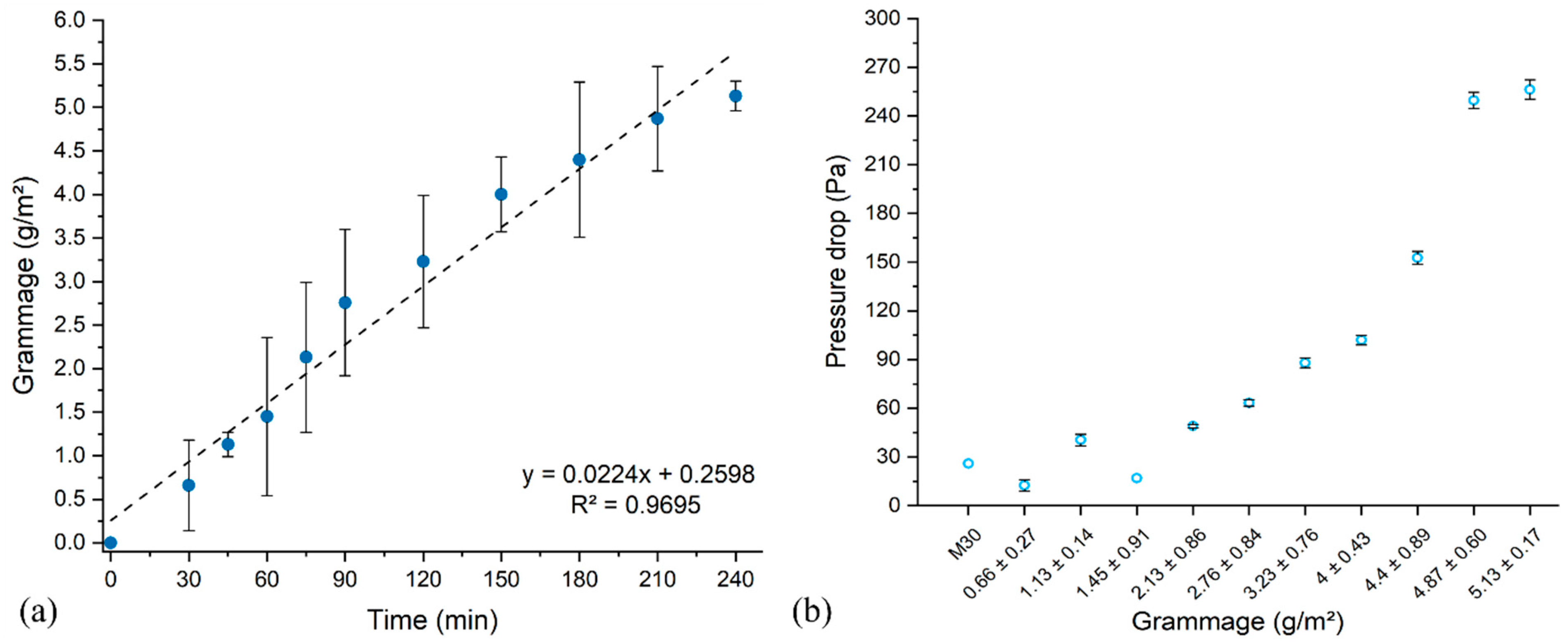
3.1. Electrospinning Parameters
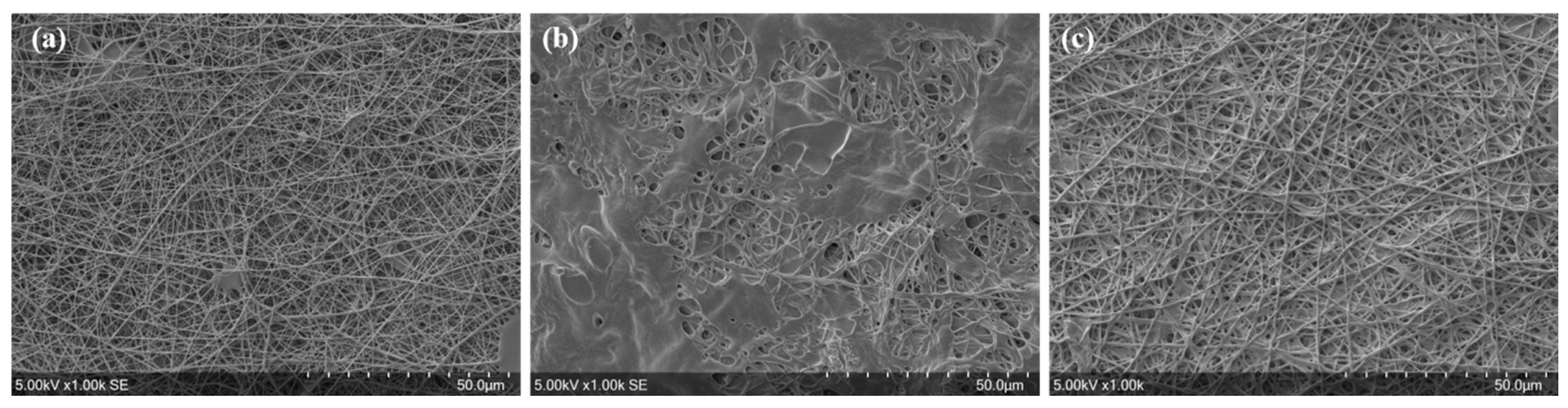
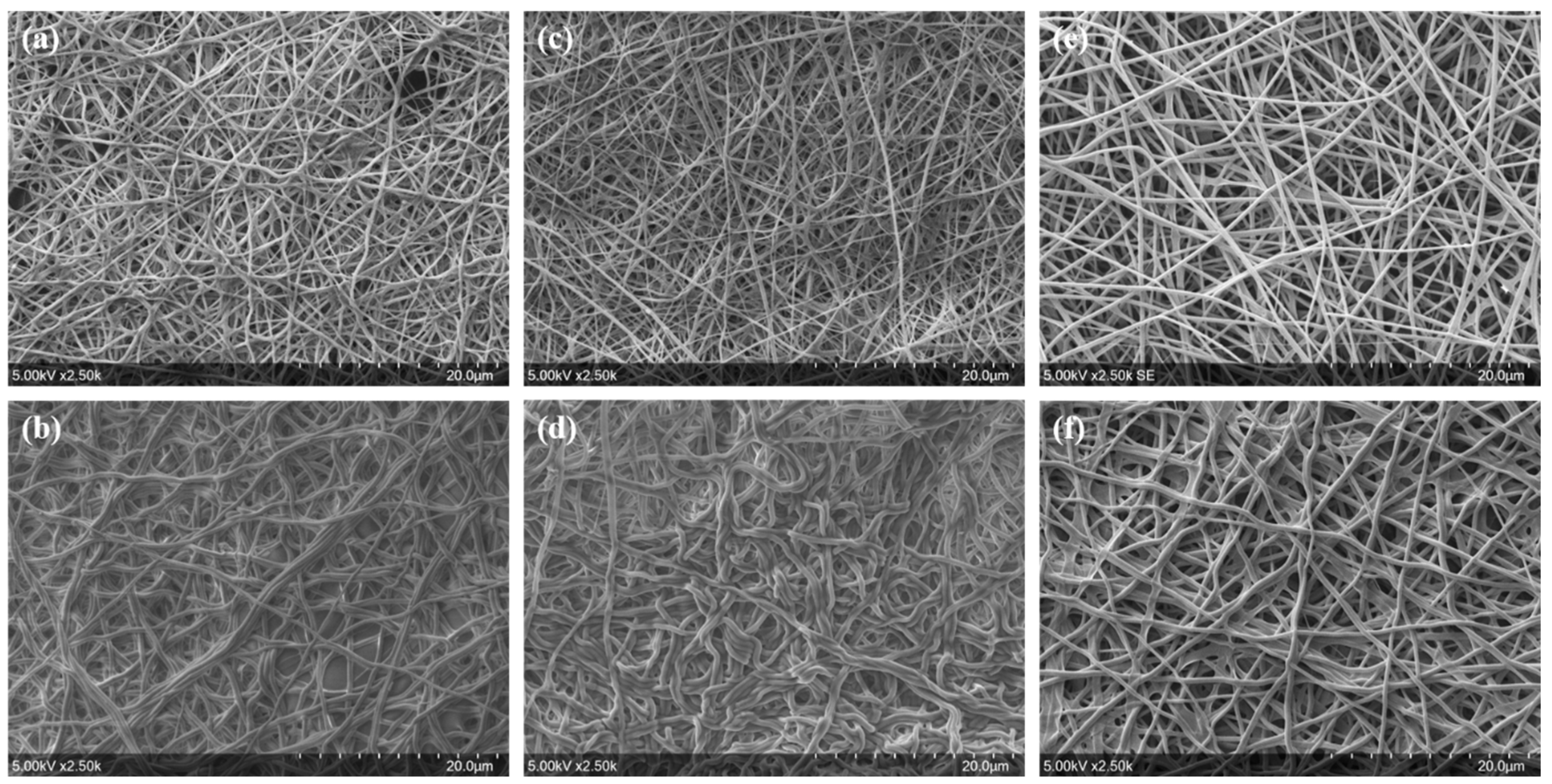
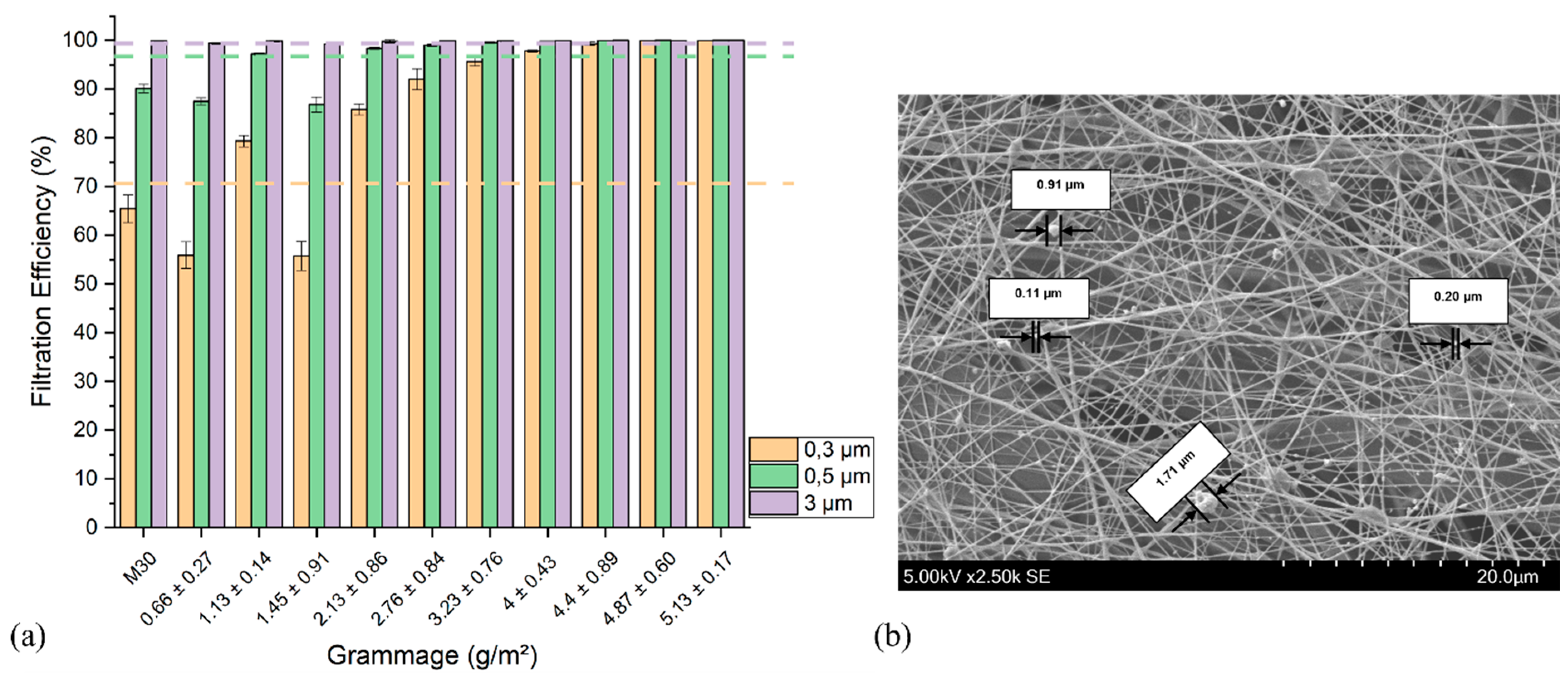
3.2. Filtration Performance of the Meltblown-Supported Electrospun Fibers
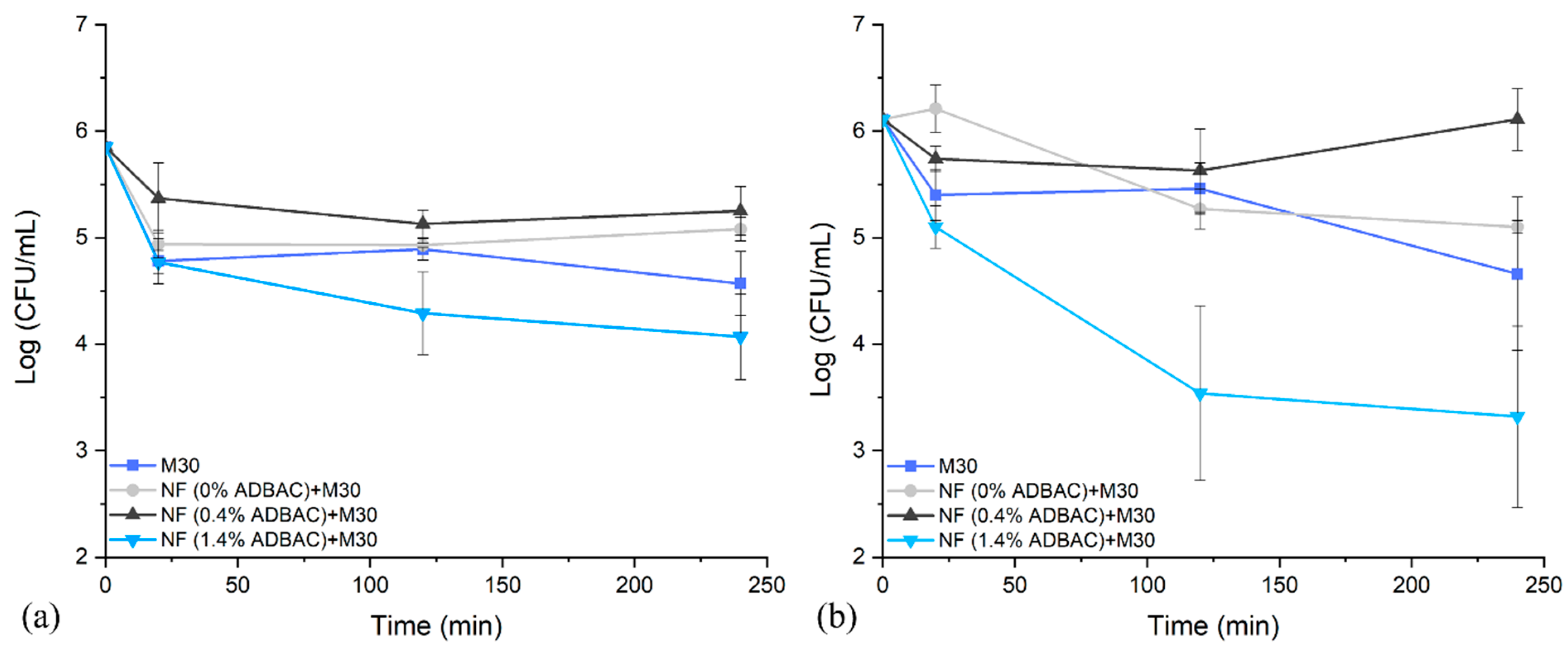
3.3. Antibacterial Activity
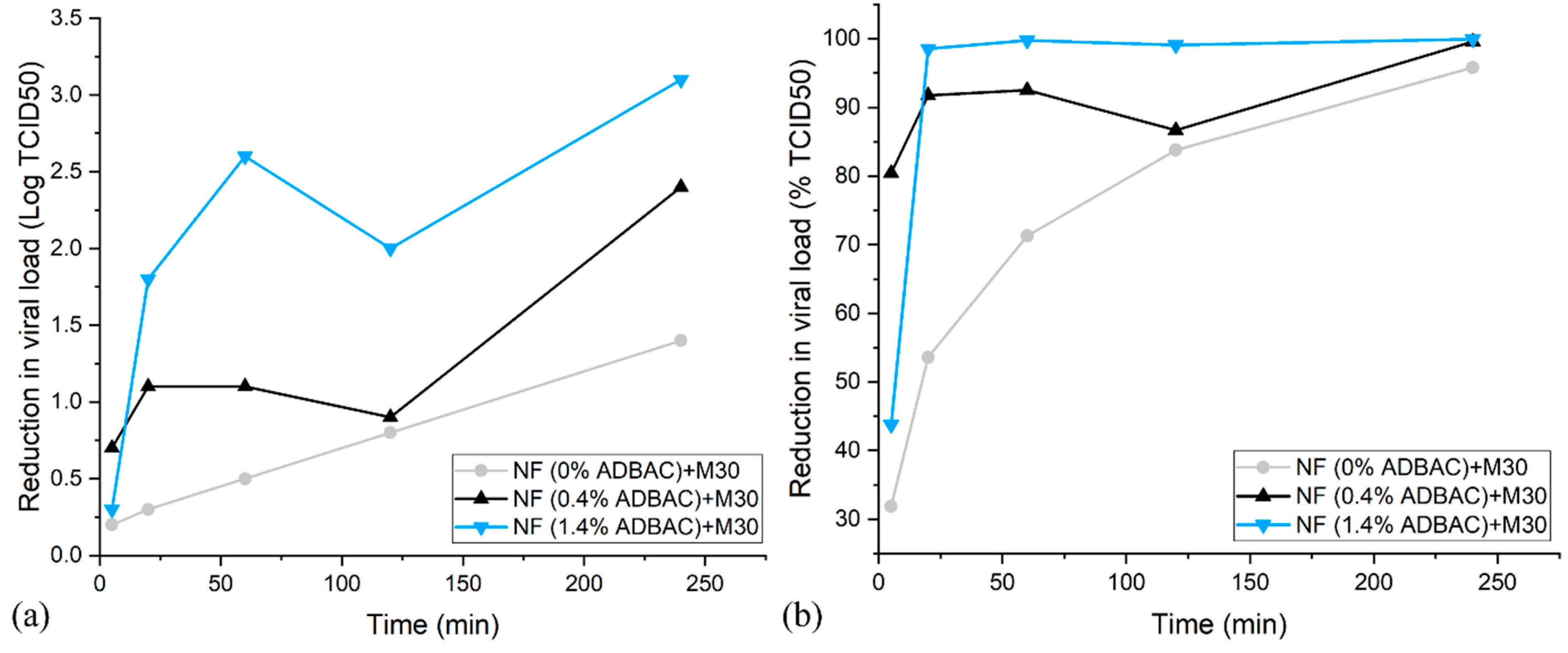
3.4. Antiviral Efficacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xue, T.; Zhu, T.; Zheng, Y.; Zhang, Q. Declines in mental health associated with air pollution and temperature variability in China. Nat. Commun. 2019, 10, 2165. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Ibrahim, M.N.M. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Aydin, O.; Emon, B.; Cheng, S.; Hong, L.; Chamorro, L.P.; Saif, M.T.A. Performance of fabrics for home-made masks against the spread of COVID-19 through droplets: A quantitative mechanistic study. Extreme Mech. Lett. 2020, 40, 100924. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.H.; Cheng, W.; Goh, S.S.; Kong, J.; Li, B.; Lim, J.Y.C.; Mao, L.; Wang, S.; Xue, K.; Yang, L.; et al. Face Masks in the New COVID-19 Normal: Materials, Testing, and Perspectives. Research 2020, 2020, 7286735. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus Cases. Available online: https://www.worldometers.info/coronavirus/ (accessed on 8 December 2021).
