Abstract
The widespread use of Ag3PO4 is not surprising when considering its higher photostability compared to other silver-based materials. The present work deals with the facile precipitation method of silver phosphate. The effects of four different phosphate sources (H3PO4, NaH2PO4, Na2HPO4, Na3PO4·12 H2O) and two different initial concentrations (0.1 M and 0.2 M) were investigated. As the basicity of different phosphate sources influences the purity of Ag3PO4, different products were obtained. Using H3PO4 did not lead to the formation of Ag3PO4, while applying NaH2PO4 resulted in Ag3PO4 and a low amount of pyrophosphate. The morphological and structural properties of the obtained samples were studied by X-ray diffractometry, diffuse reflectance spectroscopy, scanning electron microscopy, infrared spectroscopy, and X-ray photoelectron spectroscopy. The photocatalytic activity of the materials and the corresponding reaction kinetics were evaluated by the degradation of methyl orange (MO) under visible light. Their stability was investigated by reusability tests, photoluminescence measurements, and the recharacterization after degradation. The effect of as-deposited Ag nanoparticles was also highlighted on the photostability and the reusability of Ag3PO4. Although the deposited Ag nanoparticles suppressed the formation of holes and reduced the degradation of methyl orange, they did not reduce the performance of the photocatalyst.
1. Introduction
Since the discovery and first application attempts of photocatalysis, numerous materials, such as TiO2-based semiconductors and composites, other metal oxides, and salts have been investigated regarding their degradation of organic pollutants [1]. However, even the TiO2-based ”flagships” (such as P25 [2] or other commercial titania) have severe limitations due to their relatively wide band gap, poor quantum efficiency, and/or fast recombination rate of photoexcited electron─hole pairs.
Silver-based semiconductors are promising candidates among the newer claimants to reach the throne of large-scale industrial applications due to their strong visible light response. One of their main benefits is that they can provide Ag nanoparticles that can be deposited in situ on the surface of catalysts. Depending on the light source applied during photocatalytic processes, deposited nanoparticles can act as charge separators (mostly under UV irradiation) or electron carriers (under visible light irradiation). Many photocatalysts (especially the Ag-based ones) are susceptible to photochemical corrosion, resulting in poor stability [3]. However, deposited nanoparticles can be effectively used to prevent the photocorrosion of semiconductors [4].
The application of Ag3PO4 as a photocatalytic material was first reported by Yi and coworkers [5]. Their investigation focused on water-splitting and degrading organic contaminants in wastewater by utilizing visible light. Ma et al. [6] focused on the structural peculiarities of Ag3PO4. They found that it can be characterized by a body-centered cubic structure (where all oxygen atoms are adjacent with three Ag and a P atom), a lattice parameter of 6 Å, and the space group of P4-3n [7,8]. Its band gap energy is relatively low (Eg ≈ 2.4 eV), which can be mainly modified by fine-tuning its morpho-structural characteristics. During its synthesis, pH and the appearance of pyro- and polyphosphates are key parameters. It is well known that two phosphoric acid molecules can easily condensate, resulting in these phosphates. Since pH can strongly influence the formation of oxo-acid-based Ag salts, it is not surprising that the type of phosphate precursor can be decisive (as it can also strongly influence pH). Accordingly, several phosphate-containing precursors have already been investigated, focusing on the morphology of the target semiconductor, including NH4+ [9], Na+ [10], and K+ [11] based agents. Besides precursors, another decisive parameter is the applied synthesis pathway. Considering this aspect, the main methods applied to synthesize Ag3PO4 salts have been precipitation [9,10,12], ion-exchange [5,13], hydrothermal [14], ultrasonication [15], and other approaches [16]. It has been found that semiconductors prepared by ion-exchange reactions can yield electron─hole pairs with enhanced lifetime, resulting in higher photocatalytic efficiency [17]. Still, a deeper insight into the electronic structure of Ag3PO4 is necessary to understand the origin of the increased photocatalytic activity.
Thus, in this work, we focus on three main aspects related to the involvement of the Ag3PO4 semiconductor in photocatalytic approaches as follows:
- The effect of different phosphate sources on the synthesis and photocatalytic activity of Ag3PO4. For this purpose, H3PO4, NaH2PO4, Na2HPO4, and Na3PO4 · 12H2O were used as phosphate sources. In this part, we propose a mechanism for the formation of Ag3PO4.
- Investigation of the stability of Ag3PO4 via recharacterization and reusability measurements.
- The effect of Ag nanoparticles on Ag3PO4, shown through the deposition mechanism of Ag nanoparticles. This aspect was demonstrated by photoluminescence measurements and their corresponding kinetic studies.
2. Materials and Methods
2.1. Materials
All chemicals were used as received: silver nitrate (AgNO3, 99.8%, Penta industry; Prague, Czech Republic), methyl orange (MO, analytical reagent, Alfa Aesar; Tewksbury, MA, USA), Milli-Q (MQ; Budapest, Hungary) water, phosphoric acid (H3PO4, 85%, VWR Chemicals; Radnor, PA, USA), monosodium phosphate (NaH2PO4; >99.0%, Spektrum-3D; Debrecen, Hungary), disodium phosphate (Na2HPO4; >99.0%, Sigma-Aldrich; Schnelldorf, Germany), and trisodium phosphate dodecahydrate (Na3PO4·12H2O; analytical reagent, Sigma-Aldrich, Schnelldorf, Germany).
2.2. Methods
2.2.1. Synthesis of Ag3PO4 Semiconductors
A precipitation method was used [13,18] to synthesize Ag3PO4 microcrystals. Four phosphate sources (MPO4: H3PO4, NaH2PO4, Na2HPO4, Na3PO4·12 H2O) and AgNO3 were used. The weight ratio of the precursors was: MPO4:AgNO3 = 3:2.
In each case, two different initial concentrations of phosphate sources were used (0.2 M and 0.1 M). The aqueous solution of phosphate sources was stirred for 5 min then 1.247 g of AgNO3 was added. A yellow suspension formed from the colorless and transparent solution. After another 5 min of stirring, the suspensions were washed with 3 × 45 mL of MQ water (for 10 min at 4400 RPM) and dried overnight at 40 °C.
The sample abbreviations used in the manuscript were conceived as follows: Ag3PO4_source_concentration, where concentration is the initial concentration of the used phosphate precursor (example: Ag3PO4_Na3PO4_0.1M denotes the sample that was prepared using 0.1 M Na3PO4·12 H2O as the phosphate source). The word “after” was added to the names to designate samples investigated after photocatalytic tests (example: Ag3PO4_Na3PO4_0.1M_after).
2.2.2. Characterization and Instrumentation
The X-ray diffraction (XRD) measurements were performed on a Shimadzu 6000 X-ray diffractometer (Kyoto, Japan) at an accelerating voltage of 40 kV (30 mA), operated with CuKα radiation (λCuKα = 1.54 Å). The XRD patterns were recorded in 2θ range between 15 and 60°, with scan speed 1°·min−1.
Fourier transform infrared spectroscopy (FT-IR) measurements were performed on a JASCO-6200 FT-IR spectrophotometer (Jasco, Tokyo, Japan) in the 4000─400 cm−1 wavelength range, with 4 cm−1 spectral resolution, using the well-known KBr pellet technique.
The morphology of the samples was identified with a Hitachi S-4700 Type II scanning electron microscope (SEM; Hitachi, Tokyo, Japan) equipped with an Everhart—Thornley detector using an electron beam with an acceleration voltage of 10 kV.
The band structure of the semiconductors was investigated by diffuse reflectance spectroscopy (DRS). The spectra were recorded in the 250–800 nm range with a JASCO-V650 spectrophotometer (equipped with an ILV-724 integration sphere; Jasco, Wien, Austria) using BaSO4 as a reference. The band gap energy values were calculated based on the Kubelka-Munk theory [19].
X-ray photoelectron spectroscopy (XPS) measurements were carried out using a Specs Phoibos 150 MCD system (SPECS Surface Nano Analysis GmbH, Berlin, Germany) equipped with Al-Kα source (1486.6 eV) at 14 kV and 20 mA, a hemispherical analyzer, and a charge neutralization device. Care was taken to completely cover the double-sided carbon tapes with the silver—phosphate samples.
Fluorescence measurements were carried out using a Jasco LP-6500 spectrofluorometer (Jasco, Japan; PL) equipped with a Xenon lamp (excitation source) coupled to an epifluorescence accessory (EFA 383 module). Fluorescence spectra were collected with a 1 nm spectral resolution in the 350─600 nm wavelength range using a fixed excitation wavelength of 325 nm. Bandwidths of 1 nm or 10 nm were employed during excitation and emission.
2.2.3. Photocatalytic Activity
The photocatalytic investigation of the samples was carried out in a double-walled photoreactor, where MO (CMO = 125 μM) was the model pollutant. The reactor was surrounded by 6 × 15 W visible light-emitting lamps (λ > 400 nm). The system was kept at a constant temperature (25 °C), and the suspension (Csuspension = 1 g∙L−1) was continuously stirred and purged by air at constant flow (40 L∙h−1). The concentration change of MO was followed with a JASCO-V650 UV-Vis spectrophotometer (UV-Vis; Jasco, Wien, Austria) at λmax = 513 nm (using a 1 mm optical path length quartz cell). The suspension was kept in the dark for 10 min to reach the adsorption–desorption equilibrium. The experiments were conducted for 2 h. Samples were taken every 10 min in the first hour and every 20 min in the second hour. Last, the samples were centrifugated and filtrated before quantitative analysis.
The conversion of MO was calculated by the following equation:
where C120 is the concentration of MO after 120 min and C0 is the initial concentration of MO.
