A Review on Low-Dimensional Nanomaterials: Nanofabrication, Characterization and Applications
Abstract
1. Introduction
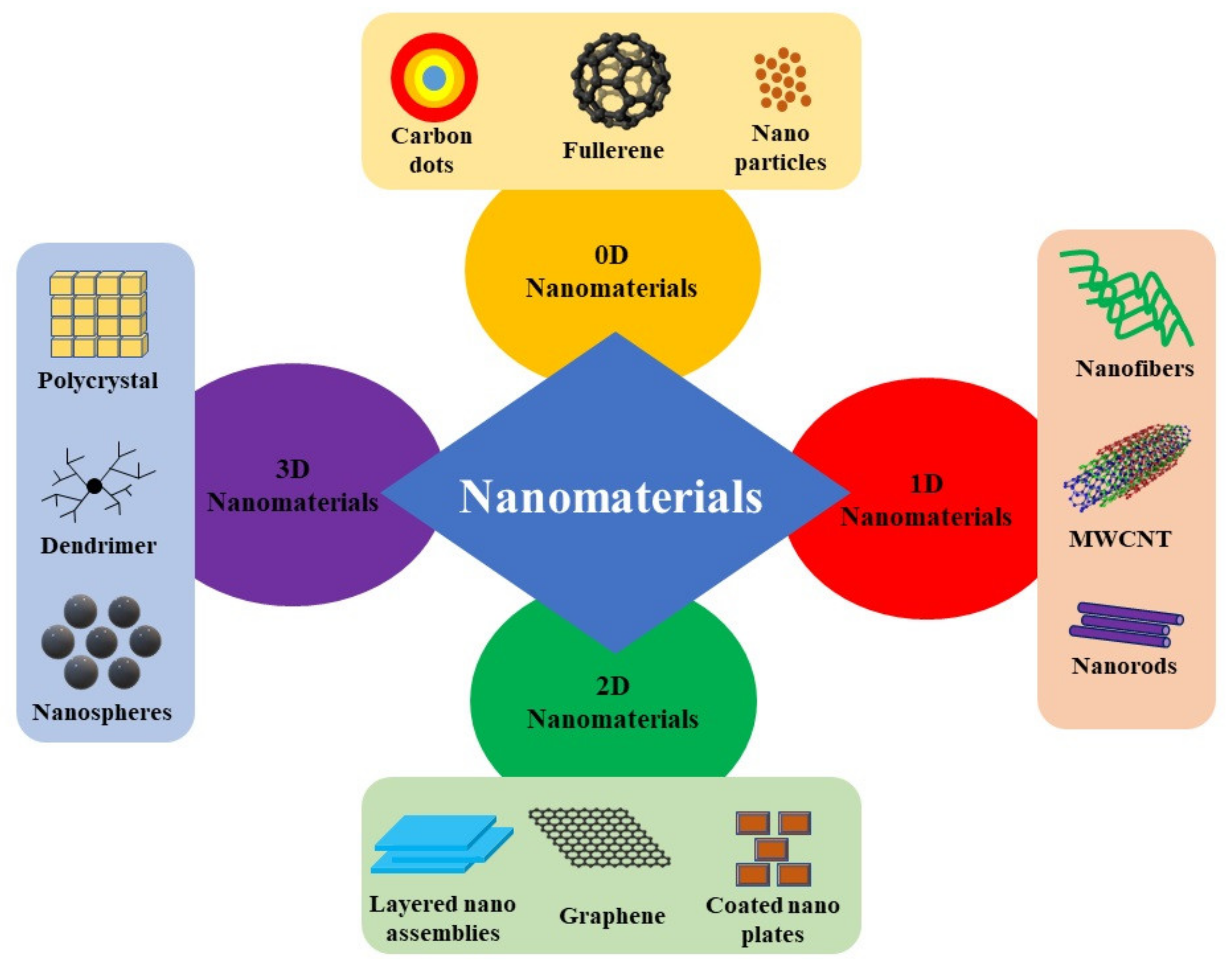
2. Classification of Low-Dimensional Nanomaterials
2.1. Zero-Dimensional (0D) Nanomaterials
2.2. One-Dimensional (1D) Nanomaterials
2.3. Two-Dimensional (2D) Nanomaterials
2.4. Three-Dimensional (3D) Nanomaterials
3. Fabrication Techniques
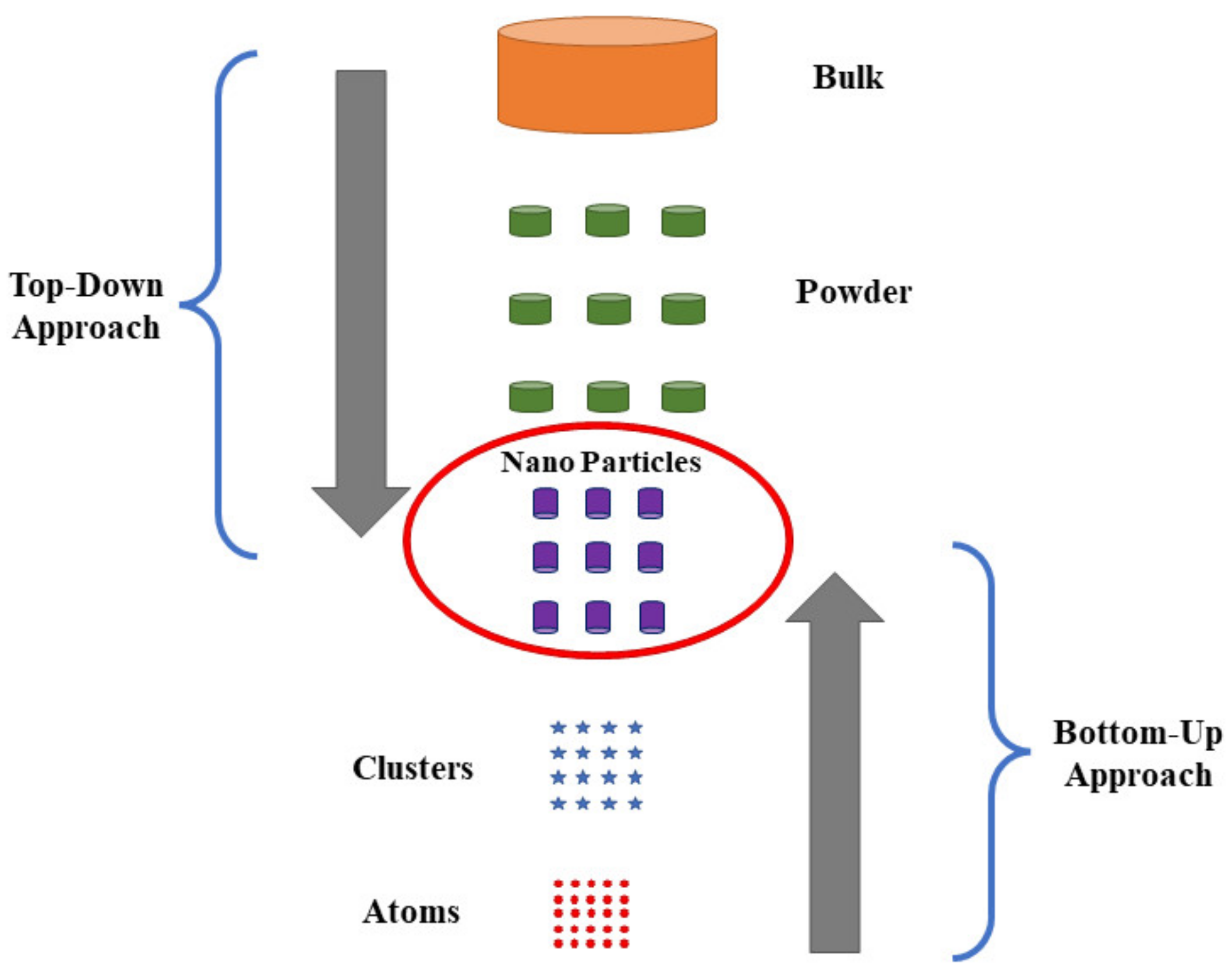
3.1. Top-Down Approach
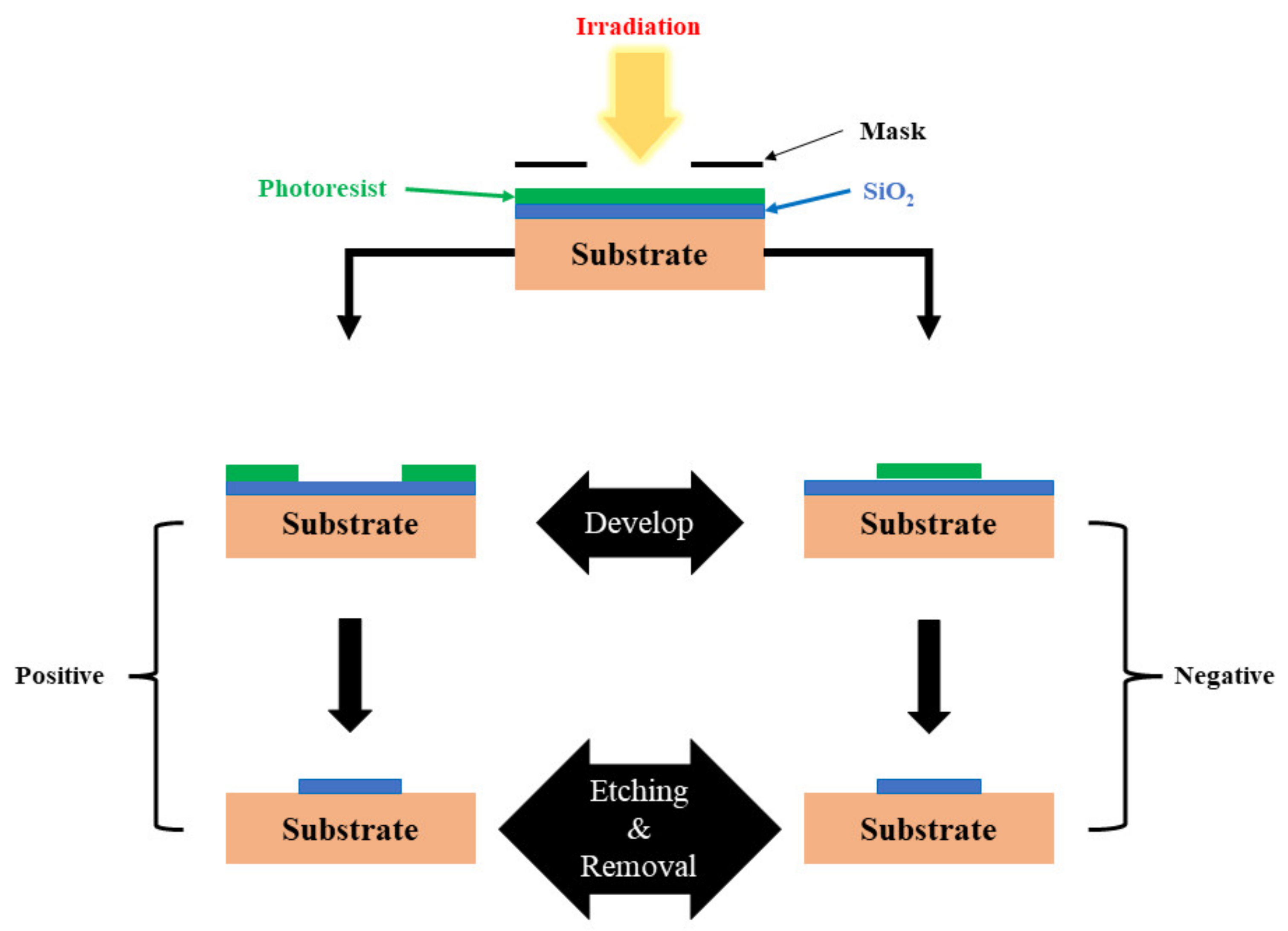
3.1.1. Lithography
Photolithography
Contact Lithography
X-Ray Lithography
Nanoimprint Lithography
Soft Lithography
Scanning Probe Lithography
Focused Ion Beam Lithography
Electron Beam Lithography
3.1.2. Sol Gel Method
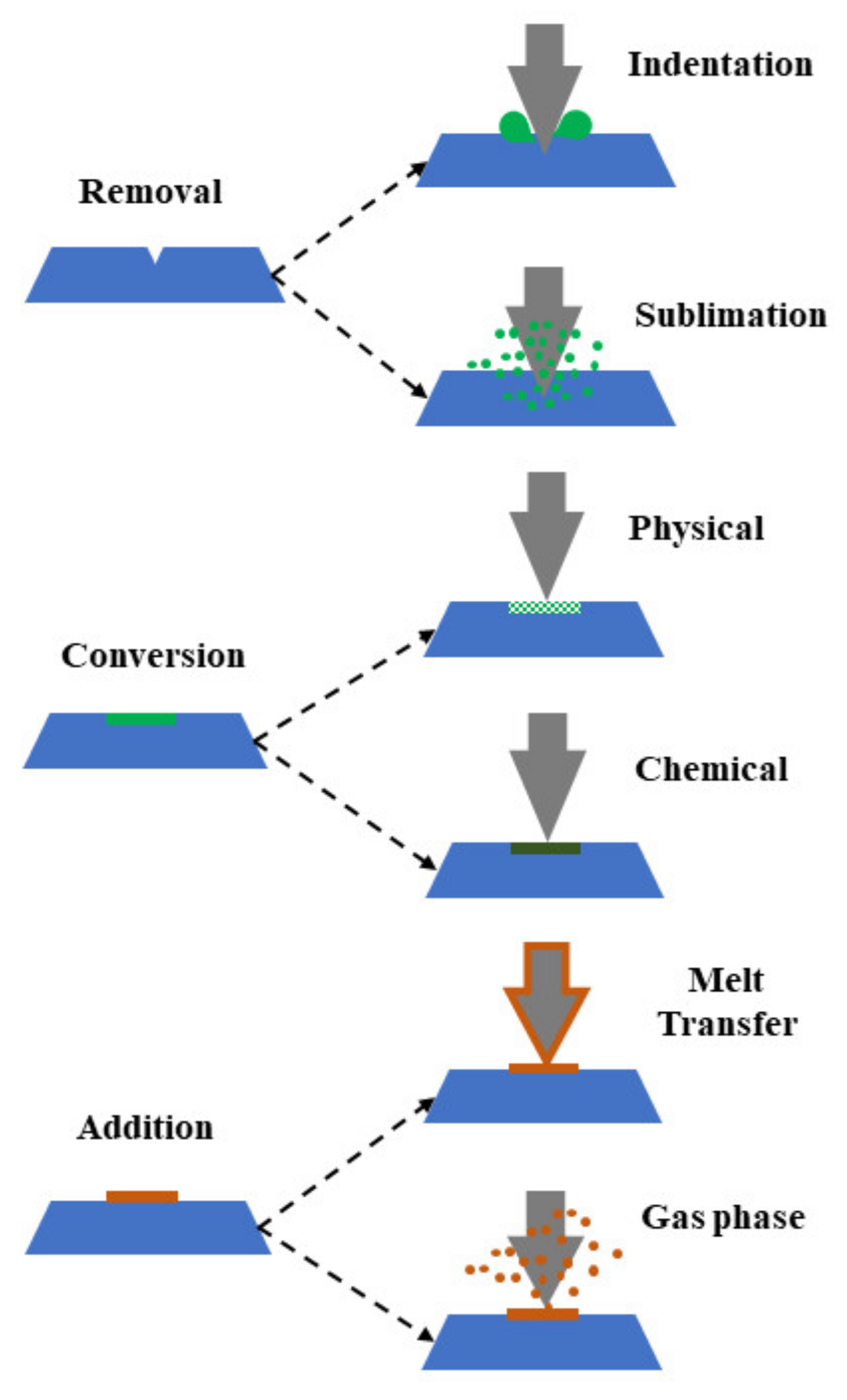
3.1.3. Template Etching
3.2. Bottom-Up Approach
3.2.1. Plasma Arching
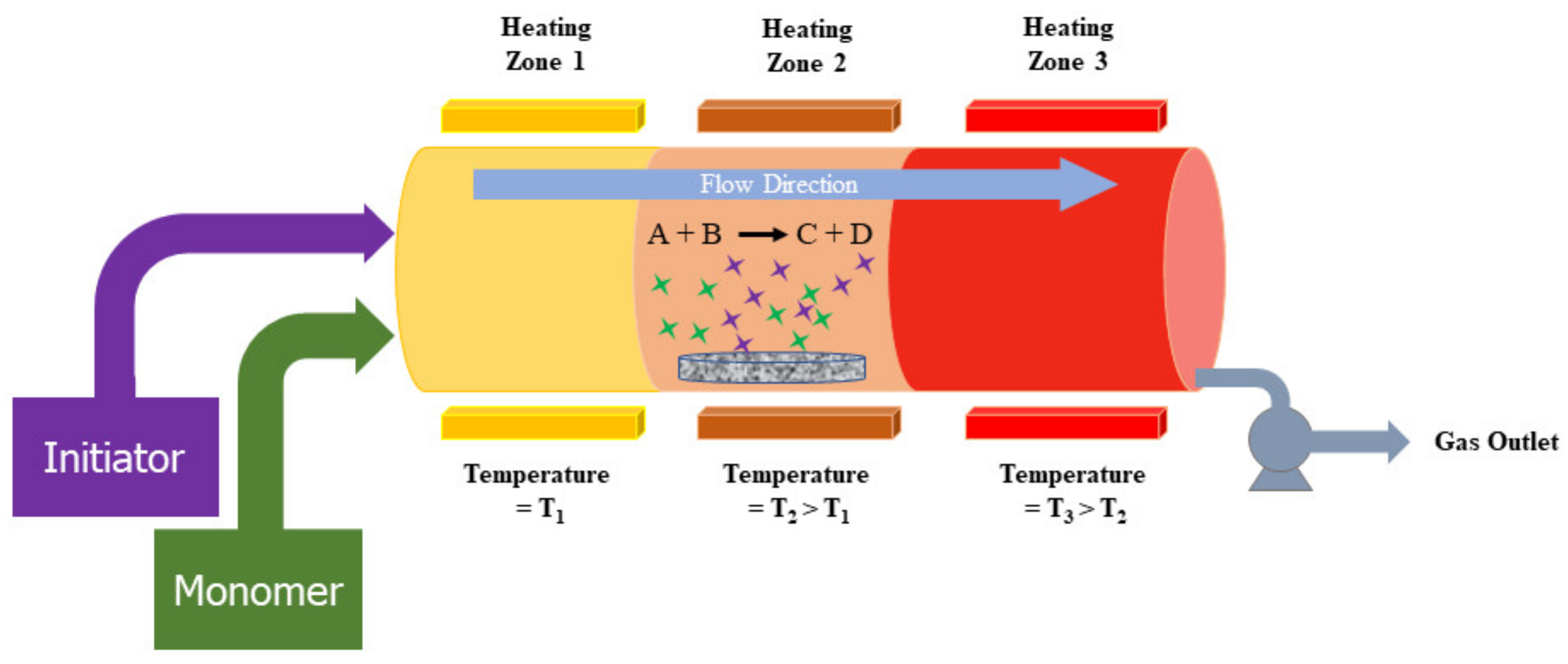
3.2.2. Chemical Vapor Deposition (CVD)
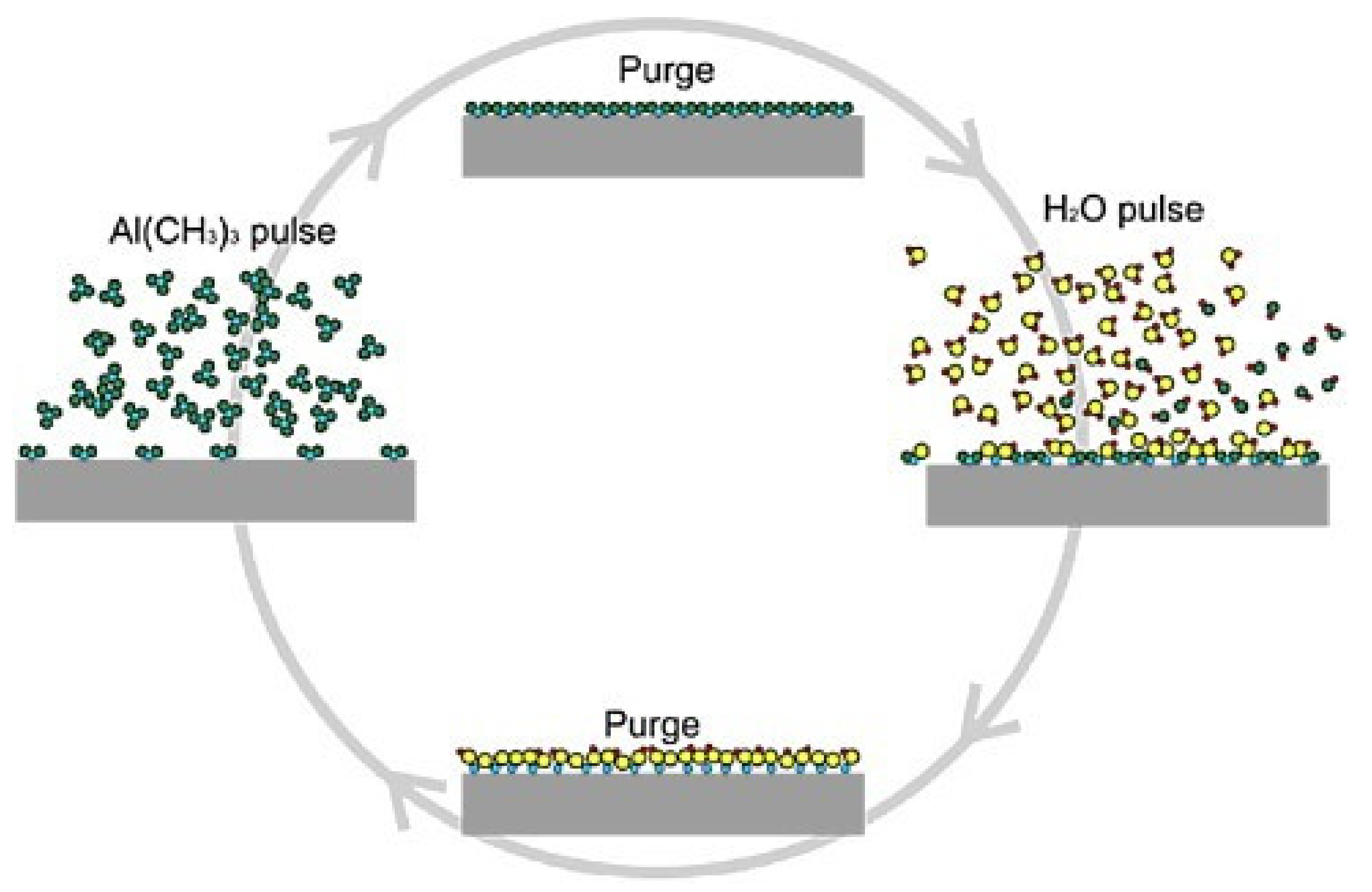
3.2.3. Atomic Layer Deposition (ALD)
3.2.4. Pulsed Laser Deposition (PLD)
3.2.5. Laser Pyrolysis
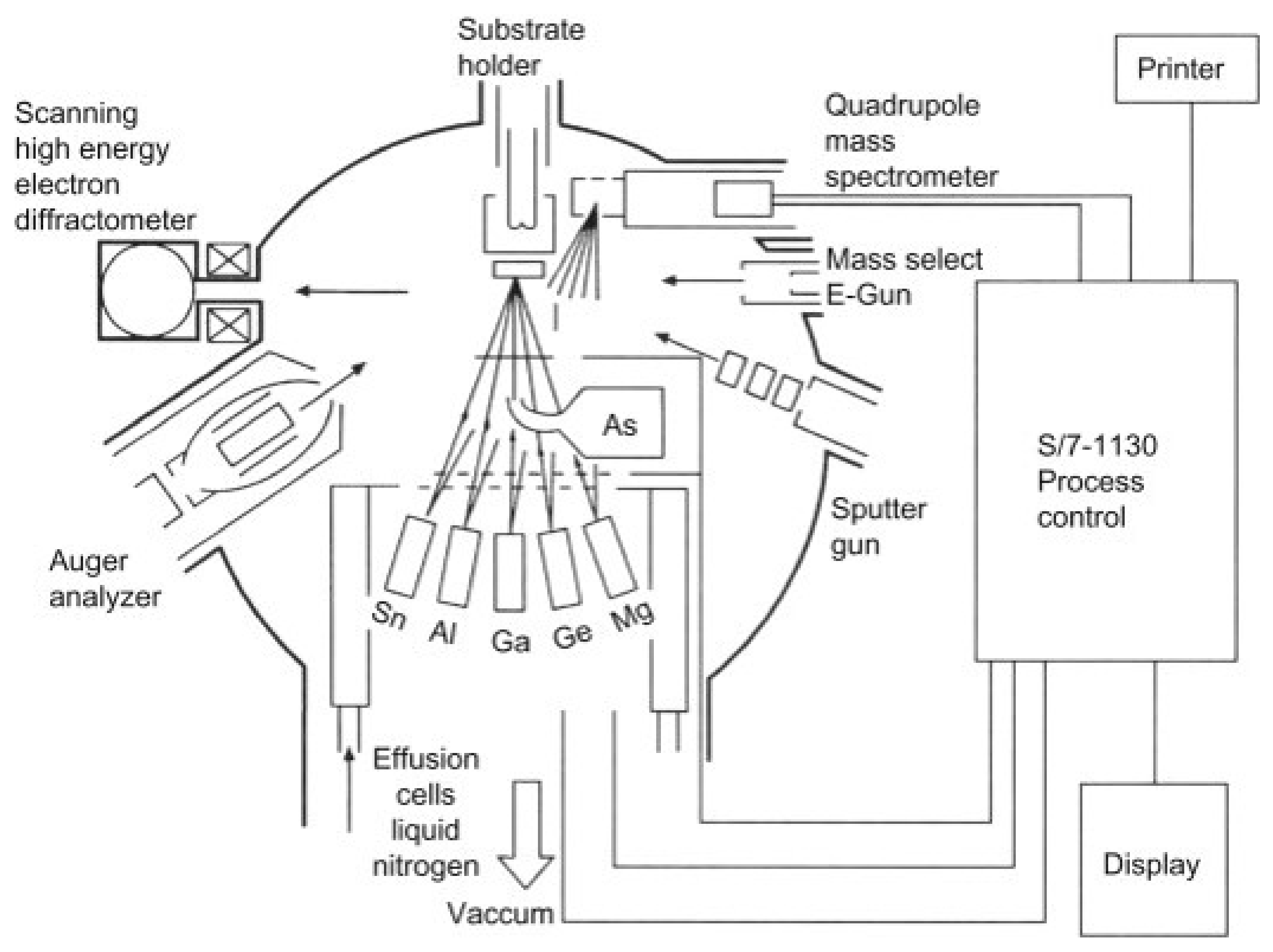
3.2.6. Molecular Beam Epitaxy (MBE)
3.2.7. Molecular Self-Assembly (MSA)
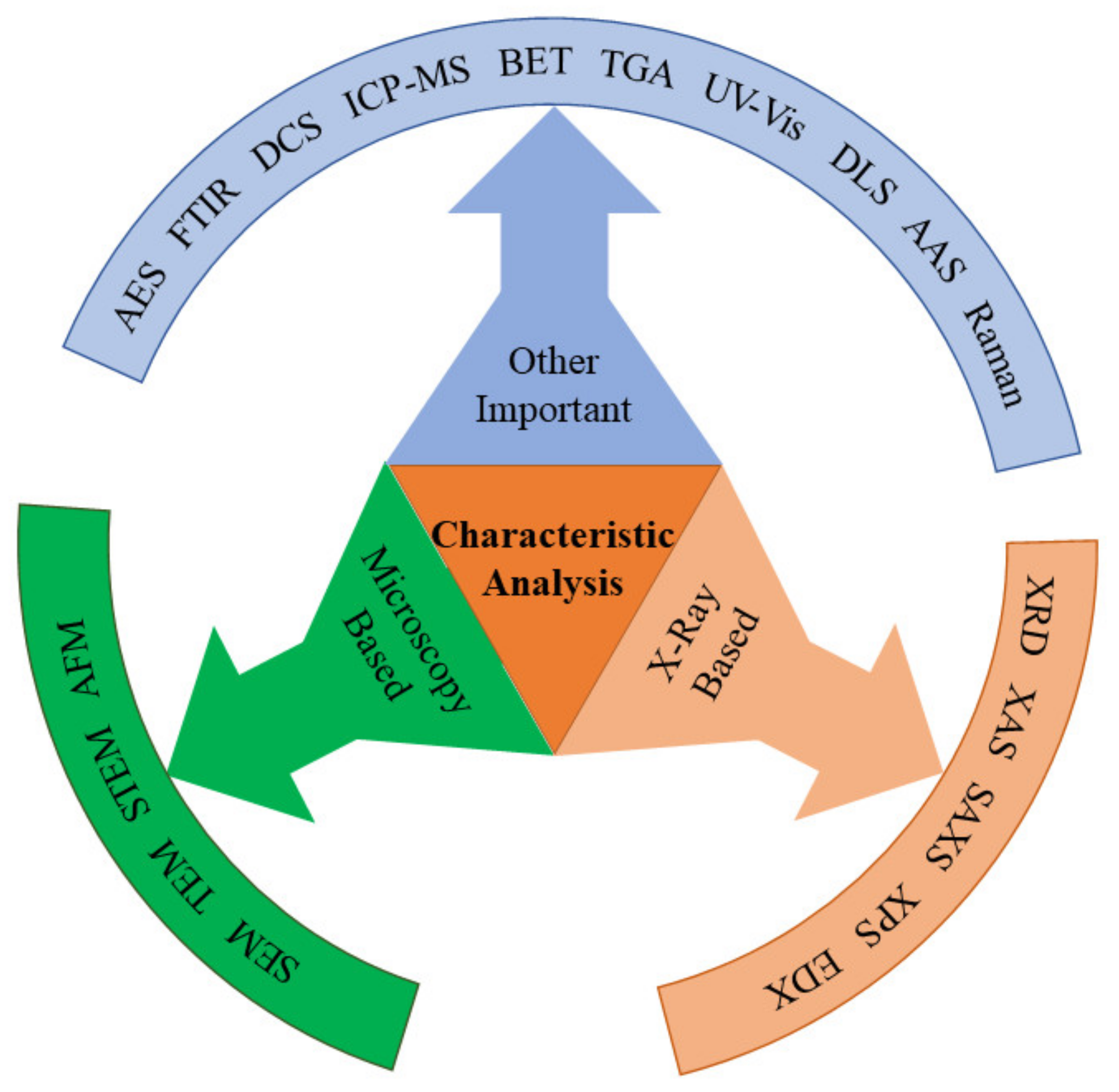
4. Characterization Techniques
4.1. X-ray-Based Characterization
4.2. Microscopy-Based Characterizations
4.3. Other Important Characterizations
5. Some Special Nanostructures
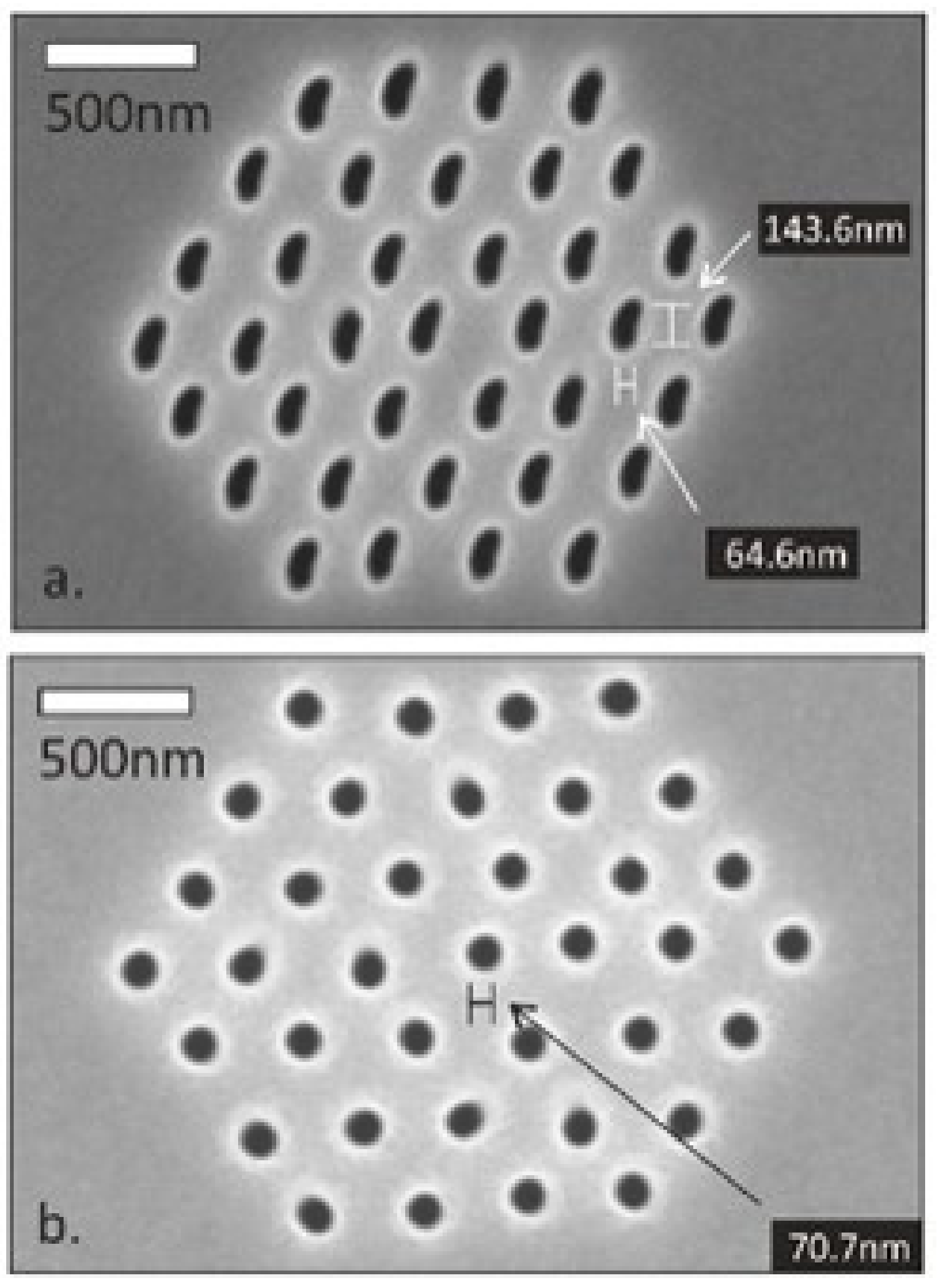
5.1. Uniformly Spaced Mushroom Structures
5.2. Semi-Circular Bumps
5.3. Uniform Silver Coating
5.4. Hierarchical Structures over Silicon Wafers
6. Applications
6.1. Bio-Sensing and Bio-Imaging
6.2. Photonics and Optoelectronics
6.3. Sensors
6.4. Energy Conversion and Storage
6.5. Functional Coatings
6.5.1. Superhydrophobic Coatings
6.5.2. Self-Cleaning Surfaces
6.5.3. Anti-corrosive Coatings
6.5.4. Antimicrobial Coating
6.6. Drug Delivery System
- Increased surface area for drug dose;
- Smaller dosage requirement;
- Increased dissolution rate.
- Promotes rapid therapeutic action;
- Increased solubility;
7. Highlighted Examples
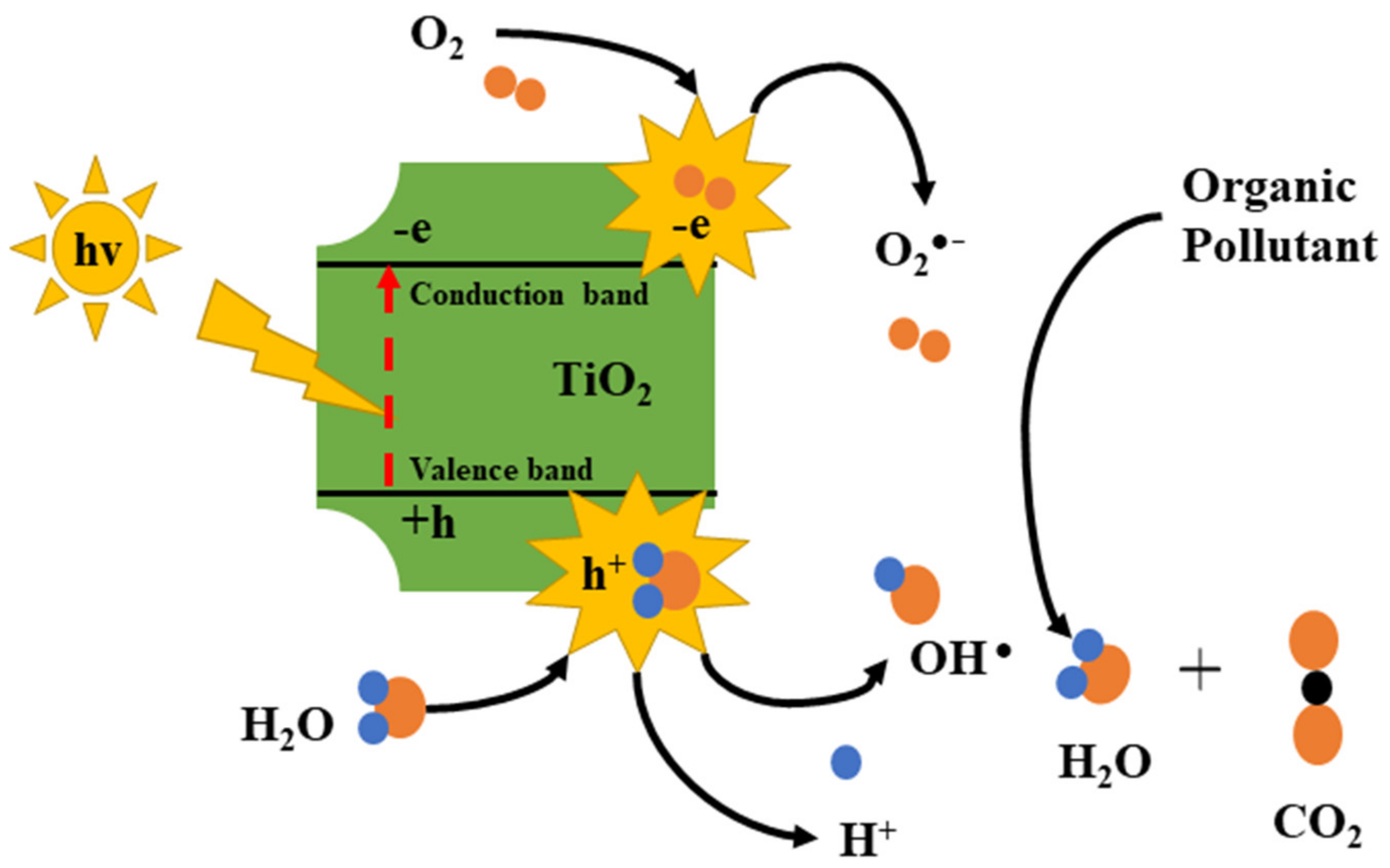
7.1. Photocatalysis
7.2. Batteries
7.3. Electrocatalysis
8. Conclusions and Future Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolahalam, L.A.; Kasi Viswanath, I.V.; Diwakar, B.S.; Govindh, B.; Reddy, V.; Murthy, Y.L.N. Review on Nanomaterials: Synthesis and Applications. Mater. Today Proc. 2019, 18, 2182–2190. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W.; Kammakakam, I. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Vollath, D. Nanomaterials: An Introduction to Synthesis, Properties and Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2013; ISBN 978-3-527-33379-0. [Google Scholar]
- Haghanifar, S.; Tomasovic, L.M.; Galante, A.J.; Pekker, D.; Leu, P.W. Stain-Resistant, Superomniphobic Flexible Optical Plastics Based on Nano-Enoki Mushroom-like Structures. J. Mater. Chem. A 2019, 7, 15698–15706. [Google Scholar] [CrossRef]
- David, N.; Djilali, N.; Wild, P. Fiber Bragg Grating Sensor for Two-Phase Flow in Microchannels. Microfluid. Nanofluidics 2012, 13, 99–106. [Google Scholar] [CrossRef]
- Qi, D.; Lu, N.; Xu, H.; Yang, B.; Huang, C.; Xu, M.; Gao, L.; Wang, Z.; Chi, L. Simple Approach to Wafer-Scale Self-Cleaning Antireflective Silicon Surfaces. Langmuir 2009, 25, 7769–7772. [Google Scholar] [CrossRef]
- Gao, Y.; Peng, Z.; Shi, T.; Tan, X.; Zhang, D.; Huang, Q.; Zou, C.; Liao, G. Bio-Inspired Fabrication of Complex Hierarchical Structure in Silicon. J. Nanosci. Nanotechnol. 2015, 15, 5918–5923. [Google Scholar] [CrossRef]
- Dao, T.C.; Luong, T.Q.N. Fabrication of Uniform Arrays of Silver Nanoparticles on Silicon by Electrodeposition in Ethanol Solution and Their Use in SERS Detection of Difenoconazole Pesticide. RSC Adv. 2020, 10, 40940–40947. [Google Scholar] [CrossRef]
- Kang, S.M.; Kim, H.; Choi, J.S.; An, J.H. Bioinspired Omniphobic Microchamber Structure. Adv. Mater. Interfaces 2021, 8, 2100027. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-Dimensional, One-Dimensional, Two-Dimensional and Three-Dimensional Nanostructured Materials for Advanced Electrochemical Energy Devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, T.; Liang, R.; Wei, M. Application of Zero-Dimensional Nanomaterials in Biosensing. Front. Chem. 2020, 8, 320. [Google Scholar] [CrossRef]
- Pandit, S.; Behera, P.; Sahoo, J.; De, M. In Situ Synthesis of Amino Acid Functionalized Carbon Dots with Tunable Properties and Their Biological Applications. ACS Appl. Bio. Mater. 2019, 2, 3393–3403. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Gong, J.; Chen, J.; Zeng, Z.; Huang, W.; Pu, K.; Liu, J.; Chen, P. Recent Advances on Graphene Quantum Dots: From Chemistry and Physics to Applications. Adv. Mater. 2019, 31, 1808283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, J.; Zhang, Y.; Chen, J.; Zhao, Y.; Niu, Y.; Yu, C. Cerium Dioxide-Doped Carboxyl Fullerene as Novel Nanoprobe and Catalyst in Electrochemical Biosensor for Amperometric Detection of the CYP2C19*2 Allele in Human Serum. Biosens. Bioelectron. 2018, 102, 94–100. [Google Scholar] [CrossRef]
- Robidillo, C.J.T.; Wandelt, S.; Dalangin, R.; Zhang, L.; Yu, H.; Meldrum, A.; Campbell, R.E.; Veinot, J.G.C. Ratiometric Detection of Nerve Agents by Coupling Complementary Properties of Silicon-Based Quantum Dots and Green Fluorescent Protein. ACS Appl. Mater. Interfaces 2019, 11, 33478–33488. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Tan, H.; Chen, Z.; Chen, X. FRET-Based Semiconducting Polymer Dots for PH Sensing. Sensors 2019, 19, 1455. [Google Scholar] [CrossRef]
- Bagheri, N.; Khataee, A.; Habibi, B.; Hassanzadeh, J. Mimetic Ag Nanoparticle/Zn-Based MOF Nanocomposite (AgNPs@ZnMOF) Capped with Molecularly Imprinted Polymer for the Selective Detection of Patulin. Talanta 2018, 179, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Sondhi, P.; Maruf, M.H.U.; Stine, K.J. Nanomaterials for Biosensing Lipopolysaccharide. Biosensors 2019, 10, 2. [Google Scholar] [CrossRef]
- Shi, X.; Meng, H.; Sun, Y.; Qu, L.; Lin, Y.; Li, Z.; Du, D. Far-Red to Near-Infrared Carbon Dots: Preparation and Applications in Biotechnology. Small 2019, 15, 1901507. [Google Scholar] [CrossRef]
- Panwar, N.; Soehartono, A.M.; Chan, K.K.; Zeng, S.; Xu, G.; Qu, J.; Coquet, P.; Yong, K.-T.; Chen, X. Nanocarbons for Biology and Medicine: Sensing, Imaging, and Drug Delivery. Chem. Rev. 2019, 119, 9559–9656. [Google Scholar] [CrossRef]
- Molaei, M.J. Carbon Quantum Dots and Their Biomedical and Therapeutic Applications: A Review. RSC Adv. 2019, 9, 6460–6481. [Google Scholar] [CrossRef]
- Su, B.; Wu, Y.; Jiang, L. The Art of Aligning One-Dimensional (1D) Nanostructures. Chem. Soc. Rev. 2012, 41, 7832–7856. [Google Scholar] [CrossRef] [PubMed]
- Gholami, T.; Pirsaheb, M. Review on Effective Parameters in Electrochemical Hydrogen Storage. Int. J. Hydrog. Energy 2021, 46, 783–795. [Google Scholar] [CrossRef]
- Cardoso, G.L.; Piquini, P.C.; Khossossi, N.; Ahuja, R. Lithium-Functionalized Boron Phosphide Nanotubes (BPNTs) as an Efficient Hydrogen Storage Carrier. Int. J. Hydrogen Energy 2021, 46, 20586–20593. [Google Scholar] [CrossRef]
- Sharma, A. Investigation on Platinum Loaded Multi-Walled Carbon Nanotubes for Hydrogen Storage Applications. Int. J. Hydrog. Energy 2020, 45, 2967–2974. [Google Scholar] [CrossRef]
- Huang, T.; Huang, X.; Hu, C.; Wang, J.; Liu, H.; Ma, Z.; Zou, J.; Ding, W. Enhancing Hydrogen Storage Properties of MgH2 through Addition of Ni/CoMoO4 Nanorods. Mater. Today Energy 2021, 19, 100613. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.; Dubonos, S.V.; Firsov, A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Fatima, J.; Shah, A.N.; Tahir, M.B.; Mehmood, T.; Shah, A.A.; Tanveer, M.; Nazir, R.; Jan, B.L.; Alansi, S. Tunable 2D Nanomaterials; Their Key Roles and Mechanisms in Water Purification and Monitoring. Front. Environ. Sci. 2022, 10, 766743. [Google Scholar] [CrossRef]
- Chhowalla, M.; Jena, D.; Zhang, H. Two-Dimensional Semiconductors for Transistors. Nat. Rev. Mater. 2016, 1, 16052. [Google Scholar] [CrossRef]
- Hu, T.; Mei, X.; Wang, Y.; Weng, X.; Liang, R.; Wei, M. Two-Dimensional Nanomaterials: Fascinating Materials in Biomedical Field. Sci. Bull. 2019, 64, 1707–1727. [Google Scholar] [CrossRef]