- Chiang, C.-H.; Chiang, C.-H.; Chiang, C.-H. Letter to Editor—Can Universal Masking Help with Our Recovery from the COVID-19 Pandemic? Int. J. Surg. 2020, 79, 125–126. [Google Scholar] [CrossRef]
- Vijayakumar, V. Personal Protection Prior to Preoperative Assessment—Little more an anaesthesiologist can do to prevent SARS-CoV-2 transmission and COVID-19 infection. Ain-Shams J. Anesthesiol. 2020, 12, 13. [Google Scholar] [CrossRef]
- Zhang, X.; Ru, Z.; Sun, Y.; Zhang, M.; Wang, J.; Ge, M.; Liu, H.; Wu, S.; Cao, C.; Ren, X.; et al. Recent advances in applications for air pollutants purification and perspectives of electrospun nanofibers. J. Clean. Prod. 2022, 378, 134567. [Google Scholar] [CrossRef]
- Shao, Z.; Chen, H.; Wang, Q.; Kang, G.; Wang, X.; Li, W.; Liu, Y.; Zheng, G. High-performance multifunctional electrospun fibrous air filter for personal protection: A review. Sep. Purif. Technol. 2022, 302, 122175. [Google Scholar] [CrossRef]
- Matulevicius, J.; Kliucininkas, L.; Martuzevicius, D.; Krugly, E.; Tichonovas, M.; Baltrusaitis, J. Design and Characterization of Electrospun Polyamide Nanofiber Media for Air Filtration Applications. J. Nanomater. 2014, 2014, 14. [Google Scholar] [CrossRef]
- Strain, I.N.; Wu, Q.; Pourrahimi, A.M.; Hedenqvist, M.S.; Olsson, R.T.; Andersson, R.L. Electrospinning of recycled PET to generate tough mesomorphic fibre membranes for smoke filtration. J. Mater. Chem. A 2014, 3, 1632–1640. [Google Scholar] [CrossRef]
- Yun, K.M.; Hogan, C.J., Jr.; Matsubayashi, Y.; Kawabe, M.; Iskandar, F.; Okuyama, K. Nanoparticle filtration by electrospun polymer fibers. Chem. Eng. Sci. 2007, 62, 4751–4759. [Google Scholar] [CrossRef]
- Zhang, J.; Gong, S.; Wang, C.; Jeong, D.-Y.; Wang, Z.L.; Ren, K. Biodegradable Electrospun Poly(lactic acid) Nanofibers for Effective PM 2.5 Removal. Macromol. Mater. Eng. 2019, 304, 1900259. [Google Scholar] [CrossRef]
- Qin, X.-H.; Wang, S.-Y. Electrospun nanofibers from crosslinked poly(vinyl alcohol) and its filtration efficiency. J. Appl. Polym. Sci. 2008, 109, 951–956. [Google Scholar] [CrossRef]
- Li, J.; Gao, F.; Liu, L.Q.; Zhang, Z. Needleless electro-spun nanofibers used for filtration of small particles. Express Polym. Lett. 2013, 7, 683–689. [Google Scholar] [CrossRef]
- Rampichova, M.; Chvojka, J.; Jencova, V.; Kubikova, T.; Tonar, Z.; Erben, J.; Buzgo, M.; Dankova, J.; Litvinec, A.; Vocetkova, K.; et al. The combination of nanofibrous and microfibrous materials for enhancement of cell infiltration and in vivo bone tissue formation. Biomed. Mater. 2017, 13, 025004. [Google Scholar] [CrossRef]
- Gu, J.; Yuan, L.; Zhang, Z.; Yang, X.; Luo, J.; Gui, Z.; Chen, S. Non-leaching bactericidal cotton fabrics with well-preserved physical properties, no skin irritation and no toxicity. Cellulose 2018, 25, 5415–5426. [Google Scholar] [CrossRef]
- Zhu, M.; Han, J.; Wang, F.; Shao, W.; Xiong, R.; Zhang, Q.; Pan, H.; Yang, Y.; Samal, S.K.; Zhang, F.; et al. Electrospun Nanofibers Membranes for Effective Air Filtration. Macromol. Mater. Eng. 2016, 302, 1600353. [Google Scholar] [CrossRef]
- Cheng, X.; Zhao, L.; Zhang, Z.; Deng, C.; Li, C.; Du, Y.; Shi, J.; Zhu, M. Highly efficient, low-resistant, well-ordered PAN nanofiber membranes for air filtration. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 655, 130302. [Google Scholar] [CrossRef]
- Kadam, V.; Kyratzis, I.L.; Truong, Y.B.; Schutz, J.; Wang, L.; Padhye, R. Electrospun bilayer nanomembrane with hierarchical placement of bead-on-string and fibers for low resistance respiratory air filtration. Sep. Purif. Technol. 2019, 224, 247–254. [Google Scholar] [CrossRef]
- Kimmer, D.; Vincent, I.; Sambaer, W.; Zatloukal, M.; Ondráček, J. The effect of combination electrospun and meltblown filtration materials on their filtration efficiency. AIP Conf. Proc. 2015, 1662, 050001. [Google Scholar] [CrossRef]
