Transfer-Printed Cuprous Iodide (CuI) Hole Transporting Layer for Low Temperature Processed Perovskite Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
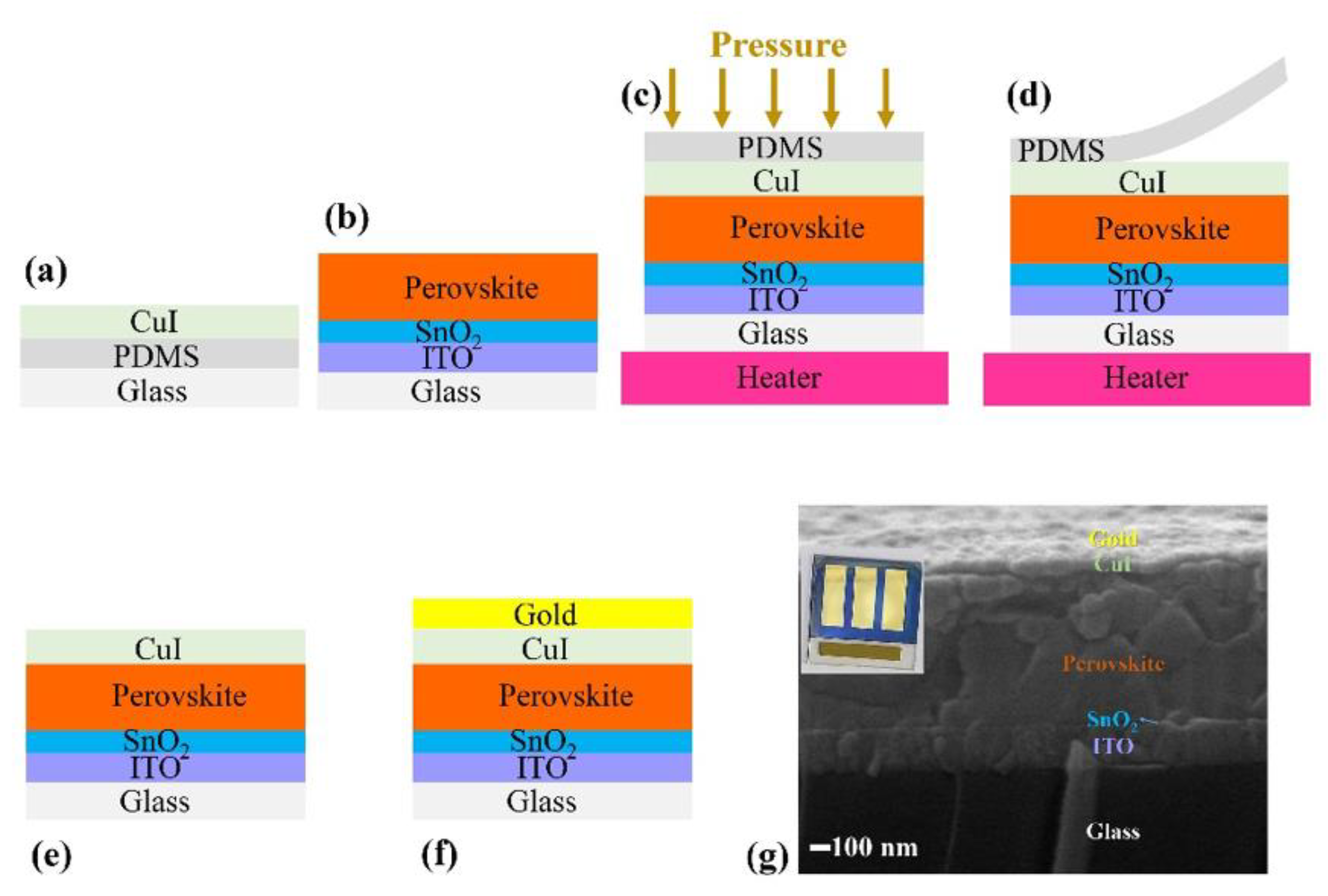
2.2. Fabrication of PDMS and Copper Iodide Stamps
2.3. Fabrication of PSCs
2.4. Fabrication of Hole-Transporting Layers
2.5. Characterizations
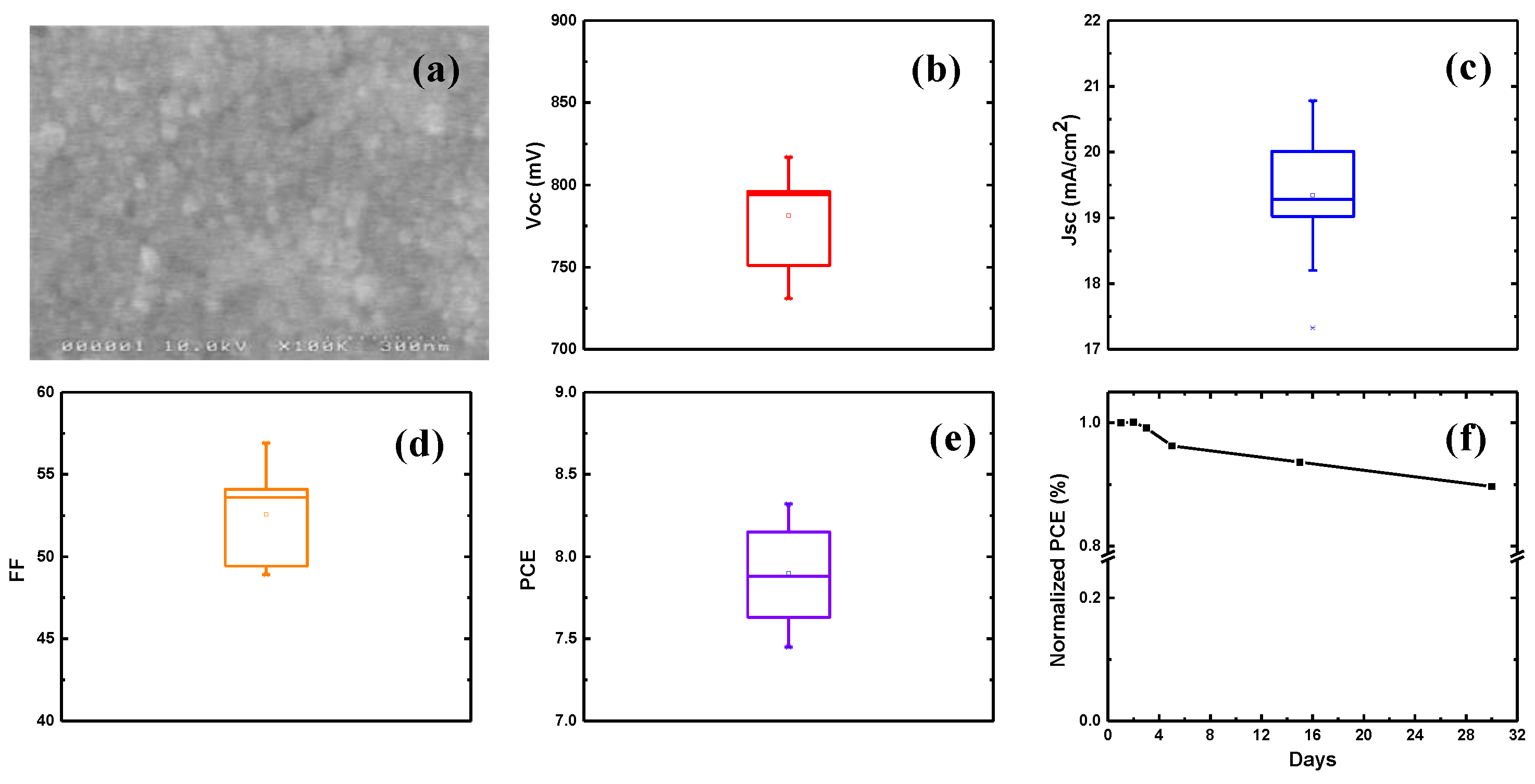
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PSCs | Perovskite solar cells |
| PCEs | Power-conversion efficiencies |
| HTL | Hole transport layer |
| ETL | Electron transport layer |
| HTM | Hole transport material |
| CuI | Cuprous iodide |
| VOC | Open-circuit voltage |
| JSC | Short-circuit current density |
| FF | Fill factor |
| H.I. | Hysteresis index |
| ITO | Indium tin oxide |
| Li-TFSI | Lithium bis- (trifluoromethylsulfonyl)imide |
| 4-tBP | 4-tert-butylpyridine |
| SnO2 | Tin (IV) oxide |
| Spiro-OMeTAD | 2,2′,7,7′-Tetrakis[N,N-di(4-methoxyphenyl)amino]-9,9′-spirobifluorene |
| PDMS | Polydimethylsiloxane |
| tCu | Thickness of copper film |
| TIod | Iodization temperature |
| tTP | Transfer-printing time |
| PTP | Transfer-printing pressure |
| TTP | Transfer-printing temperature |
| J-V | Current density–voltage |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscope |
| PL | Photoluminescence |
References
- Minami, T.; Nishi, Y.; Miyata, T. Heterojunction solar cell with 6% efficiency based on an n-type aluminum-gallium-oxide thin film and p-type sodium-doped Cu2O sheet. Appl. Phys. Express 2015, 8, 022301. [Google Scholar] [CrossRef]
- Im, J.-H.; Lee, C.-R.; Lee, J.-W.; Park, S.-W.; Park, N.-G. 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 2011, 3, 4088–4093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.M.; Alcocer, J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [Green Version]
- Steirer, K.X.; Schulz, P.; Teeter, G.; Stevanovic, V.; Yang, M.; Zhu, K.; Berry, J.J. Defect tolerance in methylammonium lead triiodide perovskite. ACS Energy Lett. 2016, 1, 360–366. [Google Scholar] [CrossRef]