- Tan, C.; Cao, X.; Wu, X.-J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.-H.; et al. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Ghosh, S.; Pradhan, B.; Dalui, A.; Shrestha, L.K.; Acharya, S.; Ariga, K. Two-Dimensional (2D) Nanomaterials towards Electrochemical Nanoarchitectonics in Energy-Related Applications. Bull. Chem. Soc. Jpn. 2017, 90, 627–648. [Google Scholar] [CrossRef]
- Fang, Z.; Xing, Q.; Fernandez, D.; Zhang, X.; Yu, G. A Mini Review on Two-Dimensional Nanomaterial Assembly. Nano Res. 2020, 13, 1179–1190. [Google Scholar] [CrossRef]
- Hang, D.R.; Huang, C.F.; Cheng, K.A. Probing semiclassical magneto-oscillations in the low-field quantum Hall effect. Phys. Rev. B 2009, 80, 085312. [Google Scholar] [CrossRef]
- Paramasivam, G.; Palem, V.V.; Sundaram, T.; Sundaram, V.; Kishore, S.C.; Bellucci, S. Nanomaterials: Synthesis and Applications in Theranostics. Nanomaterials 2021, 11, 3228. [Google Scholar] [CrossRef]
- Wang, K.; Ma, Q.; Qu, C.-X.; Zhou, H.-T.; Cao, M.; Wang, S.-D. Review on 3D Fabrication at Nanoscale. Autex Res. J. 2022. [Google Scholar] [CrossRef]
- Iqbal, P.; Preece, J.A.; Mendes, P.M. Nanotechnology: The “Top-Down” and “Bottom-Up” Approaches. Supramol. Chem. 2012. [Google Scholar] [CrossRef]
- Teo, B.K.; Sun, X.H. From Top-down to Bottom-up to Hybrid Nanotechnologies: Road to Nanodevices. J. Clust. Sci. 2006, 17, 529–540. [Google Scholar] [CrossRef]
- Ochekpe, N.A.; Olorunfemi, P.O.; Ngwuluka, N.C. Nanotechnology and Drug Delivery. Part 1: Background and Applications. Trop. J. Pharm. Res. 2009, 8, 265–274. [Google Scholar] [CrossRef]
- Thirumalai, J. Introductory Chapter: The Eminence of Lithography—New Horizons of Next-Generation Lithography. In Micro/Nanolithography: A Heuristic Aspect on the Enduring Technology; Thirumalai, J., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Wu, W.; Dey, D.; Memis, O.G.; Katsnelson, A.; Mohseni, H. Fabrication of Large Area Periodic Nanostructures Using Nanosphere Photolithography. Nanoscale Res. Lett. 2008, 3, 351–354. [Google Scholar] [CrossRef]
- Francioso, L.; Siciliano, P. Top-down Contact Lithography Fabrication of a TiO2 Nanowire Array over a SiO2 Mesa. Nanotechnology 2006, 17, 3761–3767. [Google Scholar] [CrossRef]
- Kim, K.; Park, K.; Nam, H.; Kim, G.H.; Hong, S.K.; Kim, S.; Woo, H.; Yoon, S.; Kim, J.H.; Lim, G. Fabrication of Oblique Submicron-Scale Structures Using Synchrotron Hard X-ray Lithography. Polymers 2021, 13, 1045. [Google Scholar] [CrossRef] [PubMed]
- Menumerov, E.; Golze, S.D.; Hughes, R.A.; Neretina, S. Arrays of Highly Complex Noble Metal Nanostructures Using Nanoimprint Lithography in Combination with Liquid-Phase Epitaxy. Nanoscale 2018, 10, 18186–18194. [Google Scholar] [CrossRef]
- Jang, H.I.; Yoon, H.S.; Lee, T.I.; Lee, S.; Kim, T.S.; Shim, J.; Park, J.H. Creation of Curved Nanostructures Using Soft-Materials-Derived Lithography. Nanomaterials 2020, 10, 2414. [Google Scholar] [CrossRef]
- Howell, S.T.; Grushina, A.; Holzner, F.; Brugger, J. Thermal Scanning Probe Lithography—A Review. Microsyst. Nanoeng. 2020, 6, 21. [Google Scholar] [CrossRef]
- Córdoba, R.; Orús, P.; Strohauer, S.; Torres, T.E.; De Teresa, J.M. Ultra-Fast Direct Growth of Metallic Micro- and Nano-Structures by Focused Ion Beam Irradiation. Sci. Rep. 2019, 9, 14076. [Google Scholar] [CrossRef]
- Yoon, G.; Kim, I.; So, S.; Mun, J.; Kim, M.; Rho, J. Fabrication of Three-Dimensional Suspended, Interlayered and Hierarchical Nanostructures by Accuracy-Improved Electron Beam Lithography Overlay. Sci. Rep. 2017, 7, 6668. [Google Scholar] [CrossRef]
- Ishihara, T.; Luo, X. Chapter 17 Nanophotolithography Based on Surface Plasmon Interference: Handai NanoPhotonics; Kawata, S., Masuhara, H., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2006; Volume 2, pp. 305–312. [Google Scholar]
- Tanaka, H. Epitaxial Growth of Oxide Films and Nanostructures. In Handbook of Crystal Growth, 2nd ed.; Thomas, F.K., Ed.; Elsevier: North-Holland, Netherlands, 2015; Volume 3, pp. 555–604. ISBN 9780444633057. [Google Scholar]
- Toropov, N.; Vartanyan, T. Noble Metal Nanoparticles: Synthesis and Optical Properties; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; Volumes 1–5, ISBN 9780128122952. [Google Scholar]
- Zhang, P.; Yang, G.; Li, F.; Shi, J.; Zhong, H. Direct in Situ Photolithography of Perovskite Quantum Dots Based on Photocatalysis of Lead Bromide Complexes. Nat. Commun. 2022, 13, 6713. [Google Scholar] [CrossRef]
- Wu, C.Y.; Hsieh, H.; Lee, Y.C. Contact Photolithography at Sub-Micrometer Scale Using a Soft Photomask. Micromachines 2019, 10, 547. [Google Scholar] [CrossRef]
- Seisyan, R.P. Nanolithography in Microelectronics: A Review. Tech. Phys. 2011, 56, 1061–1073. [Google Scholar] [CrossRef]
- Pugachev, M.V.; Duleba, A.I.; Galiullin, A.A.; Kuntsevich, A.Y. Micromask Lithography for Cheap and Fast 2D Materials Microstructures Fabrication. Micromachines 2021, 12, 850. [Google Scholar] [CrossRef] [PubMed]
- Cerrina, F.; Grenci, G. X-ray Lithography. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–6. ISBN 9780128035818. [Google Scholar]
- Jain, N.K.; Chaubey, S.K. 1.17 Review of Miniature Gear Manufacturing: Comprehensive Materials Finishing; Hashmi, M.S.J., Ed.; Elsevier: Oxford, UK, 2017; Volume 1, pp. 504–538. [Google Scholar] [CrossRef]
- Nguyen, N.-T. Fabrication Technologies. In Micro and Nano Technologies, Micromixers, 2nd ed.; Nguyen, N.T., Ed.; William Andrew Publishing: Norwich, NY, USA, 2012; Volume 1, pp. 113–161. ISBN 9781437735208. [Google Scholar]
- Gentselev, A.N.; Baev, S.G. Production of Planar Elements of Terahertz Optics by Means of Deep X-ray Lithography. Optoelectron. Instrum. Data Process. 2022, 58, 198–205. [Google Scholar] [CrossRef]
- Lohse, M.; Heinrich, M.; Grützner, S.; Haase, A.; Ramos, I.; Salado, C.; Thesen, M.W.; Grützner, G. Versatile Fabrication Method for Multiscale Hierarchical Structured Polymer Masters Using a Combination of Photo- and Nanoimprint Lithography. Micro Nano Eng. 2021, 10, 100079. [Google Scholar] [CrossRef]
- Choi, J.; Lee, C.C.; Park, S. Scalable Fabrication of Sub-10 nm Polymer Nanopores for DNA Analysis. Microsyst. Nanoeng. 2019, 5, 12. [Google Scholar] [CrossRef]
- Sahin, O.; Ashokkumar, M.; Ajayan, P.M. 3—Micro- and nanopatterning of biomaterial surfaces. In Woodhead Publishing Series in Biomaterials, Fundamental Biomaterials: Metals; Balakrishnan, P., Sreekala, M.S., Thomas, S., Eds.; Woodhead Publishing: Sawston, UK, 2018; Volume 1, pp. 67–78. [Google Scholar] [CrossRef]
- Kane, R.S.; Takayama, S.; Ostuni, E.; Ingber, D.E.; Whitesides, G.M. Patterning Proteins and Cells Using Soft Lithography. Biomaterials 1999, 20, 2363–2376. [Google Scholar] [CrossRef]
- Abdelgawad, M.; Watson, M.W.L.; Young, E.W.K.; Mudrik, J.M.; Ungrin, M.D.; Wheeler, A.R. Soft Lithography: Masters on Demand. Lab Chip 2008, 8, 1379–1385. [Google Scholar] [CrossRef]
- Lipomi, D.J.; Martinez, R.V.; Cademartiri, L.; Whitesides, G.M. Soft Lithographic Approaches to Nanofabrication; Elsevier B.V.: Amsterdam, The Netherlands, 2012; Volume 7, ISBN 9780080878621. [Google Scholar]
- Khademhosseini, A.; Du, Y.; Rajalingam, B.; Vacanti, J.P.; Langer, R.S. Microscale Technologies for Tissue Engineering. In Advances in Tissue Engineering; Polak, J., Mantalaris, S., Harding, S.E., Eds.; Imperial College Press: London, UK, 2008; pp. 349–369. [Google Scholar] [CrossRef]
- Gan, Z.; Najafidehaghani, E.; Han, S.H.; Shradha, S.; Abtahi, F.; Neumann, C.; Picker, J.; Vogl, T.; Hübner, U.; Eilenberger, F.; et al. Patterned Growth of Transition Metal Dichalcogenide Monolayers and Multilayers for Electronic and Optoelectronic Device Applications. Small Methods 2022, 6, 2200300. [Google Scholar] [CrossRef]
- Garcia, R.; Knoll, A.W.; Riedo, E. Advanced Scanning Probe Lithography. Nat. Nanotechnol. 2014, 9, 577–587. [Google Scholar] [CrossRef]
- Fischer, A.C.; Mäntysalo, M.; Niklaus, F. Inkjet Printing, Laser-Based Micromachining, and Micro–3D Printing Technologies for MEMS. In Handbook of Silicon Based MEMS Materials and Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 531–545. [Google Scholar]
- Bruchhaus, L.; Mazarov, P.; Bischoff, L.; Gierak, J.; Wieck, A.D.; Hövel, H. Comparison of Technologies for Nano Device Prototyping with a Special Focus on Ion Beams: A Review. Appl. Phys. Rev. 2017, 4, 011302. [Google Scholar] [CrossRef]
- Economou, N.; Ward, B.; Morgan, J.; Notte, J. Helium Ion Microscopy: An Introduction. Innov. Pharm. Technol. 2007, 020801, 28–30. [Google Scholar] [CrossRef]
- Huth, M.; Porrati, F.; Dobrovolskiy, O.V. Focused Electron Beam Induced Deposition Meets Materials Science. Microelectron. Eng. 2018, 185–186, 9–28. [Google Scholar] [CrossRef]
