Evaporative and Wicking Functionalities at Hot Airflows of Laser Nano-/Microstructured Ti-6Al-4V Material
Abstract
1. Introduction
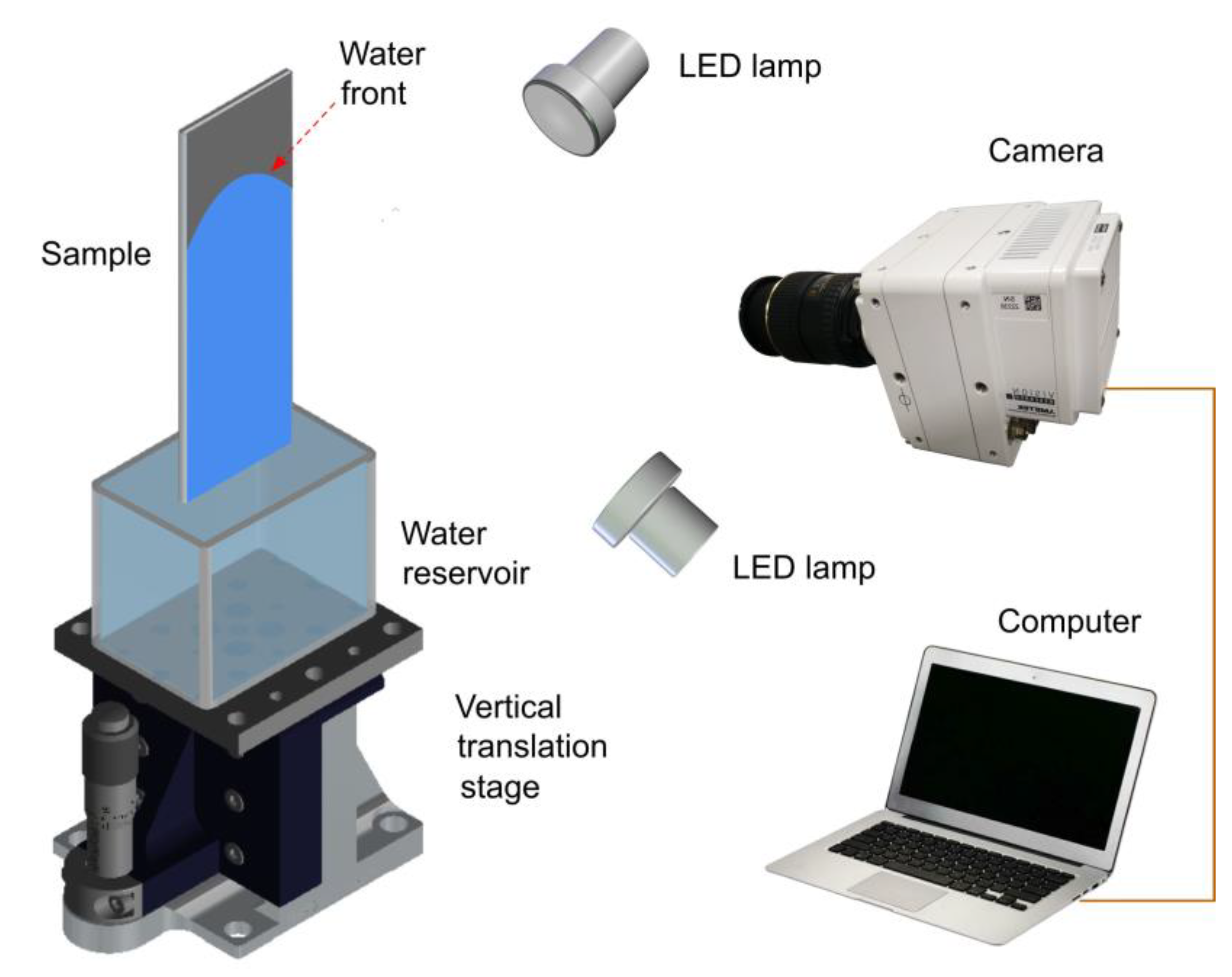
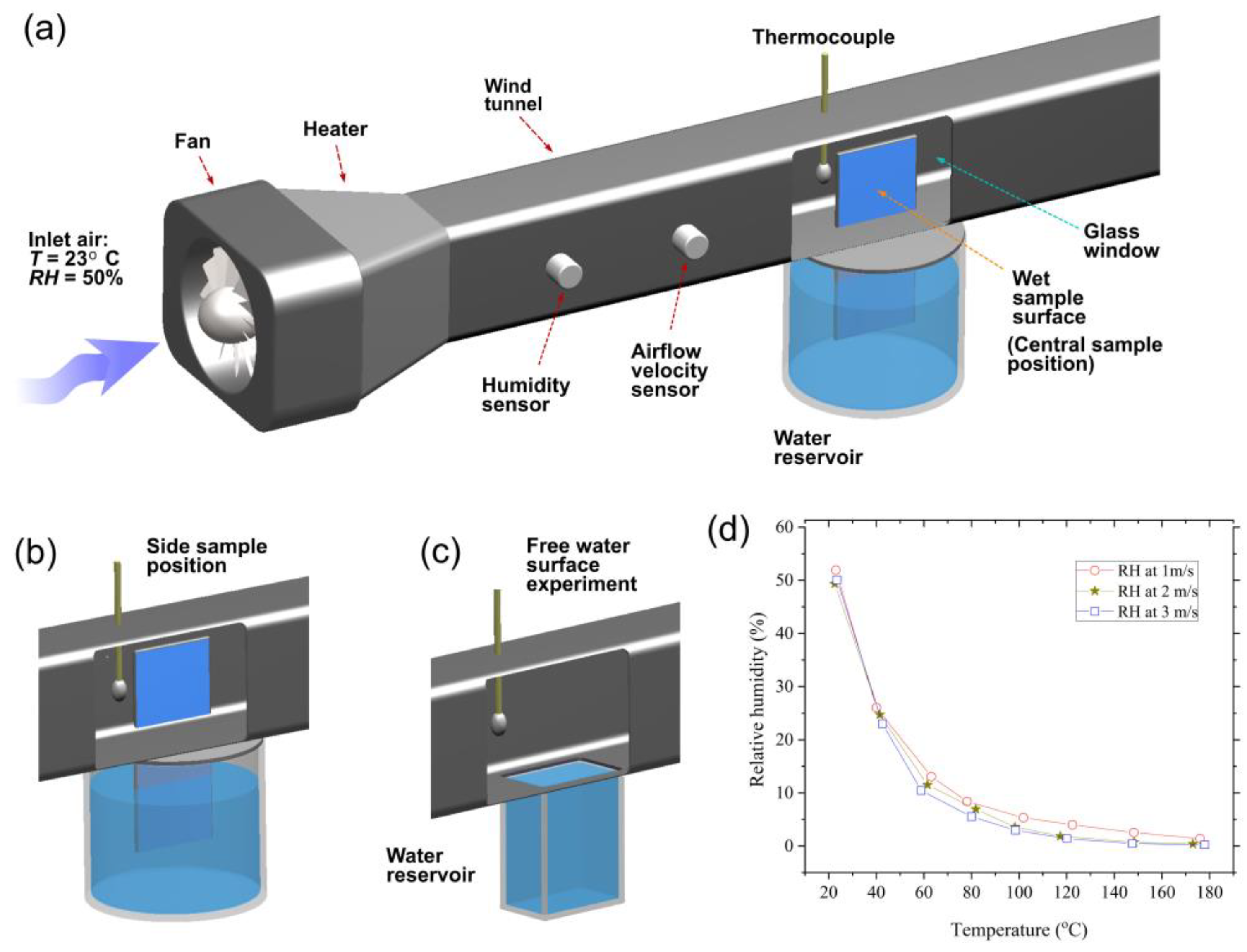
2. Experimental Techniques
3. Results and Discussion
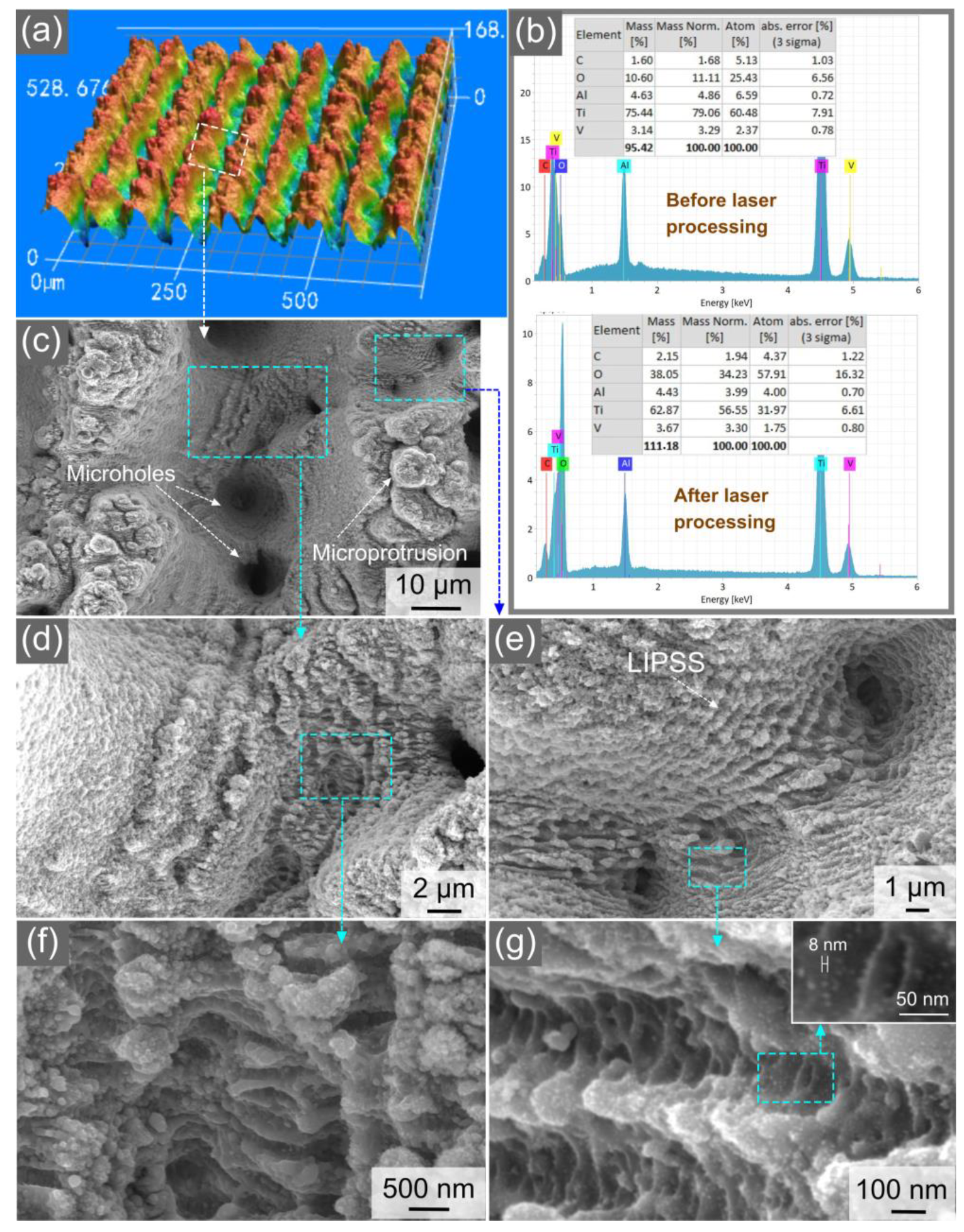
3.1. Characterization of Surface Structure
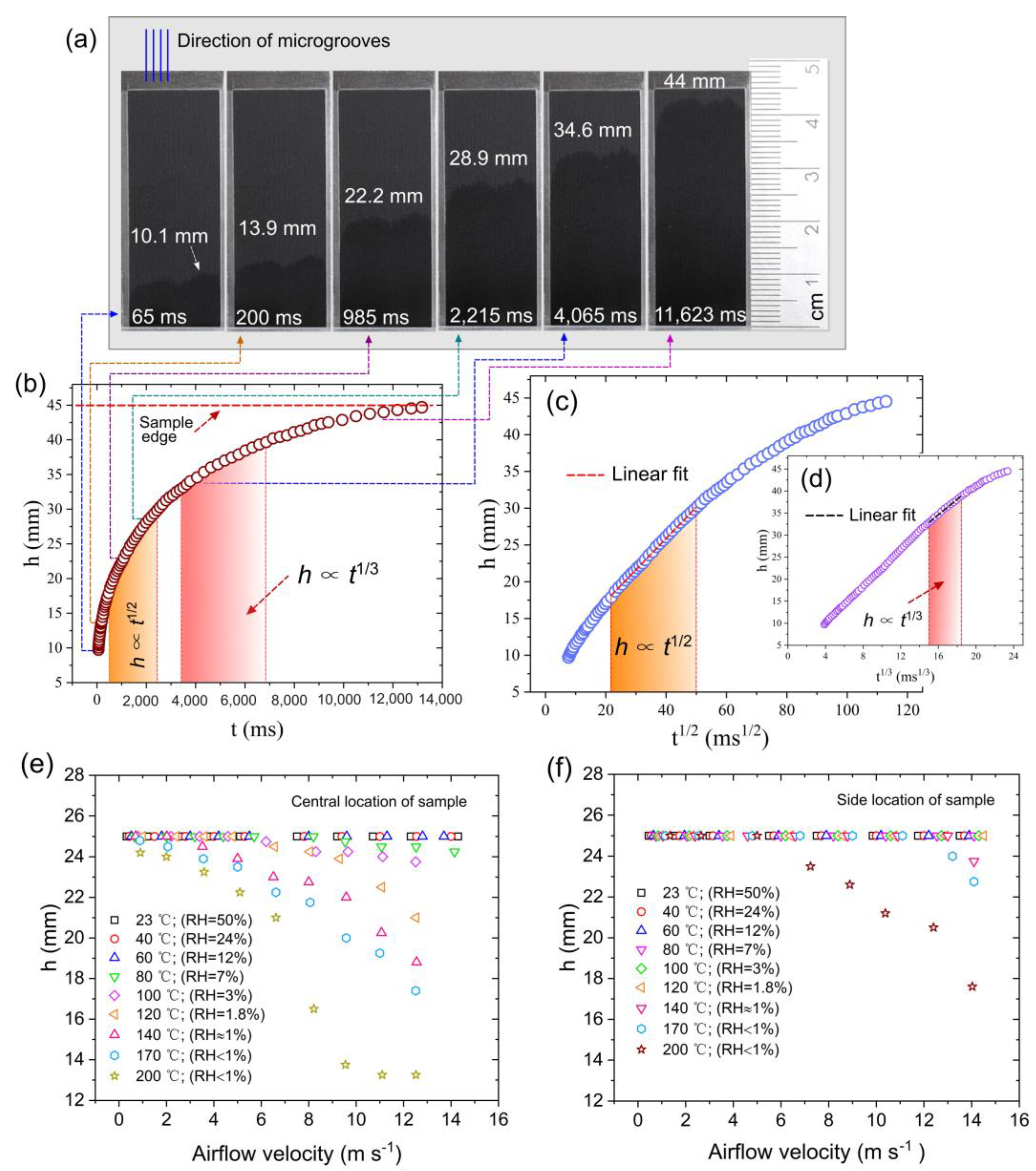
3.2. Characterization of Wicking Functionality
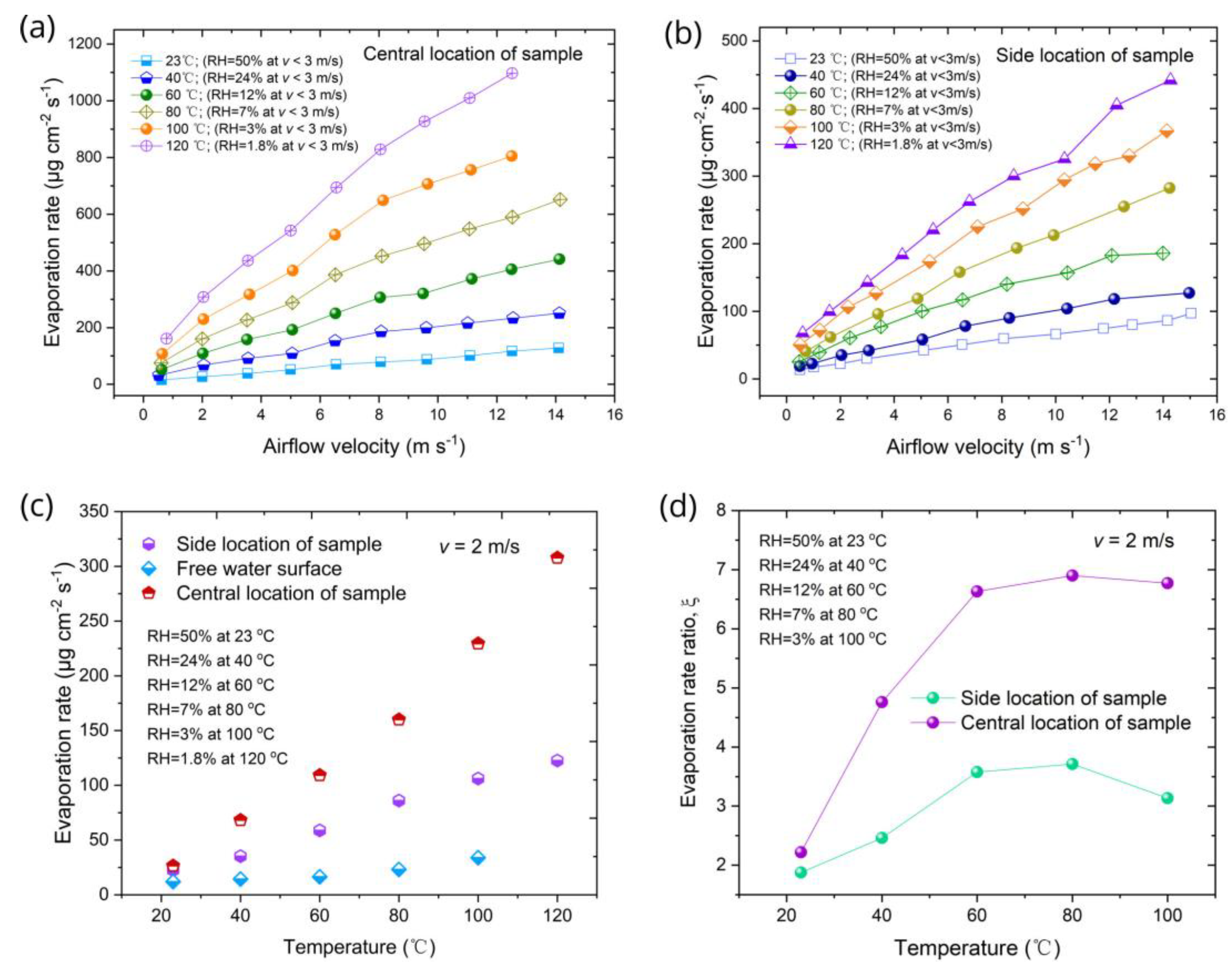
3.3. Characterization of Evaporative Functionality
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mahmood, M.H.; Sultan, M.; Miyazaki, T.; Koyama, S.; Maisotsenko, V.S. Overview of the Maisotsenko cycle–A way towards dew point evaporative cooling. Renew. Sustain. Energy Rev. 2016, 66, 537–555. [Google Scholar] [CrossRef]
- Saghafifar, M.; Gadalla, M. Analysis of Maisotsenko open gas turbine power cycle with a detailed air saturator model. Appl. Energy 2015, 149, 338–353. [Google Scholar] [CrossRef]
- Chen, L.; Shen, J.; Ge, Y.; Wu, Z.; Wang, W.; Zhu, F.; Feng, H. Power and efficiency optimization of open Maisotsenko-Brayton cycle and performance comparison with traditional open regenerated Brayton cycle. Energy Convers. Manag. 2020, 217, 113001. [Google Scholar] [CrossRef]
- Tariq, R.; Sheikh, N.A. Numerical heat transfer analysis of Maisotsenko humid air bottoming cycle–A study towards the optimization of the air-water mixture at bottoming turbine inlet. Appl. Therm. Eng. 2018, 133, 49–60. [Google Scholar] [CrossRef]
- De Paepe, W.; Pappa, A.; Carrero, M.M.; Bricteux, L.; Contino, F. Reducing waste heat to the minimum: Thermodynamic assessment of the M-power cycle concept applied to micro gas turbines. Appl. Energy 2020, 279, 115898. [Google Scholar] [CrossRef]
- Zhu, G.; Chow, T.T.; Lee, C.K. Performance analysis of biogas-fueled Maisotsenko combustion turbine cycle. Appl. Therm. Eng. 2021, 195, 117247. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, X.; Zhang, Y.; Liu, K.; Yang, H.; Li, J.; Akhlaghi, Y.G.; Liu, H.; Han, Z.; Liu, Z. Combined Rankine cycle and dew point cooler for energy efficient power generation of the power plants-A review and perspective study. Energy 2022, 238, 121688. [Google Scholar] [CrossRef]
- Pandelidis, D. Numerical study and performance evaluation of the Maisotsenko cycle cooling tower. Energy Convers. Manag. 2020, 210, 112735. [Google Scholar] [CrossRef]