- Babaahmadi, V.; Amid, H.; Naeimirad, M.; Ramakrishna, S. Biodegradable and multifunctional surgical face masks: A brief review on demands during COVID-19 pandemic, recent developments, and future perspectives. Sci. Total. Environ. 2021, 798, 149233. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Wu, Q.; Qi, Y.; Kärki, T. Electrospun nanofibers with antimicrobial properties. In Electrospun Nanofibers; Afshari, M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 551–569. [Google Scholar]
- Senthil, R.; Sumathi, V.; Tamilselvi, A.; Kavukcu, S.B.; Aruni, A.W. Functionalized electrospun nanofibers for high efficiency removal of particulate matter. Sci. Rep. 2022, 12, 8411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gu, J.; Liu, X.; Wei, D.; Zhou, H.; Xiao, H.; Zhang, Z.; Yu, H.; Chen, S. Bactericidal and antifouling electrospun PVA nanofibers modified with a quaternary ammonium salt and zwitterionic sulfopropylbetaine. Mater. Sci. Eng. C 2020, 111, 110855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, L.; Wang, L.; Nie, J.; Ma, G. Multilayer electrospun nanofibrous membranes with antibacterial property for air filtration. Appl. Surf. Sci. 2020, 515, 145962. [Google Scholar] [CrossRef]
- Lopez, B.L.; Mejia, A.I.; Sierra, L. Biodegradability of PoIy(VinyI Alcohol). Polym. Eng. Sci. 1999, 39, 1346–1352. [Google Scholar] [CrossRef]
- Deng, Y.; Zhu, M.; Lu, T.; Fan, Q.; Ma, W.; Zhang, X.; Chen, L.; Min, H.; Xiong, R.; Huang, C. Hierarchical fiber with granular-convex structure for highly efficient PM2.5 capture. Sep. Purif. Technol. 2023, 304, 122235. [Google Scholar] [CrossRef]
- Jiamjirangkul, P.; Inprasit, T.; Intasanta, V.; Pangon, A. Metal organic framework-integrated chitosan/poly(vinyl alcohol) (PVA) nanofibrous membrane hybrids from green process for selective CO2 capture and filtration. Chem. Eng. Sci. 2020, 221, 115650. [Google Scholar] [CrossRef]
- Li, S.; Zhang, R.; Xie, J.; Sameen, D.E.; Ahmed, S.; Dai, J.; Qin, W.; Li, S.; Liu, Y. Electrospun antibacterial poly(vinyl alcohol)/Ag nanoparticles membrane grafted with 3,3′,4,4′-benzophenone tetracarboxylic acid for efficient air filtration. Appl. Surf. Sci. 2020, 533, 147516. [Google Scholar] [CrossRef]
- Matulevicius, J.; Kliucininkas, L.; Prasauskas, T.; Buivydiene, D.; Martuzevicius, D. The comparative study of aerosol filtration by electrospun polyamide, polyvinyl acetate, polyacrylonitrile and cellulose acetate nanofiber media. J. Aerosol Sci. 2016, 92, 27–37. [Google Scholar] [CrossRef]
- Rynkowska, E.; Fatyeyeva, K.; Marais, S.; Kujawa, J.; Kujawski, W. Chemically and Thermally Crosslinked PVA-Based Membranes: Effect on Swelling and Transport Behavior. Polymers 2019, 11, 1799. [Google Scholar] [CrossRef]
- Afshari, E.; Mazinani, S.; Ranaei-Siadat, S.-O.; Ghomi, H. Surface modification of polyvinyl alcohol/malonic acid nanofibers by gaseous dielectric barrier discharge plasma for glucose oxidase immobilization. Appl. Surf. Sci. 2016, 385, 349–355. [Google Scholar] [CrossRef]
- Shi, J.J.; Yang, E.L. Green Electrospinning and Crosslinking of Polyvinyl Alcohol/Citric Acid. J. Nano Res. 2015, 32, 32–42. [Google Scholar] [CrossRef]
- Çay, A.; Miraftab, M. Properties of electrospun poly(vinyl alcohol) hydrogel nanofibers crosslinked with 1,2,3,4-butanetetracarboxylic acid. J. Appl. Polym. Sci. 2013, 129, 3140–3149. [Google Scholar] [CrossRef]
- Miraftab, M.; Saifullah, A.N.M.; Çay, A. Physical stabilisation of electrospun poly(vinyl alcohol) nanofibres: Comparative study on methanol and heat-based crosslinking. J. Mater. Sci. 2014, 50, 1943–1957. [Google Scholar] [CrossRef]
- Wijanarko, T.A.W.; Kusumaatmaja, A.; Chotimah; Roto; Triyana, K. Effect of heat treatment on morphology and crystallinity of electrospun Poly(vinyl alcohol) nanofibers. AIP Conf. Proc. 2016, 1755, 150010. [Google Scholar] [CrossRef]
