Lithium Vanadium Oxide/Graphene Composite as a Promising Anode for Lithium-Ion Batteries
Abstract
1. Introduction
2. Experimental
2.1. Preparation of LVO
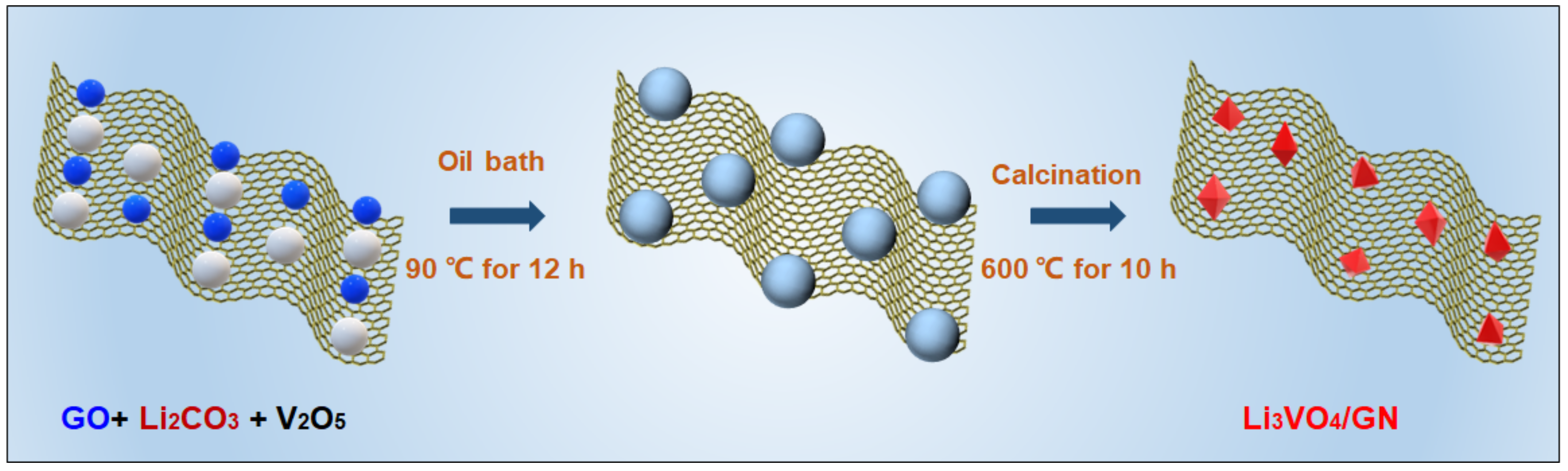
2.2. Preparation of LVO/GN Composite
2.3. Characterization
2.4. Electrochemical Measurements
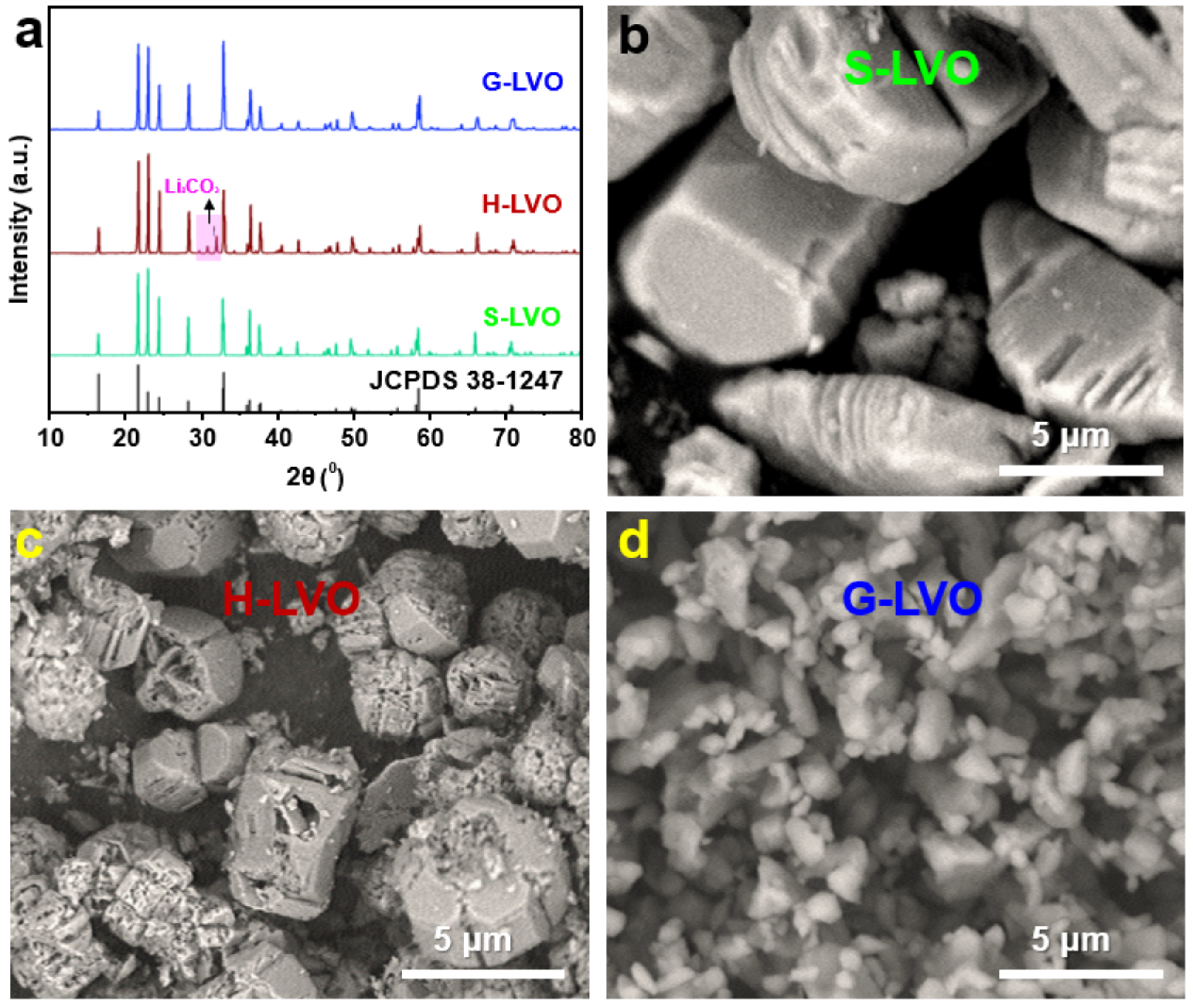
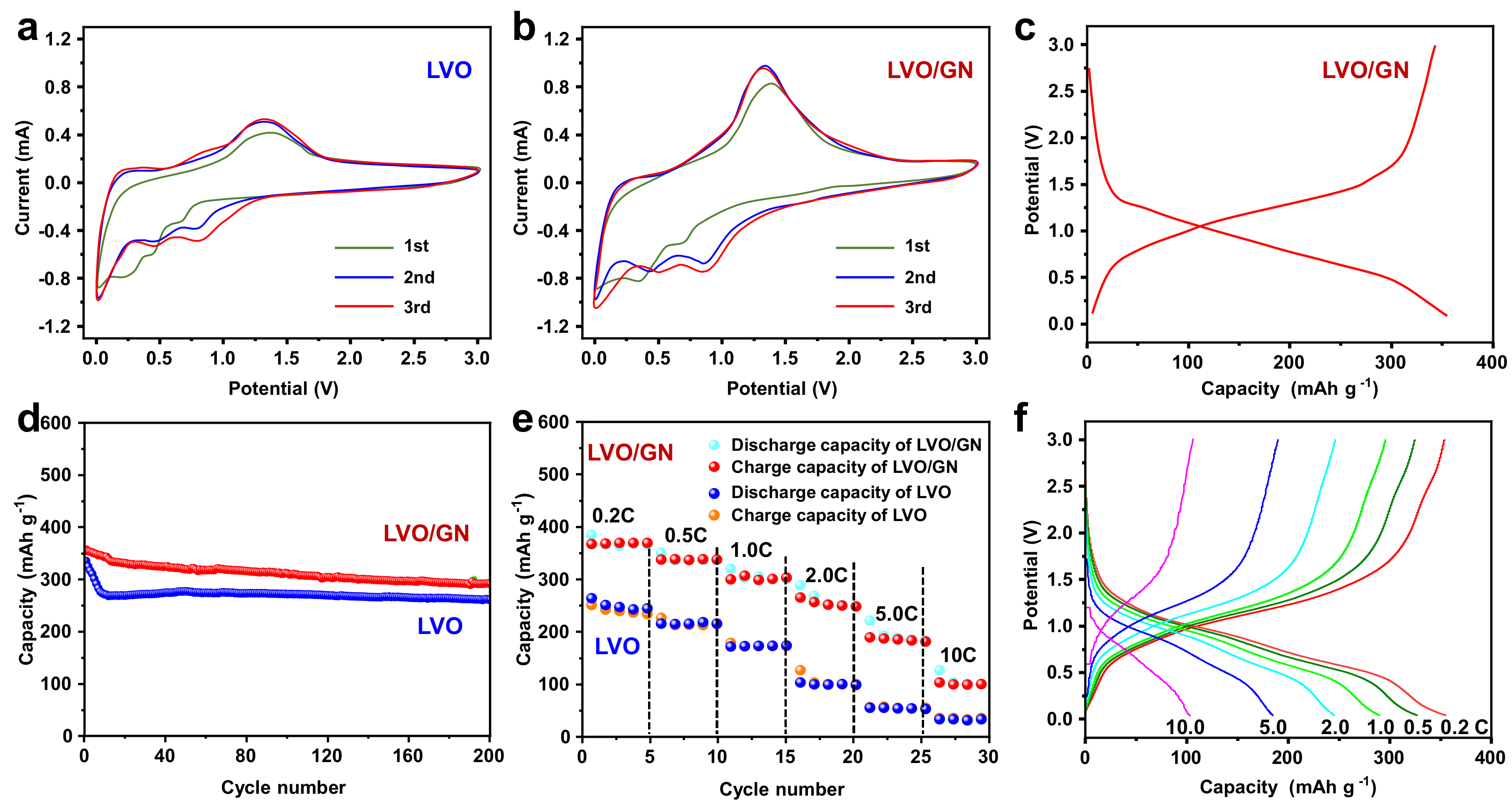
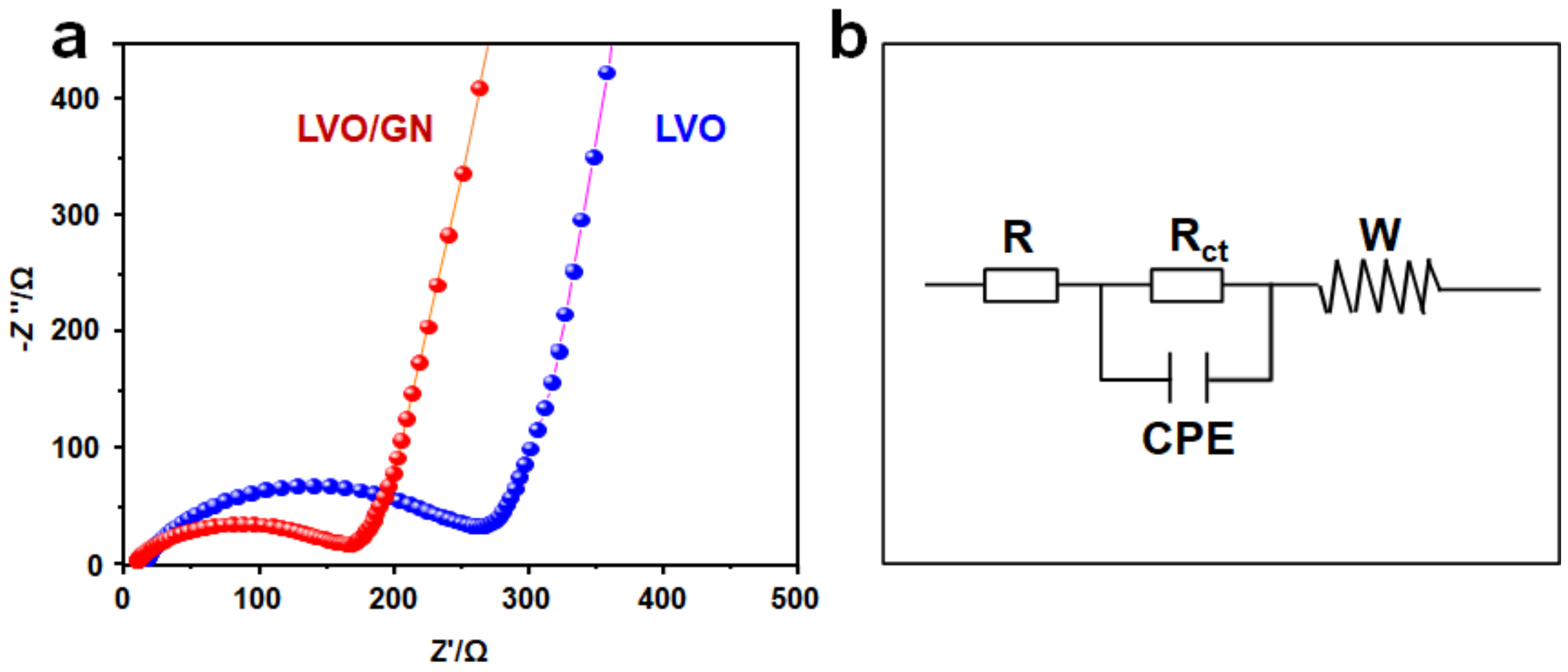
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Liu, X.; Qin, B.; Li, Z.; Zhang, Y.; Yang, W.; Fan, H. In-situ etching and ion exchange induced 2D-2D MXene@Co9S8/CoMo2S4 heterostructure for superior Na+ storage. Chem. Eng. J. 2023, 451, 138508. [Google Scholar] [CrossRef]
- Chen, B.; Wu, W.; Li, C.; Wang, Y.; Zhang, Y.; Fu, L.; Zhu, Y.; Zhang, L.; Wu, Y. Oxygen/phosphorus co-doped porous carbon from cicada slough as high-performance electrode material for supercapacitors. Sci. Rep. 2019, 9, 5431. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Bi, L.; Cheng, Y.; Xu, B.; Jen, A.K. Self-assembled monolayer enabling improved buried interfaces in blade-coated perovskite solar cells for high efficiency and stability. Nano Res. Energy 2022, 1, e9120004. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Zhao, D.; Ren, J.; Liu, S.; Tang, H.; Xu, P.; Gao, F.; Yue, X.; Yang, H.; et al. Core-shell Co2VO4/carbon composite anode for highly stable and fast-charging sodium-ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 55020–55028. [Google Scholar] [CrossRef]
- Gao, F.; Mei, B.; Xu, X.; Ren, J.; Zhao, D.; Zhang, Z.; Wang, Z.; Wu, Y.; Liu, X.; Zhang, Y. Rational design of ZnMn2O4 nanoparticles on carbon nanotubes for high-rate and durable aqueous zinc-ion batteries. Chem. Eng. J. 2022, 448, 137742. [Google Scholar] [CrossRef]