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Electron-hole diffusion lengths > 175 μm in solution-grown CH3NH3PbI3 single crystals. Science 2015, 347, 967–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amjad, M.; Khan, M.I.; Alwadai, N.; Irfan, M.; Ikram-ul-Haq; Albalawi, H.; Almuqrin, A.H.; Almoneef, M.M.; Iqbal, M. Photovoltaic Properties of ZnO Films Co-Doped with Mn and La to Enhance Solar Cell Efficiency. Nanomaterials 2022, 12, 1057. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Mukhtar, A.; Alwadai, N.; Irfan, M.; Haq, I.-U.; Albalawi, H.; Almuqrin, A.H.; Almoneef, M.M.; Iqbal, M. Improving the Structural, Optical and Photovoltaic Properties of Sb- and BiCo-Doped MAPbBr3 Perovskite Solar Cell. Coatings 2022, 12, 386. [Google Scholar] [CrossRef]
- Li, R.; Li, C.; Liu, M.; Vivo, P.; Zheng, M.; Dai, Z.; Zhan, J.; He, B.; Li, H.; Yang, W.; et al. Hydrogen-bonded dopant-free hole transport material enables efficient and stable inverted perovskite solar cells. CCS Chem. 2021, 3, 3309–3319. [Google Scholar] [CrossRef]
- Yang, W.S.; Park, B.-W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide-based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [Green Version]
- Al-Ashouri, A.; Magomedov, A.; Rob, M.; Jost, M.; Talaikis, M.; Chistiakova, G.; Bertram, T.; Marquez, J.A.; Köhnen, E.; Kasparavicius, E.; et al. Conformal monolayer contacts with lossless interfaces for perovskite single junction and monolithic tandem solar cells. Energy Environ. Sci. 2019, 12, 3356–3369. [Google Scholar] [CrossRef] [Green Version]
- Green, M.A.; Dunlop, E.D.; Ebinger, J.H.; Yoshita, M.; Kopidakis, N.; Baillie, A.W.Y.H. Solar cell efficiency tables (Version 55). Prog. Photovolt. Res. Appl. 2020, 28, 3–15. [Google Scholar] [CrossRef]
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G.; et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef]
- Conings, B.; Babayigit, A.; Klug, M.T.; Bai, S.; Gauquelin, N.; Sakai, N.; Wang, J.T.-W.; Verbeeck, J.; Boyen, H.-G.; Snaith, H.J. A universal deposition protocol for planar heterojunction solar cells with high efficiency based on hybrid lead halide perovskite families. Adv. Mater. 2016, 28, 10701–10709. [Google Scholar] [CrossRef]