- Fan, X.; Lu, X.; Nie, H.; Zhu, H.; Wang, Q.; Kang, Y.; Liu, S.; Zheng, X.; Liu, Z.; Zhang, Y.; et al. An experimental study of a novel dew point evaporative cooling tower based on M-cycle. Appl. Therm. Eng. 2021, 190, 116839. [Google Scholar] [CrossRef]
- Saghafifar, M.; Gadalla, M. A critical assessment of thermo-economic analyses of different air bottoming cycles for waste heat recovery. Int. J. Energy Res. 2019, 43, 1315–1341. [Google Scholar] [CrossRef]
- Sadighi Dizaji, H.; Hu, E.J.; Chen, L.; Pourhedayat, S. Using novel integrated Maisotsenko cooler and absorption chiller for cooling of gas turbine inlet air. Energy Convers. Manag. 2019, 195, 1067–1078. [Google Scholar] [CrossRef]
- Sohani, A.; Farasati, Y.; Sayyaadi, H. A systematic approach to find the best road map for enhancement of a power plant with dew point inlet air pre-cooling of the air compressor. Energy Convers. Manag. 2017, 150, 463–484. [Google Scholar] [CrossRef]
- Zhu, G.; Chow, T.T.; Fong, K.F.; Lee, C.K.; Luo, X.J. Design optimisation and performance appraisal of a combined cooling, heating and power system primed with Maisotsenko combustion turbine cycle. Energy Convers. Manag. 2018, 177, 91–106. [Google Scholar] [CrossRef]
- Pandelidis, D.; Pacak, A.; Anisimov, S. Energy saving potential by using Maisotsenko-cycle in different applications. Int. J. Earth Environ Sci. 2018, 3, 159. [Google Scholar] [CrossRef]
- Taler, J.; Jagiela, B.; Jaremkiewicz, M. Overview of the M-cycle technology for air conditioning and cooling applications. Energies 2022, 15, 1814. [Google Scholar] [CrossRef]
- Omar, A.; Saghafifar, M.; Gadalla, M. Thermo-economic analysis of air saturator integration in conventional combined power cycles. Appl. Therm. Eng. 2016, 107, 1104–1122. [Google Scholar] [CrossRef]
- Pacak, A.; Worek, W. Review of dew point evaporative cooling technology for air conditioning applications. Appl. Sci. 2021, 11, 934. [Google Scholar] [CrossRef]
- Duan, Z.; Zhan, C.; Zhang, X.; Mustafa, M.; Zhao, X.; Alimohammadisagvand, B.; Hasan, A. Indirect evaporative cooling: Past, present and future potentials. Renew. Sustain. Energy Rev. 2012, 16, 6823–6850. [Google Scholar] [CrossRef]
- Zhan, C.; Duan, Z.; Zhao, X.; Smith, S.; Jin, H.; Riffat, S. Comparative study of the performance of the M-cycle counter-flow and cross-flow heat exchangers for indirect evaporative cooling–Paving the path toward sustainable cooling of buildings. Energy 2011, 36, 6790–6805. [Google Scholar] [CrossRef]