- Zeng, L.; Fang, Y.; Xu, L.; Zheng, C.; Yang, M.; He, J.; Xue, H.; Qian, Q.; Wei, M.; Chen, Q. Rational design of few-layer MoSe2 confined within ZnSe-C hollow porous spheres for high-performance lithium-ion and sodium-ion batteries. Nanoscale 2019, 11, 6766–6775. [Google Scholar] [CrossRef]
- Liu, X.; Xu, F.; Li, Z.; Liu, Z.; Yang, W.; Zhang, Y.; Fan, H.; Yang, H.Y. Design strategy for MXene and metal chalcogenides/oxides hybrids for supercapacitors, secondary batteries and electro/photocatalysis. Coord. Chem. Rev. 2022, 464, 214544. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Z.; Ren, J.; Xu, Y.; Xu, X.; Zhou, J.; Gao, F.; Tang, H.; Liu, S.; Wang, Z.; et al. Fe2VO4 nanoparticles on rGO as anode material for high-rate and durable lithium and sodium ion batteries. Chem. Eng. J. 2023, 451, 138882. [Google Scholar] [CrossRef]
- Cai, Z.; Peng, Z.; Wang, M.; Wu, J.; Fan, H.; Zhang, Y. High-pseudocapacitance of porous and square NiO@NC nanosheets for high-performance lithium-ion batteries. Rare Met. 2021, 40, 1451–1458. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Hu, K.; Ren, J.; Yu, N.; Liu, X.; Wu, G.; Liu, N. Anchoring silicon on the basal plane of graphite via a three-phase heterostructure for highly reversible lithium storage. Energy Storage Mater. 2021, 34, 311–319. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, S.; Yu, S.; Ren, J.; Zhang, Z.; Liu, S.; Liu, X.; Wang, Z.; Wu, Y.; Zhang, Y. Lychee seed-derived microporous carbon for high-performance sodium-sulfur batteries. Carbon 2023, 201, 864. [Google Scholar] [CrossRef]
- Deng, W.; Li, Y.; Xu, D.; Zhou, W.; Xiang, K.; Chen, H. Three-dimensional hierarchically porous nitrogen-doped carbon from water hyacinth as selenium host for high-performance lithium–selenium batteries. Rare Met. 2022, 41, 3432–3445. [Google Scholar] [CrossRef]
- Pei, C.; Li, T.; Zhang, M.; Wang, J.; Chang, L.; Xiong, X.; Chen, W.; Huang, G.; Han, D. Synergistic effects of interface coupling and defect sites in WO3/InVO4 architectures for highly efficient nitrogen photofixation. Sep. Purif. Technol. 2022, 290, 120875. [Google Scholar] [CrossRef]