H = (100 − (C120/C0 × 100),
The reaction order (n) and apparent rate constants (k1, k2) were calculated to investigate the kinetics of MO degradation. The apparent rate constants (k1, k2) were determined by plotting the MO concentration vs. the irradiation time, where the slope was considered the apparent rate constants. For k1 values, we took the first hour (0–60 min) of the degradation process into account, whereas for k2 values, we considered the second (60–120 min) hour.
The adsorption of MO on the samples was also investigated. The photocatalysts (50 mg) and MO (50 mL; CMO = 125 μM) were added to a beaker. The beaker was stirred (500 RPR) and covered with aluminum foil to eliminate all light sources. Samples were taken every 5 min in the first 30 min, every 10 min between 30–60 min, and every 20 min between 1–2 h. Then, they were centrifuged and filtered, and the adsorption of MO was determined with a JASCO-V650 spectrophotometer.
To investigate reusability, we used the same setup for the photoactivity and adsorption measurements, but sampling times were changed to 30, 60, and 120 min. Samples collected between two cycles were washed with distilled water three times and dried at 40 °C for 12 h.
3. Results and Discussion
3.1. Characterization of Ag3PO4
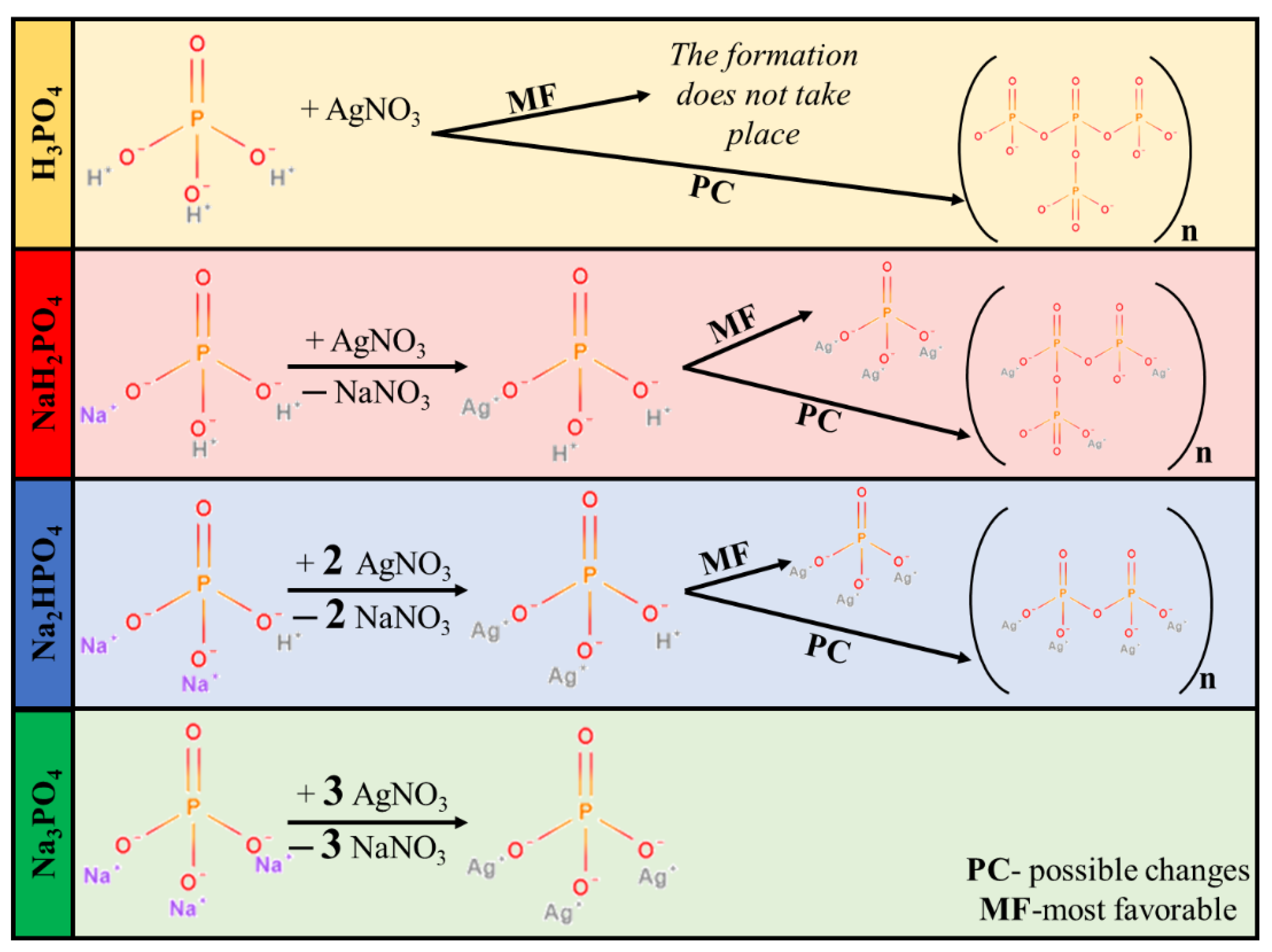
Synthesizing Ag3PO4 by using different MPO4 sources is a rather complicated process. MPO4 (used as different phosphate sources; Figure 1) has different disproportional rates in aqueous media, which resulted in different pH values (Table 1). Moreover, pH can also indirectly affect the samples’ morphological and structural properties. Using H3PO4 did not lead to the formation of Ag3PO4. The lack of Ag3PO4 can be explained by the acidic environment set by H3PO4, hindering the precipitation of Ag3PO4 (Figure 1). Moreover, the presence of H3PO4 can facilitate the formation of pyrophosphate. Thus, this sample was omitted from all the experimental work presented here.
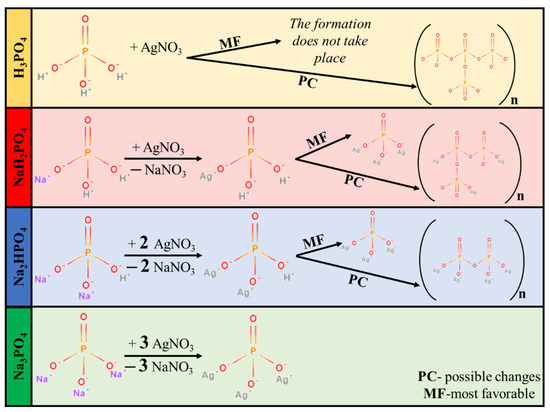
Figure 1.
Proposed mechanism of Ag3PO4 formation.

Table 1.
pH, band gap energy, absorption band maxima, average particles sizes, and conversion values of the obtained Ag3PO4 samples (n.a.—no available).
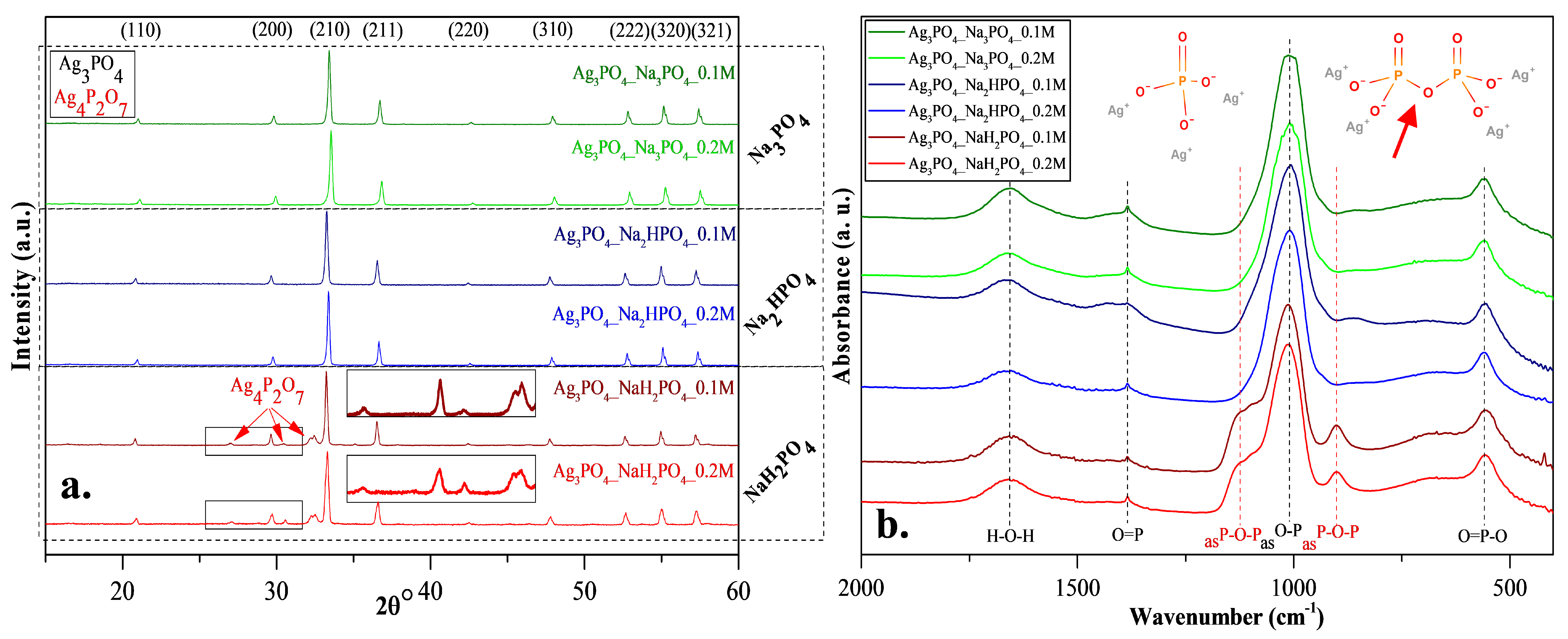
XRD patterns were recorded to elucidate the effect of the other three phosphate sources on the formation of Ag3PO4 crystals. Cubic Ag3PO4 was identified (COD 00-101-0324) in all cases: the reflections of the Ag3PO4 were located at 2θ° ≈ 21.1°, ≈29.8°, ≈33.3°, ≈36.7°, ≈42.6°, ≈47.9°, ≈52.8°, ≈55.2°, and ≈57.3°, which were assigned to (110), (200), (210), (211), (220), (310), (222), (320), and (321) crystallographic planes (Figure 2a). Additional reflections were also observed in the Ag3PO4_NaH2PO4 sample series located at 2θ° ≈ 27.0°, ≈30.6° and ≈32.1° (Figure 2a), which could be associated with the typical reflections of Ag4P2O7 [20]. These observations are consistent with the presumed mechanism (Figure 1). It must be emphasized that the pyrophosphate formation is much higher for NaH2PO4 than for the other two samples. In this case, crystallized pyrophosphate was formed since the materials have higher proton concentrations, and the possibility of condensation is much higher than in other cases. Interestingly, diffraction peaks of other Ag species, such as Ag nanoparticles or AgxO particles, were not found in the XRD patterns (Figure 2a).