- Davoodabadi, A.; Ghasemi, H. Evaporation in nano/molecular materials. Adv. Colloid Interface Sci. 2021, 290, 102385. [Google Scholar] [CrossRef]
- Farokhnia, N.; Irajizad, P.; Sajadi, S.M.; Ghasemi, H. Rational micro/nanostructuring for thin-film evaporation. J. Phys. Chem. C 2016, 120, 8742–8750. [Google Scholar] [CrossRef]
- Li, Y.; Alibakhshi, M.; Zhao, Y.; Duan, C. Exploring ultimate water capillary evaporation in nanoscale conduits. Nano Lett. 2017, 17, 4813–4819. [Google Scholar] [CrossRef] [PubMed]
- Nahar, M.M.; Ma, B.; Guye, K.; Chau, Q.H.; Padilla, J.; Iyengar, M.; Agonafer, D. Review article: Microscale evaporative cooling technologies for high heat flux microelectronics devices: Background and recent advances. Appl. Therm. Eng. 2021, 194, 117109. [Google Scholar] [CrossRef]
- Wilke, K.L.; Barabadi, B.; Lu, Z.; Zhang, T.; Wang, E.N. Parametric study of thin film evaporation from nanoporous membranes. Appl. Phys. Lett. 2017, 111, 171603. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Z.; Jiang, Y.; Xu, G.; Zhu, M.; Law, W.-C.; Yong, K.-T.; Wang, Y.; Yang, C.; Dong, B.; et al. Recent advances in solar-driven evaporation systems. J. Mater. Chem. A 2020, 8, 25571–25600. [Google Scholar] [CrossRef]
- Han, D.-D.; Chen, Z.-D.; Li, J.-C.; Mao, J.-W.; Jiao, Z.-Z.; Wang, W.; Zhang, W.; Zhang, Y.-L.; Sun, H.-B. Airflow enhanced solar evaporation based on janus graphene membranes with stable interfacial floatability. ACS Appl. Mater. Interfaces 2020, 12, 25435–25443. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, B.; Wu, W.; Yang, H.; Ning, Y.; Lai, Y.; Bradley, R. Low cost, robust, environmentally friendly geopolymer–mesoporous carbon composites for efficient solar powered steam generation. Adv. Funct. Mater. 2018, 28, 1803266. [Google Scholar] [CrossRef]
- Zhong, J.; Huang, C.; Wu, D.; Lin, Z. Influence factors of the evaporation rate of a solar steam generation system: A numerical study. Int. J. Heat Mass Transfer. 2019, 128, 860–864. [Google Scholar] [CrossRef]
- Koch, K.; Barthlott, W. Superhydrophobic and superhydrophilic plant surfaces: An inspiration for biomimetic materials. Phil. Trans. R. Soc. A 2009, 367, 1487–1509. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Barthlott, W. Multifunctional surface structures of plants: An inspiration for biomimetics. Prog. Mater. Sci. 2009, 54, 137–178. [Google Scholar] [CrossRef]