- Jiang, M.; Mu, P.; Zhang, H.; Dong, T.; Tang, B.; Qiu, H.; Chen, Z.; Cui, G. An endotenon sheath-inspired double-network binder enables superior cycling performance of silicon electrodes. Nano-Micro Lett. 2022, 14, 87. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Aldama, E.; Liu, H.; Sun, Z.; Ma, Y.; Liu, N.; Zhang, Z. Rational design of walnut-like ZnO/Co3O4 porous nanospheres with substantially enhanced lithium storage performance. Nanoscale 2022, 14, 166–174. [Google Scholar] [CrossRef]
- Ren, J.; Wang, Z.; Xu, P.; Wang, C.; Gao, F.; Zhao, D.; Liu, S.; Yang, H.; Wang, D.; Niu, C.; et al. Porous Co2VO4 nanodisk as a high-energy and fast-charging anode for lithium-ion batteries. Nano-Micro Lett. 2022, 14, 5. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Liu, L.; Zhang, Z.; Wang, J.; Chou, S.; Gao, J.; Abruña, H.D.; Li, H.; Liu, H.; et al. Rapid hydrothermal synthesis of Li3VO4 with different favored facets. J. Solid State Electrochem. 2017, 21, 2547–2553. [Google Scholar] [CrossRef]
- Shao, G.; Gan, L.; Ma, Y.; Li, H.; Zhai, T. Enhancing the performance of Li3VO4 by combining nanotechnology and surface carbon coating for lithium ion batteries. J. Mater. Chem. A 2015, 3, 11253–11260. [Google Scholar] [CrossRef]
- Zhang, C.; Song, H.; Liu, C.; Liu, Y.; Zhang, C.; Nan, X.; Cao, G. Fast and reversible Li ion insertion in carbon-encapsulated Li3VO4 as anode for lithium-ion battery. Adv. Funct. Mater. 2015, 25, 3497–3504. [Google Scholar] [CrossRef]
- Zhao, D.; Cao, M. Constructing highly graphitized carbon-wrapped Li3VO4 nanoparticles with hierarchically porous structure as a long life and high capacity anode for lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 25084–25093. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, H.; Zhang, Y.; Zhang, Y.; Wu, Y.; Tian, M.; Chen, P.; Trout, R.; Ma, Y.; Wu, T.; et al. A safe and fast-charging lithium-ion battery anode using MXene supported Li3VO4. J. Mater. Chem. A 2019, 7, 11250–11256. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Z.; Ding, G.; Xie, L.; Miao, Y.; Cao, X. Review on the recent development of Li3VO4 as anode materials for lithium-ion batteries. J. Mater. Sci. Technol. 2021, 89, 68–87. [Google Scholar] [CrossRef]
- Zhao, M.; Li, X.; Chen, X.; Li, B.; Kaskel, S.; Zhang, Q.; Huang, J. Promoting the sulfur redox kinetics by mixed organodiselenides in high-energy-density lithium-sulfur batteries. eScience 2021, 1, 44–52. [Google Scholar] [CrossRef]
- Cai, W.; Chen, R.; Yang, Y.; Yi, M.; Xiang, L. Removal of SO42− from Li2CO3 by recrystallization in Na2CO3 solution. Crystals 2018, 8, 19. [Google Scholar] [CrossRef]
- Yao, T.; Zhou, W.; Peng, W.; Zhou, J.; Li, T.; Wu, H.; Zheng, J.; Lin, N.; Liu, D.; Hou, C. Insights into concomitant enhancements of dielectric properties and thermal conductivity of PVDF composites filled with core@double-shell structured Zn@ZnO@PS particles. J. Appl. Polym. Sci. 2022, 139, e53069. [Google Scholar] [CrossRef]
- Ran, H.; Du, H.; Ma, C.; Zhao, Y.; Feng, D.; Xu, H. Effects of A/B-site Co-doping on microstructure and dielectric thermal stability of AgNbO3 ceramics. Sci. Adv. Mater. 2021, 13, 741–747. [Google Scholar] [CrossRef]
- Peng, W.; Zhou, W.; Li, T.; Zhou, J.; Yao, T.; Wu, H.; Zhao, X.; Luo, J.; Liu, J.; Zhang, D. Towards inhibiting conductivity of Mo/PVDF composites through building MoO3 shell as an interlayer for enhanced dielectric properties. J. Mater. Sci. Mater. Electron. 2022, 33, 14735–14753. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, L.; Fan, Q.; Zhang, F.; Wang, Z.; Zou, J.; Zhao, S.; Mao, J.; Guo, Z. Challenges and prospects of lithium-CO2 batteries. Nano Res. Energy 2022, 1, e9120001. [Google Scholar] [CrossRef]