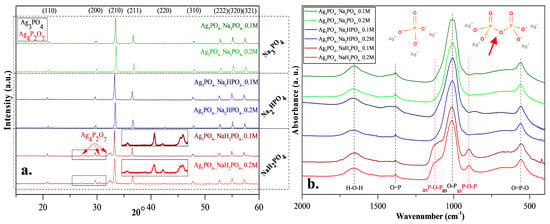
Figure 2.
(a) X-ray diffraction patterns and (b) infrared spectra of Ag3PO4 samples synthesized by using different types (Na3PO4, Na2HPO4 and NaH2PO4) and concentrations of phosphate sources (C = 0.1 and 0.2 M).
FT-IR measurements (Figure 2b) were conducted to investigate the presence of pyrophosphate. The typical bands of asymmetrical vibrations of P─O─P bonds [20] were identified at ≈902 and ≈1116 cm−1, confirming the presence of silver pyrophosphate (observed only in the Ag3PO4_NaH2PO4 sample series; Figure 2b). Additional bands were also observed [17]: at ≈554 cm−1 (O=P─O); ≈1007 cm−1 (asO─P), ≈1389 cm−1 (O=P), and H─O─H (≈1655 cm−1). Thereby, it can be concluded that the formation of Ag4P2O7 depends on the nature of the applied phosphate source (Figure 2b).
When NaH2PO4 was used as the phosphate source, the formation of Ag3PO4 was incomplete, while the formation of Ag4P2O7 was detected (Figure 1). The reason for this could be that the formation of HNO3 was not favored. This could be because, during the synthesis, Na+ exchange is more favored than that of H+. This makes the free formation of NaNO3 favorable because the electropositivity of Na+ is higher than that of H+. Against the Ag3PO4_NaH2PO4 samples, the Ag4P2O7 was not detected using Na2HPO4 or Na3PO4 as a phosphate source.
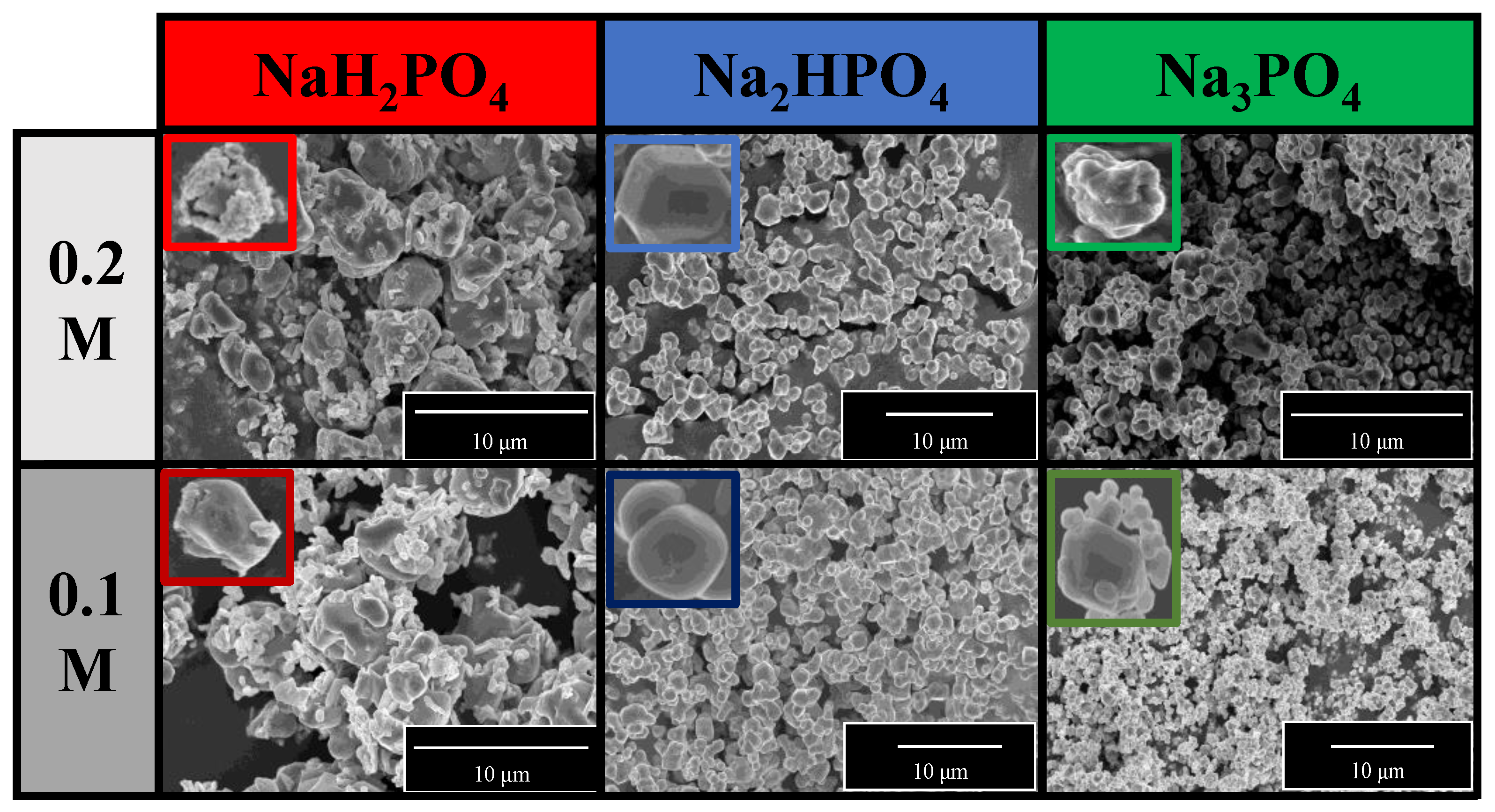
The morphological properties of the samples were analyzed using SEM. A correlation was found with the XRD measurements (Figure 2a). Two differently shaped and sized particles—spherical-like structure with 1.5 μm diameter and plates with 0.2 μm height—were obtained in the Ag3PO4_NaH2PO4 sample series (Figure 3). The particles could not be distinguished based on their elemental composition. This can be attributed to the co-presence of phosphate and pyrophosphate according to XRD patterns (Figure 2a). The samples prepared by using the other two phosphate sources had a much higher monodispersity (Figure S1) and a more defined shape (Figure 3). Their average particle size was ≈0.9 μm regardless of the used concentration. The particle size distribution was lower than for the Na3PO4 samples series; moreover, lower concentration resulted in smaller particles. The Ag3PO4_Na2HPO4 sample series contained more polyhedral particles compared to the Ag3PO4_Na3PO4 sample series.
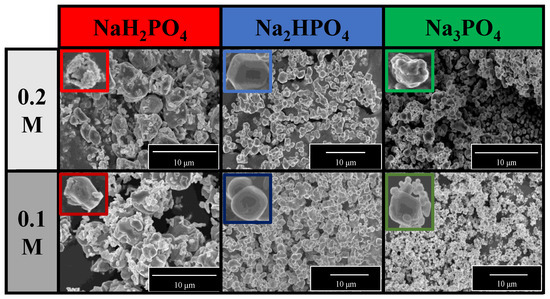
Figure 3.
SEM micrographs of Ag3PO4 samples synthesized by using different types (Na3PO4, Na2HPO4, and NaH2PO4) and concentrations of phosphate sources (C = 0.1 and 0.2 M).