- Ven Kouwen, L.; Botman, A.; Hagen, C.W. Focused Electron-Beam-Induced Deposition of 3 Nm Dots in a Scanning Electron Microscope. Nano Lett. 2009, 9, 2149–2152. [Google Scholar] [CrossRef] [PubMed]
- Fürjes, P. Controlled Focused Ion Beam Milling of Composite Solid State Nanopore Arrays for Molecule Sensing. Micromachines 2019, 10, 774. [Google Scholar] [CrossRef]
- Martinez-Chapa, S.O.; Salazar, A.; Madou, M.J. Two-Photon Polymerization as a Component of Desktop-Integrated Manufacturing Platforms; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128178270. [Google Scholar]
- Charlton, M.D.B. Photonic Crystal Nitride LEDs; Woodhead Publishing Limited: Sawston, UK, 2013; ISBN 9780857095077. [Google Scholar]
- Tu, M.; Xia, B.; Kravchenko, D.E.; Tietze, M.L.; Cruz, A.J.; Stassen, I.; Hauffman, T.; Teyssandier, J.; De Feyter, S.; Wang, Z.; et al. Direct X-ray and Electron-Beam Lithography of Halogenated Zeolitic Imidazolate Frameworks. Nat. Mater. 2021, 20, 93–99. [Google Scholar] [CrossRef]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Yilmaz, E.; Soylak, M. Functionalized Nanomaterials for Sample Preparation Methods. In Handbook of Nanomaterials in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–413. [Google Scholar]
- Pajonk, G.M. Drying methods preserving the textural Properties of gels. J. Phys. Colloques 50. 1989, 24, C4-13–C4-22. [Google Scholar] [CrossRef]
- Yu, H.D.; Regulacio, M.D.; Ye, E.; Han, M.Y. Chemical Routes to Top-down Nanofabrication. Chem. Soc. Rev. 2013, 42, 6006–6018. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, G.; Gao, L.; Yang, J.; Chhowalla, M.; Gharahcheshmeh, M.H.; Gleason, K.K.; Choi, Y.S.; Hong, B.H.; Liu, Z. Chemical Vapour Deposition. Nat. Rev. Methods Prim. 2021, 1, 5. [Google Scholar] [CrossRef]
- Oviroh, P.O.; Akbarzadeh, R.; Pan, D.; Coetzee, R.A.M.; Jen, T.C. New Development of Atomic Layer Deposition: Processes, Methods and Applications. Sci. Technol. Adv. Mater. 2019, 20, 465–496. [Google Scholar] [CrossRef]
- Leskelä, M.; Niinistö, J.; Ritala, M. Atomic Layer Deposition. In Comprehensive Materials Processing; Elsevier: Amsterdam, The Netherlands, 2014; pp. 101–123. [Google Scholar]
- Xiong, S.; Qian, X.; Zhong, Z.; Wang, Y. Atomic Layer Deposition for Membrane Modification, Functionalization and Preparation: A Review. J. Memb. Sci. 2022, 658, 120740. [Google Scholar] [CrossRef]
- Nur, O.; Willander, M. Conventional Nanofabrication Methods. In Low Temperature Chemical Nanofabrication; Elsevier: Amsterdam, The Netherlands, 2020; pp. 49–86. [Google Scholar]
- Wang, S.; Gao, L. Laser-Driven Nanomaterials and Laser-Enabled Nanofabrication for Industrial Applications. In Micro and Nano Technologies, Industrial Applications of Nanomaterials; Thomas, S., Grohens, Y., Pottathara, Y.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 181–203. ISBN 9780128157497. [Google Scholar]
- Cho, A.Y.; Arthur, J.R. Molecular Beam Epitaxy. Prog. Solid State Chem. 1975, 10, 157–191. [Google Scholar] [CrossRef]
- Adachi, H.; Wasa, K. Thin Films and Nanomaterials. In Handbook of Sputtering Technology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 3–39. [Google Scholar]
- Lombardo, D.; Kiselev, M.A.; Magazù, S.; Calandra, P. Amphiphiles Self-Assembly: Basic Concepts and Future Perspectives of Supramolecular Approaches. Adv. Condens. Matter Phys. 2015, 2015, 151683. [Google Scholar] [CrossRef]
- Rahmawan, Y.; Xu, L.; Yang, S. Self-Assembly of Nanostructures towards Transparent, Superhydrophobic Surfaces. J. Mater. Chem. A 2013, 1, 2955–2969. [Google Scholar] [CrossRef]
- Mijatovic, D.; Eijkel, J.C.T.; Van Den Berg, A. Technologies for Nanofluidic Systems: Top-down vs. Bottom-up—A Review. Lab Chip 2005, 5, 492–500. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization Techniques for Nanoparticles: Comparison and Complementarity upon Studying Nanoparticle Properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Birkholz, M. Thin Film Analysis by X-ray Scattering, 1st ed.; Wiley-VCH: Weinheim, Germany, 2006; ISBN 9783527310524. [Google Scholar]
- Yano, J.; Yachandra, V.K. X-ray Absorption Spectroscopy. Photosynth. Res. 2009, 102, 241–254. [Google Scholar] [CrossRef]
- Singh, A.K. Experimental Methodologies for the Characterization of Nanoparticles. In Engineered Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2016; pp. 125–170. [Google Scholar]
- Engelhard, M.H.; Droubay, T.C.; Du, Y. X-ray Photoelectron Spectroscopy Applications. In Encyclopedia of Spectroscopy and Spectrometry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 716–724. [Google Scholar]
- Janssens, M. Fundamental Measurement Techniques. In Flammability Testing of Materials Used in Construction, Transport and Mining; Elsevier: Amsterdam, The Netherlands, 2006; pp. 22–62. [Google Scholar]
- Koutcher, J.A.; Burt, C.T. Principles of Nuclear Magnetic Resonance. J. Nucl. Med. 1984, 25, 101–111. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Atarod, M.; Sajjadi, M.; Sajadi, S.M.; Issaabadi, Z. Plant-Mediated Green Synthesis of Nanostructures: Mechanisms, Characterization, and Applications. Interface Sci. Technol. 2019, 28, 199–322. [Google Scholar]
- Bottom, R. Thermogravimetric Analysis. In Principles and Applications of Thermal Analysis; Blackwell Publishing Ltd.: Oxford, UK, 2008; pp. 87–118. [Google Scholar]
- Venkatachalam, S. Ultraviolet and Visible Spectroscopy Studies of Nanofillers and Their Polymer Nanocomposites. In Spectroscopy of Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2016; pp. 130–157. [Google Scholar]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic Light Scattering: A Practical Guide and Applications in Biomedical Sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- Minelli, C.; Sikora, A.; Garcia-Diez, R.; Sparnacci, K.; Gollwitzer, C.; Krumrey, M.; Shard, A.G. Measuring the Size and Density of Nanoparticles by Centrifugal Sedimentation and Flotation. Anal. Methods 2018, 10, 1725–1732. [Google Scholar] [CrossRef]
- Goenaga-Infante, H.; Bartczak, D. Single Particle Inductively Coupled Plasma Mass Spectrometry (SpICP-MS). In Characterization of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 65–77. [Google Scholar]
- Zhao, J.; Liu, X. Electron Microscopic Methods (TEM, SEM and Energy Dispersal Spectroscopy). In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Vlasov, I.I.; Turner, S.; Van Tendeloo, G.; Shiryaev, A.A. Recent Results on Characterization of Detonation Nanodiamonds. In Ultananocrystalline Diamond; Elsevier: Amsterdam, The Netherlands, 2012; pp. 291–326. [Google Scholar]
- Mehrpouya, M.; Lavvafi, H.; Darafsheh, A. Microstructural Characterization and Mechanical Reliability of Laser-Machined Structures. In Advances in Laser Materials Processing; Elsevier: Amsterdam, The Netherlands, 2018; pp. 731–761. [Google Scholar]
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Importance of Physicochemical Characterization of Nanoparticles in Pharmaceutical Product Development. In Basic Fundamentals of Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 369–400. [Google Scholar]
- Vahabi, S.; Nazemi Salman, B.; Javanmard, A. Atomic Force Microscopy Application in Biological Research: A Review Study. Iran. J. Med. Sci. 2013, 38, 76–83. [Google Scholar] [PubMed]
- Ilyin, A.M. Auger Electron Spectroscopy. In Microscopy Methods in Nanomaterials Characterization; Elsevier: Amsterdam, The Netherlands, 2017; pp. 363–381. [Google Scholar]
- Rades, S.; Wirth, T.; Unger, W. Investigation of Silica Nanoparticles by Auger Electron Spectroscopy (AES). Surf. Interface Anal. 2014, 46, 952–956. [Google Scholar] [CrossRef]
- Xu, M.; Fujita, D.; Gao, J.; Hanagata, N. Auger Electron Spectroscopy: A Rational Method for Determining Thickness of Graphene Films. ACS Nano 2010, 4, 2937–2945. [Google Scholar] [CrossRef] [PubMed]
- Vladitsi, M.; Nikolaou, C.; Kalogiouri, N.P.; Samanidou, V.F. Analytical Methods for Nanomaterial Determination in Biological Matrices. Methods Protoc. 2022, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Kees, K.; Leopold, K. Characterization of Various Metal Nanoparticles by Graphite Furnace Atomic Absorption Spectrometry: Possibilities and Limitations with Regard to Size and Shape. J. Anal. At. Spectrom. 2020, 35, 2536–2544. [Google Scholar] [CrossRef]