- Hong, K.H.; Park, J.L.; Sul, I.H.; Youk, J.H.; Kang, T.J. Preparation of antimicrobial poly(vinyl alcohol) nanofibers containing silver nanoparticles. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 2468–2474. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, J.H. Preparation and properties of antibacterial Poly(vinyl alcohol) nanofibers by nanoparticles. Fibers Polym. 2011, 12, 602–609. [Google Scholar] [CrossRef]
- Huang, C.-L.; Lee, K.-M.; Liu, Z.-X.; Lai, R.-Y.; Chen, C.-K.; Chen, W.-C.; Hsu, J.-F. Antimicrobial Activity of Electrospun Polyvinyl Alcohol Nanofibers Filled with Poly[2-(tert-butylaminoethyl) Methacrylate]-Grafted Graphene Oxide Nanosheets. Polymers 2020, 12, 1449. [Google Scholar] [CrossRef]
- Hauser, E.D.W.; Cutter, L.W.W. Cationic Detergents as Antiseptics. Am. J. Surg. 1944, 64, 352–358. [Google Scholar] [CrossRef]
- Kang, J.-H.; Park, J.-B.; Bin Song, K. Inhibitory activities of quaternary ammonium surfactants against Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes inoculated on spinach leaves. LWT 2018, 102, 284–290. [Google Scholar] [CrossRef]
- Nicosia, A.; Gieparda, W.; Foksowicz-Flaczyk, J.; Walentowska, J.; Wesołek, D.; Vazquez, B.; Prodi, F.; Belosi, F. Air filtration and antimicrobial capabilities of electrospun PLA/PHB containing ionic liquid. Sep. Purif. Technol. 2015, 154, 154–160. [Google Scholar] [CrossRef]
- Tsao, I.-F.; Wang, H.Y.; Shipman, C., Jr. Interaction of infectious viral particles with a quaternary ammonium chlorid (QAC) surface. Biotechnol. Bioeng. 1989, 34, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Caschera, A.; McAuley, J.; Kim, Y.; Purcell, D.; Rymenants, J.; Foucher, D.A. Evaluation of Virucidal Activity of Residual Quaternary Ammonium-treated Surfaces on SARS-CoV-2. Am. J. Infect. Control 2021, 50, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, B.; Solis-Leal, A.; Lopez, J.; Poole, B.; Robison, R.; Berges, B. Alcohol-free hand sanitizer and other quaternary ammonium disinfectants quickly and effectively inactivate SARS-CoV-2. J. Hosp. Infect. 2020, 108, 142–145. [Google Scholar] [CrossRef] [PubMed]
- El Hage, S.; Lajoie, B.; Stigliani, J.-L.; Furiga-Chusseau, A.; Roques, C.; Baziard, G. Synthesis, antimicrobial activity and physico-chemical properties of some n-alkyldimethylbenzylammonium halides. J. Appl. Biomed. 2014, 12, 245–253. [Google Scholar] [CrossRef]
- Hinchliffe, D.J.; Condon, B.D.; Slopek, R.P.; Reynolds, M. The adsorption of alkyl-dimethyl-benzyl-ammonium chloride onto cotton nonwoven hydroentangled substrates at the solid–liquid interface is minimized by additive chemistries. Text. Res. J. 2016, 87, 70–80. [Google Scholar] [CrossRef]
- Sutka, A.; Sutka, A.; Gaidukov, S.; Timusk, M.; Gravitis, J.; Kukle, S. Enhanced stability of PVA electrospun fibers in water by adding cellulose nanocrystals. Holzforschung 2015, 69, 737–743. [Google Scholar] [CrossRef]
- Yang, C.Q. Infrared spectroscopy studies of the cyclic anhydride as the intermediate for the ester crosslinking of cotton cellulose by polycarboxylic acids. I. Identification of the cyclic anhydride intermediate. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 1187–1193. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, G. Catalytic Actions of Sodium Salts in Direct Esterification of 3,3′4,4′-Benzophenone Tetracarboxylic Acid with Cellulose. Ind. Eng. Chem. Res. 2015, 54, 10553–10559. [Google Scholar] [CrossRef]
- Ji, X.; Guo, J.; Guan, F.; Liu, Y.; Yang, Q.; Zhang, X.; Xu, Y. Preparation of Electrospun Polyvinyl Alcohol/Nanocellulose Composite Film and Evaluation of Its Biomedical Performance. Gels 2021, 7, 223. [Google Scholar] [CrossRef]
- Limpan, N.; Prodpran, T.; Benjakul, S.; Prasarpran, S. Influences of degree of hydrolysis and molecular weight of poly(vinyl alcohol) (PVA) on properties of fish myofibrillar protein/PVA blend films. Food Hydrocoll. 2012, 29, 226–233. [Google Scholar] [CrossRef]
