Self-Healing Thiolated Pillar[5]arene Films Containing Moxifloxacin Suppress the Development of Bacterial Biofilms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization
2.2. Fluorescence Spectroscopy
2.3. UV–Visible Spectroscopy
2.4. Dynamic Light Scattering (DLS)
2.5. Transmission Electron Microscopy (TEM)
2.6. Gel Permeation Chromatography (GPC)
2.7. Simultaneous Thermogravimetry and Differential Scanning Calorimetry (TG–DSC)
2.8. X-ray Diffraction Analysis
2.9. Computational Method
2.10. Biological Experiments
2.11. Synthesis
2.11.1. Synthesis of 4,8,14,18,23,26,28,31,32,35-deca-[Acylthioethoxy]-pillar[5]arene (2)
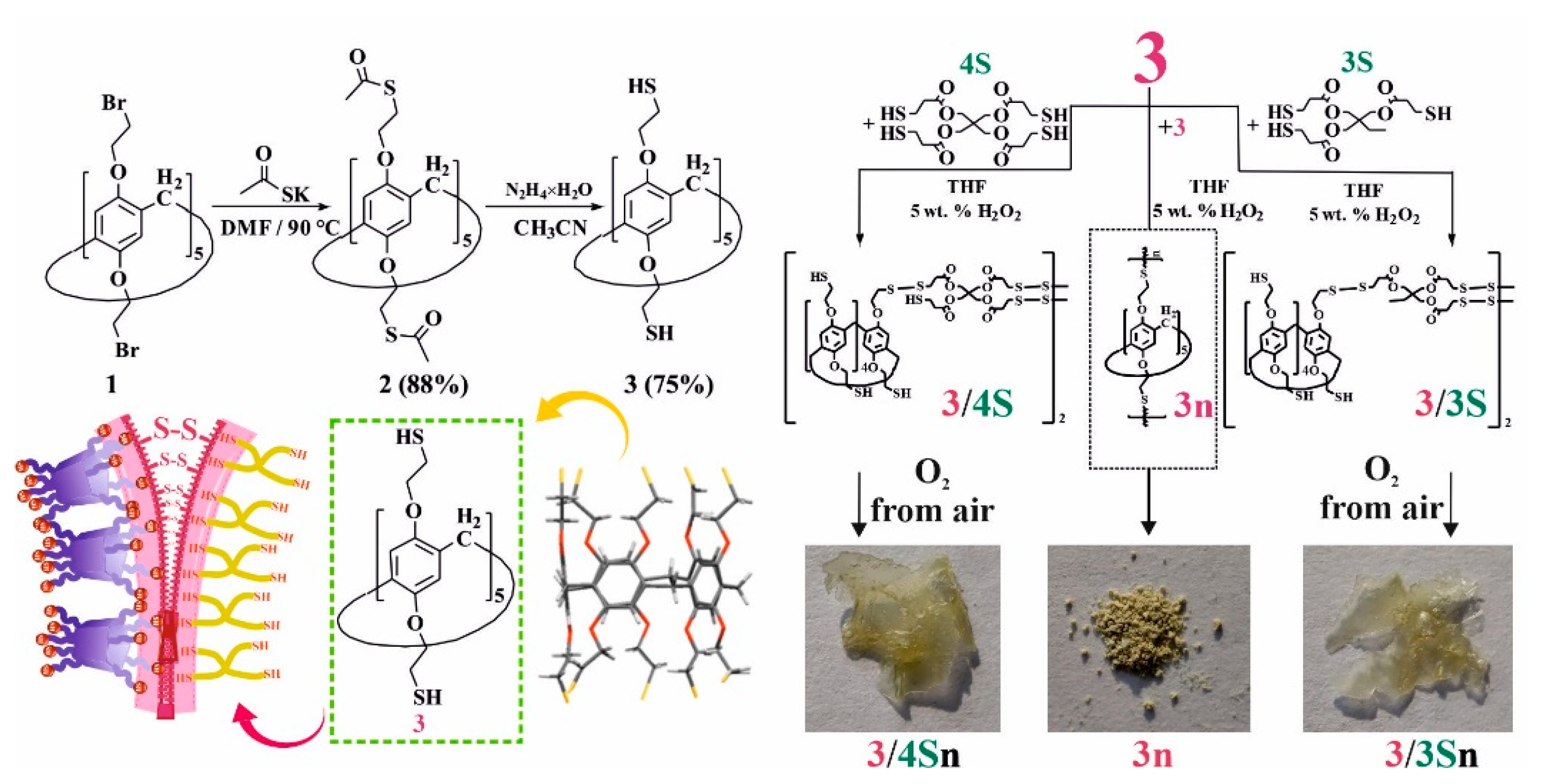
2.11.2. Synthesis of 4,8,14,18,23,26,28,31,32,35-deca-[2-Mercaptoethoxy]-pillar[5]arene (3)
2.11.3. General Procedure for the Synthesis of 3n, 3/3S, 3/4S
2.11.4. General Procedure for the Synthesis of Cross-Linked Supramolecular Polymers 3/3Sn, 3/4Sn
3. Results
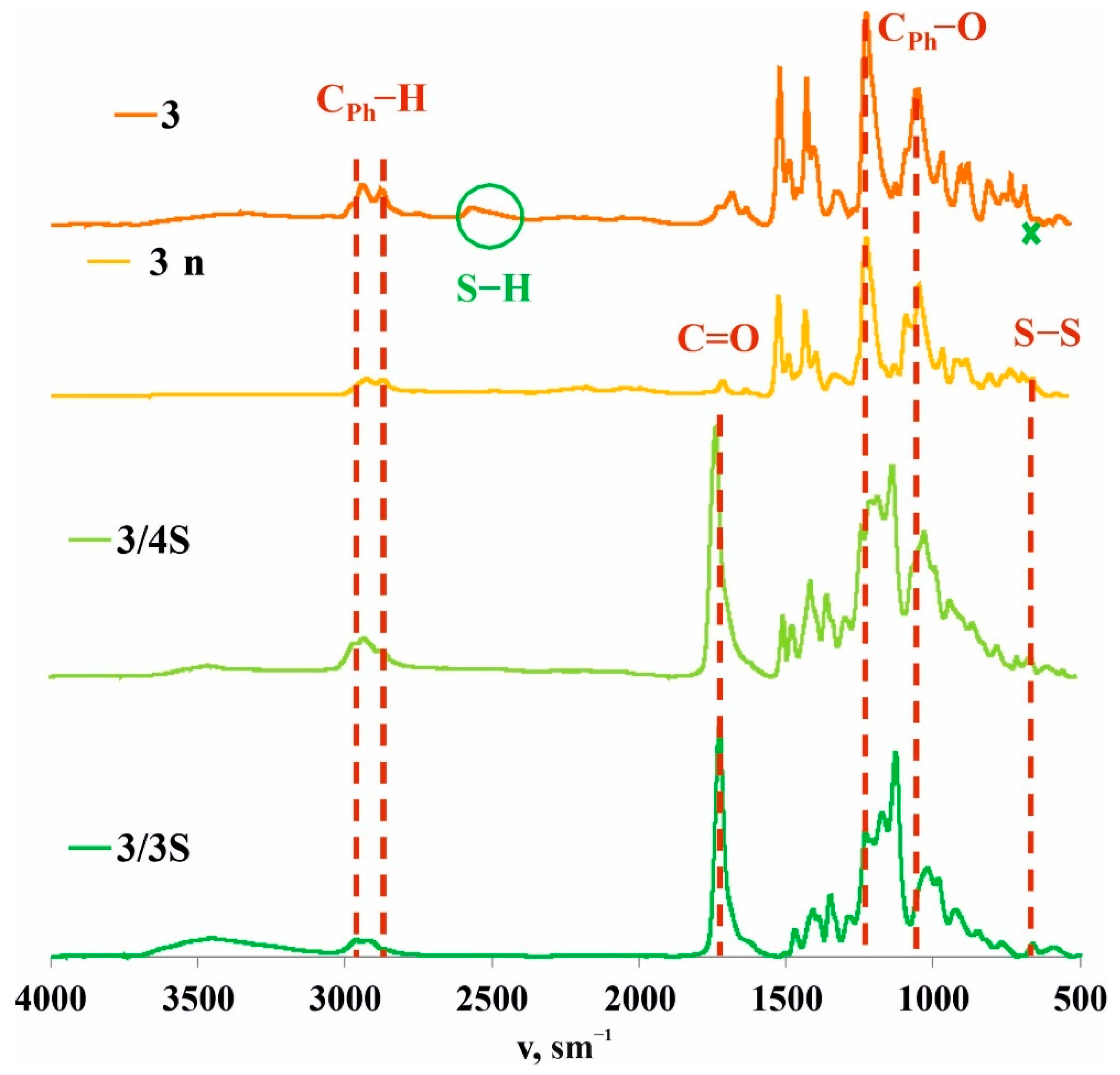
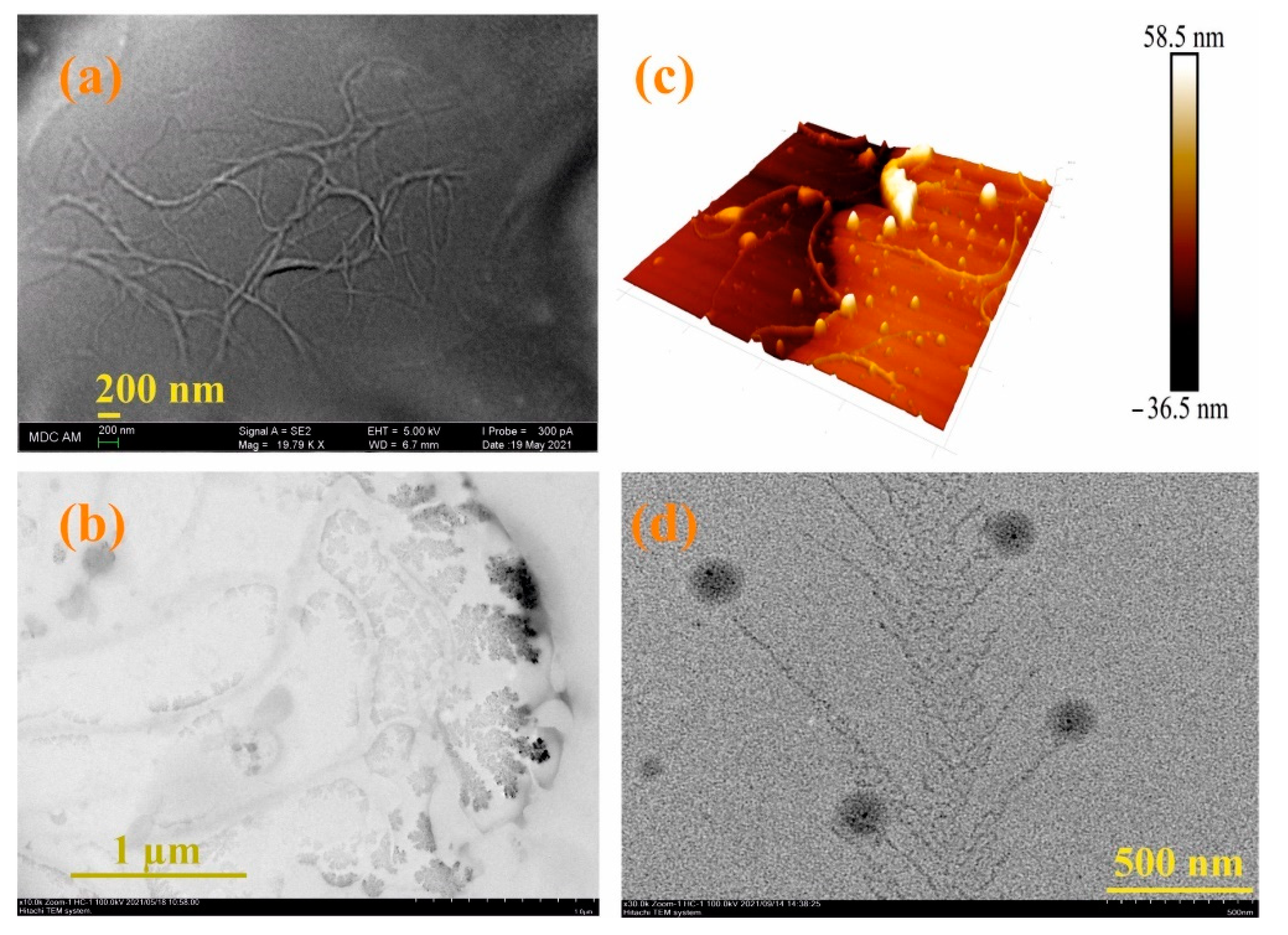
3.1. Synthesis and Polymerization of Pillar[5]arene Containing Mercapto Groups
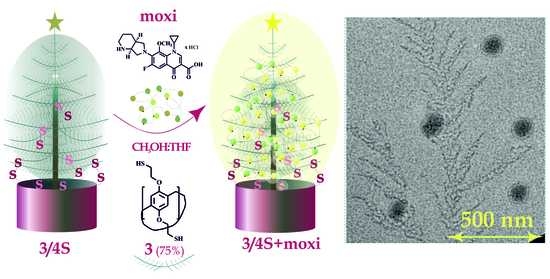
3.2. Interaction of Macrocycle 3 with the Antibiotic Moxifloxacin Hydrochloride
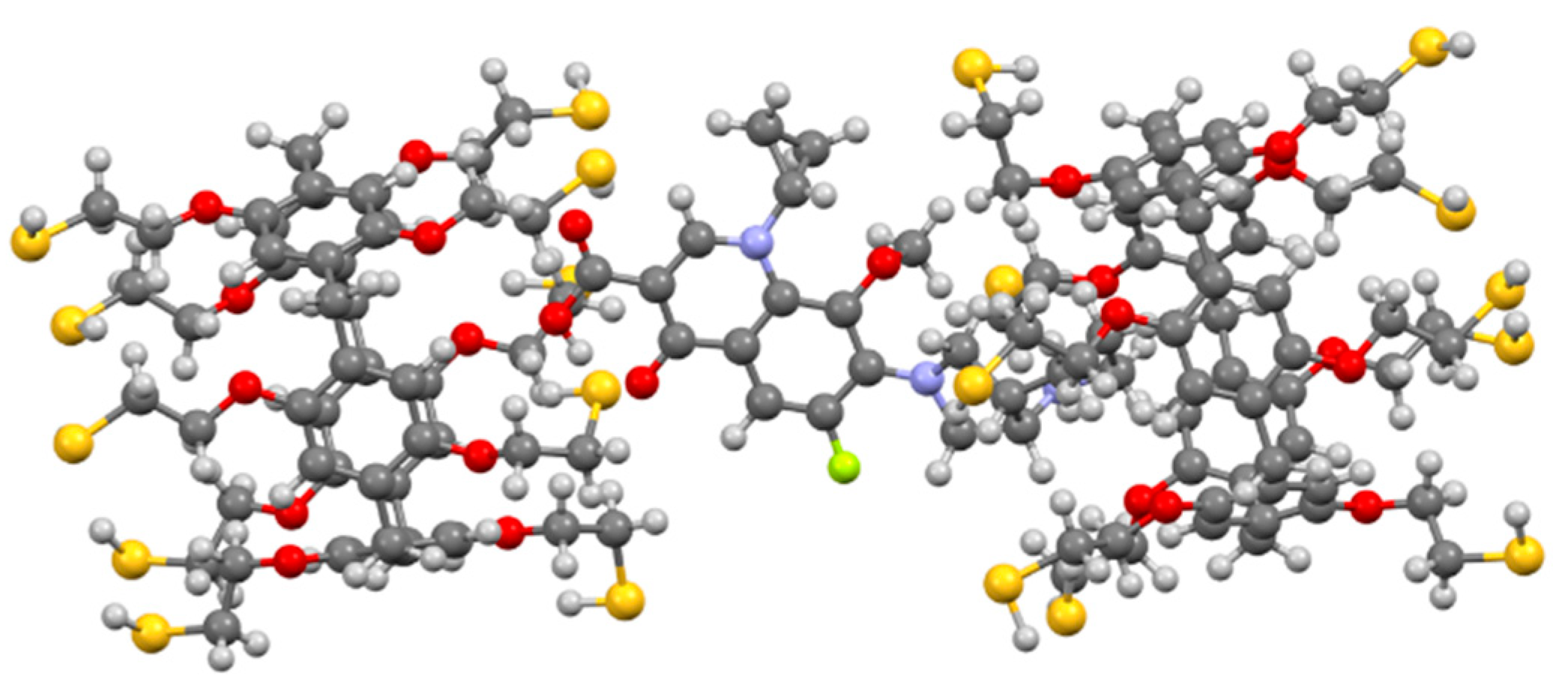
3.3. Formation of Supramolecular Polymer Networks
3.4. Interaction of 3/3S, 3/4S and Supramolecular Copolymers 3/3Sn, 3/4Sn with the Antibiotic Moxifloxacin Hydrochloride
