Synthesis of CuO/GO-DE Catalyst and Its Catalytic Properties and Mechanism on Ciprofloxacin Degradation
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
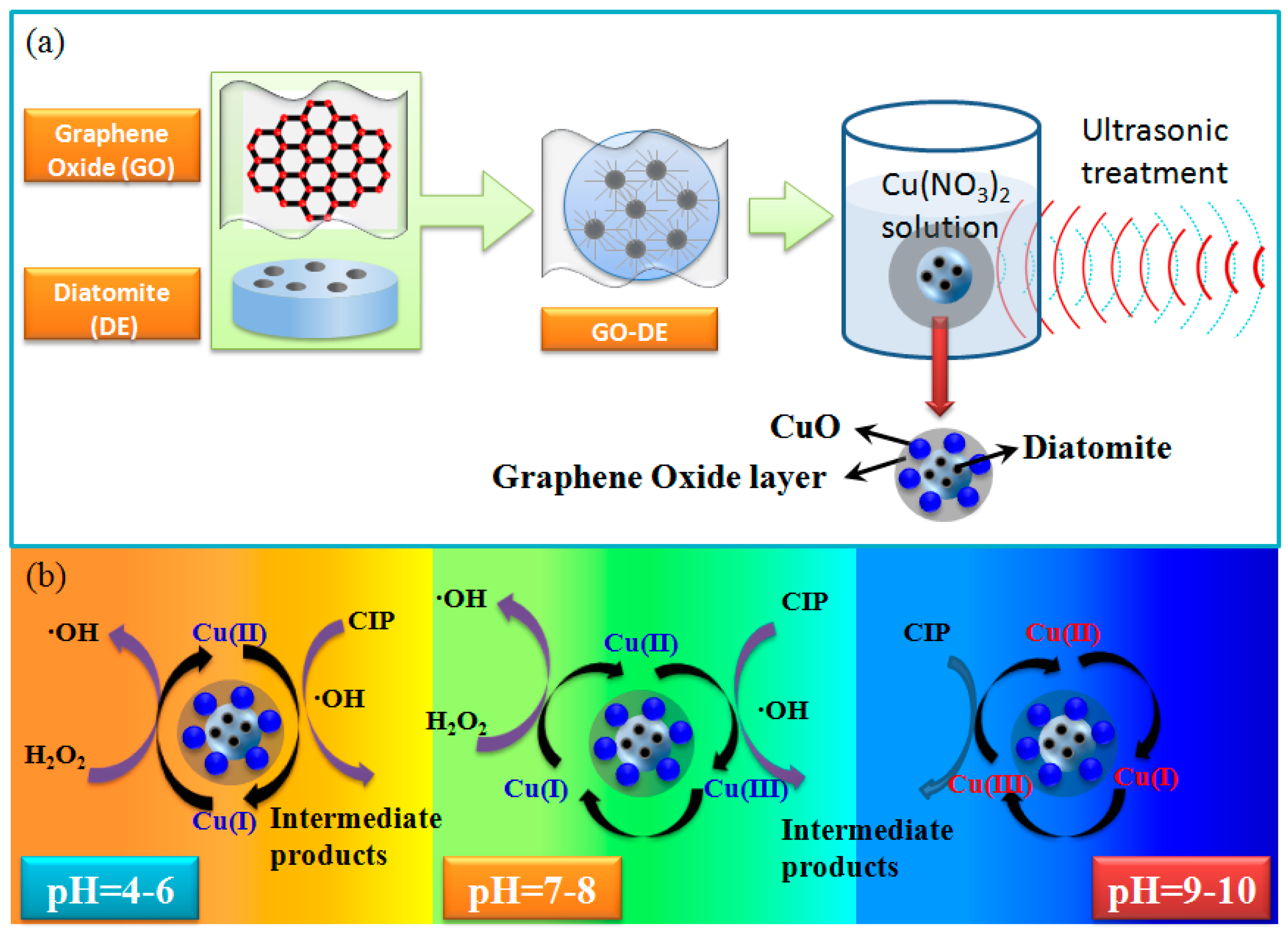
2.2. Synthesis of Metal-Loaded GO-DE Composites
2.3. Characterization
2.4. CIP Degradation
2.5. Measurement of ·OH Concentration and ·OH Scavenging Experiment
2.6. Capture of Cu(III) in Alkaline Condition
3. Result and Discussion
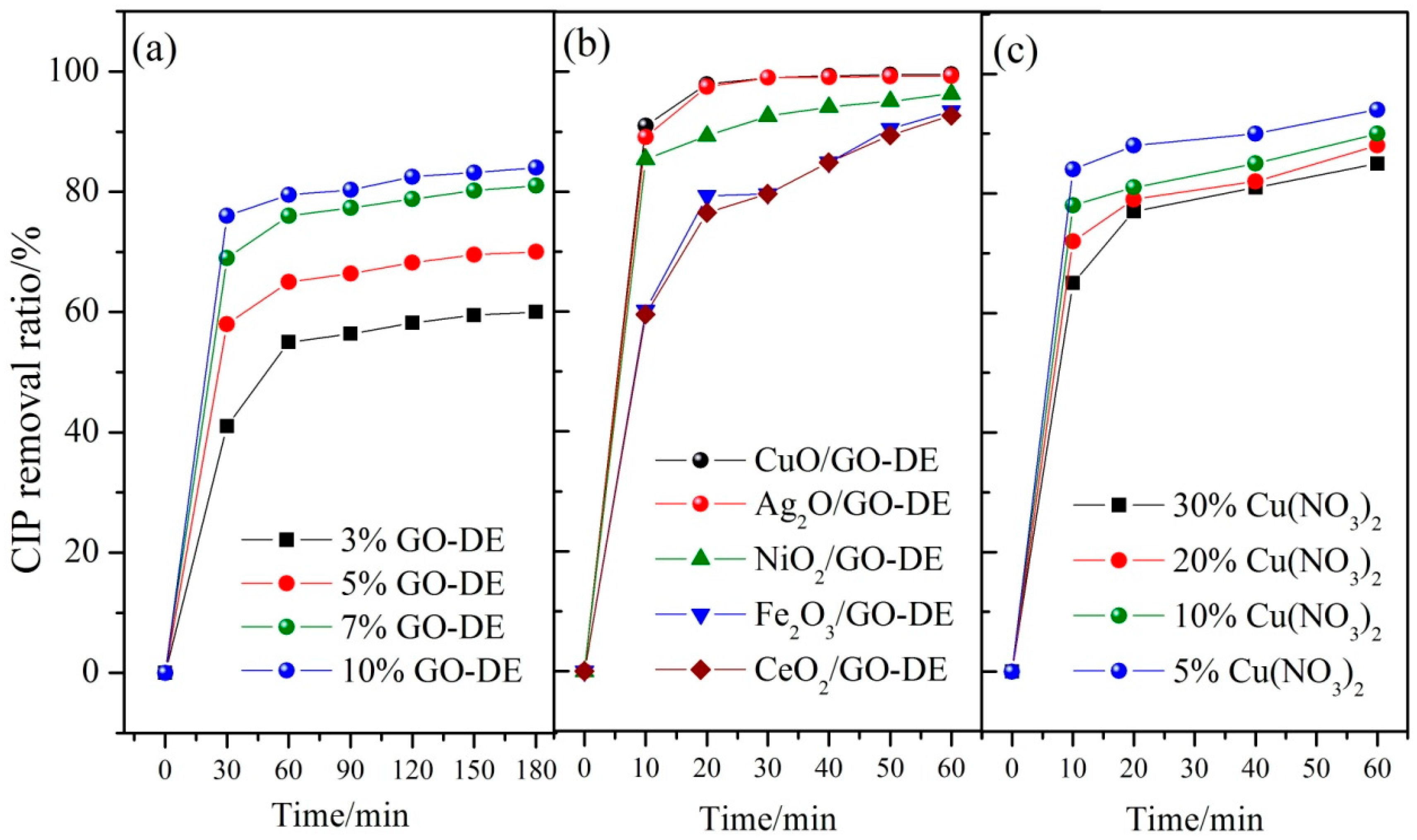
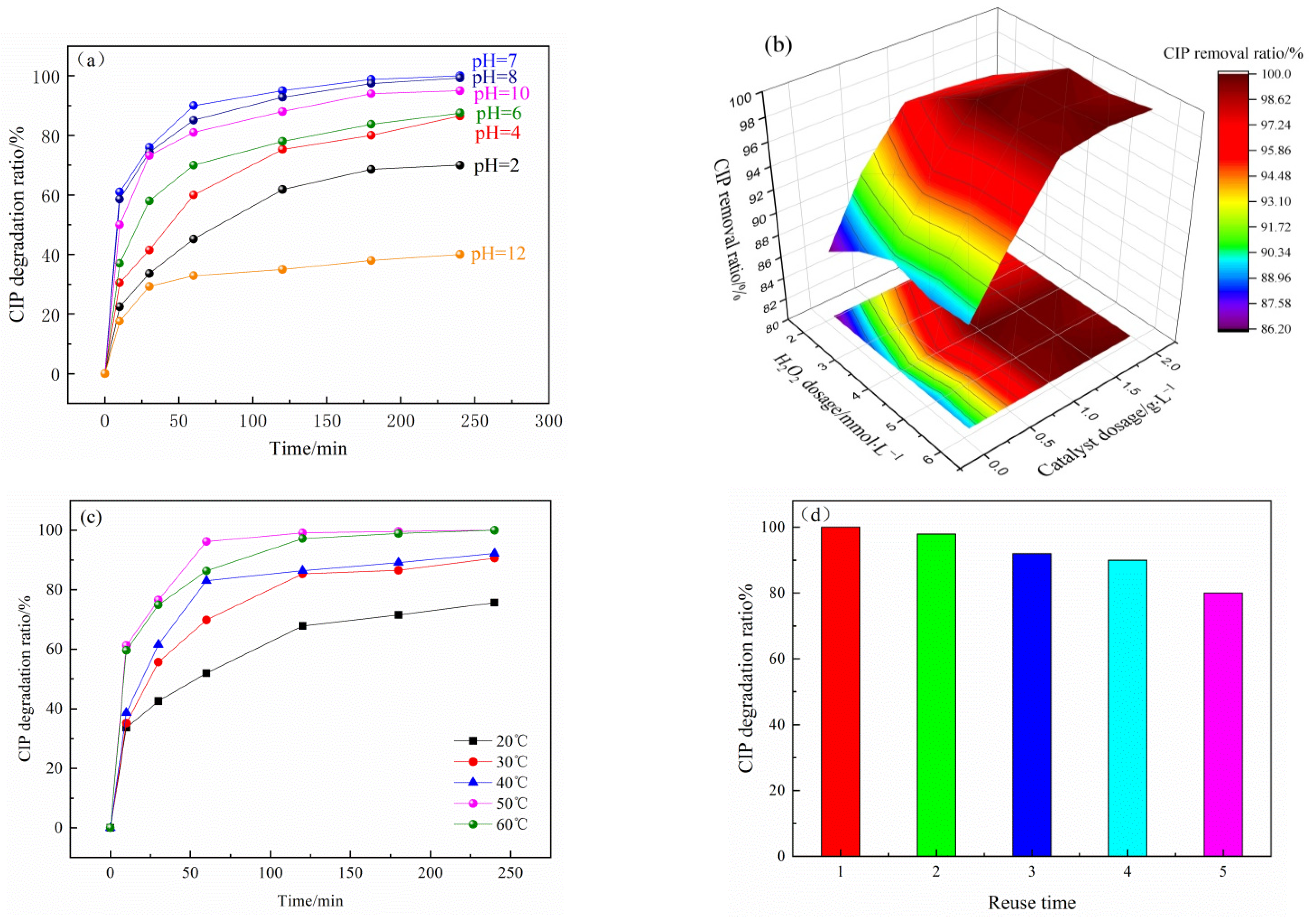
3.1. Optimization of Catalyst Preparation
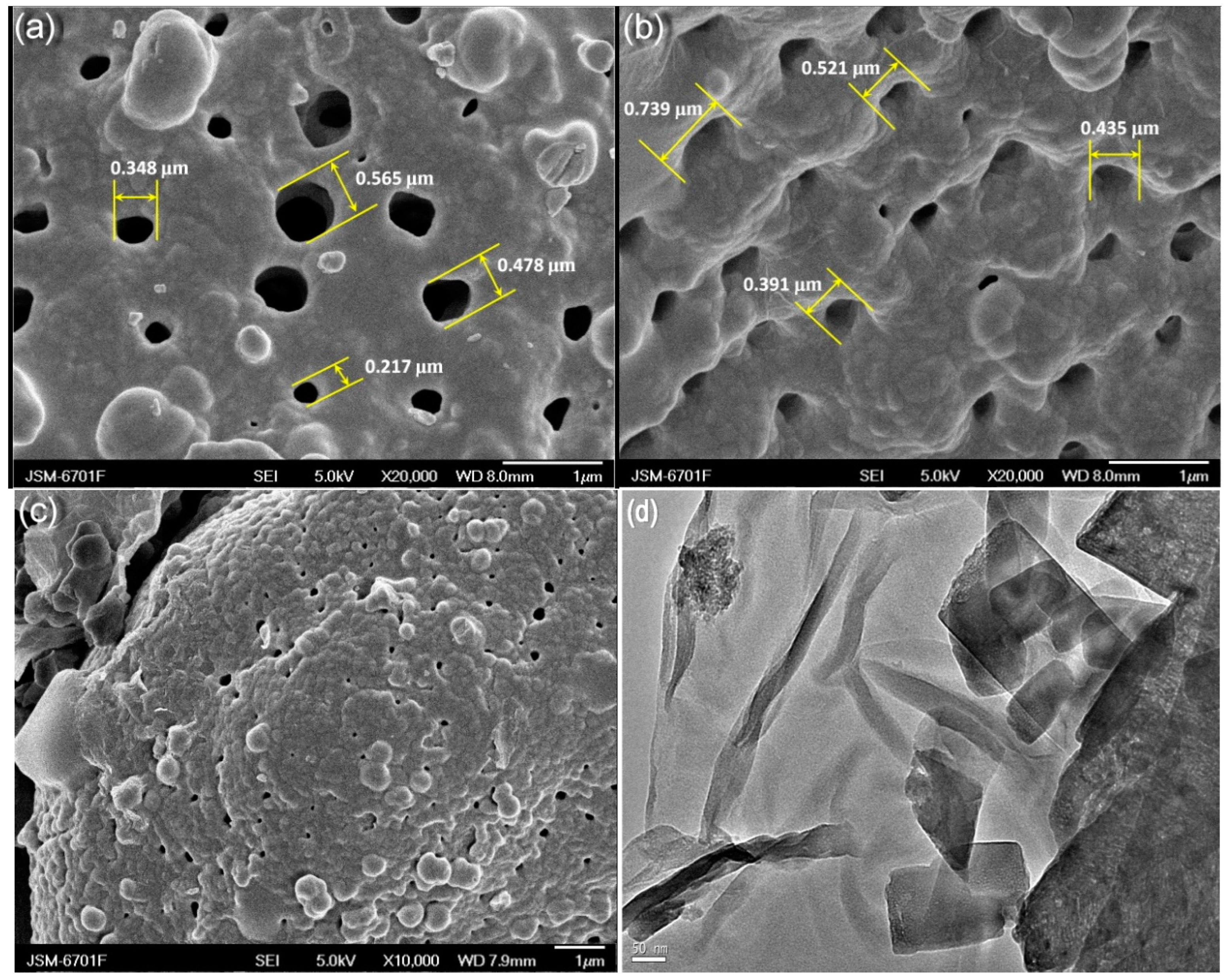
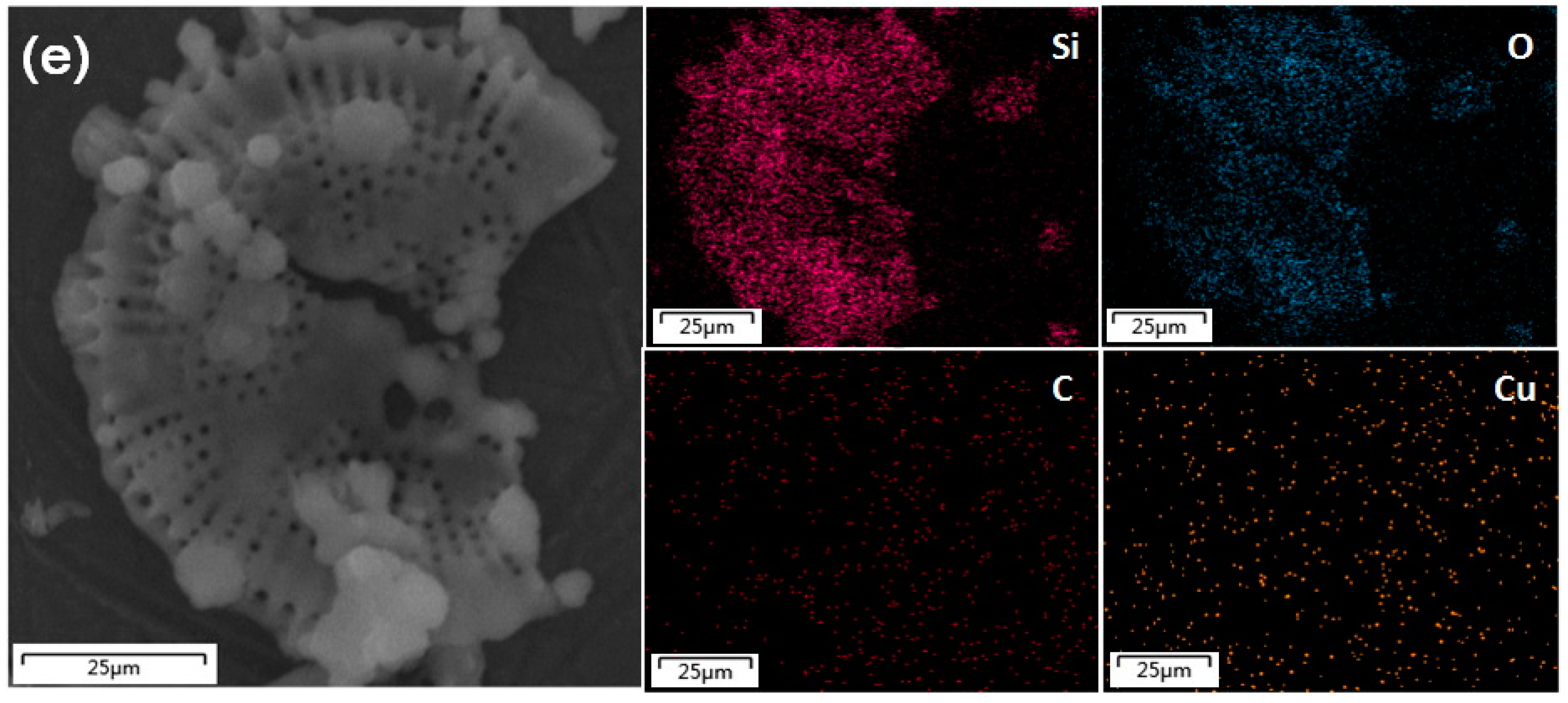
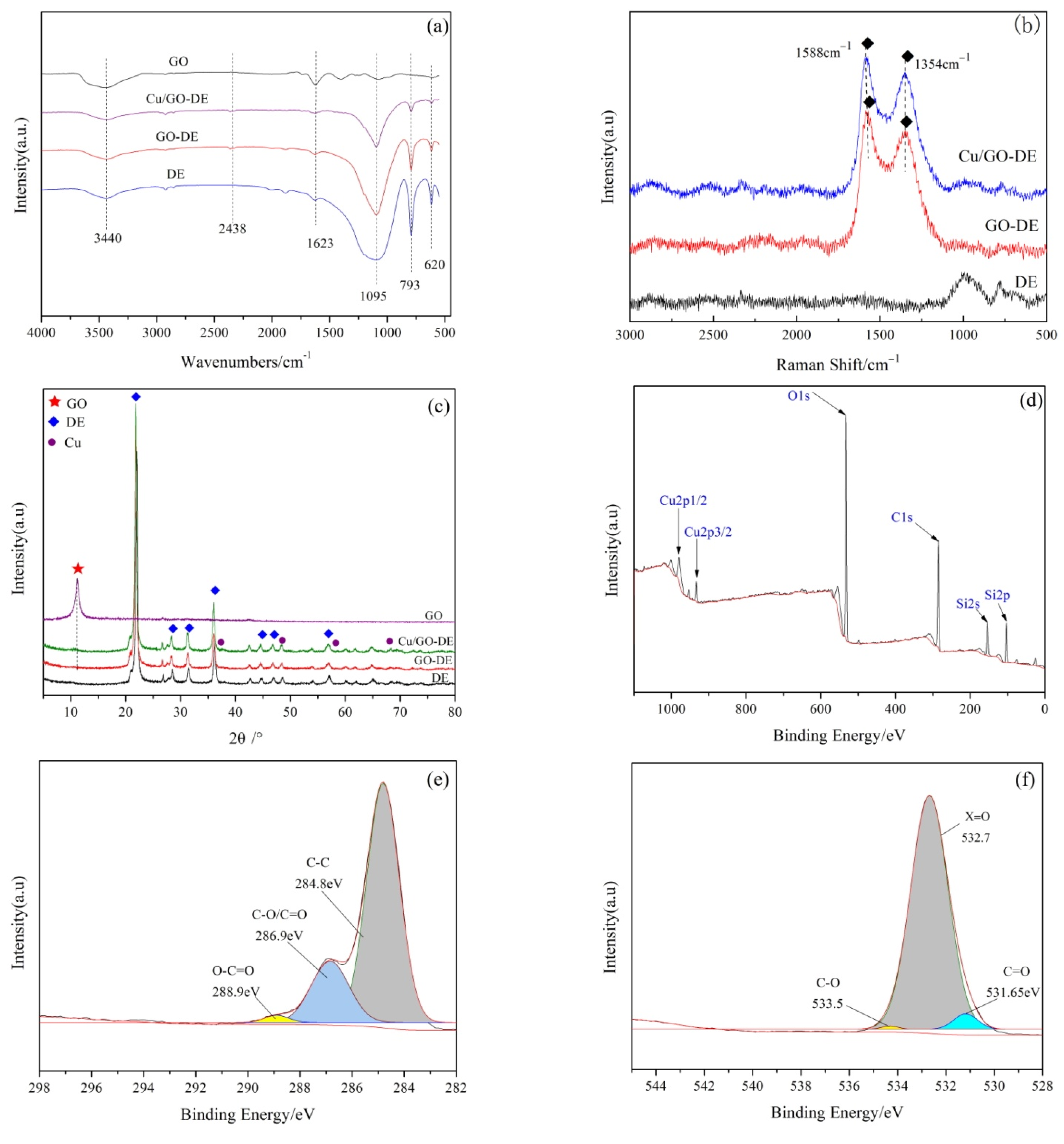
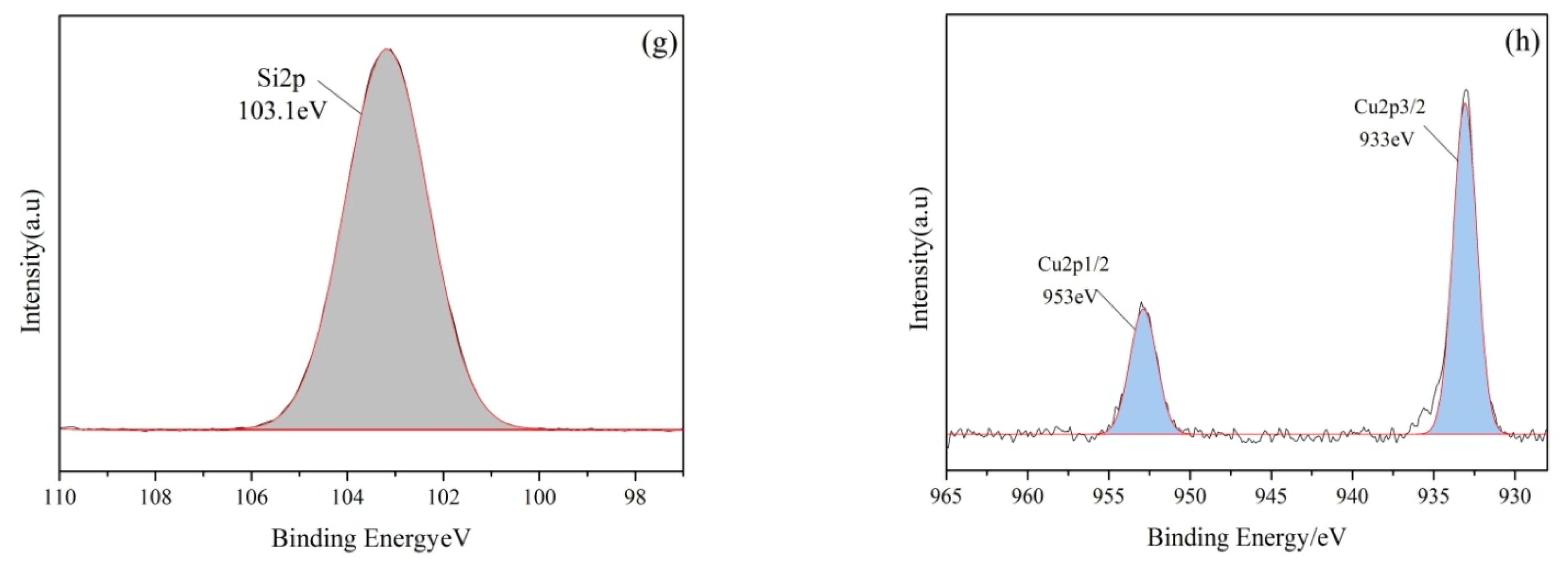
3.2. Characterization of CuO/GO-DE
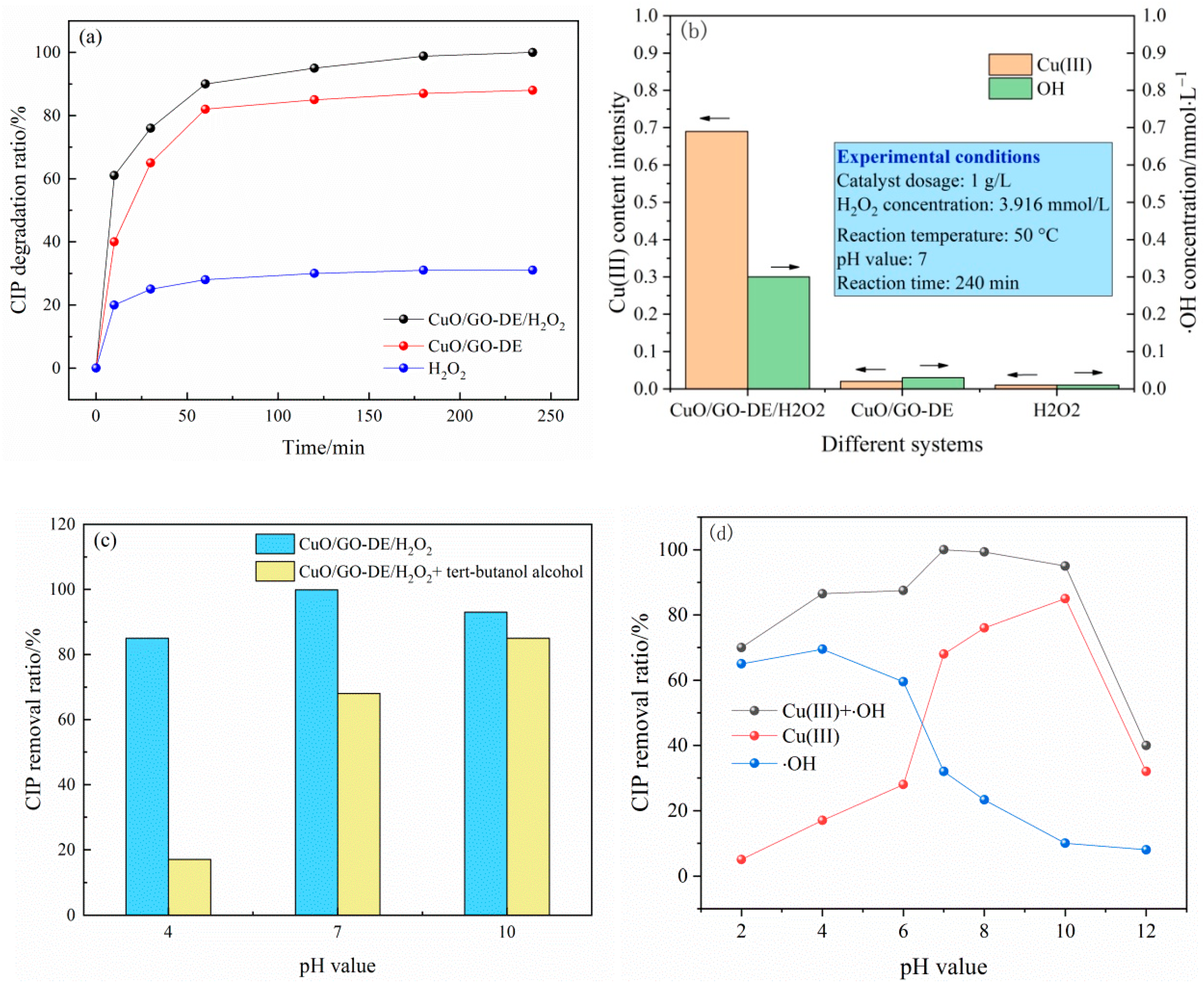
3.3. Degradation of CIP
3.4. Degradation Mechanism of Ciprofloxacin
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Przybylska, N.; Śliwińska-Bartkowiak, M.; Kościński, M.; Rotnicki, K.; Bartkowiak, M.; Jurga, S. Confined effect of water solution of ciprofloxacin in carbon nanotubes studied by Raman and Fourier Transform Infrared Spectroscopy methods. J. Mol. Liq. 2021, 336, 115938. [Google Scholar] [CrossRef]
- Du, Y.; Dai, M.; Naz, I.; Hao, X.; Wei, X.; Rong, R.; Peng, C.; Ali, I. Carbothermal reduction synthesis of zero-valent iron and its application as a persulfate activator for ciprofloxacin degradation. Sep. Purif. Technol. 2021, 275, 119201. [Google Scholar] [CrossRef]
- HGull, H.; Karadag, C.; Senger, B.; Sorg, R.V.; Möller, P.; Mellert, K.; Steiger, H.; Hänggi, D.; Cornelius, J.F. Ciprofloxacin enhances phototoxicity of 5-aminolevulinic acid mediated photodynamic treatment for chordoma cell lines. J. Photodiagnosis Photodyn. Ther. 2021, 35, 102346. [Google Scholar] [CrossRef]
- Zhou, M.; Li, C.; Zhao, L.; Ning, J.; Pan, X.; Cai, G.; Zhu, G. Synergetic effect of nano zero-valent iron and activated carbon on high-level ciprofloxacin removal in hydrolysis-acidogenesis of anaerobic digestion. J. Sci. Total. Environ. 2021, 752, 142261. [Google Scholar] [CrossRef] [PubMed]