- Luo, D.; Yang, W.; Wang, Z.; Sadhanala, A.; Hu, Q.; Su, R.; Shivanna, R.; Trindade, G.F.; Watts, J.F.; Xu, Z.; et al. Enhanced photovoltage for inverted planar heterojunction perovskite solar cells. Science 2018, 360, 1442–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saragi, T.P.I.; Spehr, T.; Siebert, A.; Fuhrmann-Lieker, T.; Salbeck, J. Spiro compounds for organic optoelectronics. Chem. Rev. 2007, 107, 1011–1065. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, A.; Yanagida, M.; Shirai, Y.; Miyano, K. Degradation of perovskite solar cells by the doping level decrease of HTL revealed by capacitance spectroscopy. Sol. Energy Mater. Sol. Cells 2021, 220, 110854. [Google Scholar] [CrossRef]
- Zhou, W.; Wen, Z.; Gao, P. Less is more: Dopant-free hole transporting materials for high-efficiency perovskite solar cells. Adv. Energy Mater. 2018, 8, 1702512. [Google Scholar] [CrossRef]
- Pham, H.D.; Yang, T.C.-J.; Jain, S.M.; Wilson, G.J.; Sonar, P. Development of dopant-free organic hole transporting materials for perovskite solar cells. Adv. Energy Mater. 2020, 10, 1903326. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Wu, B.; Wang, Z.; Sun, Z.; Xue, S.; Wu, Y.; Hagfeldt, A.; Liang, M. Indeno [1,2-b]carbazole as methoxy-free donor group: Constructing efficient and stable hole-transporting materials for perovskite solar cells. Angew. Chem. Int. Ed. 2019, 58, 15721–15725. [Google Scholar] [CrossRef]
- Shen, C.; Wu, Y.; Zhang, H.; Li, E.; Zhang, W.; Xu, X.; Wu, W.; Tian, H.; Zhu, W.-H. Semi-locked tetrathienylethene as a building block for hole transporting materials: Toward efficient and stable perovskite solar cells. Angew. Chem. Int. Ed. 2019, 58, 3784–3789. [Google Scholar] [CrossRef]
- Dong, Z.; Yin, X.; Ali, A.; Zhou, J.; Bista, S.S.; Chen, C.; Yan, Y.; Tang, W. A dithieno[3,2-b:2′,3′-d]pyrrole-cored four-arm hole transporting material for over 19% efficiency dopant-free perovskite solar cells. J. Mater. Chem. C 2019, 7, 9455–9459. [Google Scholar] [CrossRef]