- Darienzo, R.E.; Wang, J.; Chen, O.; Sullivan, M.; Mironava, T.; Kim, H.; Tannenbaum, R. Surface-Enhanced Raman Spectroscopy Characterization of Breast Cell Phenotypes: Effect of Nanoparticle Geometry. ACS Appl. Nano Mater. 2019, 2, 6960–6970. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Velavan, S.; Amargeetha, A. X-ray Diffraction (XRD) and Energy Dispersive Spectroscopy (EDS) Analysis of Silver Nanoparticles Synthesized from Erythrina Indica Flowers. Nanosci. Technol. Open Access 2018, 5, 1–5. [Google Scholar] [CrossRef]
- De Groot, F. High-Resolution X-ray Emission and X-ray Absorption Spectroscopy. Chem. Rev. 2001, 101, 1779–1808. [Google Scholar] [CrossRef]
- Hummer, A.A.; Rompel, A. X-ray Absorption Spectroscopy: A Tool to Investigate the Local Structure of Metal-Based Anticancer Compounds In Vivo, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 93, ISBN 9780124165960. [Google Scholar]
- Lopes, C.W.; Cerrillo, J.L.; Palomares, A.E.; Rey, F.; Agostini, G. An in Situ XAS Study of the Activation of Precursor-Dependent Pd Nanoparticles. Phys. Chem. Chem. Phys. 2018, 20, 12700–12709. [Google Scholar] [CrossRef]
- Li, T.; Senesi, A.J.; Lee, B. Small Angle X-ray Scattering for Nanoparticle Research. Chem. Rev. 2016, 116, 11128–11180. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Ma, Z.; Bruce, P.G. Ordered Mesoporous Metal Oxides: Synthesis and Applications. Chem. Soc. Rev. 2012, 41, 4909. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.R.A.F.; Prymak, O.; Grasmik, V.; Pappert, K.; Wlysses, W.; Otubo, L.; Epple, M.; Oliveira, C.L.P. An in Situ SAXS Investigation of the Formation of Silver Nanoparticles and Bimetallic Silver–Gold Nanoparticles in Controlled Wet-Chemical Reduction Synthesis. Nanoscale Adv. 2020, 2, 225–238. [Google Scholar] [CrossRef]
- Oswald, S. X-ray Photoelectron Spectroscopy in Analysis of Surfaces; Elsevier Inc.: Amsterdam, The Netherlands, 2013; ISBN 9780470027318. [Google Scholar]
- Baer, D.R. Guide to Making XPS Measurements on Nanoparticles. J. Vac. Sci. Technol. A 2020, 38, 031201. [Google Scholar] [CrossRef]
- Korin, E.; Froumin, N.; Cohen, S. Surface Analysis of Nanocomplexes by X-ray Photoelectron Spectroscopy (XPS). ACS Biomater. Sci. Eng. 2017, 3, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Sublemontier, O.; Nicolas, C.; Aureau, D.; Patanen, M.; Kintz, H.; Liu, X.; Gaveau, M.-A.; Le Garrec, J.-L.; Robert, E.; Barreda, F.-A.; et al. X-ray Photoelectron Spectroscopy of Isolated Nanoparticles. J. Phys. Chem. Lett. 2014, 5, 3399–3403. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, N.M.; Kuipers, J.; Giepmans, B.N.G. Sample Preparation for Energy Dispersive X-ray Imaging of Biological Tissues, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; Volume 162, ISBN 9780128220580. [Google Scholar]
- Girão, A.V.; Caputo, G.; Ferro, M.C. Application of Scanning Electron Microscopy–Energy Dispersive X-ray Spectroscopy (SEM-EDS). Compr. Anal. Chem. 2017, 75, 153–168. [Google Scholar] [CrossRef]
- Scimeca, M.; Bischetti, S.; Lamsira, H.K.; Bonfiglio, R.; Bonanno, E. Energy Dispersive X-ray (EDX) Microanalysis: A Powerful Tool in Biomedical Research and Diagnosis. Eur. J. Histochem. 2018, 62, 89–99. [Google Scholar] [CrossRef]
- Hamuyuni, J.; Daramola, M.O.; Oluwasina, O.O. Energy-Dispersive X-ray Spectroscopy: Theory and Application in Engineering and Science. In Encyclopedia of Physical Organic Chemistry, 1st ed.; Wang, U.W., Juaristi, E., Eds.; Wiley: Aalto, Finland, 2017; Volume 1, pp. 1–23. ISBN 9781118470459. [Google Scholar]
- Patri, A.; Umbreit, T.; Zheng, J.; Nagashima, K.; Goering, P.; Francke-Carroll, S.; Gordon, E.; Weaver, J.; Miller, T.; Sadrieh, N.; et al. Energy Dispersive X-ray Analysis of Titanium Dioxide Nanoparticle Distribution after Intravenous and Subcutaneous Injection in Mice. J. Appl. Toxicol. 2009, 29, 662–672. [Google Scholar] [CrossRef]
- Vladár, A.E.; Hodoroaba, V.-D. Characterization of Nanoparticles by Scanning Electron Microscopy. In Characterization of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 7–27. ISBN 9780128141830. [Google Scholar]
- Vernon-Parry, K.D. Scanning Electron Microscopy: An Introduction. III-Vs Rev. 2000, 13, 40–44. [Google Scholar] [CrossRef]
- Suga, M.; Asahina, S.; Sakuda, Y.; Kazumori, H.; Nishiyama, H.; Nokuo, T.; Alfredsson, V.; Kjellman, T.; Stevens, S.M.; Cho, H.S.; et al. Recent Progress in Scanning Electron Microscopy for the Characterization of Fine Structural Details of Nano Materials. Prog. Solid State Chem. 2014, 42, 1–21. [Google Scholar] [CrossRef]
- Mohammed, A.; Abdullah, A. Scanning Electron Microscopy (Sem): A Review. In Proceedings of the 2018 International Conference on Hydraulics and Pneumatics—HERVEX, Băile Govora, Romania, 7–9 November 2018; pp. 77–85. [Google Scholar]
- Zheng, J.; Nagashima, K.; Parmiter, D.; de la Cruz, J.; Patri, A.K. SEM X-ray Microanalysis of Nanoparticles Present in Tissue or Cultured Cell Thin Sections. Methods Mol. Biol. 2011, 697, 93–99. [Google Scholar] [PubMed]
- Malatesta, M. Transmission Electron Microscopy as a Powerful Tool to Investigate the Interaction of Nanoparticles with Subcellular Structures. Int. J. Mol. Sci. 2021, 22, 12789. [Google Scholar] [CrossRef] [PubMed]
- Tushar, R.G.; Babita, A.R. Transmission Electron Microscopy-An Overview. Int. Res. J. Invent. Pharm. Sci. 2013, 1, 1–7. [Google Scholar]
- Cheng, Z.; Wang, C.; Wu, X.; Chu, J. Review in Situ Transmission Electron Microscope with Machine Learning. J. Semicond. 2022, 43, 081001. [Google Scholar] [CrossRef]
- Franken, L.E.; Grünewald, K.; Boekema, E.J.; Stuart, M.C.A. A Technical Introduction to Transmission Electron Microscopy for Soft-Matter: Imaging, Possibilities, Choices, and Technical Developments. Small 2020, 16, 1906198. [Google Scholar] [CrossRef] [PubMed]
- Walther, T. Transmission Electron Microscopy of Nanostructures. In Microscopy Methods in Nanomaterials Characterization; Elsevier: Amsterdam, The Netherlands, 2017; pp. 105–134. [Google Scholar]
- Ghosh, S.; Basu, R.N. Nanoscale Characterization. In Noble Metal-Metal Oxide Hybrid Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 65–93. [Google Scholar]
- Aliofkhazraei, M.; Ali, N. AFM Applications in Micro/Nanostructured Coatings. In Comprehensive Materials Processing; Elsevier: Amsterdam, The Netherlands, 2014; pp. 191–241. [Google Scholar]
- Farré, M.; Barceló, D. Introduction to the Analysis and Risk of Nanomaterials in Environmental and Food Samples. Compr. Anal. Chem. 2012, 59, 1–32. [Google Scholar]
- Sinha Ray, S. Structure and Morphology Characterization Techniques. In Clay-Containing Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2013; pp. 39–66. [Google Scholar]
- Rao, A.; Schoenenberger, M.; Gnecco, E.; Glatzel, T.; Meyer, E.; Brändlin, D.; Scandella, L. Characterization of Nanoparticles Using Atomic Force Microscopy. J. Phys. Conf. Ser. 2007, 61, 971–976. [Google Scholar] [CrossRef]
- Groarke, R.; Vijayaraghavan, R.K.; Powell, D.; Rennie, A.; Brabazon, D. Powder Characterization—Methods, Standards, and State of the Art. In Fundamentals of Laser Powder Bed Fusion of Metals; Elsevier: Amsterdam, The Netherlands, 2021; pp. 491–527. [Google Scholar]
- Sindhu, R.; Binod, P.; Pandey, A. Microbial Poly-3-Hydroxybutyrate and Related Copolymers. In Industrial Biorefineries & White Biotechnology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 575–605. [Google Scholar]
- Torres-Rivero, K.; Bastos-Arrieta, J.; Fiol, N.; Florido, A. Metal and Metal Oxide Nanoparticles: An Integrated Perspective of the Green Synthesis Methods by Natural Products and Waste Valorization: Applications and Challenges. Compr. Anal. Chem. 2021, 94, 433–469. [Google Scholar]
- Devaraj, P.; Kumari, P.; Aarti, C.; Renganathan, A. Synthesis and Characterization of Silver Nanoparticles Using Cannonball Leaves and Their Cytotoxic Activity against MCF-7 Cell Line. J. Nanotechnol. 2013, 2013, 598328. [Google Scholar] [CrossRef]
- Plüisch, C.S.; Stuckert, R.; Wittemann, A. Direct Measurement of Sedimentation Coefficient Distributions in Multimodal Nanoparticle Mixtures. Nanomaterials 2021, 11, 1027. [Google Scholar] [CrossRef]
- Laborda, F.; Bolea, E.; Jimenez, M.S. Inductively Coupled Plasma Mass Spectrometry for Nanomaterial Analysis. In 21st Century Nanoscience—A Handbook; CRC Press: Boca Raton, FL, USA, 2020; pp. 1–15. ISBN 9780429340420. [Google Scholar]