- Sar, A.B.; Shabani, E.G.; Haghighi, M.; Shabani, M. Synergistic catalytic degradation of ciprofloxacin using magnetic carbon nanomaterial/NiFe2O4 promoted cold atmospheric pressure plasma jet: Influence of charcoal, multi walled carbon nanotubes and walnut shell. J. Taiwan Inst. Chem. Eng. 2022, 132, 104131. [Google Scholar] [CrossRef]
- Ude, Z.; Flothkötter, N.; Sheehan, G.; Brennan, M.; Kavanagh, K.; Marmion, C.J. Multi-targeted metallo-ciprofloxacin derivatives rationally designed and developed to overcome antimicrobial resistance. Int. J. Antimicrob. Agents 2021, 58, 106449. [Google Scholar] [CrossRef]
- Mahmoudpour, M.; Kholafazad-Kordasht, H.; Dolatabadi, J.E.N.; Hasanzaden, M.; Rad, A.H.; Torbati, M. Sensitive aptasensing of ciprofloxacin residues in raw milk samples using reduced graphene oxide and nanogold-functionalized poly (amidoamine) dendrimer: An innovative apta-platform towards electroanalysis of antibiotics. Anal. Chim. Acta 2021, 1174, 338736. [Google Scholar] [CrossRef]
- Yu, F.; Yang, Z.; Zhang, X.; Yang, P.; Ma, J. Lanthanum modification κ-carrageenan/sodium alginate dual-network aerogels for efficient adsorption of ciprofloxacin hydrochloride. Environ. Technol. Innov. 2021, 24, 102052. [Google Scholar] [CrossRef]
- Krishnan, S.; Shriwastav, A. Chlorophyll sensitized and salicylic acid functionalized TiO2 nanoparticles as a stable and efficient catalyst for the photocatalytic degradation of ciprofloxacin with visible light. Environ. Res. 2023, 216, 114568. [Google Scholar] [CrossRef]
- Li, X.; Wang, W.; Dou, J.; Gao, J.; Chen, S.; Quan, X.; Zhao, H. Dynamic adsorption of ciprofloxacin on carbon nanofibers: Quantitative measurement by in situ fluorescence. J. Water Process. Eng. 2016, 9, 14–20. [Google Scholar] [CrossRef]
- Huang, Y.; Nengzi, L.C.; Zhang, X.; Gou, J.; Gao, Y.; Zhu, G.; Cheng, Q.; Cheng, X. Catalytic degradation of ciprofloxacin by magnetic CuS/Fe2O3/Mn2O3 nanocomposite activated peroxymonosulfate: Influence factors, degradation pathways and reaction mechanism. Chem. Eng. J. 2020, 388, 124274. [Google Scholar] [CrossRef]
- Yu, F.; Sun, S.; Han, S.; Zheng, J.; Ma, J. Adsorption removal of ciprofloxacin by multi-walled carbon nanotubes with different oxygen contents from aqueous solutions. Chem. Eng. J. 2016, 285, 588–595. [Google Scholar] [CrossRef]