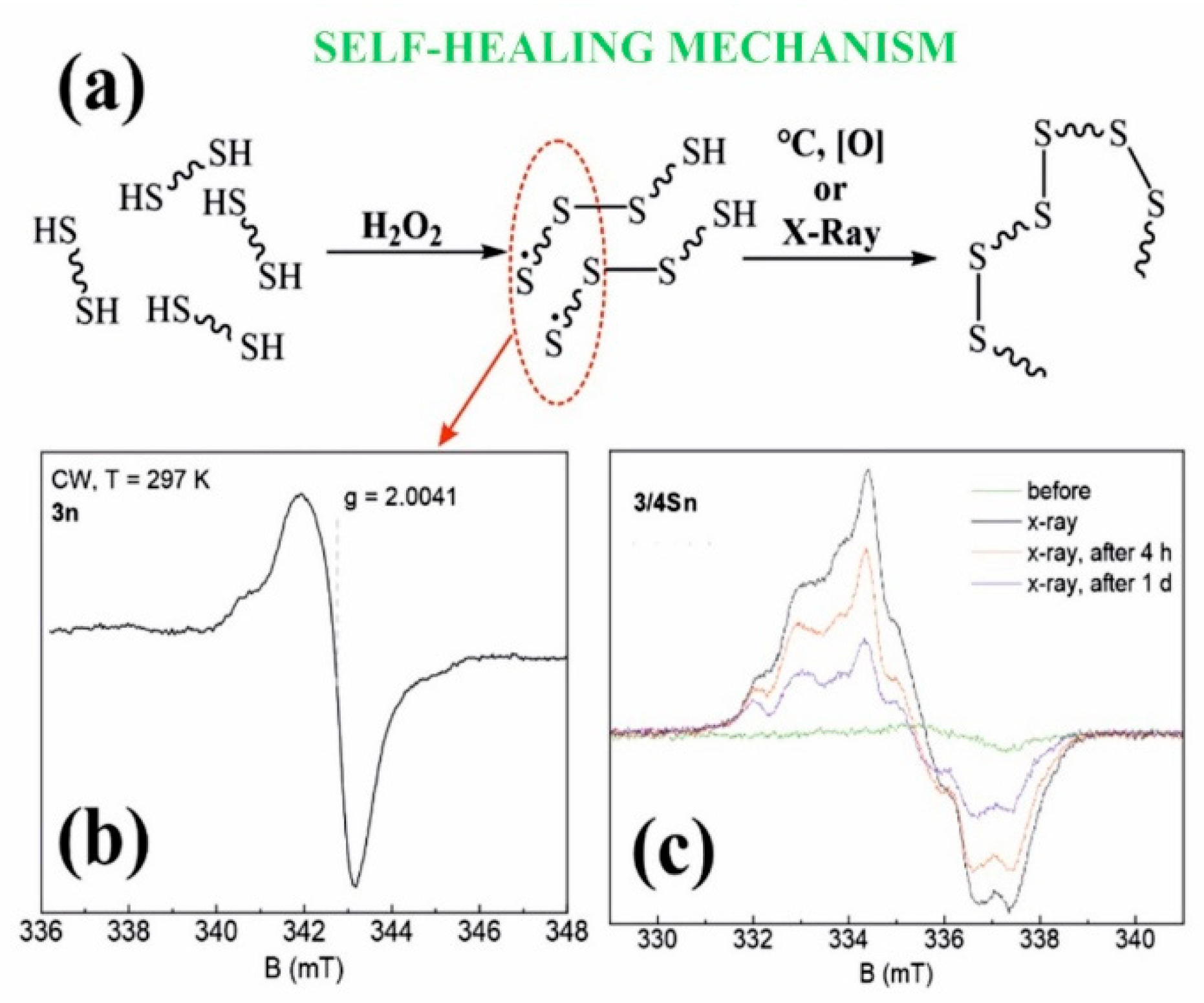
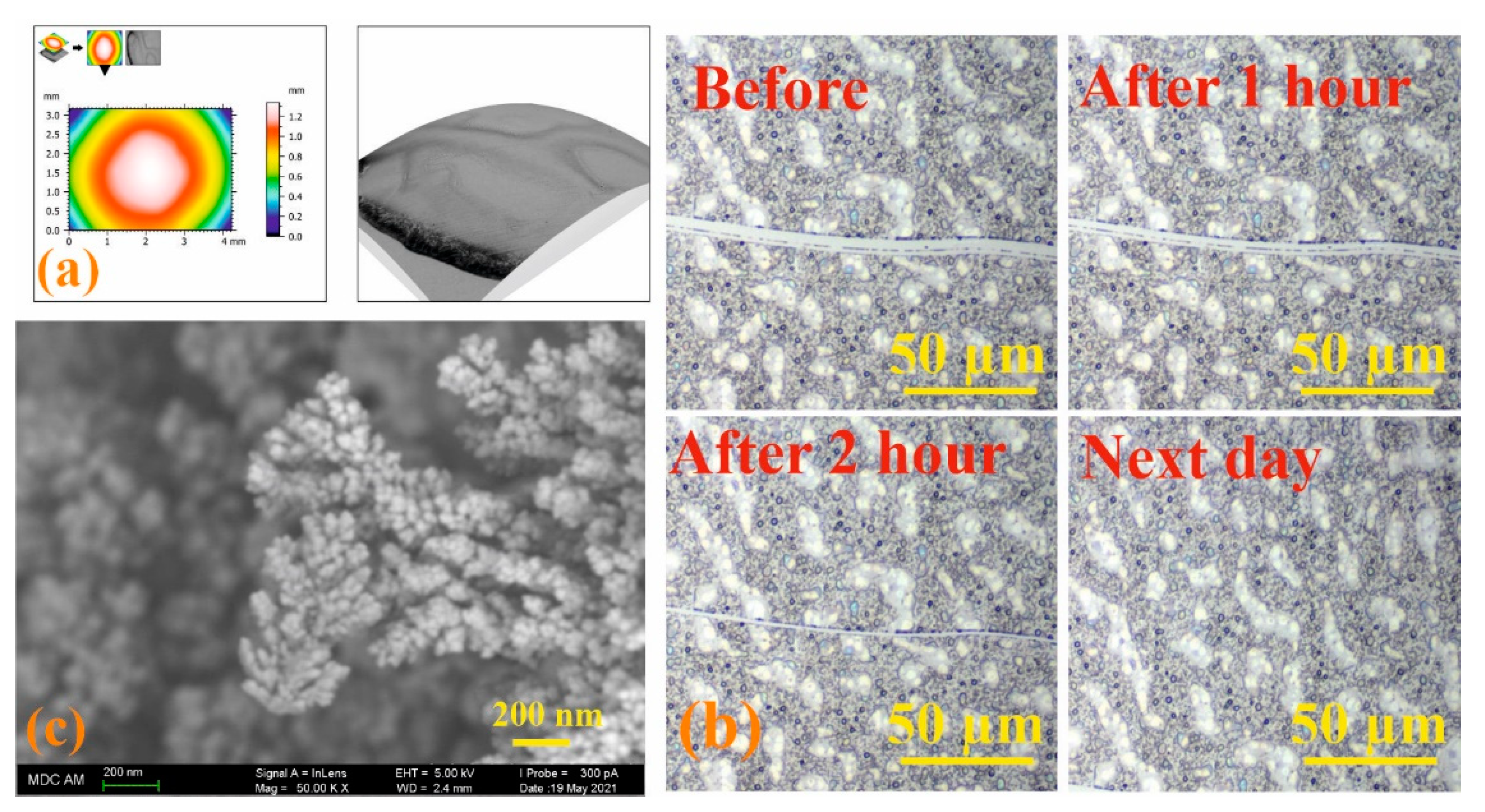
3.5. Study of the Process of Self-Regeneration of Films 3/3Sn, 3/4Sn
3.6. Antibacterial Properties of Self-Regenerating Films 3/3Sn, 3/4Sn
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parsek, M.R.; Singh, P.K. Bacterial biofilms: An emerging link to disease pathogenesis. Annu. Rev. Microbiol. 2003, 57, 677–701. [Google Scholar] [CrossRef] [PubMed]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial biofilm eradication agents: A current review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scharnow, A.M.; Solinski, A.E.; Wuest, W.M. Targeting S. mutans biofilms: A perspective on preventing dental caries. MedChemComm 2019, 10, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, V. Impact of environmental biofilms: Industrial components and its remediation. J. Basic Microbiol. 2020, 60, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Cheng, E.; Whitley, J.W.; Horne, R.R.; Leigh, B.; Xu, L.; Jones, B.D.; Guymon, C.A.; Hansen, M.R. Photograftable zwitterionic coatings prevent Staphylococcus aureus and Staphylococcus epidermidis adhesion to PDMS surfaces. ACS Appl. Bio Mater. 2021, 4, 1283–1293. [Google Scholar] [CrossRef]
- Berne, C.; Ellison, C.K.; Ducret, A.; Brun, Y.V. Bacterial adhesion at the single-cell level. Nat. Rev. Microbiol. 2018, 16, 616–627. [Google Scholar] [CrossRef]