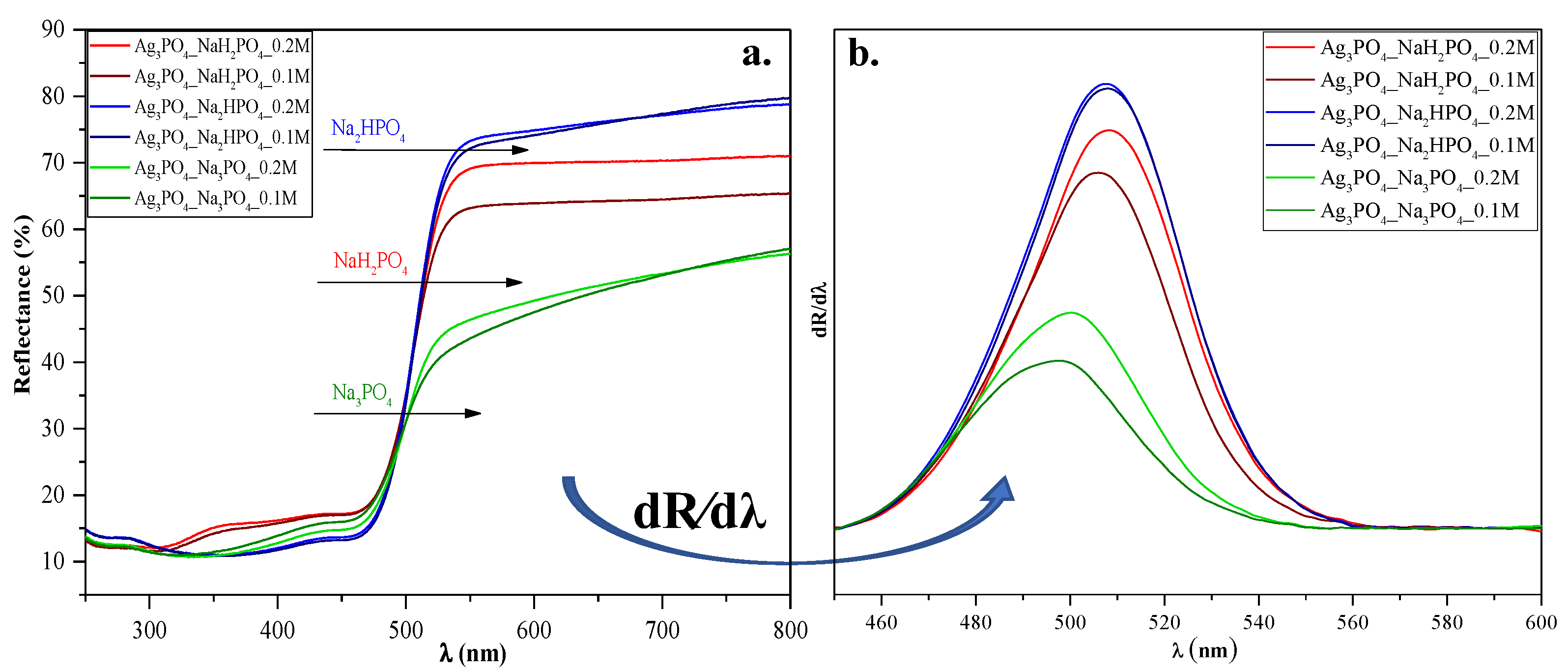
Diffuse reflectance spectroscopy was used to analyze the optical properties of the materials and to understand the light absorption properties of Ag3PO4_NaH2PO4 (which contained Ag4P2O7, a stand-alone photocatalyst [20]). As shown in Figure 4a, using different phosphate sources significantly influenced the visible light absorption properties of the samples, whose reflectance intensities decreased in the following order:
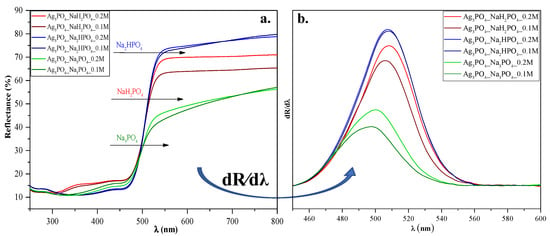
Figure 4.
(a) Diffuse reflectance spectra and (b) their first derivative order of Ag3PO4 samples synthesized by using different types (Na3PO4, Na2HPO4, and NaH2PO4) and concentrations of phosphate sources (C = 0.1 and 0.2 M).
Ag3PO4_Na2HPO4 > Ag3PO4_NaH2PO4 > Ag3PO4_Na3PO4. These differences may result in different photocatalytic performances. On the other hand, no specific plasmon resonance band of Ag was detected (which could be found at ≈320 nm [21]). These observations agreement with the XRD results, where the reflection of Ag could not be detected.
The Kubelka-Munk theory was used to calculate the indirect band gap energies of the samples. No significant changes could be observed between them (Eg ≈ 2.22–2.34 eV; Table 1). Hence no clear conclusions could be drawn. To understand the relationship between the samples’ light absorption and photocatalytic activity, we analyzed their first derivative spectra (Figure 4b). Still, the λmax values were almost identical (λmax ≈ 495─507 nm; Figure 4). Since Ag4P2O7 can be photoactive as well [20], the derivative DRS of sample Ag3PO4_NaH2PO4 should result in two specific electron transition peaks: (i) one corresponding to Ag4P2O7 (observed at ≈300 nm [20]) and (ii) one corresponding to Ag3PO4, (observed at ≈548 nm (2.26 eV [11])).
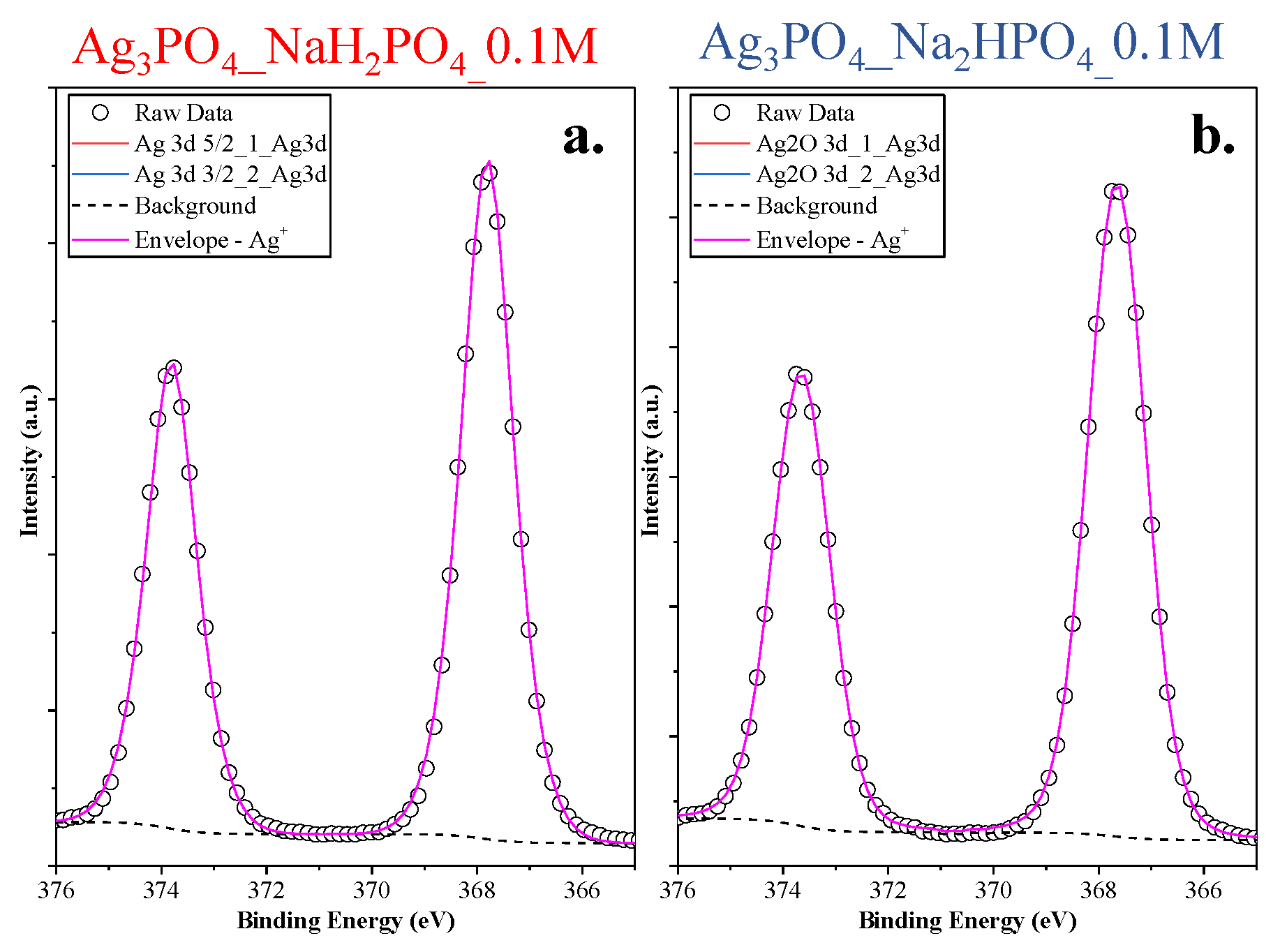
The lack of the Ag nanoparticles was also demonstrated with high-resolution XPS (Figure 5). Symmetrical peaks were found in the Ag3d spectra (Ag 3d5/2 and 3d3/2 of Ag3PO4 corresponding to the peaks 373.67 and 367.67 eV, respectively [22]), which could be associated with Ag+ from Ag3PO4.
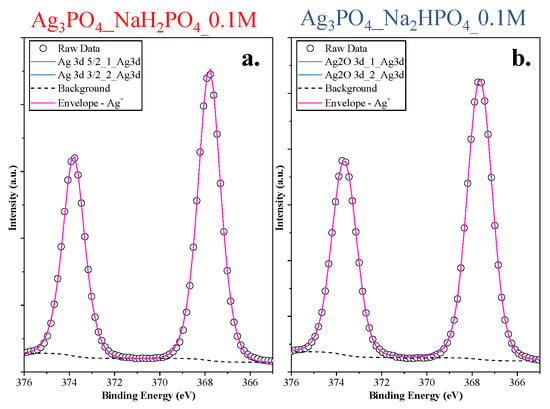
Figure 5.
Ag3d XPS spectra of the Ag 3d (a) Ag3PO4_NaH2PO4_0.1M and (b) Ag3PO4_Na2HPO4_0.1M samples.