- Jiang, Y.; Chai, L.; Zhang, D.; Ouyang, F.; Zhou, X.; Alhassan, S.I.; Liu, S.; He, Y.; Yan, L.; Wang, H.; et al. Facet-controlled LiMn2O4/C as deionization electrode with enhanced stability and high desalination performance. Nano-Micro Lett. 2022, 14, 176. [Google Scholar] [CrossRef]
- Li, X.; Liang, H.; Liu, X.; Sun, R.; Qin, Z.; Fan, H.; Zhang, Y. Ion-exchange strategy of CoS2/Sb2S3 hetero-structured nanocrystals encapsulated into 3D interpenetrating dual-carbon framework for high-performance Na+/K+ batteries. Chem. Eng. J. 2021, 425, 130657. [Google Scholar] [CrossRef]
- Jia, X.; Yu, X.; Lu, B. Fe0.8CoSe2 nanosphere coated by N-doped carbon for ultra-high rate potassium selenium battery. Rare Met. 2021, 40, 2455–2463. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, J.; Wang, X.; Chen, L.; Cao, G.; Wang, J.; Zhang, H.; He, X. Insights for understanding multiscale degradation of LiFePO4 cathodes. eScience 2022, 2, 125–137. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, R.; King, S.; Yin, F.; Peng, H.; Wang, G. Dual active and kinetically inter-promoting Li3VO4/graphene anode enabling printable high energy density lithium ion micro capacitors. Energy Storage Mater. 2021, 43, 482–491. [Google Scholar] [CrossRef]
- Gou, W.; Zhou, S.; Cao, X.; Luo, Y.; Kong, X.; Chen, J.; Xie, X.; Pan, A. Agitation drying synthesis of porous carbon supported Li3VO4 as advanced anode material for lithium-ion batteries. Rare Met. 2021, 40, 3466–3476. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, C.; Cao, B.; Xu, Y.; Zhang, D.; Li, A.; Zhou, J.; Ma, Z.; Chen, X.; Song, H. One-step synthesis of spherical Si/C composites with onion-like buffer structure as high-performance anodes for lithium-ion batteries. Energy Storage Mater. 2020, 24, 312–318. [Google Scholar] [CrossRef]