- Ahmmed, K.M.; Grambow, C.; Kietzig, A.M. Fabrication of micro/nano structures on metals by femtosecond laser micromachining. Micromachines 2014, 5, 1219–1253. [Google Scholar] [CrossRef]
- Bonse, J.; Gräf, S. Maxwell meets marangoni—A review of theories on laser-induced periodic surface structures. Laser Photonics Rev. 2020, 14, 2000215. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, C.S.; Shang, S.; Liu, D.; Perrie, W.; Dearden, G.; Watkins, K. A review of ultrafast laser materials micromachining. Opt. Laser Technol. 2013, 46, 88–102. [Google Scholar] [CrossRef]
- Fang, R.; Vorobyev, A.; Guo, C. Direct visualization of the complete evolution of femtosecond laser-induced surface structural dynamics of metals. Light Sci. Appl. 2017, 6, e16256. [Google Scholar] [CrossRef]
- Gattass, R.R.; Mazur, E. Femtosecond laser micromachining in transparent materials. Nat. Photonics 2008, 2, 219–225. [Google Scholar] [CrossRef]
- Povarnitsyn, M.E.; Fokin, V.B.; Levashov, P.R.; Itina, T.E. Molecular dynamics simulation of subpicosecond double-pulse laser ablation of metals. Phys. Rev. B 2015, 92, 174104. [Google Scholar] [CrossRef]
- Sedao, X.; Shugaev, M.V.; Wu, C.; Douillard, T.; Esnouf, C.; Maurice, C.; Reynaud, S.; Pigeon, F.; Garrelie, F.; Zhigilei, L.V.; et al. Growth twinning and generation of high-frequency surface nanostructures in ultrafast laser-induced transient melting and resolidification. ACS Nano 2016, 10, 6995–7007. [Google Scholar] [CrossRef]
- Shugaev, M.V.; Wu, C.; Armbruster, O.; Naghilou, A.; Brouwer, N.; Ivanov, D.S.; Derrien, T.J.Y.; Bulgakova, N.M.; Kautek, W.; Rethfeld, B.; et al. Fundamentals of ultrafast laser–material interaction. MRS Bull. 2016, 41, 960–968. [Google Scholar] [CrossRef]
- Sugioka, K.; Cheng, Y. Ultrafast lasers—Reliable tools for advanced materials processing. Light Sci. Appl. 2014, 3, e149. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C. Direct femtosecond laser surface nano/microstructuring and its applications. Laser Photonics Rev. 2013, 7, 385–407. [Google Scholar] [CrossRef]
- Fang, R.; Zhang, X.; Zheng, J.; Pan, Z.; Yang, C.; Deng, L.; Li, R.; Lai, C.; Yan, W.; Maisotsenko, V.S.; et al. Superwicking functionality of femtosecond laser textured aluminum at high temperatures. Nanomaterials 2021, 11, 2964. [Google Scholar] [CrossRef] [PubMed]