- Çay, A.; Kumbasar, E.P.A.; Keskin, Z.; Akduman, Ç.; Ürkmez, A.Ş. Crosslinking of poly(vinyl alcohol) nanofibres with polycarboxylic acids: Biocompatibility with human skin keratinocyte cells. J. Mater. Sci. 2017, 52, 12098–12108. [Google Scholar] [CrossRef]
- Leung, W.W.F.; Sun, Q. Electrostatic charged nanofiber filter for filtering airborne novel coronavirus (COVID-19) and nano-aerosols. Sep. Purif. Technol. 2020, 250, 116886. [Google Scholar] [CrossRef]
- Choi, H.-J.; Kumita, M.; Hayashi, S.; Yuasa, H.; Kamiyama, M.; Seto, T.; Tsai, C.-J.; Otani, Y. Filtration Properties of Nanofiber/Microfiber Mixed Filter and Prediction of its Performance. Aerosol Air Qual. Res. 2017, 17, 1052–1062. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Y.; Xiong, J.; Shao, W.; Cui, C.; Sun, N.; Zhang, Y.; Chang, S.; Han, P.; Liu, F.; et al. Biodegradable and high-performance multiscale structured nanofiber membrane as mask filter media via poly(lactic acid) electrospinning. J. Colloid Interface Sci. 2021, 606, 961–970. [Google Scholar] [CrossRef]
- Zhang, P.; Wan, D.; Zhang, Z.; Wang, G.; Hu, J.; Shao, G. RGO-functionalized polymer nanofibrous membrane with exceptional surface activity and ultra-low airflow resistance for PM2.5 filtration. Environ. Sci. Nano 2018, 5, 1813–1820. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Hao, X.; Teng, D.; Zhao, T.; Zeng, Y. Micro/nanofibrous nonwovens with high filtration performance and radiative heat dissipation property for personal protective face mask. Chem. Eng. J. 2021, 423, 130175. [Google Scholar] [CrossRef]
- He, R.; Li, J.; Chen, M.; Zhang, S.; Cheng, Y.; Ning, X.; Wang, N. Tailoring moisture electroactive Ag/Zn@cotton coupled with electrospun PVDF/PS nanofibers for antimicrobial face masks. J. Hazard. Mater. 2022, 428, 128239. [Google Scholar] [CrossRef]
- Ding, X.; Li, Y.; Si, Y.; Yin, X.; Yu, J.; Ding, B. Electrospun polyvinylidene fluoride/SiO2 nanofibrous membranes with enhanced electret property for efficient air filtration. Compos. Commun. 2019, 13, 57–62. [Google Scholar] [CrossRef]
- Rajak, A.; Hapidin, D.A.; Iskandar, F.; Munir, M.M.; Khairurrijal, K. Electrospun nanofiber from various source of expanded polystyrene (EPS) waste and their characterization as potential air filter media. Waste Manag. 2020, 103, 76–86. [Google Scholar] [CrossRef]
- Yang, X.; Pu, Y.; Li, S.; Liu, X.; Wang, Z.; Yuan, D.; Ning, X. Electrospun Polymer Composite Membrane with Superior Thermal Stability and Excellent Chemical Resistance for High-Efficiency PM2.5 Capture. ACS Appl. Mater. Interfaces 2019, 11, 43188–43199. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, F.; Pei, H.; Yan, K.; Cui, Z.; He, B.; Fang, K.; Li, J. Environmentally-friendly halloysite nanotubes@chitosan/polyvinyl alcohol/non-woven fabric hybrid membranes with a uniform hierarchical porous structure for air filtration. J. Membr. Sci. 2019, 594, 117445. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, C.; Hsu, P.-C.; Zhang, C.; Liu, N.; Zhang, J.; Lee, H.R.; Lu, Y.; Qiu, Y.; Chu, S.; et al. Nanofiber Air Filters with High-Temperature Stability for Efficient PM2.5 Removal from the Pollution Sources. Nano Lett. 2016, 16, 3642–3649. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wang, Y.; Lu, T.; Liu, K.; Huang, C. High performance, environmentally friendly and sustainable nanofiber membrane filter for removal of particulate matter 1.0. J. Colloid Interface Sci. 2021, 597, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Cvelbar, U.; Strazar, P.; Vossebein, L.; Zille, A. Chemical, Thermo-Mechanical and Antimicrobial Properties of DBD Plasma Treated Disinfectant-Impregnated Wipes during Storage. Polymers 2019, 11, 1769. [Google Scholar] [CrossRef]
- Vu, N.-N.; Venne, C.; Ladhari, S.; Saidi, A.; Moskovchenko, L.; Lai, T.T.; Xiao, Y.; Barnabe, S.; Barbeau, B.; Nguyen-Tri, P. Rapid Assessment of Biological Activity of Ag-Based Antiviral Coatings for the Treatment of Textile Fabrics Used in Protective Equipment Against Coronavirus. ACS Appl. Bio Mater. 2022, 5, 3405–3417. [Google Scholar] [CrossRef]