- Fréchette-Viens, L.; Hadioui, M.; Wilkinson, K.J. Quantification of ZnO Nanoparticles and Other Zn Containing Colloids in Natural Waters Using a High Sensitivity Single Particle ICP-MS. Talanta 2019, 200, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.E.; Liu, J.; Montoro Bustos, A.R.; Johnson, M.E.; Winchester, M.R. Characterization of Nanoparticle Suspensions Using Single Particle Inductively Coupled Plasma Mass Spectrometry. In National Institute of Standards and Technology Special Publication 1200-21; NIST: Gaithersburg, MD, USA, 2016; Volume 29, pp. 1–29. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, F.; Guo, B.; Gao, N.; Zhang, X. Synthesis of N-Doped Micropore Carbon Quantum Dots with High Quantum Yield and Dual-Wavelength Photoluminescence Emission from Biomass for Cellular Imaging. Nanomaterials 2019, 9, 495. [Google Scholar] [CrossRef]
- Sabri, M.; Habibi-Yangjeh, A.; Vadivel, S. Activation of Persulfate Ions by TiO2/Carbon Dots Nanocomposite under Visible Light for Photocatalytic Degradations of Organic Contaminants. J. Mater. Sci. Mater. Electron. 2019, 30, 12510–12522. [Google Scholar] [CrossRef]
- Mohan, V.B.; Jayaraman, K.; Bhattacharyya, D. Brunauer–Emmett–Teller (BET) Specific Surface Area Analysis of Different Graphene Materials: A Comparison to Their Structural Regularity and Electrical Properties. Solid State Commun. 2020, 320, 114004. [Google Scholar] [CrossRef]
- Saadatkhah, N.; Carillo Garcia, A.; Ackermann, S.; Leclerc, P.; Latifi, M.; Samih, S.; Patience, G.S.; Chaouki, J. Experimental Methods in Chemical Engineering: Thermogravimetric Analysis—TGA. Can. J. Chem. Eng. 2020, 98, 34–43. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee Recommendations for Performing Kinetic Computations on Thermal Analysis Data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Wu, Y.; Chen, J.; Zhao, J.; Jin, F.; Na, P. Carbon Dots-TiO2 Nanosheets Composites for Photoreduction of Cr(VI) under Sunlight Illumination: Favorable Role of Carbon Dots. Appl. Catal. B Environ. 2018, 224, 508–517. [Google Scholar] [CrossRef]
- Bannov, A.G.; Popov, M.V.; Kurmashov, P.B. Thermal Analysis of Carbon Nanomaterials: Advantages and Problems of Interpretation. J. Therm. Anal. Calorim. 2020, 142, 349–370. [Google Scholar] [CrossRef]
- Farivar, F.; Lay Yap, P.; Karunagaran, R.U.; Losic, D. Thermogravimetric Analysis (TGA) of Graphene Materials: Effect of Particle Size of Graphene, Graphene Oxide and Graphite on Thermal Parameters. C 2021, 7, 41. [Google Scholar] [CrossRef]
- John, B.K.; Abraham, T.; Mathew, B. A Review on Characterization Techniques for Carbon Quantum Dots and Their Applications in Agrochemical Residue Detection. J. Fluoresc. 2022, 32, 449–471. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Gao, L.; Sun, J. Production of Aqueous Colloidal Dispersions of Carbon Nanotubes. J. Colloid Interface Sci. 2003, 260, 89–94. [Google Scholar] [CrossRef]
- Pu, N.W.; Wang, C.A.; Liu, Y.M.; Sung, Y.; Wang, D.S.; Ger, M. Der Dispersion of Graphene in Aqueous Solutions with Different Types of Surfactants and the Production of Graphene Films by Spray or Drop Coating. J. Taiwan Inst. Chem. Eng. 2012, 43, 140–146. [Google Scholar] [CrossRef]
- Rashmi, W.; Ismail, A.F.; Sopyan, I.; Jameel, A.T.; Yusof, F.; Khalid, M.; Mubarak, N.M. Stability and Thermal Conductivity Enhancement of Carbon Nanotube Nanofluid Using Gum Arabic. J. Exp. Nanosci. 2011, 6, 567–579. [Google Scholar] [CrossRef]
- Abdolkarimi-Mahabadi, M.; Bayat, A.; Mohammadi, A. Use of UV-Vis Spectrophotometry for Characterization of Carbon Nanostructures: A Review. Theor. Exp. Chem. 2021, 57, 191–198. [Google Scholar] [CrossRef]
- Liu, J.C.; Wang, Y.J.; Liu, L.; Sun, D.D. High-Quality Reduced Graphene Oxide-Nanocrystalline Platinum Hybrid Materials Prepared by Simultaneous Co-Reduction of Graphene Oxide and Chloroplatinic Acid. Nanoscale Res. Lett. 2011, 6, 241. [Google Scholar]
- Wang, G.; Shen, X.; Yao, J.; Park, J. Graphene Nanosheets for Enhanced Lithium Storage in Lithium Ion Batteries. Carbon N. Y. 2009, 47, 2049–2053. [Google Scholar] [CrossRef]
- Wang, G.; Wang, B.; Park, J.; Yang, J.; Shen, X.; Yao, J. Synthesis of Enhanced Hydrophilic and Hydrophobic Graphene Oxide Nanosheets by a Solvothermal Method. Carbon N. Y. 2009, 47, 68–72. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascón, J.M.D. Graphene Oxide Dispersions in Organic Solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Dager, A.; Uchida, T.; Maekawa, T.; Tachibana, M. Synthesis and Characterization of Mono-Disperse Carbon Quantum Dots from Fennel Seeds: Photoluminescence Analysis Using Machine Learning. Sci. Rep. 2019, 9, 14004. [Google Scholar] [CrossRef] [PubMed]
- Reinert, L.; Zeiger, M.; Suárez, S.; Presser, V.; Mücklich, F. Dispersion Analysis of Carbon Nanotubes, Carbon Onions, and Nanodiamonds for Their Application as Reinforcement Phase in Nickel Metal Matrix Composites. RSC Adv. 2015, 5, 95149–95159. [Google Scholar] [CrossRef]
- Smith, B.; Wepasnick, K.; Schrote, K.E.; Bertele, A.R.; Ball, W.P.; O’Melia, C.; Fairbrother, D.H. Colloidal Properties of Aqueous Suspensions of Acid-Treated, Multi-Walled Carbon Nanotubes. Environ. Sci. Technol. 2009, 43, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cheng, X.; Zhong, J.; Meng, J.; Yang, M.; Jia, F.; Xu, Z.; Kong, H. Characterization of Multiwalled Carbon Nanotubes Dispersing in Water and Association with Biological Effects. J. Nanomater. 2011, 2011, 938491. [Google Scholar] [CrossRef]
- Wang, Y.M.; Zhou, D.M.; Yuan, X.Y.; Zhang, X.H.; Li, Y. Modeling the Interaction and Toxicity of Cu-Cd Mixture to Wheat Roots Affected by Humic Acids, in Terms of Cell Membrane Surface Characteristics. Chemosphere 2018, 199, 76–83. [Google Scholar] [CrossRef]
- Kumar, P.; Chakraborty, S. Rice Husk-Derived Silica Nanoparticles Using Optimized Titrant Concentration for the One-Step Nanofluid Preparation. In Sustainable Chemical, Mineral and Material Processing; Chinthapudi, E., Basu, S., Narayan, B., Eds.; Springer: Singapore, 2023; ISBN 978-981-19-7263-8. [Google Scholar]
- Amaro-Gahete, J.; Benítez, A.; Otero, R.; Esquivel, D.; Jiménez-Sanchidrián, C.; Morales, J.; Caballero, Á.; Romero-Salguero, F. A Comparative Study of Particle Size Distribution of Graphene Nanosheets Synthesized by an Ultrasound-Assisted Method. Nanomaterials 2019, 9, 152. [Google Scholar] [CrossRef]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical Evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the Measurement of Nanoparticles and Protein Aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef]
- Lin, P.C.; Lin, S.; Wang, P.C.; Sridhar, R. Techniques for Physicochemical Characterization of Nanomaterials. Biotechnol. Adv. 2014, 32, 711–726. [Google Scholar] [CrossRef]
- Edwards, E.R.; Antunes, E.F.; Botelho, E.C.; Baldan, M.R.; Corat, E.J. Evaluation of Residual Iron in Carbon Nanotubes Purified by Acid Treatments. Appl. Surf. Sci. 2011, 258, 641–648. [Google Scholar] [CrossRef]
- Pourjavid, M.R.; Arabieh, M.; Yousefi, S.R.; Akbari Sehat, A. Interference Free and Fast Determination of Manganese(II), Iron(III) and Copper(II) Ions in Different Real Samples by Flame Atomic Absorption Spectroscopy after Column Graphene Oxide-Based Solid Phase Extraction. Microchem. J. 2016, 129, 259–267. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, X.; Pang, A.; Yang, J. Facile Synthesis and Photoluminescence Characteristics of Blue-Emitting Nitrogen-Doped Graphene Quantum Dots. Nanotechnology 2016, 27, 165704. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, A.; Shinohara, H. Control of Diameter Distribution of Single-Walled Carbon Nanotubes Using the Zeolite-CCVD Method at Atmospheric Pressure. Carbon 2005, 43, 431–436. [Google Scholar] [CrossRef]
- Autumn, K.; Liang, Y.A.; Hsieh, S.T.; Zesch, W.; Chan, W.P.; Kenny, T.W.; Fearing, R.; Full, R.J. Adhesive Force of a Single Gecko Foot-Hair. Nature 2000, 405, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Gorb, S.N.; Varenberg, M. Mushroom-Shaped Geometry of Contact Elements in Biological Adhesive Systems. J. Adhes. Sci. Technol. 2007, 21, 1175–1183. [Google Scholar] [CrossRef]
- Gorb, S.; Varenberg, M.; Peressadko, A.; Tuma, J. Biomimetic Mushroom-Shaped Fibrillar Adhesive Microstructure. J. R. Soc. Interface 2007, 4, 271–275. [Google Scholar] [CrossRef]
- Carbone, G.; Pierro, E.; Gorb, S.N. Origin of the Superior Adhesive Performance of Mushroom-Shaped Microstructured Surfaces. Soft Matter 2011, 7, 5545–5552. [Google Scholar] [CrossRef]
- Shao, J.; Ding, Y.; Wang, W.; Mei, X.; Zhai, H.; Tian, H.; Li, X.; Liu, B. Generation of Fully-Covering Hierarchical Micro-/Nano- Structures by Nanoimprinting and Modified Laser Swelling. Small 2014, 10, 2595–2601. [Google Scholar] [CrossRef]