- Vorobyev, A.Y.; Guo, C. Laser turns silicon superwicking. Opt. Express 2010, 18, 6455–6460. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Yang, J.; Ngo, C.-V. The effect of femtosecond laser fluence and pitches between v-shaped microgrooves on the dynamics of capillary flow. Results Phys. 2020, 19, 103606. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Duan, J.A.; Sun, X.; Wang, C.; Luo, Z. Formation of superwetting surface with line-patterned nanostructure on sapphire induced by femtosecond laser. Appl. Phys. A 2015, 119, 69–74. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, L.; Tan, B.; Chen, Z.; Zhang, W.; Liu, Z.; Peng, J. Directional liquid spreading on laser textured aluminum surface. Microsyst. Technol. 2020, 26, 2767–2776. [Google Scholar] [CrossRef]
- Fang, R.; Li, Z.; Zhang, X.; Zhu, X.; Zhang, H.; Li, J.; Pan, Z.; Huang, Z.; Yang, C.; Zheng, J.; et al. Spreading and drying dynamics of water drop on hot surface of superwicking Ti-6Al-4V alloy material fabricated by femtosecond laser. Nanomaterials 2021, 11, 899. [Google Scholar] [CrossRef]
- Fang, R.; Zhu, H.; Li, Z.; Zhu, X.; Zhang, X.; Huang, Z.; Li, K.; Yan, W.; Huang, Y.; Maisotsenko, V.S.; et al. Temperature effect on capillary flow dynamics in 1D array of open nanotextured microchannels produced by femtosecond laser on silicon. Nanomaterials 2020, 10, 796. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C. Nanochemical effects in femtosecond laser ablation of metals. Appl. Phys. Lett. 2013, 102, 074107. [Google Scholar] [CrossRef]
- Landis, E.C.; Phillips, K.C.; Mazur, E.; Friend, C.M. Formation of nanostructured TiO2 by femtosecond laser irradiation of titanium in O2. J. Appl. Phys. 2012, 112, 063108. [Google Scholar] [CrossRef]
- Drelich, J.; Chibowski, E. Superhydrophilic and superwetting surfaces: Definition and mechanisms of control. Langmuir 2010, 26, 18621–18623. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Makin, V.S.; Guo, C. Periodic ordering of random surface nanostructures induced by femtosecond laser pulses on metals. J. Appl. Phys. 2007, 101, 034903. [Google Scholar] [CrossRef]
- Stange, M.; Dreyer, M.E.; Rath, H.J. Capillary driven flow in circular cylindrical tubes. Phys. Fluids 2003, 15, 2587–2601. [Google Scholar] [CrossRef]
- Quéré, D. Inertial capillarity. Europhys. Lett. 1997, 39, 533–538. [Google Scholar] [CrossRef]
- Rye, R.R.; Mann, J.A.; Yost, F.G. The flow of liquids in surface grooves. Langmuir 1996, 12, 555–565. [Google Scholar] [CrossRef]