3.2. Photocatalytic Degradation
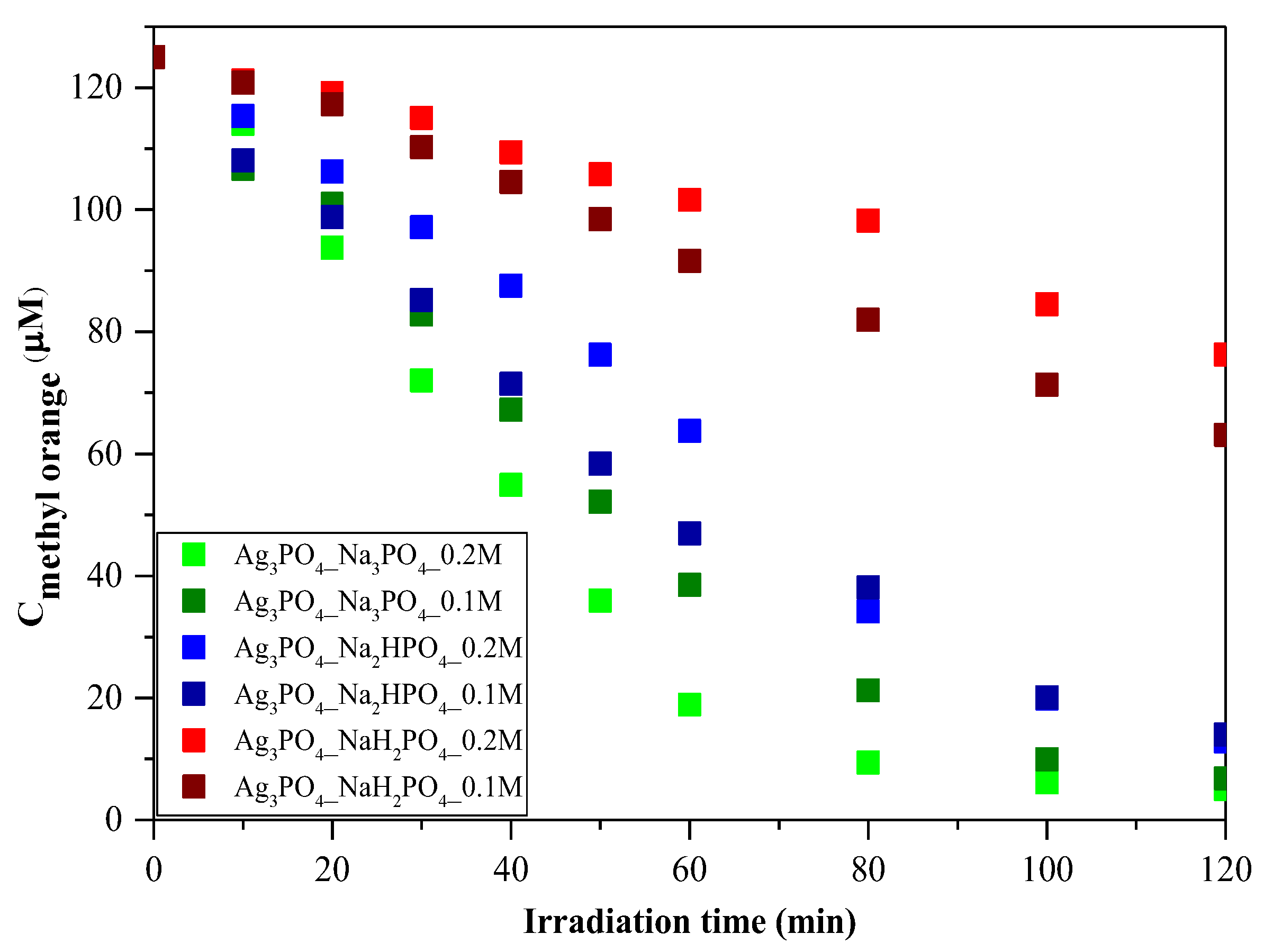
MO was employed as a model pollutant to investigate photoactivity. As shown in Figure 6, the photocatalytic activity of the samples can be correlated with the phosphate source used in the synthesis process. Regardless of the concentration of the phosphate source, the order was as follows: Ag3PO4_Na3PO4 > Ag3PO4_Na2HPO4 > Ag3PO4_NaH2PO4 (Figure 6). Because the samples had different absorption properties, similar photocatalytic activation was also expected to differ. According to our assumptions, samples containing Ag4P2O7, a stand-alone photocatalyst, should have higher photocatalytic activity. Moreover, a photojunction between Ag4P2O7 and Ag3PO4 could also form [23]. Ag4P2O7 did not show any spectral features, that is, its characteristic secondary bands were not found in the DR and first-order derivate spectra (Figure 4). It could inhibit the photocatalyst and absorb electrons, which in turn could not be used in the photocatalytic process. As a result, a photojunction did not form. Similar to the DRS measurements, regardless of the used phosphate source, different photoactivity was observed (Figure 6). Using different phosphate sources resulted in different optical and structural properties, which influenced the degradation of MO. To clarify the degradation of MO, we evaluated the MO conversion values presented in Section 2.2.3. After two hours, 95.94 and 94.53% conversions were obtained for Ag3PO4_Na3PO4_0.2M and Ag3PO4_Na3PO4_0.1M, respectively (Table 1). It could also be noticed that the most photoactive materials (Ag3PO4_Na3PO4; Figure 6) had the highest degradation yield of MO degradation after one hour. On the other hand, the second-highest MO decolorization was achieved by using Na2HPO4 as a phosphate source. Based on these findings, we ascertained that the proposed mechanism of Ag3PO4 formation (Figure 1) is in good agreement with the photocatalytic performance. The more complete the transformation, the higher the photoactivity because when Na3PO4 was used during the synthesis, no Ag4P2O7 or Ag nanoparticles could be observed.
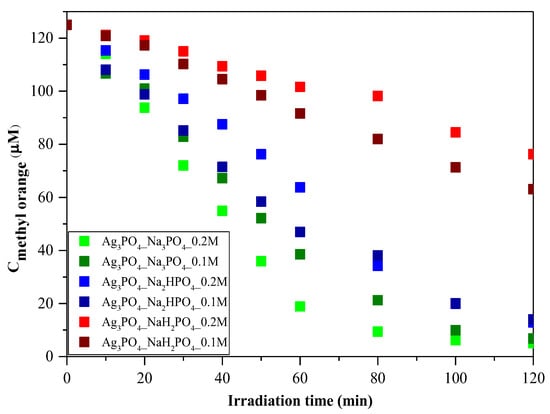
Figure 6.
Photocatalytic investigation of Ag3PO4 samples using C = 125 μM of MO as a model pollutant and visible light (λ > 400 nm) as a light source.
As a parallel measurement, the adsorption capacity of the two most efficient photocatalysts was also investigated without using any light source (Figure S2). Adsorption did not occur throughout the process. Thus, it could be concluded that the photocatalytic degradation of MO could indeed be performed using these Ag-based materials.
Amornpitoksuk et al. [10] investigate the effect of different phosphates on the photocatalytic degradation of methylene blue. They found that using Na2HPO4 resulted in the highest photocatalytic activity. They also mentioned that the samples synthesized from Na3PO4 contained not only Ag3PO4 but Ag2O as well, which inhibited photoactivity. Similarly, in our case, samples that did not contain Ag2O had the highest photocatalytic activity. However, it cannot be conclusively declared which phosphate source is the most suitable for achieving high photoactivity. In our case, the samples containing pyrophosphate proved to be less effective. Thus, it can be concluded that synthesizing pure Ag3PO4 is the best approach since both Ag2O and Ag4P2O7 are stand-alone photocatalysts that do not improve the efficiency of Ag3PO4.
Regardless of the employed phosphate source, after one hour (Figure 6), a change in the photocatalytic reaction was observed during MO removal. A kinetic study of the MO degradation curves was carried out.
Regarding the kinetics of MO degradation, a two-step mechanism was proposed (Table 2). The first step is the zero-order decolorization of azo dye bonds (R1─N=N─R2), probably by direct hole oxidation (0–60 min) since this is the thermodynamic and electrochemical facile pathway [24]. The second step (k2) is the first-order mineralization (60–120 min) of the intermediates (benzenesulfonic acid; 4-hydrazinylaniline; phenyl diazene) by reactive radicals (•OH; O2•─) [25]. The k1 values are in excellent agreement with the correlation coefficients (R2) regarding the MO conversion values after 1 h of visible light irradiation. The proposed mechanism can be applied to most samples; however, for Ag3PO4-Na3PO4-0.2M and Ag3PO4-Na3PO4-0.1M (Table 2), the degradation of intermediates follows second-order kinetics [26]. This could mean that the relatively fast mineralization of intermediates could also occur by direct hole oxidation and not by reactive radicals [27,28].

Table 2.
Kinetics of methyl orange degradation of the Ag3PO4 samples.