- Paras; Kumar, A. Smart Bioinspired Anti-Wetted Surfaces: Perspectives, Fabrication, Stability and Applications. Curr. Res. Green Sustain. Chem. 2021, 4, 100139. [Google Scholar] [CrossRef]
- Shateri-Khalilabad, M.; Yazdanshenas, M.E.; Etemadifar, A. Fabricating Multifunctional Silver Nanoparticles-Coated Cotton Fabric. Arab. J. Chem. 2017, 10, S2355–S2362. [Google Scholar] [CrossRef]
- Bindra, H.S.; Krishna, J.; Kumeria, T.; Nayak, R. Rapid and Facile Fabrication of Wafer Scale Silicon Hierarchical Structures with Broadband Ultra High Anti-Reflection Property. In Proceedings of the 3rd International Electronic Conference on Materials Sciences, Basel, Switzerland, 14–28 May 2018; p. 5223. [Google Scholar]
- Cho, S.J.; Seok, S.Y.; Kim, J.Y.; Lim, G.; Lim, H. One-Step Fabrication of Hierarchically Structured Silicon Surfaces and Modification of Their Morphologies Using Sacrificial Layers. J. Nanomater. 2013, 2013, 289256. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Song, S.; Li, D.; Fan, C. Biomolecular Sensing via Coupling DNA-Based Recognition with Gold Nanoparticles. J. Phys. D. Appl. Phys. 2009, 42, 203001. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble Metals on the Nanoscale: Optical and Photothermal Properties and Some Applications in Imaging, Sensing, Biology, and Medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, E. Nanomaterials with Enzyme-like Characteristics (Nanozymes): Next-Generation Artificial Enzymes. Chem. Soc. Rev. 2013, 42, 6060. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.; Xiao, Y.; Shlyahovsky, B.; Willner, I. Aptamer-Functionalized Au Nanoparticles for the Amplified Optical Detection of Thrombin. J. Am. Chem. Soc. 2004, 126, 11768–11769. [Google Scholar] [CrossRef] [PubMed]
- Leggett, R.; Lee-Smith, E.E.; Jickells, S.M.; Russell, D.A. “Intelligent” Fingerprinting: Simultaneous Identification of Drug Metabolites and Individuals by Using Antibody-Functionalized Nanoparticles. Angew. Chemie 2007, 119, 4178–4181. [Google Scholar] [CrossRef]
- Hawkins, M.J.; Soon-Shiong, P.; Desai, N. Protein Nanoparticles as Drug Carriers in Clinical Medicine. Adv. Drug Deliv. Rev. 2008, 60, 876–885. [Google Scholar] [CrossRef]
- Ryan, S.; Kell, A.J.; van Faassen, H.; Tay, L.-L.; Simard, B.; MacKenzie, R.; Gilbert, M.; Tanha, J. Single-Domain Antibody-Nanoparticles: Promising Architectures for Increased Staphylococcus Aureus Detection Specificity and Sensitivity. Bioconjug. Chem. 2009, 20, 1966–1974. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Pandey, S.; Sharma, K.H.; Sharma, A.K.; Nerthigan, Y.; Hang, D.-R.; Wu, H.-F. Comparative photothermal performance among various sub-stoichiometric 2D oxygen-deficient molybdenum oxide nanoflakes and in vivo toxicity. Chem. Eur. J. 2018, 24, 7417–7427. [Google Scholar] [CrossRef]
- Bhaisare, M.L.; Sharma, K.H.; Lee, J.-Y.; Hang, D.-R.; Wu, H.-F. Synthesis and characterization of two-dimensional carbon dots decorated with molybdenum oxide nanoflakes with various phases. New J. Chem. 2016, 40, 8954–8960. [Google Scholar] [CrossRef]
- Nerthigan, Y.; Sharma, A.K.; Pandey, S.; Sharma, K.H.; Khan, M.S.; Hang, D.-R.; Wu, H.-F. Glucose oxidase assisted visual detection of glucose using oxygen deficient α-MoO3-x nanoflakes. Microchim. Acta 2018, 185, 65. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Pandey, S.; Sharma, K.H.; Nerthigan, Y.; Khan, M.S.; Hang, D.-R.; Wu, H.-F. Two dimensional α-MoO3-x nanoflakes as bare eye probe for hydrogen peroxide in biological fluids. Anal. Chim. Acta 2018, 1015, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Sharma, A.K.; Sharma, K.H.; Nerthigan, Y.; Khan, M.S.; Hang, D.-R.; Wu, H.-F. Rapid naked eye detection of alkaline phosphatase using α-MoO3-x nano-flakes. Sens. Actuators B Chem. 2018, 254, 514–518. [Google Scholar] [CrossRef]
- Brueck, S.R. Photonics in Nanotechnology. In Conference on Lasers and Electro-Optics/International Quantum Electronics Conference and Photonic Applications Systems Technologies; Optica Publishing Group: San Francisco, CA, USA, 2004. [Google Scholar]
- Hang, D.-R.; Islam, S.E.; Sharma, K.H.; Chen, C.; Liang, C.-T.; Chou, M.M.C. Optical characteristics of nonpolar a-plane ZnO thin film on (010) LiGaO2 substrate. Semicond. Sci. Technol. 2014, 29, 085004. [Google Scholar] [CrossRef]
- Hang, D.R.; Liang, C.-T.; Huang, C.F.; Chang, Y.H.; Chen, Y.F.; Jiang, H.X.; Lin, J.Y. Effective mass of two-dimensional electron gas in an Al0.2Ga0.8N/GaN heterojunction. Appl. Phys. Lett. 2001, 79, 66–68. [Google Scholar] [CrossRef]
- Hang, D.-R.; Chen, Y.F.; Jiang, H.X.; Lin, J.Y. AlxGa1-xN/GaN band offsets determined by deep-level emission. J. Appl. Phys. 2001, 90, 1887–1890. [Google Scholar] [CrossRef]
- Hang, D.-R.; Huang, C.F.; Chen, Y.F. Two-subband-populated AlGaN/GaN heterostructures probed by electrically detected and microwave-modulated magnetotransport measurements. Appl. Phys. Lett. 2006, 89, 092116. [Google Scholar] [CrossRef]
- Chou, M.M.C.; Chen, C.; Hang, D.-R.; Yang, W.-T. Growth of nonpolar m-plane GaN epitaxial film on a lattice-matched (100) β-LiGaO2 substrate by chemical vapor deposition. Thin Solid Films 2011, 519, 5066–5069. [Google Scholar] [CrossRef]
- Chou, M.M.C.; Hang, D.-R.; Kalisch, H.; Jansen, R.H.; Dikme, Y.; Heuken, M.; Yablonskii, G.P. Crystal growth and properties of LiAlO2 and nonpolar GaN on LiAlO2 substrate. J. Appl. Phys. 2007, 101, 103106. [Google Scholar] [CrossRef]
- Hang, D.-R.; Chou, M.M.C.; Chang, L.; Dikme, Y.; Heuken, M. Growth and characterization of m-plane GaN-based layers on LiAlO2 (1 0 0) grown by MOVPE. J. Crystal Growth 2009, 311, 452–455. [Google Scholar] [CrossRef]
- Hang, D.-R.; Chou, M.M.C.; Chang, L.; Lin, J.L.; Heuken, M. Optical characteristics of m-plane InGaN/GaN multiple quantum well grown on LiAlO2 (1 0 0) by MOVPE. J. Crystal Growth 2009, 311, 2919–2922. [Google Scholar] [CrossRef]
- Hang, D.-R.; Chou, M.M.C.; Mauder, C.; Heuken, M. MOVPE growth and properties of non-polar InGaN/GaN multiple quantum wells on γ-LiAlO2 substrates. J. Crystal Growth 2010, 312, 1329–1333. [Google Scholar] [CrossRef]
- Hang, D.-R.; Islam, S.E.; Sharma, K.H.; Kuo, S.-W.; Zhang, C.-Z.; Wang, J.-J. Annealing effects on the optical and morphological properties of ZnO nanorods on AZO substrate by using aqueous solution method at low temperature. Nanoscale Res. Lett. 2014, 9, 632. [Google Scholar] [CrossRef] [PubMed]
- Hang, D.-R.; Islam, S.E.; Chen, C.-H.; Sharma, K.H. Full solution-processed synthesis and mechanisms of a recyclable and bifunctional Au/ZnO plasmonic platform for enhanced UV/Vis photocatalysis and optical properties. Chem. Eur. J. 2016, 22, 14950–14961. [Google Scholar] [CrossRef]
- Chen, W.-J.; Wu, J.-K.; Lin, J.-C.; Lo, S.-T.; Lin, H.-D.; Hang, D.-R.; Shin, M.-F.; Liang, C.-T.; Chang, Y.H. Room-temperature violet luminescence and ultraviolet photodetection of Sb-doped ZnO/Al-doped ZnO homojunction array. Nanoscale Res. Lett. 2013, 8, 313. [Google Scholar] [CrossRef]
- Wu, J.-K.; Chen, W.-J.; Chang, Y.H.; Chen, Y.F.; Hang, D.-R.; Liang, C.-T.; Lu, J.-Y. Fabrication and photo-response of ZnO nanowiress/CuO coaxial heterojunction. Nanoscale Res. Lett. 2013, 8, 387. [Google Scholar] [CrossRef]
- Chou, M.M.C.; Hang, D.-R.; Wang, S.C.; Chen, C.; Lee, C.-Y. Growth and characterizations of nonpolar [1 1 −2 0] ZnO on [1 0 0] (La,Sr)(Al,Ta)O3 substrate by chemical vapor deposition. J. Crystal Growth 2010, 312, 1170–1174. [Google Scholar] [CrossRef]
- Hang, D.-R.; Sharma, K.H.; Islam, S.E.; Chen, C.; Chou, M.M.C. Resonant Raman scattering and photoluminescent properties of nonpolar a-plane ZnO thin film on LiGaO2 substrate. Appl. Phys. Express 2014, 7, 041101. [Google Scholar] [CrossRef]
- Nguyen, H.P.T.; Arafin, S.; Piao, J.; Cuong, T.V. Nanostructured Optoelectronics: Materials and Devices. J. Nanomater. 2016, 2016, 2–4. [Google Scholar] [CrossRef]
- Aamir Iqbal, M.; Ashraf, N.; Shahid, W.; Awais, M.; Khan Durrani, A.; Shahzad, K.; Ikram, M. Nanophotonics: Fundamentals, Challenges, Future Prospects and Applied Applications. In Nonlinear Optics—Nonlinear Nanophotonics and Novel Materials for Nonlinear Optics; IntechOpen: London, UK, 2021; pp. 225–240. [Google Scholar]
- Ye, Y.; Wong, Z.J.; Lu, X.; Ni, X.; Zhu, H.; Chen, X.; Wang, Y.; Zhang, X. Monolayer Excitonic Laser. Nat. Photonics 2015, 9, 733–737. [Google Scholar] [CrossRef]