- Deng, C.; Seidi, F.; Yong, Q.; Jin, X.; Li, C.; Zhang, X.; Han, J.; Liu, Y.; Huang, Y.; Wang, Y.; et al. Antiviral/antibacterial biodegradable cellulose nonwovens as environmentally friendly and bioprotective materials with potential to minimize microplastic pollution. J. Hazard. Mater. 2021, 424, 127391. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Yim, S.-L.; Wong, C.-H.; Kan, C.-W. Development of Antiviral CVC (Chief Value Cotton) Fabric. Polymers 2021, 13, 2601. [Google Scholar] [CrossRef]
- Chughtai, A.A.; Stelzer-Braid, S.; Rawlinson, W.; Pontivivo, G.; Wang, Q.; Pan, Y.; Zhang, D.; Zhang, Y.; Li, L.; MacIntyre, C.R. Contamination by respiratory viruses on outer surface of medical masks used by hospital healthcare workers. BMC Infect. Dis. 2019, 19, 491. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR Spectroscopy Characterization of Poly (Vinyl Alcohol) Hydrogel with Different Hydrolysis Degree and Chemically Crosslinked with Glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Q.; Zhang, L.; Wang, S.; Harper, D.P.; Wu, Q.; Young, T.M. Preparation of Electrospun Nanofibrous Poly(Vinyl Alcohol)/Cellulose Nanocrystals Air Filter for Efficient Particulate Matter Removal with Repetitive Usage Capability via Facile Heat Treatment. Chem. Eng. J. 2020, 399, 125768. [Google Scholar] [CrossRef]
- Peppas, N. Infrared Spectroscopy of Semicrystalline Poly(Vinyl Alcohol) Networks. Die Makromol. Chem. 1977, 178, 595–601. [Google Scholar] [CrossRef]
- Krimm, S. Infrared Spectra of High Polymers; Springer: Berlin/Heidelberg, Germany, 1960; Volume 2/1, ISBN 978-3-540-02494-1. [Google Scholar]
- Qashou, S.I.; El-Zaidia, E.F.M.; Darwish, A.A.A.; Hanafy, T.A. Methylsilicon Phthalocyanine Hydroxide Doped PVA Films for Optoelectronic Applications: FTIR Spectroscopy, Electrical Conductivity, Linear and Nonlinear Optical Studies. Phys. B Condens. Matter 2019, 571, 93–100. [Google Scholar] [CrossRef]
- Tretinnikov, O.N.; Zagorskaya, S.A. Determination of the Degree of Crystallinity of Poly(Vinyl Alcohol) by Ftir Spectroscopy. J. Appl. Spectrosc. 2012, 79, 521–526. [Google Scholar] [CrossRef]
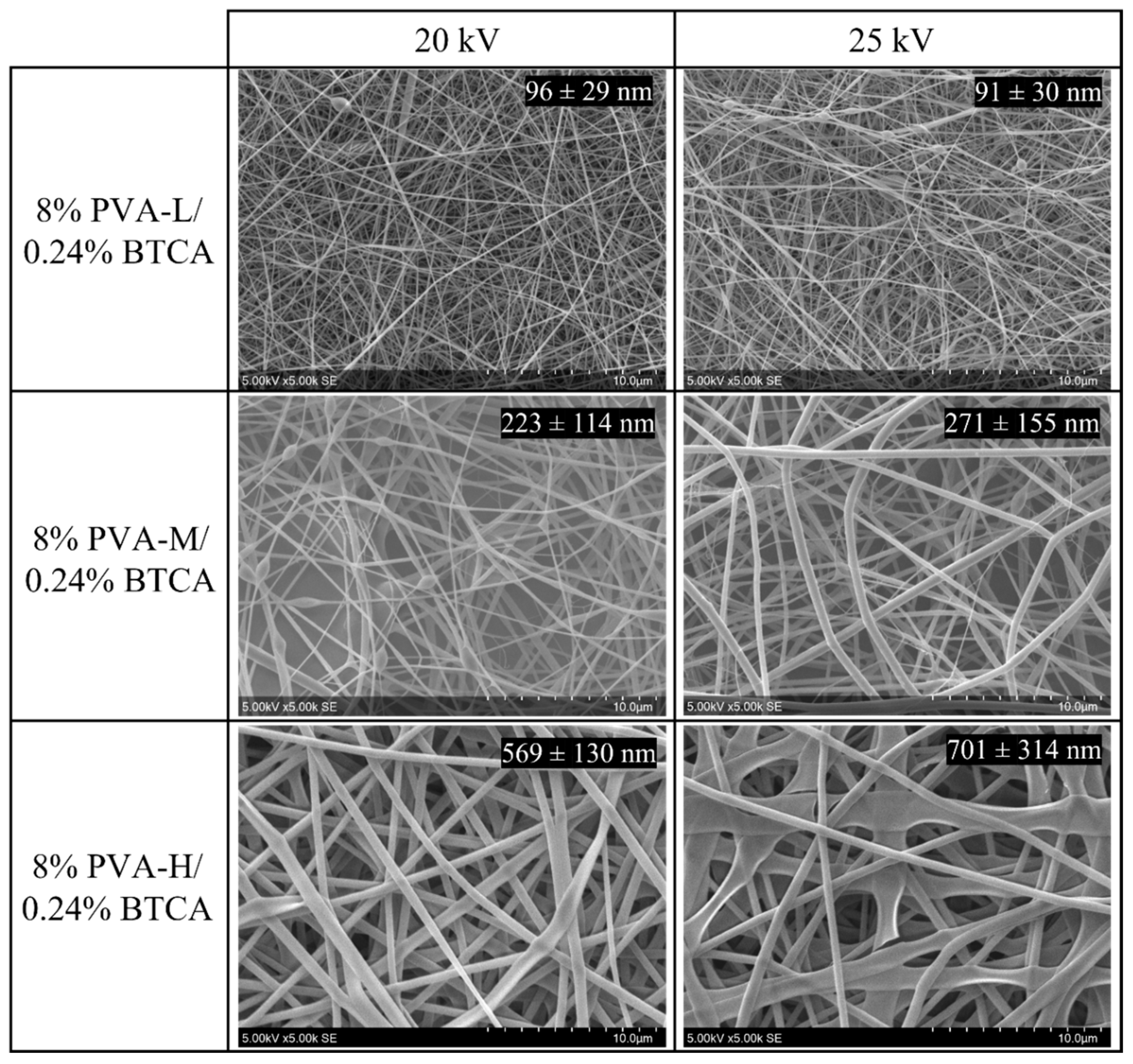
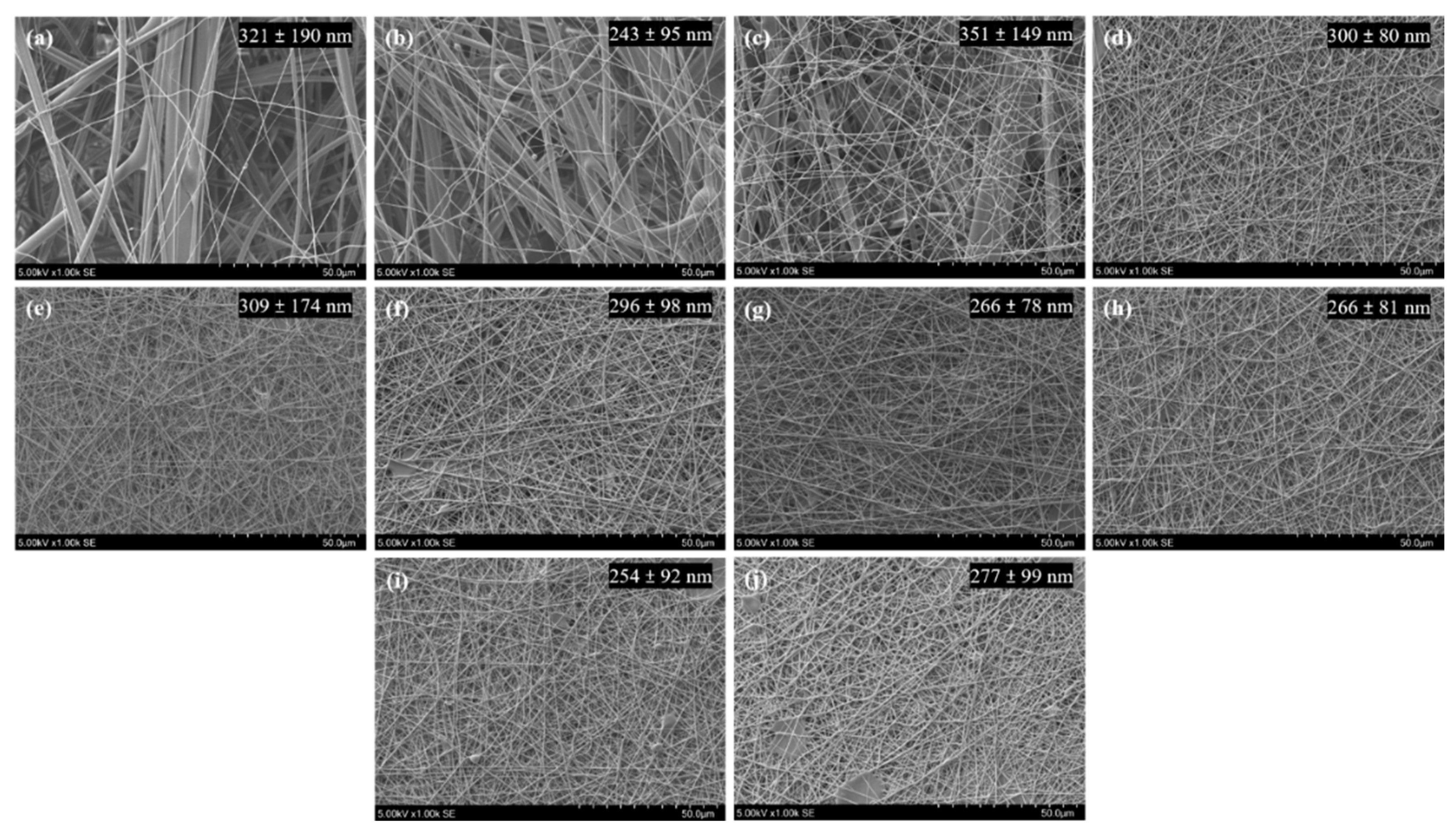
| Nomenclature | MW (g/mol) | Degree of Hydrolysis (DH) (%) |
|---|---|---|
| PVA-L | 31,000–50,000 | 87–89 |
| PVA-M | 85,000–124,000 | 87–89 |
| PVA-H | 146,000–186,000 | 87–89 |
| PVA (8% w/v) | BTCA (% w/v) | ADBAC (% w/v) | AH (% w/v) |
|---|---|---|---|
| PVA-L PVA-M PVA-H | 0.24 0.64 0.81 0.96 | 0.4 1.4 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muniz, N.O.; Gabut, S.; Maton, M.; Odou, P.; Vialette, M.; Pinon, A.; Neut, C.; Tabary, N.; Blanchemain, N.; Martel, B. Electrospun Filtering Membrane Designed as Component of Self-Decontaminating Protective Masks. Nanomaterials 2023, 13, 9. https://doi.org/10.3390/nano13010009
Muniz NO, Gabut S, Maton M, Odou P, Vialette M, Pinon A, Neut C, Tabary N, Blanchemain N, Martel B. Electrospun Filtering Membrane Designed as Component of Self-Decontaminating Protective Masks. Nanomaterials. 2023; 13(1):9. https://doi.org/10.3390/nano13010009
Chicago/Turabian StyleMuniz, Nathália Oderich, Sarah Gabut, Mickael Maton, Pascal Odou, Michèle Vialette, Anthony Pinon, Christel Neut, Nicolas Tabary, Nicolas Blanchemain, and Bernard Martel. 2023. "Electrospun Filtering Membrane Designed as Component of Self-Decontaminating Protective Masks" Nanomaterials 13, no. 1: 9. https://doi.org/10.3390/nano13010009
APA StyleMuniz, N. O., Gabut, S., Maton, M., Odou, P., Vialette, M., Pinon, A., Neut, C., Tabary, N., Blanchemain, N., & Martel, B. (2023). Electrospun Filtering Membrane Designed as Component of Self-Decontaminating Protective Masks. Nanomaterials, 13(1), 9. https://doi.org/10.3390/nano13010009