3.3. Stability Investigation of Ag3PO4
3.3.1. Recharacterization of the Investigated Ag3PO4
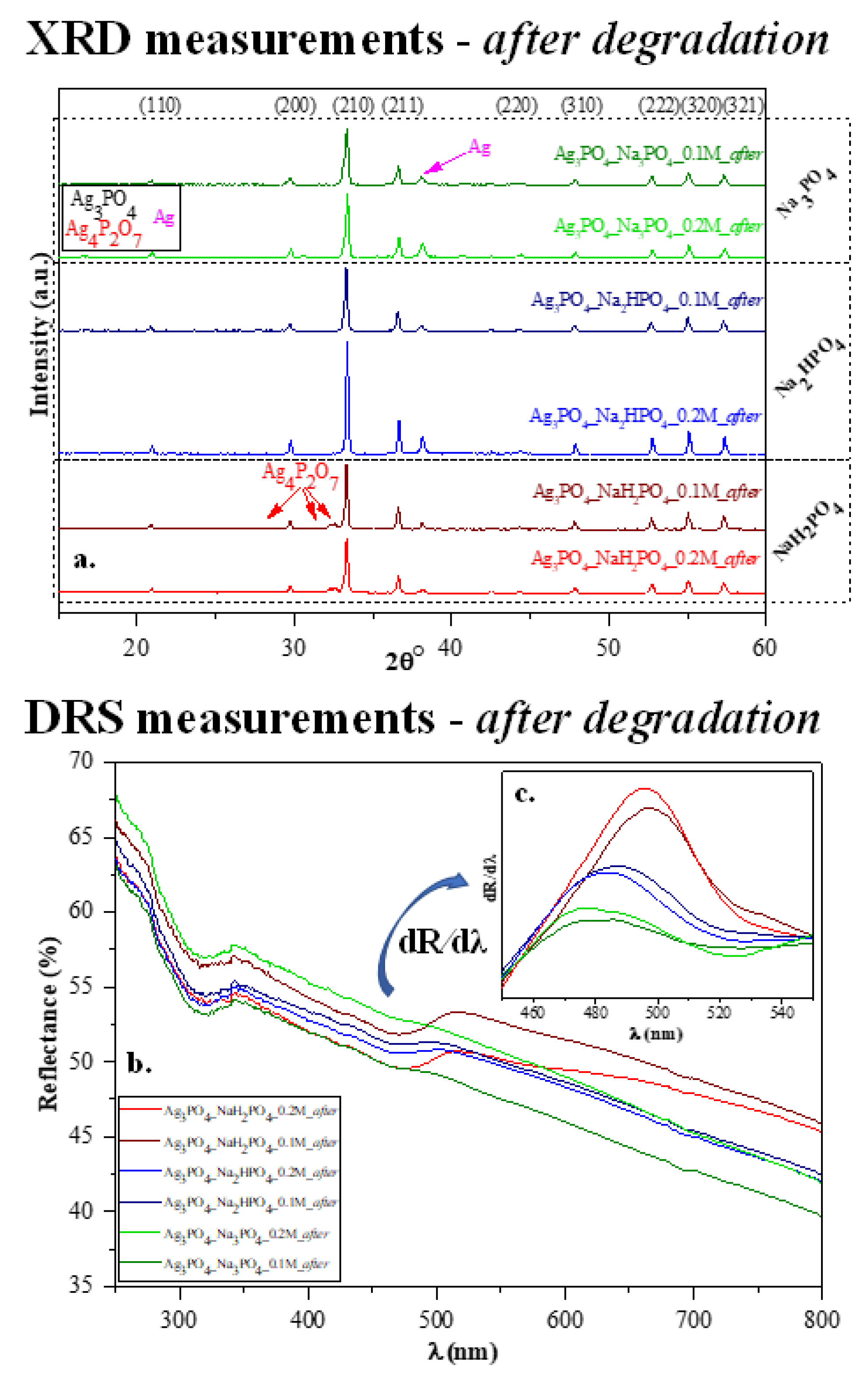
After the photocatalytic tests, we once again investigated the structural and optical parameters of the samples to examine the stability of the photocatalysts. After the photocatalytic activity, a slight modification was observed in the XRD patterns (Figure 7a) because not only the typical Ag3PO4 reflections (presented in Figure 2a), but a specific reflection of Ag (nano)particles was also observed at 38.1° (COD-00-110-0136). Based on our previous measurements [29,30], a silver-based material might lose photocatalytic activity due to the presence of Ag nanoparticles on the surface. Still, several publications in the literature state that Ag-based materials can be used quasi-unlimited times in (photo)catalytic processes [10]. Besides the deposited Ag nanoparticles, the typical reflection of Ag4P2O7 was also observed after the photocatalytic degradation, which was noticed before the degradation. Moreover, the optical parameters (Figure 7b–c) were also evaluated, and the decreasing intensity of the absorption band of Ag3PO4 was observed. The typical plasmon resonance band could not be clearly identified. Thus, it can be concluded that the amount of Ag nanoparticles was lower than the detection limit of the device applied. The appearance of AgxO is also possible because their reflection is close to that of Ag, and they do not have plasmon resonance bands in the reflectance spectra.
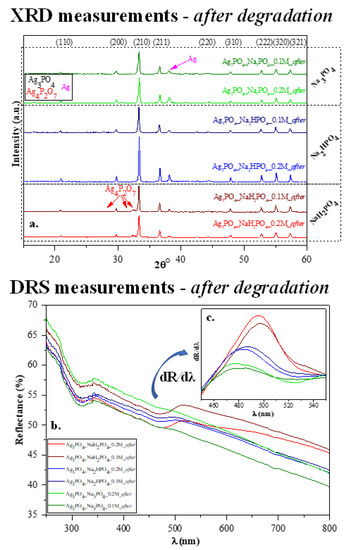
Figure 7.
Stability measurements carried out after photocatalytic degradation: (a) XRD patterns and (b) DR spectra and (c) insert figure: their first derivative order. The term “after” was used in the sample names to indicate that the results were obtained after MO degradation.
3.3.2. Reusability of Ag3PO4 Samples: Recycling Tests and Photoluminescence Measurements
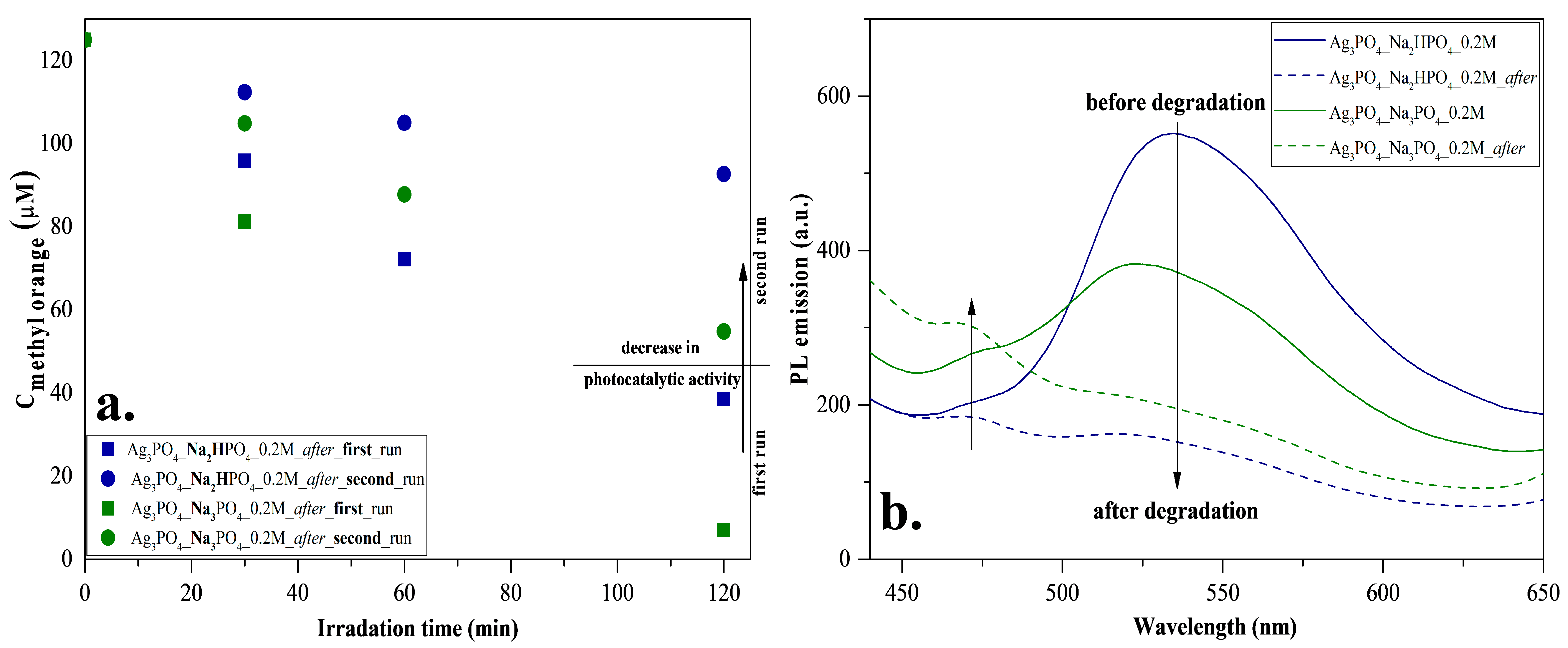
To investigate reusability, we have chosen the Ag3PO4_Na2HPO4_0.2M and Ag3PO4_Na3PO4_0.2M samples because these were the most efficient photocatalysts with a degradation of 89% and 95%, respectively (Table 1). The samples had photocatalytic activity after the second round as well (Figure 8a); however, a decrease in the degradation of MO was observed. The reason for this decrease was investigated by photoluminescence measurements (Figure 8b) to examine the recombination properties of Ag3PO4. Similar to Mohammadreza Batvandi et al. [31], two of the observed emission bands in our samples were in the violet and blue-cyan wavelength regions. The emission band observed in the violet region corresponds to charge transfer and self-trapping [32]. The blue-cyan wavelength can be assigned to surface oxygen vacancies and defects. After degradation, the intensity of these bands decreased, and in parallel, the band in the violet region increased slightly. The reason for the decreasing bands may be the disappearance of surface oxygen vacancies and defects, which may result from the deposition of Ag nanoparticles on the semiconductor surface. Based on our interpretation, the disappearance of oxygens vacancies and significant disappearances of defects resulted in a decrease in photocatalytic activity.
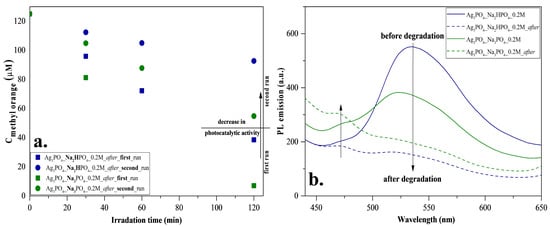
Figure 8.