- Alshaikh, H.; Shawky, A.; Mohamed, R.M.; Knight, J.G.; Selva, R.L. Solution-based synthesis of Co3O4/ZnO pn heterojunctions for rapid visible-light-driven oxidation of ciprofloxacin. J. Mol. Liq. 2021, 334, 116092. [Google Scholar] [CrossRef]
- Chafyq, E.H.; Legrouri, K.; Aghrouch, M.; Oumam, M.; Mansouri, S.; Khouya, E.H.; Hannache, H. Adsorption of ciprofloxacin antibiotic on materials prepared from Moroccan oil shales. Chem. Phys. Lett. 2021, 778, 138707. [Google Scholar] [CrossRef]
- Devi, M.G.; Dutta, S.; Al Hinai, A.T.; Feroz, S. Nano engineered biodegradable capsules for the encapsulation and kinetic release studies of ciprofloxacin hydrochloride. J. Indian Chem. Soc. 2021, 98, 100109. [Google Scholar] [CrossRef]
- Nie, X.; Li, G.Y.; Li, S.S.; Luo, Y.M.; Wan, Q.; An, T.C. Highly efficient adsorption and catalytic degradation of ciprofloxacin by a novel heterogeneous Fenton catalyst of hexapod-like pyrite nanosheets mineral clusters. Appl. Catal. B Environ. 2022, 300, 120734. [Google Scholar] [CrossRef]
- van Garderen, N.; Clemens, F.J.; Kaufmann, J.; Urbanek, M.; Binkowski, M.; Graule, T.; Aneziris, C.G. Pore analyses of highly porous diatomite and clay based materials for fluidized bed reactors. Microporous Mesoporous Mater. 2012, 151, 255–263. [Google Scholar] [CrossRef]
- Lv, Z.Q.; Jiang, A.N.; Jin, J.X. Influence of ultrafine diatomite on cracking behavior of concrete: An acoustic emission analysis. Constr. Build. Mater. 2012, 308, 124993. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Wei, W.; Wang, Y.; Ni, B.J. Enhancing methane production from algae anaerobic digestion using diatomite. J. Clean. Prod. 2021, 315, 128138. [Google Scholar] [CrossRef]
- Taoukil, D.; Meski, Y.E.; Lahlaouti, M.L.; Djedjig, R.; Bouardi, A.E. Effect of the use of diatomite as partial replacement of sand on thermal and mechanical properties of mortars. J. Build. Eng. 2021, 42, 103038. [Google Scholar] [CrossRef]
- Chen, J.; Ren, Q.F.; Ding, Y.; Xiong, C.Y.; Guo, W.M. Synthesis of bifunctional composites Ag/BiOCl/diatomite: Degradation of tetracycline and evaluation of antimicrobial activity. J. Environ. Chem. Eng. 2021, 9, 106476. [Google Scholar] [CrossRef]