- Romero, L.A.; Yost, F.G. Flow in an open channel capillary. J. Fluid Mech. 1996, 322, 109–129. [Google Scholar] [CrossRef]
- Deng, D.; Tang, Y.; Zeng, J.; Yang, S.; Shao, H. Characterization of capillary rise dynamics in parallel micro V-grooves. Int. J. Heat Mass Transfer. 2014, 77, 311–320. [Google Scholar] [CrossRef]
- Lade, R.K.; Hippchen, E.J.; Macosko, C.W.; Francis, L.F. Dynamics of capillary-driven flow in 3D printed open microchannels. Langmuir 2017, 33, 2949–2964. [Google Scholar] [CrossRef]
- Fang, R.; Zhu, H.; Li, Z.; Yan, W.; Zhang, X.; Zhu, X.; Maisotsenko, V.S.; Vorobyev, A.Y. Capillary Nylon 6 polymer material produced by femtosecond laser processing. Opt. Express 2019, 27, 36066–36074. [Google Scholar] [CrossRef]
- Washburn, E.W. The dynamics of capillary flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef]
- Mai, T.T.; Lai, C.Q.; Zheng, H.; Balasubramanian, K.; Leong, K.C.; Lee, P.S.; Lee, C.; Choi, W.K. Dynamics of wicking in silicon nanopillars fabricated with interference lithography and metal-assisted chemical etching. Langmuir 2012, 28, 11465–11471. [Google Scholar] [CrossRef]
- Obara, N.; Okumura, K. Imbibition of a textured surface decorated by short pillars with rounded edges. Phys. Rev. E 2012, 86, 020601. [Google Scholar] [CrossRef] [PubMed]
- Tani, M.; Kawano, R.; Kamiya, K.; Okumura, K. Towards combinatorial mixing devices without any pumps by open-capillary channels: Fundamentals and applications. Sci. Rep. 2015, 5, 10263. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Liu, Q.; Li, Y. Capillary filling flows inside patterned-surface microchannels. Chem. Eng. Technol. 2006, 29, 716–723. [Google Scholar] [CrossRef]
- Ponomarenko, A.; QuÉRÉ, D.; Clanet, C. A universal law for capillary rise in corners. J. Fluid Mech. 2011, 666, 146–154. [Google Scholar] [CrossRef]
- Ben Neriah, A.; Assouline, S.; Shavit, U.; Weisbrod, N. Impact of ambient conditions on evaporation from porous media. Water Resour. Res. 2014, 50, 6696–6712. [Google Scholar] [CrossRef]
- Shokri-Kuehni, S.M.S.; Norouzi Rad, M.; Webb, C.; Shokri, N. Impact of type of salt and ambient conditions on saline water evaporation from porous media. Adv. Water Resour. 2017, 105, 154–161. [Google Scholar] [CrossRef]