Stability measurements for Ag3PO4_Na2HPO4_0.2M and Ag3PO4_Na3PO4_0.2M: (a) reusability test with MO degradation under visible light irradiation and (b) photoluminescence spectra at 325 nm excitation.
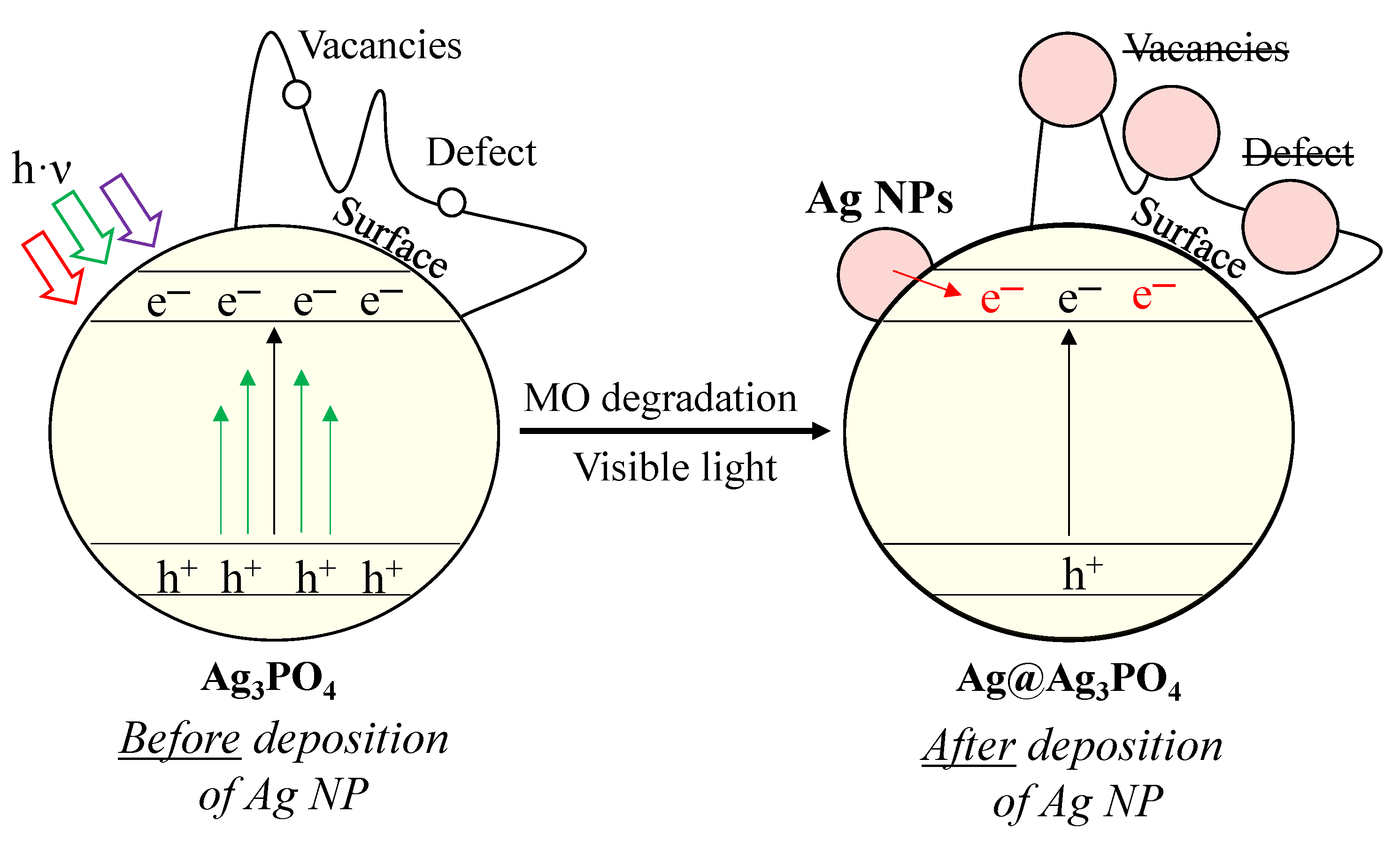
Considering all the results presented above, we proposed a possible charge transfer mechanism (Figure 9). Before elaborating on the mechanism, we summarized the main conclusions that are indispensable for understanding the mechanism as follows:
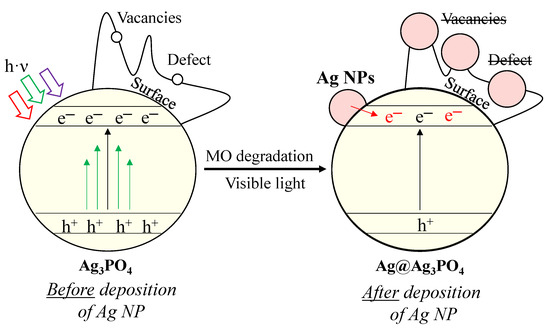
Figure 9.
Proposed degradation mechanism of MO with Ag3PO4 photocatalysts.
- Besides the Ag3PO4, only Ag4P2O7 was observed after the synthesis. Typical bands for surface oxygen vacancies and defects were also observed in the PL spectra.
- Two different pathways were observed using MO as a model pollutant, and the kinetics parameters changed after the first hour.
- The deposition of Ag nanoparticles was observed after MO degradation, which resulted in the lack of surface oxygen vacancies and defects.
Defects and vacancies are essential for the degradation of MO. The degradation of MO occurs by reactive radicals/h+ formed in the valence bands of materials. After irradiation, structural changes were observed in our materials. We assume that Ag nanoparticles (Figure 9) overlap with or replace vacancies and defects (demonstrated by PL measurements; Figure 8b). These nanoparticles change the reaction mechanism, which was confirmed by the change in the kinetic parameters (from 0th→1st and 2nd order reaction; Table 2). This assumption was confirmed by the typical band observed in the PL spectra, attributed to charge transfer and self-trapping (Figure 8b). This also caused fewer holes to form. Thus, the charge transfer between Ag nanoparticles and Ag3PO4 occurs by utilizing their localized surface plasmon resonance effect (Figure 9). Thereby, it can be deduced that the formed Ag nanoparticles do not modify the photocatalysts; they only change the mechanism of MO degradation by promoting the transfer of electrons to the semiconductor conduction band. Consequently, the formation of holes is no longer favored. The lack of charge carrier holes also results in a lower photocatalytic efficiency, supported by the MO degradation rate slowing down during the 2-h-long experiment. This is also supported by the lower MO degradation in the second run of the reusability tests. In addition, the formation of Ag NPs could also degrade the Ag3PO4 and could damage the semiconductor via Ag+ ions being reduced into Ag NPs. These are observable by the change in the DR spectra of the samples before (Figure 4a) and after (Figure 7b) the degradation.
Although Ag nanoparticles were formed on the surface, they participated in electron transfer (transferring electrons to the conduction band of the semiconductor) processes, which did not promote the degradation of MO.
4. Conclusions
This paper examined the effect of different phosphate sources on the synthesis and photocatalytic activity of Ag3PO4. We proved that the formation of Ag4P2O7 depends on the nature of the phosphate source. The type of phosphate sources influenced the light absorption properties and photocatalytic activity of the samples. We concluded that Ag4P2O7 inhibits the photocatalytic activity of Ag3PO4. In addition to other similar publications in the literature, we also investigated the stability and reusability of Ag3PO4. We concluded that Ag species were formed on the Ag3PO4, which resulted in a slightly lower methyl orange degradation during the reusability processes. The difference could be attributed to the localized surface plasmon resonance of Ag nanoparticles, promoting the transfer of electrons within the semiconductor and preventing hole formation. This fact was supported by PL measurements. Considering the characterization results obtained before and after the photocatalytic tests, we concluded that Ag3PO4-based materials could be reliably used for the degradation of MO as they mostly retain their photoactivity during second recycling test.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano13010089/s1, Figure S1: The particle size distribution of Ag3PO4 materials; Figure S2: The adsorption test of the Ag3PO4_Na3PO4_0.2M and Ag3PO4_Na3PO4_0.1M on MO (C = 125 μM).
Author Contributions
Conceptualization, Z.-R.T., G.K., Z.P. and D.D.; methodology, D.D., T.G. and K.M.; formal analysis, G.K.; investigation, M.T. and M.F.; resources, L.B. and K.H.; writing—original draft preparation, D.D., Z.-R.T. and G.K.; writing—review and editing L.B., K.H., I.S., G.K., Z.P., K.M. and T.G.; visualization, Z.-R.T.; supervision, K.H. and Z.P.; project administration, K.H., G.K., K.M. and Z.P. All authors have read and agreed to the published version of the manuscript.