- Shen, T.M.; Xu, H.X.; Miao, Y.; Ma, L.L.; Chen, N.C.; Xie, Q.L. Study on the adsorption process of Cd(II) by Mn-diatomite modified adsorbent. Mater. Lett. 2021, 300, 130087. [Google Scholar] [CrossRef]
- Xiong, C.Y.; Ren, Q.F.; Chen, S.H.; Liu, X.Y.; Jin, Z.; Ding, Y. A multifunctional Ag3PO4/Fe3O4/Diatomite composites: Photocatalysis, adsorption and sterilization. Mater. Today Commun. 2021, 28, 102695. [Google Scholar] [CrossRef]
- Sun, Y.X.; Zhou, J.B.; Liu, D.; Liu, X.J.; Leng, C.H. Highly efficient removal of tetracycline hydrochloride under neutral conditions by visible photo-Fenton process using novel MnFe2O4/diatomite composite. J. Water Process. Eng. 2021, 43, 102307. [Google Scholar] [CrossRef]
- Dang, T.D.; Banerjee, A.N.; Tran, Q.T.; Roy, S. Fast degradation of dyes in water using manganese-oxide-coated diatomite for environmental remediation. J. Phys. Chem. Solids 2016, 98, 50–58. [Google Scholar] [CrossRef]
- Wang, M.F.; Xu, H.L.; Huang, C.N.; Cui, Z.T.; Li, M.L.; Song, B.; Shao, G.; Wang, H.L.; Lu, H.X.; Zhang, R. Preparation of g-C3N4/diatomite composite with improved visible light photocatalytic activity. Inorg. Chem. Commun. 2021, 129, 108645. [Google Scholar] [CrossRef]
- Ulloa-Ovares, D.; Rodríguez-Rodríguez, C.E.; Masís-Mora, M.; Durán, J.E. Simultaneous degradation of pharmaceuticals in fixed and fluidized bed reactors using iron-modified diatomite as heterogeneous Fenton catalyst. Process. Saf. Environ. Prot. 2021, 152, 97–107. [Google Scholar] [CrossRef]
- Noreen, S.; Tahira, M.; Ghamkhar, M.; Hafiz, I.; Bhatti, H.N.; Nadeem, R.; Murtaza, M.A.; Yaseen, M.; Sheikh, A.A.; Naseem, Z.; et al. Treatment of textile wastewater containing acid dye using novel polymeric graphene oxide nanocomposites (GO/PAN, GO/PPy, GO/PSty). J. Mater. Res. Technol. 2021, 14, 25–35. [Google Scholar] [CrossRef]
- Brodie, B.C., XIII. On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar] [CrossRef]
- Chung, C.; Kim, Y.K.; Shin, D.; Ryoo, S.R.; Hong, B.H.; Min, D.H. Biomedical applications of graphene and graphene oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, W.; Wu, M.Y.; Qi, G.S.; Yuan, Y.; Li, J.; Su, H.L.; Zhang, H. Preparation and properties of cellulosenanofiber (CNF)/polyvinyl alcohol (PVA)/graphene oxide (GO): Application of CO2 absorption capacity and molecular dynamics simulation. J. Environ. Manag. 2022, 302, 114044. [Google Scholar] [CrossRef]