- Sapozhnikov, S.V.; Shtyrlin, N.V.; Kayumov, A.R.; Zamaldinova, A.E.; Iksanova, A.G.; Nikitina, E.V.; Krylova, E.S.; Grishaev, D.Y.; Balakin, K.V.; Shtyrlin, Y.G. New quaternary ammonium pyridoxine derivatives: Synthesis and antibacterial activity. Med. Chem. Res. 2017, 26, 3188–3202. [Google Scholar] [CrossRef]
- Trizna, E.Y.; Yarullina, M.N.; Baidamshina, D.R.; Mironova, A.V.; Akhatova, F.S.; Rozhina, E.V.; Fakhrullin, R.F.; Khabibrakhmanova, A.M.; Kurbangalieva, A.R.; Bogachev, M.I.; et al. Bidirectional alterations in antibiotics susceptibility in Staphylococcus aureus—Pseudomonas aeruginosa dual-species biofilm. Sci. Rep. 2020, 10, 14849. [Google Scholar] [CrossRef]
- Shtyrlin, N.V.; Sapozhnikov, S.V.; Galiullina, A.S.; Kayumov, A.R.; Bondar, O.V.; Mirchink, E.P.; Isakova, E.B.; Firsov, A.A.; Balakin, K.V.; Shtyrlin, Y.G. Synthesis and antibacterial activity of quaternary ammonium 4-deoxypyridoxine derivatives. BioMed Res. Int. 2016, 2016, 3864193. [Google Scholar] [CrossRef] [Green Version]
- Boudarel, H.; Mathias, J.D.; Blaysat, B.; Grédiac, M. Towards standardized mechanical characterization of microbial biofilms: Analysis and critical review. NPJ Biofilms Microbiomes 2018, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.; Zheng, W.; Prananty, D.; Li, J.; Koh, C.H.; Kang, E.T.; Pethe, K.; Chan-Park, M.B. Polymers as advanced antibacterial and antibiofilm agents for direct and combination therapies. Chem. Sci. 2022, 13, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, S.; Lu, D.; Shi, Y.; Yao, Y. Water-soluble supramolecular polymers constructed by macrocycle-based host-guest interactions. Chin. Chem. Lett. 2019, 30, 37–43. [Google Scholar] [CrossRef]
- Dong, S.; Zheng, B.; Wang, F.; Huang, F. Supramolecular polymers constructed from macrocycle-based host–guest molecular recognition motifs. Acc. Chem. Res. 2014, 47, 1982–1994. [Google Scholar] [CrossRef]
- Han, W.; Xiang, W.; Li, Q.; Zhang, H.; Yang, Y.; Shi, J.; Ji, Y.; Wang, S.; Ji, X.; Khashab, N.M.; et al. Water compatible supramolecular polymers: Recent progress. Chem. Soc. Rev. 2021, 50, 10025–10043. [Google Scholar] [CrossRef]
- Correia, H.D.; Chowdhury, S.; Ramos, A.P.; Guy, L.; Demets, G.J.F.; Bucher, C. Dynamic supramolecular polymers built from cucurbit[n]urils and viologens. Polym. Int. 2019, 68, 572–588. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.S.; Liu, Y. Calixarene-based supramolecular polymerization in solution. Chem. Soc. Rev. 2012, 41, 5907–5921. [Google Scholar] [CrossRef]
- Shurpik, D.N.; Padnya, P.L.; Stoikov, I.I.; Cragg, P.J. Antimicrobial activity of calixarenes and related macrocycles. Molecules 2020, 25, 5145. [Google Scholar] [CrossRef]
- Gao, L.; Wang, H.; Zheng, B.; Huang, F. Combating antibiotic resistance: Current strategies for the discovery of novel antibacterial materials based on macrocycle supramolecular chemistry. Giant 2021, 7, 100066. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Y.W. Functional materials with pillarene struts. Acc. Mater. Res. 2021, 2, 292–305. [Google Scholar] [CrossRef]