- Lu, S.; Zhu, T.; Li, Z.; Pang, Y.; Shi, L.; Ding, S.; Gao, G. Ordered mesoporous carbon supported Ni3V2O8 composites for lithium-ion batteries with long-term and high-rate performance. J. Mater. Chem. A 2018, 6, 7005–7013. [Google Scholar] [CrossRef]
- Kim, J.; Jeghan, S.; Lee, G. Superior fast-charging capability of graphite anode via facile surface treatment for lithium-ion batteries. Microporous Mesoporous Mater. 2020, 305, 110325. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, Z.; Yang, Y.; Zhang, Z.; Chen, W.; Yan, R.; Jin, Y.; Zhang, J. Defective WO3 nanoplates controllably decorated with MIL-101(Fe) nanoparticles to efficiently remove tetracycline hydrochloride by S-scheme mechanism. Sep. Purif. Technol. 2022, 300, 121846. [Google Scholar] [CrossRef]
- Su, D.; Pei, Y.; Liu, L.; Liu, Z.; Liu, J.; Yang, M.; Wen, J.; Dai, J.; Deng, H.; Cao, G. Wire-in-Wire TiO2/C Nanofibers Free-Standing Anodes for Li-Ion and K-Ion Batteries with Long Cycling Stability and High Capacity. Nano-Micro Lett. 2021, 13, 107. [Google Scholar] [CrossRef]
- Zhou, W.; Cao, G.; Yuan, M.; Zhong, S.; Wang, Y.; Liu, X.; Cao, D.; Peng, W.; Liu, J.; Wang, G.; et al. Core-shell engineering of conductive fillers toward enhanced dielectric properties: A universal polarization mechanism in polymer conductor composites. Adv. Mater. 2022, 2207829. [Google Scholar] [CrossRef]
- Bo, R.; Zhang, F.; Bu, S.; Nasiri, N.; Di Bernardo, I.; Tran-Phu, T.; Shrestha, A.; Chen, H.; Taheri, M.; Qi, S.; et al. One-step synthesis of porous transparent conductive oxides by hierarchical self-assembly of aluminum-doped ZnO nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 9589–9599. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Ge-Zhang, S.; Zhang, Z.; Tang, H.; Xu, Y.; Gao, F.; Liu, S.; Zhou, J.; Wang, Z.; Wu, Y.; et al. Three-Dimensional Honeycomb-Like Carbon as Sulfur Host for Sodium–Sulfur Batteries without the Shuttle Effect. ACS Appl. Mater. Interfaces 2022, 14, 54662–54669. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, Y.; Yu, X.; Qin, Y.; Meng, T.; Hu, X. The pursuit of commercial silicon-based microparticle anodes for advanced lithium-ion batteries: A review. Nano Res. Energy 2022, 1, e9120037. [Google Scholar] [CrossRef]
- Luo, F.; Feng, X.; Zeng, L.; Lin, L.; Li, X.; Kang, B.; Xiao, L.; Chen, Q.; Wei, M.; Qian, Q. In situ simultaneous encapsulation of defective MoS2 nanolayers and sulfur nanodots into SPAN fibers for high rate sodium-ion batteries. Chem. Eng. J. 2021, 404, 126430. [Google Scholar] [CrossRef]
- Lin, C.; Yang, X.; Xiong, P.; Lin, H.; He, L.; Yao, Q.; Wei, M.; Qian, Q.; Chen, Q.; Zeng, L. High-rate, large capacity, and long life dendrite-free Zn metal anode enabled by trifunctional electrolyte additive with a wide temperature range. Adv. Sci. 2022, 9, 2201433. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, L.; Peng, J.; Zhang, Y.; Cui, Y.; An, L.; Chen, P.; Zhang, F. Lithium Vanadium Oxide/Graphene Composite as a Promising Anode for Lithium-Ion Batteries. Nanomaterials 2023, 13, 43. https://doi.org/10.3390/nano13010043
Meng L, Peng J, Zhang Y, Cui Y, An L, Chen P, Zhang F. Lithium Vanadium Oxide/Graphene Composite as a Promising Anode for Lithium-Ion Batteries. Nanomaterials. 2023; 13(1):43. https://doi.org/10.3390/nano13010043
Chicago/Turabian StyleMeng, Leichao, Jianhong Peng, Yi Zhang, Yongfu Cui, Lingyun An, Peng Chen, and Fan Zhang. 2023. "Lithium Vanadium Oxide/Graphene Composite as a Promising Anode for Lithium-Ion Batteries" Nanomaterials 13, no. 1: 43. https://doi.org/10.3390/nano13010043
APA StyleMeng, L., Peng, J., Zhang, Y., Cui, Y., An, L., Chen, P., & Zhang, F. (2023). Lithium Vanadium Oxide/Graphene Composite as a Promising Anode for Lithium-Ion Batteries. Nanomaterials, 13(1), 43. https://doi.org/10.3390/nano13010043