- Li, Y.; Jin, X.; Li, W.; Niu, J.; Han, X.; Yang, X.; Wang, W.; Lin, T.; Zhu, Z. Biomimetic hydrophilic foam with micro/nano-scale porous hydrophobic surface for highly efficient solar-driven vapor generation. Sci. China Mater. 2022, 65, 1057–1067. [Google Scholar] [CrossRef]
- Choi, J.; Lee, H.; Sohn, B.; Song, M.; Jeon, S. Highly efficient evaporative cooling by all-day water evaporation using hierarchically porous biomass. Sci. Rep. 2021, 11, 16811. [Google Scholar] [CrossRef]
- Pandelidis, D.; Pacak, A.; Cichoń, A.; Gizicki, W.; Worek, W.; Cetin, S. Experimental study of plate materials for evaporative air coolers. Int. Commun. Heat Mass Transfer 2021, 120, 105049. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Riffat, S.B. Comparative study of heat and mass exchanging materials for indirect evaporative cooling systems. Build. Environ. 2008, 43, 1902–1911. [Google Scholar] [CrossRef]
- Xu, P.; Ma, X.; Zhao, X.; Fancey, K.S. Experimental investigation on performance of fabrics for indirect evaporative cooling applications. Build. Environ. 2016, 110, 104–114. [Google Scholar] [CrossRef]
- Doğramacı, P.A.; Aydın, D. Comparative experimental investigation of novel organic materials for direct evaporative cooling applications in hot-dry climate. J. Build. Eng. 2020, 30, 101240. [Google Scholar] [CrossRef]
- Niyomvas, B.P.B. Performance study of cooling pads. Adv. Mater. Res. 2013, 664, 931–935. [Google Scholar] [CrossRef]
- Lv, J.; Xu, H.; Zhu, M.; Dai, Y.; Liu, H.; Li, Z. The performance and model of porous materials in the indirect evaporative cooling system: A review. J. Build. Eng. 2021, 41, 102741. [Google Scholar] [CrossRef]
- Davarzani, H.; Smits, K.; Tolene, R.M.; Illangasekare, T. Study of the effect of wind speed on evaporation from soil through integrated modeling of the atmospheric boundary layer and shallow subsurface. Water Resour. Res. 2014, 50, 661–680. [Google Scholar] [CrossRef]
- Omeje, I.S.; Itina, T.E. Numerical study of the wetting dynamics of droplet on laser textured surfaces: Beyond classical wenzel and cassie-baxter model. Appl. Surf. Sci. Adv. 2022, 9, 100250. [Google Scholar] [CrossRef]
- Wang, Q.-h.; Wang, H.-X. Laser surface functionalization to achieve extreme surface wetting conditions and resultant surface functionalities. J. Cent. South Univ. 2022, 29, 3217–3247. [Google Scholar] [CrossRef]
- Hrabovsky, J.; Liberatore, C.; Mirza, I.; Sladek, J.; Beranek, J.; Bulgakov, A.V.; Bulgakova, N.M. Surface structuring of kapton polyimide with femtosecond and picosecond ir laser pulses. Interfacial Phenom. Heat Transf. 2019, 7, 113–121. [Google Scholar] [CrossRef]