- Zhou, J.; Yin, X.; Dong, Z.; Ali, A.; Song, Z.; Shrestha, N.; Bista, S.S.; Bao, Q.; Ellingson, R.J.; Yan, Y.; et al. Dithieno[3,2-b:2′,3′-d]pyrrole cored p-type semiconductors enabling 20% efficiency dopant-free perovskite solar cells. Angew. Chem. Int. Ed. 2019, 58, 13717–13721. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, X.; Wang, Y.; Chen, Z.; Yao, F.; Zhang, Q.; Fang, G.; Chen, Z.-K.; Huang, W.; Xu, Z.-X. Dopant-free hole-transport materials based on methoxytriphenylamine-substituted indacenodithienothiophene for solution-processed perovskite solar cells. ChemSusChem 2017, 10, 2833–2838. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.-B.; Han, Y.; Yang, N.; Yang, S.; Zhang, L.; Wang, Y.; Jia, Y.; Zhao, L.; Zhong, Y.-W.; Chen, Q. Propeller-shaped, triarylamine-rich, and dopant-free hole transporting materials for efficient n–i–p perovskite solar cells. ACS Appl. Mater. Interfaces 2018, 10, 41592–41598. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Rezaee, E.; Shan, H.; Xu, J.; Zhang, Y.; Feng, Y.; Dai, J.; Chen, Z.-K.; Huang, W.; Xu, Z.-X. Dopant-free hole transport materials based on alkyl-substituted indacenodithiophene for planar perovskite solar cells. J. Mater. Chem. C 2018, 6, 4706–4713. [Google Scholar] [CrossRef]
- Sun, X.; Xue, Q.; Zhu, Z.; Xiao, Q.; Jiang, K.; Yip, H.-L.; Yan, H.; Li, Z. Fluoranthene-based dopant-free hole transporting materials for efficient perovskite solar cells. Chem. Sci. 2018, 9, 2698–2704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braukyla, T.; Xia, R.; Daskeviciene, M.; Malinauskas, T.; Grudis, A.; Jankauskas, V.; Fei, Z.; Momblona, C.; Carmona, C.R.; Dyson, P.J.; et al. Inexpensive hole-transporting materials derived from troger’s base afford efficient and stable perovskite solar cells. Angew. Chem. Int. Ed. 2019, 58, 11266–11272. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, Q.; Dong, L.; Zhang, Z.; Li, C.; Yang, S.; Cai, S.; Xu, Z.-X. Carbon-chain length substituent effects on Cu(II) phthalocyanines as dopant free hole-transport materials for perovskite solar cells. Sol. Energy 2019, 184, 649–656. [Google Scholar] [CrossRef]