- Fa, S.; Kakuta, T.; Yamagishi, T.A.; Ogoshi, T. One-, two-, and three-dimensional supramolecular assemblies based on tubular and regular polygonal structures of pillar [n] arenes. CCS Chem. 2019, 1, 50–63. [Google Scholar] [CrossRef] [Green Version]
- Shurpik, D.N.; Aleksandrova, Y.I.; Zelenikhin, P.V.; Subakaeva, E.V.; Cragg, P.J.; Stoikov, I.I. Towards new nanoporous biomaterials: Self-assembly of sulfopillar[5]arenes with vitamin D3 into supramolecular polymers. Org. Biomol. Chem. 2020, 18, 4210–4216. [Google Scholar] [CrossRef] [PubMed]
- Shurpik, D.N.; Mostovaya, O.A.; Sevastyanov, D.A.; Lenina, O.A.; Sapunova, A.S.; Voloshina, A.D.; Petrov, K.A.; Kovyazina, I.V.; Cragg, P.J.; Stoikov, I.I. Supramolecular neuromuscular blocker inhibition by a pillar[5]arene through aqueous inclusion of rocuronium bromide. Org. Biomol. Chem. 2019, 17, 9951–9959. [Google Scholar] [CrossRef]
- Shurpik, D.N.; Yakimova, L.S.; Gorbachuk, V.V.; Sevastyanov, D.A.; Padnya, P.L.; Bazanova, O.B.; Stoikov, I.I. Hybrid multicyclophanes based on thiacalix[4]arene and pillar[5]arene: Synthesis and influence on the formation of polyaniline. Org. Chem. Front. 2018, 5, 2780–2786. [Google Scholar] [CrossRef]