- Majumdar, S.; Ghosh, M.; Mukherjee, S.; Satpati, B.; Biswajit, D. DNA mediated graphene oxide (GO)-nanosheets dispersed supramolecular GO-DNA hydrogel: An efficient soft-milieu for simplistic synthesis of Ag-NPs@ GO-DNA and Gram +ve/−ve bacteria-based Ag-NPs@ GO-DNA-bacteria nano-bio composites. J. Mol. Liq. 2021, 342, 117482. [Google Scholar] [CrossRef]
- Raza, M.M.H.; Khan, S.; Aalam, S.M.; Sadiq, M.; Sarvar, M.; Zulfequar, M.; Husain, S.; Ali, J. Study the electron field emission properties of plasma-based reduction of graphene oxide (GO): An ex-situ plasma approach. Carbon Trends 2021, 5, 100127. [Google Scholar] [CrossRef]
- Grajek, H.; Jonik, J.; Witkiewicz, Z.; Wawer, T.; Purchaa, M. Applications of graphene and its derivatives in chemical analysis. Crit. Rev. Anal. Chem. 2020, 50, 445–471. [Google Scholar] [CrossRef]
- Hassan, N.S.; Jalil, A.A.; Azami, M.S.; Rahman, A.F.A.; Firmansyah, M.L.; Nabgan, W. Photodegradation of bisphenol A from aqueous solution over reduced graphene oxide supported on tetragonal silica-zirconia nanocatalysts: Optimization using RSM. Process. Saf. Environ. 2021, 156, 496–507. [Google Scholar] [CrossRef]
- Liu, M.S.; Wang, J.; Wang, X.; Zhu, W.X.; Yao, X.L.; Su, L.H.; Sun, J.; Yue, T.L.; Wang, J.L. Highly efficient and cost-effective removal of patulin from apple juice by surface engineering of diatomite with sulfur-functionalized graphene oxide. Food Chem. 2019, 300, 125111. [Google Scholar] [CrossRef]
- Zhang, T.; Qian, C.Y.; Guo, P.R.; Gan, S.C.; Dong, L.Y.; Bai, G.; Guo, Q.Y. A novel reduced graphene oxide-attapulgite (RGO-ATP) supported Fe2O3 catalyst for heterogeneous Fenton-like oxidation of ciprofloxacin: Degradation mechanism and pathway. Catalysts 2020, 10, 189. [Google Scholar] [CrossRef]