- Wu, J.; Liu, C.; Li, B.; Gu, F.; Zhang, L.; Hu, M.; Deng, X.; Qiao, Y.; Mao, Y.; Tan, W.; et al. Side-chain polymers as dopant-free hole-transporting materials for perovskite solar cells-the impact of substituents’ positions in carbazole on device performance. ACS Appl. Mater. Interfaces 2019, 11, 26928–26937. [Google Scholar] [CrossRef]
- Kim, G.-W.; Lee, J.; Kang, G.; Kim, T.; Park, T. Donor–acceptor type dopant-free, polymeric hole transport material for planar perovskite solar cells (19.8%). Adv. Energy Mater. 2018, 8, 1701935. [Google Scholar] [CrossRef]
- Qin, P.; Tanaka, S.; Ito, S.; Tetreault, N.; Manabe, K.; Nishino, H.; Nazeeruddin, M.K.; Grätzel, M. Inorganic hole conductor-based lead halide perovskite solar cells with 12.4% conversion efficiency. Nat. Commun. 2014, 5, 3834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christians, J.A.; Fung, R.C.M.; Kamat, P.V. An inorganic hole conductor for organo-lead halide perovskite solar cells improved hole conductivity with copper iodide. J. Am. Chem. Soc. 2013, 136, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Sun, W.; Li, Y.; Yan, W.; Peng, H.; Bian, Z.; Liu, Z.; Huang, C. CuSCN-based inverted planar perovskite solar cell with an average PCE of 15.6%. Nano Lett. 2015, 15, 3723–3728. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, A.S.; Halder, A.; Ghosh, S.; Mahuli, N.; Hodes, G.; Sarkar, S.K. Inorganic hole conducting layers for perovskite-based solar cells. J. Phys. Chem. Lett. 2014, 5, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, Z.; Zuo, Z.; Zhang, M.; Zhao, Z.; Shen, Y.; Zhou, H.; Chen, Q.; Yang, Y.; Wang, M. Hole selective NiO contact for efficient perovskite solar cells with carbon electrode. Nano Lett. 2015, 15, 2402–2408. [Google Scholar] [CrossRef]
- Yamada, N.; Ino, R.; Ninomiya, Y. Truly Transparent p-Type γ-CuI thin films with high hole mobility. Chem. Mater. 2016, 28, 4971–4981. [Google Scholar] [CrossRef]
- Xu, B.; Sheibani, E.; Liu, P.; Zhang, J.; Tian, H.; Vlachopoulos, N.; Boschloo, G.; Kloo, L.; Hagfeldt, A.; Sun, L. Carbazole-based hole-transport materials for efficient solid-state dye-sensitized solar cells and perovskite solar cells. Adv. Mater. 2014, 26, 6629–6634. [Google Scholar] [CrossRef]