- Antipin, I.S.; Alfimov, M.V.; Arslanov, V.V.; Burilov, V.A.; Vatsadze, S.Z.; Voloshin, Y.Z.; Volcho, K.P.; Gorbatchuk, V.V.; Gorbunova, Y.G.; Gromov, S.P.; et al. Functional supramolecular systems: Design and applications. Russ. Chem. Rev. 2021, 90, 895. [Google Scholar] [CrossRef]
- Feng, W.; Jin, M.; Yang, K.; Pei, Y.; Pei, Z. Supramolecular delivery systems based on pillararenes. Chem. Commun. 2018, 54, 13626–13640. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Lou, X.Y.; Yang, Y.W. Pillararene-based molecular-scale porous materials. Chem. Commun. 2021, 57, 13429–13447. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, Y.; Xu, F.; Liang, T.; Wen, H.; Tian, W. Pillararene-based supramolecular polymers. Chem. Commun. 2019, 55, 271–285. [Google Scholar] [CrossRef]
- Yang, H.; Jin, L.; Zhao, D.; Lian, Z.; Appu, M.; Huang, J.; Zhang, Z. Antibacterial and Antibiofilm Formation Activities of Pyridinium-Based Cationic Pillar[5]arene Against Pseudomonas aeruginosa. J. Agric. Food Chem. 2021, 69, 4276–4283. [Google Scholar] [CrossRef]
- Guo, S.; Huang, Q.; Chen, Y.; Wei, J.; Zheng, J.; Wang, L.; Wang, Y.; Wang, R. Synthesis and Bioactivity of Guanidinium-Functionalized Pillar[5]arene as a Biofilm Disruptor. Angew. Chem. Int. Ed. 2021, 60, 618–623. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2007, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Van De Streek, J. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Spartan ’20, version 1.1.1; Wavefunction Inc.: Irvine, CA, USA, 2020.