- Meng, Z.; Stolz, R.M.; Mendecki, L.; Mirica, K.A. Electrically-Transduced Chemical Sensors Based on Two-Dimensional Nanomaterials. Chem. Rev. 2019, 119, 478–598. [Google Scholar] [CrossRef] [PubMed]
- Steinhubl, S.R.; Muse, E.D.; Topol, E.J. The Emerging Field of Mobile Health. Sci. Transl. Med. 2015, 7, 283rv3. [Google Scholar] [CrossRef] [PubMed]
- Mu, B.; Zhang, J.; McNicholas, T.P.; Reuel, N.F.; Kruss, S.; Strano, M.S. Recent Advances in Molecular Recognition Based on Nanoengineered Platforms. Acc. Chem. Res. 2014, 47, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y. Recent advances in SnO2 nanostructure based gas sensors. Sens. Actuators B Chem. 2022, 364, 131876. [Google Scholar] [CrossRef]
- Jiahua, F.; Qiulin, T.; Wenyi, L. Preparation of CNT-SnO2 Composites and Study on Formaldehyde Gas Sensitivity. Sci. Technol. Eng. 2016, 1, 53–57. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, K. Recent Progress on Graphene-Analogous 2D Nanomaterials: Properties, Modeling and Applications. Prog. Mater. Sci. 2019, 100, 99–169. [Google Scholar] [CrossRef]
- Wen, W.; Song, Y.; Yan, X.; Zhu, C.; Du, D.; Wang, S.; Asiri, A.M.; Lin, Y. Recent Advances in Emerging 2D Nanomaterials for Biosensing and Bioimaging Applications. Mater. Today 2018, 21, 164–177. [Google Scholar] [CrossRef]
- Hang, D.-R.; Pan, Y.-Q.; Sharma, K.H.; Chou, M.M.C.; Islam, S.E.; Wu, H.-F.; Liang, C.-T. 2D CTAB-MoSe2 nanosheets and 0D MoSe2 quantum dots: Facile top-down preparations and their peroxidase-like catalytic activity for colorimetric detection of hydrogen peroxide. Nanomaterials 2020, 10, 2045. [Google Scholar] [CrossRef]
- Hang, D.-R.; Sun, D.-Y.; Chen, C.-H.; Wu, H.-F.; Chou, M.M.C.; Islam, S.E.; Sharma, K.H. Facile bottom-up preparation of WS2-based water-soluble quantum dots as luminescent probes for hydrogen peroxide and glucose. Nanoscale Res. Lett. 2019, 14, 271. [Google Scholar] [CrossRef]
- Avasare, V.; Zhang, Z.; Avasare, D.; Khan, I.; Qurashi, A. Room-Temperature Synthesis of TiO2 Nanospheres and Their Solar Driven Photoelectrochemical Hydrogen Production. Int. J. Energy Res. 2015, 39, 1714–1719. [Google Scholar] [CrossRef]
- Mueller, N.C.; Nowack, B. Exposure Modeling of Engineered Nanoparticles in the Environment. Environ. Sci. Technol. 2008, 42, 4447–4453. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Dong, C.-K.; Han, L.; Yang, J.; Du, X.-W. Top-Down Preparation of Active Cobalt Oxide Catalyst. ACS Catal. 2016, 6, 6699–6703. [Google Scholar] [CrossRef]
- Wang, D.-W.; Su, D. Heterogeneous Nanocarbon Materials for Oxygen Reduction Reaction. Energy Environ. Sci. 2014, 7, 576. [Google Scholar] [CrossRef]
- Maarisetty, D.; Reeba, M.; Hang, D.-R.; Mohapatra, P.; Baral, S.S. The role of material defects in the photocatalytic CO2 reduction: Interfacial properties, thermodynamics, kinetics and mechanism. J. CO2 Util. 2022, 64, 102175. [Google Scholar] [CrossRef]
- Li, D.; Baydoun, H.; Verani, C.N.; Brock, S.L. Efficient Water Oxidation Using CoMnP Nanoparticles. J. Am. Chem. Soc. 2016, 138, 4006–4009. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.-Q.; Liu, J.-X.; Gupta, V. Fundamental Formulations and Recent Achievements in Piezoelectric Nano-Structures: A Review. Nanoscale 2013, 5, 1716. [Google Scholar] [CrossRef]
- Lei, Y.-M.; Huang, W.-X.; Zhao, M.; Chai, Y.-Q.; Yuan, R.; Zhuo, Y. Electrochemiluminescence Resonance Energy Transfer System: Mechanism and Application in Ratiometric Aptasensor for Lead Ion. Anal. Chem. 2015, 87, 7787–7794. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef]
- Greeley, J.; Markovic, N.M. The Road from Animal Electricity to Green Energy: Combining Experiment and Theory in Electrocatalysis. Energy Environ. Sci. 2012, 5, 9246. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.-T.; Zhong, J.; Kang, Z. Metal-Free Efficient Photocatalyst for Stable Visible Water Splitting via a Two-Electron Pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Maarisetty, D.; Hang, D.-R.; Chou, M.M.C.; Parida, S. Tuning the Ni/Co ratios and surface concentration of reduced molybdenum states for enhanced electrocatalytic performance in trimetallic molybdates: OER, HER, and MOR activity. ACS Appl. Energy Mater. 2022, 5, 14059–14070. [Google Scholar] [CrossRef]
- Hang, D.-R.; Sharma, K.H.; Chen, C.-H.; Islam, S.E. Enhanced photocatalytic performance of ZnO nanorods coupled by two-dimensional α-MoO3 nanoflakes under UV and visible light irradiation. Chem. Eur. J. 2016, 22, 12777–12784. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.E.; Hang, D.-R.; Chen, C.-H.; Sharma, K.H. Facile and cost-efficient synthesis of quasi-0D/2D ZnO/MoS2 nanocomposites for highly enhanced visible-light-driven photocatalytic degradation of organic pollutants and antibiotics. Chem. Eur. J. 2018, 24, 9305–9315. [Google Scholar] [CrossRef]
- Islam, S.E.; Hang, D.-R.; Chen, C.-H.; Chou, M.M.C.; Liang, C.-T.; Sharma, K.H. Rational design of hetero-dimensional C-ZnO/MoS2 nanocomposite anchored on 3D mesoporous carbon framework towards synergistically enhanced stability and efficient visible-light-driven photocatalytic activity. Chemosphere 2021, 266, 129148. [Google Scholar] [CrossRef]
- Sharma, K.H.; Hang, D.-R.; Bolloju, S.; Lee, J.-T.; Wu, H.-F.; Islam, S.E.; Chou, M.M.C.; Liang, C.-T.; Srivastava, R.R. Two-dimensional molybdenum trioxide nanoflakes wrapped with interlayer-expanded molybdenum disulfide nanosheets: Superior performances in supercapacitive energy storage and visible-light-driven photocatalysis. Int. J. Hydrog. Energy 2021, 46, 34663–34678. [Google Scholar] [CrossRef]
- Ning, F.; Shao, M.; Xu, S.; Fu, Y.; Zhang, R.; Wei, M.; Evans, D.G.; Duan, X. TiO2/Graphene/NiFe-Layered Double Hydroxide Nanorod Array Photoanodes for Efficient Photoelectrochemical Water Splitting. Energy Environ. Sci. 2016, 9, 2633–2643. [Google Scholar] [CrossRef]
- Ulaeto, S.B.; Pancrecious, J.K.; Rajan, T.P.D.; Pai, B.C. Smart Coatings. In Noble Metal-Metal Oxide Hybrid Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 341–372. [Google Scholar]
- Zou, L.; Lan, C.; Li, X.; Zhang, S.; Qiu, Y.; Ma, Y. Superhydrophobization of Cotton Fabric with Multiwalled Carbon Nanotubes for Durable Electromagnetic Interference Shielding. Fibers Polym. 2015, 16, 2158–2164. [Google Scholar] [CrossRef]
- Aminayi, P.; Abidi, N. Ultra-Oleophobic Cotton Fabric Prepared Using Molecular and Nanoparticle Vapor Deposition Methods. Surf. Coatings Technol. 2015, 276, 636–644. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Jung, Y.C.; Barthlott, W. Fabrication of Artificial Lotus Leaves and Significance of Hierarchical Structure for Superhydrophobicity and Low Adhesion. Soft Matter 2009, 5, 1386. [Google Scholar] [CrossRef]
- Jung, Y.C.; Bhushan, B. Mechanically Durable Carbon Nanotube−Composite Hierarchical Structures with Superhydrophobicity, Self-Cleaning, and Low-Drag. ACS Nano 2009, 3, 4155–4163. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.; Zhai, L.; Wu, Z.; Cohen, R.E.; Rubner, M.F. Transparent Superhydrophobic Films Based on Silica Nanoparticles. Langmuir 2007, 23, 7293–7298. [Google Scholar] [CrossRef] [PubMed]
- Ubaid, F.; Radwan, A.B.; Naeem, N.; Shakoor, R.A.; Ahmad, Z.; Montemor, M.F.; Kahraman, R.; Abdullah, A.M.; Soliman, A. Multifunctional Self-Healing Polymeric Nanocomposite Coatings for Corrosion Inhibition of Steel. Surf. Coatings Technol. 2019, 372, 121–133. [Google Scholar] [CrossRef]
- Birkett, M.; Dover, L.; Cherian Lukose, C.; Wasy Zia, A.; Tambuwala, M.M.; Serrano-Aroca, Á. Recent Advances in Metal-Based Antimicrobial Coatings for High-Touch Surfaces. Int. J. Mol. Sci. 2022, 23, 1162. [Google Scholar] [CrossRef]
- Salesa, B.; Assis, M.; Andrés, J.; Serrano-Aroca, Á. Carbon Nanofibers versus Silver Nanoparticles: Time-Dependent Cytotoxicity, Proliferation, and Gene Expression. Biomedicines 2021, 9, 1155. [Google Scholar] [CrossRef]
- Zhao, J.; Cai, X.M.; Tang, H.Q.; Liu, T.; Gu, H.Q.; Cui, R.Z. Bactericidal and Biocompatible Properties of TiN/Ag Multilayered Films by Ion Beam Assisted Deposition. J. Mater. Sci. Mater. Med. 2009, 20, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Mejía, H.D.; Echavarría, A.M.; Bejarano, G.G. Influence of Ag-Cu Nanoparticles on the Microstructural and Bactericidal Properties of TiAlN(Ag,Cu) Coatings for Medical Applications Deposited by Direct Current (DC) Magnetron Sputtering. Thin Solid Films 2019, 687, 137460. [Google Scholar] [CrossRef]