- Takahashi, K.; Suzuki, Y. Perovskite solar cells with CuI inorganic hole conductor. Jpn. J. Appl. Phys. 2017, 56, 08MC04. [Google Scholar] [CrossRef] [Green Version]
- Huangfu, M.; Shen, Y.; Zhu, G.; Xu, K.; Cao, M.; Gu, F.; Wang, L. Copper iodide as inorganic hole conductor for perovskite solar cells with different thickness of mesoporous layer and hole transport layer. Appl. Surf. Sci. 2015, 357, 2234–2240. [Google Scholar] [CrossRef]
- Nazari, P.; Ansari, F.; Nejand, B.A.; Ahmadi, V.; Payandeh, M.; Niasari, M.S. Physicochemical interface engineering of CuI/Cu as advanced potential hole-transporting materials/metal contact couples in hysteresis-free ultralow-cost and large-area perovskite solar cells. J. Phys. Chem. C 2017, 121, 21935–21944. [Google Scholar] [CrossRef]
- Shi, B.; Jia, J.; Feng, X.; Ma, G.; Wu, Y.; Cao, B. Thermal evaporated CuI film thickness-dependent performance of perovskite solar cells. Vacuum 2021, 187, 110076. [Google Scholar] [CrossRef]
- Uthayaraj, S.; Karunarathne, D.G.B.C.; Kumara, G.R.A.; Murugathas, T.; Rasalingam, S.; Rajapakse, R.M.G.; Ravirajan, P.; Velauthapillai, D. Powder pressed cuprous iodide (CuI) as a hole transporting material for perovskite solar cells. Materials 2019, 12, 2037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sepalage, G.A.; Meyer, S.; Pascoe, A.; Scully, A.D.; Huang, F.; Bach, U.; Cheng, Y.-B.; Spiccia, L. Copper(I) iodide as hole-conductor in planar perovskite solar cells: Probing the origin of J-V hysteresis. Adv. Funct. Mater. 2015, 25, 5650–5661. [Google Scholar] [CrossRef]
- Srivastava, R.P.; Lee, J.; Khang, D.-Y. Ambient environment induced synergetic improvement in morphology and iodine vacancy passivation by MAI surface engineering in mixed-cation lead mixed-halide (FA0.85MA0.15PbI0.55Br0.45) perovskite solar cells. Surf. Interfaces 2022, 29, 101703. [Google Scholar] [CrossRef]