- Yao, Y.; Xue, M.; Chi, X.; Ma, Y.; He, J.; Abliz, Z.; Huang, F. A new water-soluble pillar[5]arene: Synthesis and application in the preparation of gold nanoparticles. Chem. Commun. 2012, 48, 6505. [Google Scholar] [CrossRef] [PubMed]
- Pena-Francesch, A.; Jung, H.; Demirel, M.C.; Sitti, M. Biosynthetic self-healing materials for soft machines. Nat. Mater. 2020, 19, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Liu, J.; Gan, L.; Long, M. Research progress in bio-based self-healing materials. Eur. Polym. J. 2020, 129, 109651. [Google Scholar] [CrossRef]
- Thakur, V.K.; Kessler, M.R. Self-healing polymer nanocomposite materials: A review. Polymer 2015, 69, 369–383. [Google Scholar] [CrossRef] [Green Version]
- An, S.Y.; Arunbabu, D.; Noh, S.M.; Song, Y.K.; Oh, J.K. Recent strategies to develop self-healable crosslinked polymeric networks. Chem. Commun. 2015, 51, 13058–13070. [Google Scholar] [CrossRef]
- Yoon, J.A.; Kamada, J.; Koynov, K.; Mohin, J.; Nicolaÿ, R.; Zhang, Y.; Balazs, A.C.; Kowalewski, T.; Matyjaszewski, K. Self-healing polymer films based on thiol–disulfide exchange reactions and self-healing kinetics measured using atomic force microscopy. Macromolecules 2012, 45, 142–149. [Google Scholar] [CrossRef]
- Martin, R.; Rekondo, A.; De Luzuriaga, A.R.; Casuso, P.; Dupin, D.; Cabañero, G.; Grande, H.J.; Odriozola, I. Dynamic sulfur chemistry as a key tool in the design of self-healing polymers. Smart Mater. Struct. 2016, 25, 084017. [Google Scholar] [CrossRef]
- Tyuftin, A.A.; Solovieva, S.E.; Murav’ev, A.A.; Polyantsev, F.M.; Latypov, S.K.; Antipin, I.S. Thiacalix[4]arenes with terminal thiol groups at the lower rim: Synthesis and structure. Russ. Chem. Bull. 2009, 58, 145–151. [Google Scholar] [CrossRef]
- Naga, N.; Moriyama, K.; Furukawa, H. Synthesis and properties of multifunctional thiol crosslinked gels containing disulfide bond in the network structure. J. Polym. Sci. A Polym. Chem. 2017, 55, 3749–3756. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, L.; Li, Y.; Li, Q. Effects of networks composed of epoxy/dual thiol-curing agents on properties of shape memory polymers. J. Appl. Polym. Sci. 2022, 139, 51548. [Google Scholar] [CrossRef]
- Felton, L.A. Mechanisms of polymeric film formation. Int. J. Pharm. 2013, 457, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, R.P.; Romanowski, E.G.; Mah, F.S.; Yates, K.A.; Gordon, Y.J. Intracameral Vigamox ® (moxifloxacin 0.5%) is non-toxic and effective in preventing endophthalmitis in a rabbit model. Am. J. Ophthalmol. 2005, 140, 497. [Google Scholar] [CrossRef]
- Bindfit. Available online: http://supramolecular.org (accessed on 3 March 2022).