- Starinskiy, S.V.; Rodionov, A.A.; Shukhov, Y.G.; Safonov, A.I.; Maximovskiy, E.A.; Sulyaeva, V.S.; Bulgakov, A.V. Formation of periodic superhydrophilic microstructures by infrared nanosecond laser processing of single-crystal silicon. Appl. Surf. Sci. 2020, 512, 145753. [Google Scholar] [CrossRef]
- Yuan, G.; Liu, Y.; Xie, F.; Guo, C.; Ngo, C.-V.; Li, W. Fabrication of superhydrophobic gully-structured surfaces by femtosecond laser and imprinting for high-efficiency self-cleaning rain collection. Langmuir 2022, 38, 2720–2728. [Google Scholar] [CrossRef]
- Zhan, Z.; Garcell, E.M.; Guo, C. Robust mold fabricated by femtosecond laser pulses for continuous thermal imprinting of superhydrophobic surfaces. Mater. Res. Express 2019, 6, 075011. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Xu, Y.; Ding, T.; Li, J.; Yin, J.; Fei, W.; Guo, W. Water-evaporation-induced electricity with nanostructured carbon materials. Nat. Nanotechnol. 2017, 12, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Cavusoglu, A.H.; Chen, X.; Gentine, P.; Sahin, O. Potential for natural evaporation as a reliable renewable energy resource. Nat. Commun. 2017, 8, 617. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, R.; Pan, Z.; Zheng, J.; Wang, X.; Li, R.; Yang, C.; Deng, L.; Vorobyev, A.Y. Evaporative and Wicking Functionalities at Hot Airflows of Laser Nano-/Microstructured Ti-6Al-4V Material. Nanomaterials 2023, 13, 218. https://doi.org/10.3390/nano13010218
Fang R, Pan Z, Zheng J, Wang X, Li R, Yang C, Deng L, Vorobyev AY. Evaporative and Wicking Functionalities at Hot Airflows of Laser Nano-/Microstructured Ti-6Al-4V Material. Nanomaterials. 2023; 13(1):218. https://doi.org/10.3390/nano13010218
Chicago/Turabian StyleFang, Ranran, Zhonglin Pan, Jiangen Zheng, Xiaofa Wang, Rui Li, Chen Yang, Lianrui Deng, and Anatoliy Y. Vorobyev. 2023. "Evaporative and Wicking Functionalities at Hot Airflows of Laser Nano-/Microstructured Ti-6Al-4V Material" Nanomaterials 13, no. 1: 218. https://doi.org/10.3390/nano13010218
APA StyleFang, R., Pan, Z., Zheng, J., Wang, X., Li, R., Yang, C., Deng, L., & Vorobyev, A. Y. (2023). Evaporative and Wicking Functionalities at Hot Airflows of Laser Nano-/Microstructured Ti-6Al-4V Material. Nanomaterials, 13(1), 218. https://doi.org/10.3390/nano13010218