- Zhang, T.; Dong, L.Y.; Du, J.H.; Qian, C.Y.; Wang, Y. CuO and CeO2 assisted Fe2O3/attapulgite catalyst for heterogeneous Fenton-like oxidation of methylene blue. RSC Adv. 2020, 10, 23431–23439. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G.Q. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Xu, Z.H.; Jing, C.Y.; Li, F.S.; Meng, X.G. Mechanisms of photocatalytical degradation of monomethylarsonic and dimethylarsinic acids using nanocrystalline titanium dioxide. Environ. Sci. Technol. 2008, 42, 2349–2354. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Xu, H.D.; Jiang, N.; Wang, Z.M.; Jiang, J.; Zhang, T. Trace cupric species triggered decomposition of peroxymonosulfate and degradation of organic pollutants: Cu (III) being the primary and selective intermediate oxidant. Environ. Sci. Technol. 2020, 54, 4686–4694. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Goetz, V.; Chiron, S. Peroxydisulfate activation process on copper oxide: Cu (III) as the predominant selective intermediate oxidant for phenol and waterborne antibiotics removal. J. Environ. Chem. Eng. 2021, 9, 105145. [Google Scholar] [CrossRef]
- Chua, C.K.; Sofer, Z.; Pumera, M. Graphite oxides: Effects of permanganate and chlorate oxidants on the oxygen composition. Chem. A Eur. J. 2012, 18, 13453–13459. [Google Scholar] [CrossRef]
- Li, M.; Shi, J.B. Mechanical and thermal performance assessment of paraffin/expanded vermiculite-diatomite composite phase change materials integrated mortar: Experimental and numerical approach. Sol. Energy. 2021, 227, 343–353. [Google Scholar] [CrossRef]
- Cheng, G.; Liu, Y.L.; Wang, Z.G.; Zhang, J.L.; Sun, D.H.; Ni, J.Z. The GO/rGO–Fe3O4 composites with good water-dispersibility and fast magnetic response for effective immobilization and enrichment of biomolecules. J. Mater. Chem. 2012, 22, 21998–22004. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Zhou, T.; Huang, Y.; Zhao, L.; Ren, Z.; Liu, X.; Chen, L.; Shao, Y.; Ma, X.; et al. Photocatalytic removal of hexavalent chromium by Fe doped g-C3N4 loaded with dispersed diatomite modified by electron beam bombardment. J. Clean. Prod. 2021, 315, 128219. [Google Scholar] [CrossRef]
- Singhal, S.; Kaur, J.; Namgyal, T.; Sharma, R. Cu-doped ZnO nanoparticles: Synthesis, structural and electrical properties. J. Physica. B. 2012, 407, 1223–1226. [Google Scholar] [CrossRef]