- Esteban-Tejeda, L.; Prado, C.; Cabal, B.; Sanz, J.; Torrecillas, R.; Moya, J.S. Antibacterial and Antifungal Activity of ZnO Containing Glasses. PLoS ONE 2015, 10, e0132709. [Google Scholar] [CrossRef]
- Hizal, F.; Rungraeng, N.; Lee, J.; Jun, S.; Busscher, H.J.; van der Mei, H.C.; Choi, C.-H. Nanoengineered Superhydrophobic Surfaces of Aluminum with Extremely Low Bacterial Adhesivity. ACS Appl. Mater. Interfaces 2017, 9, 12118–12129. [Google Scholar] [CrossRef]
- Bartlet, K.; Movafaghi, S.; Dasi, L.P.; Kota, A.K.; Popat, K.C. Antibacterial Activity on Superhydrophobic Titania Nanotube Arrays. Colloids Surf. B Biointerfaces 2018, 166, 179–186. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.; Lv, J.; Huang, F.; An, Y.; Shi, L.; Ma, R. Glucose and H2O2 Dual-Responsive Polymeric Micelles for the Self-Regulated Release of Insulin. ACS Appl. Bio Mater. 2020, 3, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yin, Z.; Du, Y.; Huang, X.; Zeng, Z.; Fan, Z.; Liu, H.; Wang, J.; Zhang, H. Synthesis of Few-Layer MoS2 Nanosheet-Coated TiO2 Nanobelt Heterostructures for Enhanced Photocatalytic Activities. Small 2013, 9, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Nasir, J.A.; Rehman, Z.U.; Shah, S.N.A.; Khan, A.; Butler, I.S.; Catlow, C.R.A. Recent Developments and Perspectives in CdS-Based Photocatalysts for Water Splitting. J. Mater. Chem. A 2020, 8, 20752–20780. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Yao, W. CdS@MoS2 Hetero-structured Nanocomposites Are Highly Effective Photo-Catalysts for Organic Dye Degradation. ACS Omega 2020, 5, 27463–27469. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xiong, F.; Meng, J.; Wang, X.; Niu, C.; An, Q.; Mai, L. Vanadium-Based Nanomaterials: A Promising Family for Emerging Metal-Ion Batteries. Adv. Funct. Mater. 2020, 30, 1904398. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, X.; Wang, C.; Rao, A.; Lu, B. Sulfur-Assisted Large-Scale Synthesis of Graphene Microspheres for Superior Potassium-Ion Batteries. Energy Environ. Sci. 2021, 14, 965–974. [Google Scholar] [CrossRef]
- Desalegn, B.Z.; Jadhav, H.S.; Seo, J.G. Highly efficient g-C3N4 nanorods with dual active sites as an electrocatalyst for the oxygen evolution reaction. ChemCatChem 2019, 11, 2870–2878. [Google Scholar] [CrossRef]



| Top-Down Approach | Bottom-Up Approach |
|---|---|
| Fabrication over a large-scale substrate is possible | Ultra-fine nanoparticles, nano shells, nanotubes, nano arrays can be prepared. |
| Process of chemical purification is not required | Deposition parameters can be easily controlled by varying reaction temperature, precursor concentration, etc. |
| Comparatively cheaper technique. |
| Top-Down Approach | Bottom-Up Approach |
|---|---|
| Size distribution is broad (10–100 nm). | Scale-up of the process is difficult. |
| Control over deposition parameters is tedious. | Chemical purification of final product is required. |
| Often expensive technique. |
| S. No. | Characterization Technique | Principle | Entity Identified | Reference |
|---|---|---|---|---|
| 1 | X-ray Diffraction (XRD) | Constructive interference of monochromatic X-ray | Size (structural properties), elemental-chemical composition, crystal structure | [96] |
| 2 | X-ray Absorption Spectroscopy (XAS) | X-ray absorption and fluorescence, Multiple Scattering | Chemical state, oxidation state | [97] |
| 3 | Small Angle X-ray Scattering (SAXS) | Collisions between an incoming wave and a surface particle results in wave scattering in all direction | Size (structural properties), size distribution, growth kinetics | [98] |
| 4 | X-ray Photoelectron Spectroscopy (XPS) | Electrons within a sample absorb photons of a particular energy and then emerge from the solid | Ligand binding, surface composition, chemical state-oxidation state, Elemental-chemical composition | [99] |
| 5 | Fourier Transform Infrared Spectroscopy (FTIR) | Gases absorb IR radiation at species-specific frequencies | Ligand binding, composition, density, arrangement, mass, surface composition | [100] |
| 6 | Nuclear Magnetic Resonance (NMR) Spectroscopy | Transfer of energy is possible from base energy to higher energy levels when an external magnetic field is applied | Size (structural properties), elemental-chemical composition, crystal structure, growth kinetics, ligand binding, composition, density, arrangement, mass, surface composition, surface area, specific surface area | [101] |
| 7 | Brunauer-Emmett-Teller (BET) | Physical adsorption of gas molecules on a solid surface | Surface area, specific surface area | [102] |
| 8 | Thermogravimetric Analysis (TGA) | Dynamic relationship between temperature with a change in physical property like mass change or enthalpy change etc. | Ligand binding, composition, density, arrangement, mass, surface composition | [103] |
| 9 | UV-visible Spectroscopy | Absorption of ultraviolet light or visible light by materials | Optical properties, concentration, size (structural properties) | [104] |
| 10 | Dynamic Light Scattering (DLS) | Brownian motion of macromolecules in solution that arises due to bombardment from solvent molecules | Size (structural properties), size distribution, agglomeration state | [105] |
| 11 | Differential Centrifugal Sedimentation (DCS) | Time taken by nanoparticles to sediment through a fluid when exposed to centrifugal field | Size (structural properties), size distribution, agglomeration state, density | [106] |
| 12 | Inductively Coupled Plasma Mass Spectrometry (ICP-MS) | Ions of analyte atoms are sorted and quantified based on their mass-to-charge ratio | Size (structural properties), elemental-chemical composition, size distribution, concentration, single particle properties | [107] |
| 13 | Transmission Electron Spectroscopy (TEM) | Beam of electrons is transmitted through a specimen to form an image | Structural defects, size, shape, 3D visualization, single particle properties, agglomeration state | [108] |
| 14 | Scanning Electron Microscopy (SEM) | Beam of secondary electrons emitted from a specimen to form an image | Size (structural properties), size distribution, 3D visualization, NPs detection | [109] |
| 15 | Scanning Transmission Electron microscopy (STEM) | Properties of both SEM and TEM | Crystal structure, NPs detection, optical properties | [110] |
| 16 | Energy Dispersive X-Ray (EDX) | Generation of characteristic X-rays from a specimen through the electron beam | Elemental-chemical composition | [111] |
| 17 | Atomic Force Microscopy (AFM) | Surface interaction between sharp tip and surface atoms | Structural defects, size, shape, 3D visualization | [112] |
| 18 | Auger Electron Spectroscopy (AES) | Auger effects: a chain of radiation-less transitions in an atom in which one of its inner levels is ionized | Elemental-chemical composition, thickness | [113,114,115] |
| 19 | Atomic Absorption Spectroscopy (AAS) | Atoms (and ions) can absorb light at a specific, unique wavelength | NPs detection, elemental-chemical composition | [116,117] |
| 20 | Raman Spectroscopy | Inelastic scattering of laser light | Fingerprint of chemical species, bonding nature | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paras; Yadav, K.; Kumar, P.; Teja, D.R.; Chakraborty, S.; Chakraborty, M.; Mohapatra, S.S.; Sahoo, A.; Chou, M.M.C.; Liang, C.-T.; et al. A Review on Low-Dimensional Nanomaterials: Nanofabrication, Characterization and Applications. Nanomaterials 2023, 13, 160. https://doi.org/10.3390/nano13010160
Paras, Yadav K, Kumar P, Teja DR, Chakraborty S, Chakraborty M, Mohapatra SS, Sahoo A, Chou MMC, Liang C-T, et al. A Review on Low-Dimensional Nanomaterials: Nanofabrication, Characterization and Applications. Nanomaterials. 2023; 13(1):160. https://doi.org/10.3390/nano13010160
Chicago/Turabian StyleParas, Kushal Yadav, Prashant Kumar, Dharmasanam Ravi Teja, Sudipto Chakraborty, Monojit Chakraborty, Soumya Sanjeeb Mohapatra, Abanti Sahoo, Mitch M. C. Chou, Chi-Te Liang, and et al. 2023. "A Review on Low-Dimensional Nanomaterials: Nanofabrication, Characterization and Applications" Nanomaterials 13, no. 1: 160. https://doi.org/10.3390/nano13010160
APA StyleParas, Yadav, K., Kumar, P., Teja, D. R., Chakraborty, S., Chakraborty, M., Mohapatra, S. S., Sahoo, A., Chou, M. M. C., Liang, C.-T., & Hang, D.-R. (2023). A Review on Low-Dimensional Nanomaterials: Nanofabrication, Characterization and Applications. Nanomaterials, 13(1), 160. https://doi.org/10.3390/nano13010160