- Hibbert, D.B.; Thordarson, P. The death of the Job plot, transparency, open science and online tools, uncertainty estimation methods and other developments in supramolecular chemistry data analysis. Chem. Commun. 2016, 52, 12792–12805. [Google Scholar] [CrossRef] [Green Version]
- Barbera, L.; De Plano, L.M.; Franco, D.; Gattuso, G.; Guglielmino, S.P.; Lando, G.; Notti, A.; Parisi, M.F.; Pisagatti, I. Antiadhesive and antibacterial properties of pillar[5]arene-based multilayers. Chem. Commun. 2018, 54, 10203–10206. [Google Scholar] [CrossRef]
- Cash, J.J.; Kubo, T.; Bapat, A.P.; Sumerlin, B.S. Room-temperature self-healing polymers based on dynamic-covalent boronic esters. Macromolecules 2015, 48, 2098–2106. [Google Scholar] [CrossRef]
- Blaiszik, B.J.; Caruso, M.M.; McIlroy, D.A.; Moore, J.S.; White, S.R.; Sottos, N.R. Microcapsules filled with reactive solutions for self-healing materials. Polymer 2009, 50, 990–997. [Google Scholar] [CrossRef]
- Ye, G.; Jiang, T. Preparation and Properties of Self-Healing Waterborne Polyurethane Based on Dynamic Disulfide Bond. Polymers 2021, 13, 2936. [Google Scholar] [CrossRef]
- Zheng, S.; Brook, M.A. Reversible Redox Crosslinking of Thiopropylsilicones. Macromol. Rapid Commun. 2021, 42, 2000375. [Google Scholar] [CrossRef] [PubMed]
- Nevejans, S.; Ballard, N.; Miranda, J.I.; Reck, B.; Asua, J.M. The underlying mechanisms for self-healing of poly(disulfide)s. Phys. Chem. Chem. Phys. 2016, 18, 27577–27583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, M.; Grande, A.M.; Dierkes, W.; Bijleveld, J.; Van Der Zwaag, S.; García, S.J. Turning vulcanized natural rubber into a self-healing polymer: Effect of the disulfide/polysulfide ratio. ACS Sustain. Chem. Eng. 2016, 4, 5776–5784. [Google Scholar] [CrossRef]
- Murzakhanov, F.F.; Grishin, P.O.; Goldberg, M.A.; Yavkin, B.V.; Mamin, G.V.; Orlinskii, S.B.; Komlev, V.S. Radiation-induced stable radicals in calcium phosphates: Results of multifrequency epr, ednmr, eseem, and endor studies. Appl. Sci. 2021, 11, 7727. [Google Scholar] [CrossRef]
- Hu, J.; Mo, R.; Jiang, X.; Sheng, X.; Zhang, X. Structural design and antimicrobial properties of polypeptides and saccharide–polypeptide conjugates. Polymer 2019, 183, 1904683. [Google Scholar]
- Shurpik, D.N.; Aleksandrova, Y.I.; Mostovaya, O.A.; Nazmutdinova, V.A.; Zelenikhin, P.V.; Subakaeva, E.V.; Mukhametzyanov, T.A.; Cragg, P.J.; Stoikov, I.I. Water-soluble pillar[5]arene sulfo-derivatives self-assemble into biocompatible nanosystems to stabilize therapeutic proteins. Bioorg. Chem. 2021, 117, 105415. [Google Scholar] [CrossRef]
- Friedrich, A.W. Control of hospital acquired infections and antimicrobial resistance in Europe: The way to go. Wien. Med. Wochenschr. 2019, 169, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Chen, S.; Gou, X.; Yang, J.; An, J.; Jin, X.; Yang, Y.-W.; Chen, L.; Gao, H. Biodegradable supramolecular materials based on cationic polyaspartamides and pillar[5]arene for targeting gram-positive bacteria and mitigating antimicrobial resistance. Adv. Funct. Mater. 2019, 29, 1904683. [Google Scholar] [CrossRef]
- Pluth, M.D.; Raymond, K.N. Reversible guest exchange mechanisms in supramolecular host–guest assemblies. Chem. Soc. Rev. 2007, 36, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Bojtár, M.; Paudics, A.; Hessz, D.; Kubinyi, M.; Bitter, I. Amino acid recognition by fine tuning the association constants: Tailored naphthalimides in pillar[5]arene-based indicator displacement assays. RSC Adv. 2016, 6, 86269–86275. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shurpik, D.N.; Aleksandrova, Y.I.; Mostovaya, O.A.; Nazmutdinova, V.A.; Tazieva, R.E.; Murzakhanov, F.F.; Gafurov, M.R.; Zelenikhin, P.V.; Subakaeva, E.V.; Sokolova, E.A.; et al. Self-Healing Thiolated Pillar[5]arene Films Containing Moxifloxacin Suppress the Development of Bacterial Biofilms. Nanomaterials 2022, 12, 1604. https://doi.org/10.3390/nano12091604
Shurpik DN, Aleksandrova YI, Mostovaya OA, Nazmutdinova VA, Tazieva RE, Murzakhanov FF, Gafurov MR, Zelenikhin PV, Subakaeva EV, Sokolova EA, et al. Self-Healing Thiolated Pillar[5]arene Films Containing Moxifloxacin Suppress the Development of Bacterial Biofilms. Nanomaterials. 2022; 12(9):1604. https://doi.org/10.3390/nano12091604
Chicago/Turabian StyleShurpik, Dmitriy N., Yulia I. Aleksandrova, Olga A. Mostovaya, Viktoriya A. Nazmutdinova, Regina E. Tazieva, Fadis F. Murzakhanov, Marat R. Gafurov, Pavel V. Zelenikhin, Evgenia V. Subakaeva, Evgenia A. Sokolova, and et al. 2022. "Self-Healing Thiolated Pillar[5]arene Films Containing Moxifloxacin Suppress the Development of Bacterial Biofilms" Nanomaterials 12, no. 9: 1604. https://doi.org/10.3390/nano12091604
APA StyleShurpik, D. N., Aleksandrova, Y. I., Mostovaya, O. A., Nazmutdinova, V. A., Tazieva, R. E., Murzakhanov, F. F., Gafurov, M. R., Zelenikhin, P. V., Subakaeva, E. V., Sokolova, E. A., Gerasimov, A. V., Gorodov, V. V., Islamov, D. R., Cragg, P. J., & Stoikov, I. I. (2022). Self-Healing Thiolated Pillar[5]arene Films Containing Moxifloxacin Suppress the Development of Bacterial Biofilms. Nanomaterials, 12(9), 1604. https://doi.org/10.3390/nano12091604