- Wang, J.X.; Zhang, G.Q.; Qiao, S.; Zhou, J.T. Magnetic Fe0/iron oxide-coated diatomite as a highly efficient adsorbent for recovering phosphorus from water. Chem. Eng. J. 2021, 412, 128696. [Google Scholar] [CrossRef]
- Tajmiri, S.; Hosseini, M.R.; Azimi, E. Combined photocatalytic-adsorptive removal of water contaminants using a biologically prepared CdS-diatomite nanocomposite. Mater. Chem. Phys. 2021, 258, 123913. [Google Scholar] [CrossRef]
- Chio, J.; Oh, H.; Han, S.W.; Ahn, S.; Noh, J.; Park, J.B. Preparation and characterization of graphene oxide supported Cu, Cu2O, and CuO nanocomposites and their high photocatalytic activity for organic dye molecule. Curr. Appl. Phys. 2017, 17, 137–145. [Google Scholar] [CrossRef]
- Tang, G.Q.; Jiang, Z.G.; Li, X.F.; Zhang, H.B.; Dasari, A.; Yu, Z.Z. Three dimensional graphene aerogels and their electrically conductive composites. Carbon 2014, 77, 592–599. [Google Scholar] [CrossRef]
- Kim, H.E.; Nguyen, T.T.M.; Lee, H.; Lee, C. Enhanced inactivation of Escherichia coli and MS2 coliphage by cupric ion in the presence of hydroxylamine: Dual microbicidal effects. Environ. Sci. Technol. 2015, 49, 14416–14423. [Google Scholar] [CrossRef]
- Pham, A.N.; Xing, G.; Miller, C.J.; Waite, T.D. Fenton-like copper redox chemistry revisited: Hydrogen peroxide and superoxide mediation of copper-catalyzed oxidant production. J. Catal. 2013, 301, 54–64. [Google Scholar] [CrossRef]
- Ulanski, P.; Sonntag, C.V. Stability Constants and Decay of Aqua-Copper (III)–A Study by Pulse Radiolysis with Conductometric and Optical Detection. Eur. J. Inorg. Chem. 2000, 2000, 1211–1217. [Google Scholar] [CrossRef]
- Deng, Y.; Handoko, A.D.; Du, Y.; Xi, S.; Yeo, B.S. In situ Raman spectroscopy of copper and copper oxide surfaces during electrochemical oxygen evolution reaction: Identification of CuIII oxides as catalytically active species. ACS. Catal. 2016, 6, 2473–2481. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, H.J.; Sedlak, D.L.; Lee, C.H. PH-Dependent reactivity of oxidants formed by iron and copper-catalyzed decomposition of hydrogen peroxide. Chemosphere 2013, 92, 652–658. [Google Scholar] [CrossRef]
- Chen, J.B.; Zhou, X.F.; Sun, P.Z.; Zhang, Y.L.; Huang, C.H. Complexation enhances Cu (II)-activated peroxydisulfate: A novel activation mechanism and Cu (III) contribution. Environ. Sci. Technol. 2019, 53, 11774–11782. [Google Scholar] [CrossRef]