Funding
K. Magyari: Zs. Pap and G. Kovács acknowledge the financial support of the Bolyai János fellowship.
Institutional Review Board Statement
Not relevant.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- al Kausor, M.; sen Gupta, S.; Chakrabortty, D. Ag3PO4-Based Nanocomposites and Their Applications in Photodegradation of Toxic Organic Dye Contaminated Wastewater: Review on Material Design to Performance Enhancement. J. Saudi Chem. Soc. 2020, 24, 20–41. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What Is Degussa (Evonic) P25? Crystalline Composition Analysis, Reconstruction from Isolated Pure Particles and Photocatalytic Activity Test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Feng, C.; Li, G.; Ren, P.; Wang, Y.; Huang, X.; Li, D. Effect of Photo-Corrosion of Ag2CO3 on Visible Light Photocatalytic Activity of Two Kinds of Ag2CO3/TiO2 Prepared from Different Precursors. Appl. Catal. B 2014, 158–159, 224–232. [Google Scholar] [CrossRef]
- Li, J.; Fang, W.; Yu, C.; Zhou, W.; Zhu, L.; Xie, Y. Ag-Based Semiconductor Photocatalysts in Environmental Purification. Appl. Surf. Sci. 2015, 358, 46–56. [Google Scholar] [CrossRef]
- Yi, Z.; Ye, J.; Kikugawa, N.; Kako, T.; Ouyang, S.; Stuart-Williams, H.; Yang, H.; Cao, J.; Luo, W.; Li, Z.; et al. An Orthophosphate Semiconductor with Photooxidation Properties under Visible-Light Irradiation. Nat. Mater. 2010, 9, 559–564. [Google Scholar] [CrossRef]
- Ma, X.; Lu, B.; Li, D.; Shi, R.; Pan, C.; Zhu, Y. Origin of Photocatalytic Activation of Silver Orthophosphate from First-Principles. J. Phys. Chem. C 2011, 115, 4680–4687. [Google Scholar] [CrossRef]
- Huang, G.F.; Ma, Z.L.; Huang, W.Q.; Tian, Y.; Jiao, C.; Yang, Z.M.; Wan, Z.; Pan, A. Ag3PO4 Semiconductor Photocatalyst: Possibilities and Challenges. J. Nanomater. 2013, 2013, 371356. [Google Scholar] [CrossRef]
- Ng, H.N.; Calvo, C.; Faggiani, R. A New Investigation of the Structure of Silver Orthophosphate. Acta Cryst. 1978, B34, 898–899. [Google Scholar] [CrossRef]
- Song, X.; Li, R.; Xiang, M.; Hong, S.; Yao, K.; Huang, Y. Morphology and Photodegradation Performance of Ag3PO4 Prepared by (NH4)3PO4, (NH4)2HPO4 and NH4H2PO4. Ceram. Int. 2017, 43, 4692–4701. [Google Scholar] [CrossRef]
- Amornpitoksuk, P.; Intarasuwan, K.; Suwanboon, S.; Baltrusaitis, J. Effect of Phosphate Salts (Na3PO4, Na2HPO4, and NaH2PO4) on Ag3PO4 Morphology for Photocatalytic Dye Degradation under Visible Light and Toxicity of the Degraded Dye Products. Ind. Eng. Chem. Res. 2013, 52, 17369–17375. [Google Scholar] [CrossRef]
- Qin, L.; Tao, P.; Zhou, X.; Pang, Q.; Liang, C.; Liu, K.; Luo, X. Synthesis and Characterization of High Efficiency and Stable Spherical Ag3PO4 Visible Light Photocatalyst for the Degradation of Methylene Blue Solutions. J. Nanomater. 2015, 2015, 258342. [Google Scholar] [CrossRef]
- Yang, Z.M.; Tian, Y.; Huang, G.F.; Huang, W.Q.; Liu, Y.Y.; Jiao, C.; Wan, Z.; Yan, X.G.; Pan, A. Novel 3D Flower-like Ag3PO4 Microspheres with Highly Enhanced Visible Light Photocatalytic Activity. Mater. Lett. 2014, 116, 209–211. [Google Scholar] [CrossRef]
- Raudoniene, J.; Skaudzius, R.; Zarkov, A.; Selskis, A.; Karlsson, O.; Kareiva, A.; Garskaite, E. Wet-Chemistry Synthesis of Shape-Controlled Ag3PO4 Crystals and Their 3D Surface Reconstruction from SEM Imagery. Powder Technol. 2019, 345, 26–34. [Google Scholar] [CrossRef]
- Krungchanuchat, S.; Ekthammathat, N.; Phuruangrat, A.; Thongtem, S.; Thongtem, T. High UV-Visible Photocatalytic Activity of Ag3PO4 Dodecahedral Particles Synthesized by a Simple Hydrothermal Method. Mater. Lett. 2017, 201, 58–61. [Google Scholar] [CrossRef]
- Dong, P.; Wang, Y.; Li, H.; Li, H.; Ma, X.; Han, L. Shape-Controllable Synthesis and Morphology-Dependent Photocatalytic Properties of Ag3PO4 Crystals. J. Mater. Chem. A Mater. 2013, 1, 4651–4656. [Google Scholar] [CrossRef]
- Bi, Y.; Hu, H.; Jiao, Z.; Yu, H.; Lu, G.; Ye, J. Two-Dimensional Dendritic Ag3PO4 Nanostructures and Their Photocatalytic Properties. Phys. Chem. Chem. Phys. 2012, 14, 14486–14488. [Google Scholar] [CrossRef]
- Dhanabal, R.; Chithambararaj, A.; Velmathi, S.; Bose, A.C. Visible Light Driven Degradation of Methylene Blue Dye Using Ag3PO4. J. Environ. Chem. Eng. 2015, 3, 1872–1881. [Google Scholar] [CrossRef]
- Tóth, Z.R.; Feraru, A.; Debreczeni, D.; Todea, M.; Popescu, R.A.; Gyulavári, T.; Sesarman, A.; Negrea, G.; Vodnar, D.C.; Hernadi, K.; et al. Influence of Different Silver Species on the Structure of Bioactive Silicate Glasses. J. Non Cryst. Solids 2022, 583, 121498. [Google Scholar] [CrossRef]
- Tauc, J. Optical Properties and Electronic Structure of Amorphous Ge and Si. Mat. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- da Pereira, W.S.; Gozzo, C.B.; Longo, E.; Leite, E.R.; Sczancoski, J.C. Investigation on the Photocatalytic Performance of Ag4P2O7 Microcrystals for the Degradation of Organic Pollutants. Appl. Surf. Sci. 2019, 493, 1195–1204. [Google Scholar] [CrossRef]
- Huang, K.; Lv, Y.; Zhang, W.; Sun, S.; Yang, B.; Chi, F.; Ran, S.; Liu, X. One-Step Synthesis of Ag3PO4/Ag Photocatalyst with Visible-Light Photocatalytic Activity. Mater. Res. 2015, 18, 939–945. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, L.; Shi, J.; Deng, H. Synthesis of Ag3PO4/g-C3N4 Composite with Enhanced Photocatalytic Performance for the Photodegradation of Diclofenac under Visible Light Irradiation. Catalysts 2018, 8, 45. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Shi, Y.; Wang, Q.; Wang, A.; Gao, S. Effect of Blending Manner on Composition and Photocatalytic Performance of Ag/Ag3PO4/Ag4P2O7 Composites via an in-Situ Reduction-Precipitation Route. Inorg. Chem. Commun. 2021, 130, 108675. [Google Scholar] [CrossRef]
- Nie, C.; Dong, J.; Sun, P.; Yan, C.; Wu, H.; Wang, B. An Efficient Strategy for Full Mineralization of an Azo Dye in Wastewater: A Synergistic Combination of Solar Thermo- and Electrochemistry plus Photocatalysis. RSC Adv. 2017, 7, 36246–36255. [Google Scholar] [CrossRef]
- Putri, R.A.; Safni, S.; Jamarun, N.; Septiani, U. Kinetics Study and Degradation Pathway of Methyl Orange Photodegradation in the Presence of C-N-Codoped TiO2 Catalyst. Egypt. J. Chem. 2020, 63, 563–575. [Google Scholar] [CrossRef]
- Rodríguez-Cabo, B.; Rodríguez-Palmeiro, I.; Corchero, R.; Rodil, R.; Rodil, E.; Arce, A.; Soto, A. Photocatalytic Degradation of Methyl Orange, Methylene Blue and Rhodamine B with AgCl Nanocatalyst Synthesised from Its Bulk Material in the Ionic Liquid [P6 6 6 14]Cl. Water Sci. Technol. 2017, 75, 128–140. [Google Scholar] [CrossRef]
- Hou, M.; Li, F.; Liu, X.; Wang, X.; Wan, H. The Effect of Substituent Groups on the Reductive Degradation of Azo Dyes by Zerovalent Iron. J. Hazard. Mater. 2007, 145, 305–314. [Google Scholar] [CrossRef]
- Székely, I.; Baia, M.; Magyari, K.; Boga, B.; Pap, Z. The Effect of the PH Adjustment upon the WO3-WO3·0.33H2O-TiO2 Ternary Composite Systems’ Photocatalytic Activity. Appl. Surf. Sci. 2019, 490, 469–480. [Google Scholar] [CrossRef]
- Tóth, Z.R.; Hernadi, K.; Baia, L.; Kovács, G.; Pap, Z. Controlled Formation of Ag-AgxO Nanoparticles on the Surface of Commercial TiO2 Based Composites for Enhanced Photocatalytic Degradation of Oxalic Acid and Phenol. Catal. Today 2020. [Google Scholar] [CrossRef]
- Tóth, Z.R.; Pap, Z.; Kiss, J.; Baia, L.; Gyulavári, T.; Czekes, Z.; Todea, M.; Magyari, K.; Kovács, G.; Hernadi, K. Shape Tailoring of AgBr Microstructures: Effect of the Cations of Different Bromide Sources and Applied Surfactants. RSC Adv. 2021, 11, 9709–9720. [Google Scholar] [CrossRef]
- Batvandi, M.; Haghighatzadeh, A.; Mazinani, B. Synthesis of Ag3PO4 Microstructures with Morphology-Dependent Optical and Photocatalytic Behaviors. Appl. Phys. A Mater. Sci. Process. 2020, 126, 571. [Google Scholar] [CrossRef]
- Botelho, G.; Andres, J.; Gracia, L.; Matos, L.S.; Longo, E. Photoluminescence and Photocatalytic Properties of Ag3PO4 Microcrystals: An Experimental and Theoretical Investigation. Chempluschem 2016, 81, 202–212. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).