- Xing, G.W.; Pham, A.N.; Miller, C.J.; Waite, T.D. PH-dependence of production of oxidants (Cu (III) and/or HO•) by copper-catalyzed decomposition of hydrogen peroxide under conditions typical of natural saline waters. Geochim. Cosmochim. Acta. 2018, 232, 30–47. [Google Scholar] [CrossRef]
- Gupta, B.; Gupta, A.K. Photocatalytic performance of 3D engineered chitosan hydrogels embedded with sulfur-doped C3N4/ZnO nanoparticles for Ciprofloxacin removal: Degradation and mechanistic pathways. Int. J. Biol. Macromol. 2022, 198, 87–100. [Google Scholar] [CrossRef]
- Wang, A.Q.; Wang, H.; Deng, H.; Wang, S.; Shi, W.; Yi, Z.X.; Qiu, R.L.; Yan, K. Controllable synthesis of mesoporous manganese oxide microsphere efficient for photo-Fenton-like removal of fluoroquinolone antibiotics. Appl. Catal. B 2019, 248, 298–308. [Google Scholar] [CrossRef]

| Samples | BET Specific Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (μm) |
|---|---|---|---|
| DE | 10.7239 | 0.021179 | 0.483013 |
| GO-DE | 18.7833 | 0.027155 | 0.553611 |
| CuO/GO-DE | 15.4166 | 0.025061 | 0.526992 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Zhang, J.; Yu, Y.; Li, J.; Zhou, Z.; Li, C. Synthesis of CuO/GO-DE Catalyst and Its Catalytic Properties and Mechanism on Ciprofloxacin Degradation. Nanomaterials 2022, 12, 4305. https://doi.org/10.3390/nano12234305
Zhang T, Zhang J, Yu Y, Li J, Zhou Z, Li C. Synthesis of CuO/GO-DE Catalyst and Its Catalytic Properties and Mechanism on Ciprofloxacin Degradation. Nanomaterials. 2022; 12(23):4305. https://doi.org/10.3390/nano12234305
Chicago/Turabian StyleZhang, Ting, Jingjing Zhang, Yinghao Yu, Jinxu Li, Zhifang Zhou, and Chunlei Li. 2022. "Synthesis of CuO/GO-DE Catalyst and Its Catalytic Properties and Mechanism on Ciprofloxacin Degradation" Nanomaterials 12, no. 23: 4305. https://doi.org/10.3390/nano12234305
APA StyleZhang, T., Zhang, J., Yu, Y., Li, J., Zhou, Z., & Li, C. (2022). Synthesis of CuO/GO-DE Catalyst and Its Catalytic Properties and Mechanism on Ciprofloxacin Degradation. Nanomaterials, 12(23), 4305. https://doi.org/10.3390/nano12234305