- Baek, S.-D.; Kwon, D.-K.; Kim, Y.C.; Myoung, J.-M. Violet light-emitting diodes based on p-CuI thin film/n-MgZnO quantum dot heterojunction. ACS Appl. Mater. Interfaces 2020, 12, 6037–6047. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-H.; Chen, S.-S.; Liu, H.-M.; Jang, L.-W.; Chang, S.-Y. Glycine–Nitrate combustion synthesis of Cu-based nanoparticles for NP9EO degradation applications. Catalysts 2020, 10, 1061. [Google Scholar] [CrossRef]
- Zhu, B.L.; Zhao, X.Z. Transparent conductive CuI thin films prepared by pulsed laser deposition. Phys. Status Solidi A 2011, 208, 91–96. [Google Scholar] [CrossRef]
- Cao, Q.; Li, Y.; Zhang, H.; Yang, J.; Han, J.; Xu, T.; Wang, S.; Wang, Z.; Gao, B.; Zhao, J.; et al. Efficient and stable inverted perovskite solar cells with very high fill factors via incorporation of star-shaped polymer. Sci. Adv. 2021, 7, eabg0633. [Google Scholar] [CrossRef]
- Proctor, C.M.; Nguyen, T.-Q. Effect of leakage current and shunt resistance on the light intensity dependence of organic solar cells. Appl. Phys. Lett. 2015, 106, 083301. [Google Scholar] [CrossRef]
- Schubert, B.E.; Floreano, D. Variable stiffness material based on rigid low-melting-point-alloy microstructures embedded in soft poly(dimethylsiloxane) (PDMS). RSC Adv. 2013, 3, 24671–24679. [Google Scholar] [CrossRef] [Green Version]
- Gharibzadeh, S.; Nejand, B.A.; Moshaii, A.; Mohammadian, N.; Alizadeh, A.H.; Mohammadpour, R.; Ahmadi, V.; Alizadeh, A. Two-step physical deposition of a compact CuI holetransport layer and the formation of an interfacial species in perovskite solar cells. ChemSusChem 2016, 9, 1921–1937. [Google Scholar] [CrossRef] [PubMed]


| Cu Film Thickness (nm) | TTP (°C) | Scan Direction | Voc (mV) | Jsc (mA/cm2) | FF (%) | PCE (%) | H.I. |
|---|---|---|---|---|---|---|---|
| 30 | 30 | Forward Reverse | 557 669 | 18.1 18.8 | 32.9 42.1 | 3.3 5.3 | 0.37 |
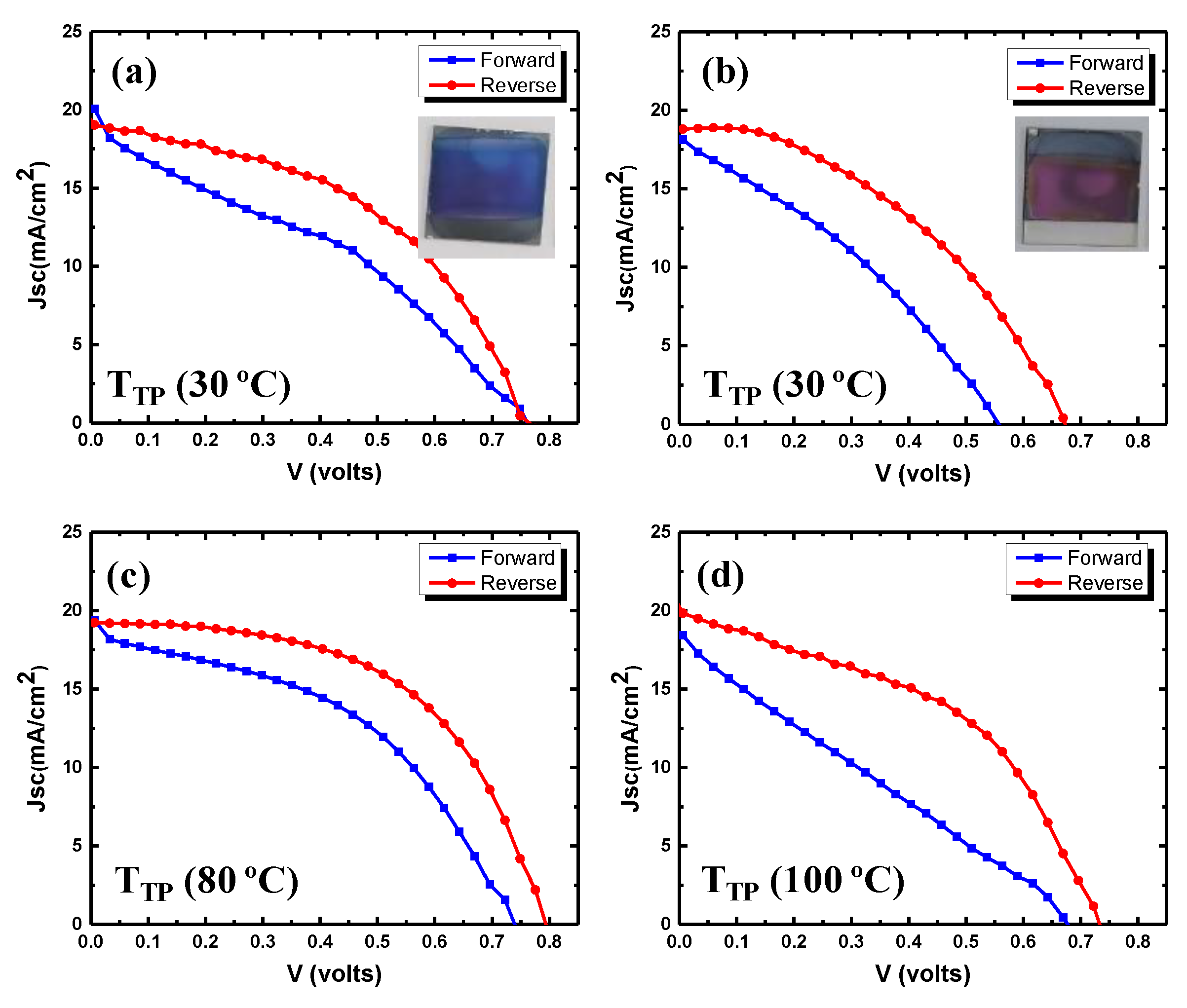
| 20 | 30 | Forward Reverse | 758 764 | 19.3 19.0 | 35.9 45.4 | 5.3 6.6 | 0.20 |
| 20 | 80 | Forward Reverse | 742 795 | 18.8 19.3 | 44.1 53.8 | 6.3 8.3 | 0.24 |
| 20 | 100 | Forward Reverse | 675 728 | 18.4 19.9 | 25.3 45.2 | 3.2 6.6 | 0.31 |
| HTL Material | Scan Direction | Voc (mV) | Jsc (mA/cm2) | FF (%) | PCE (%) | H.I. |
|---|---|---|---|---|---|---|
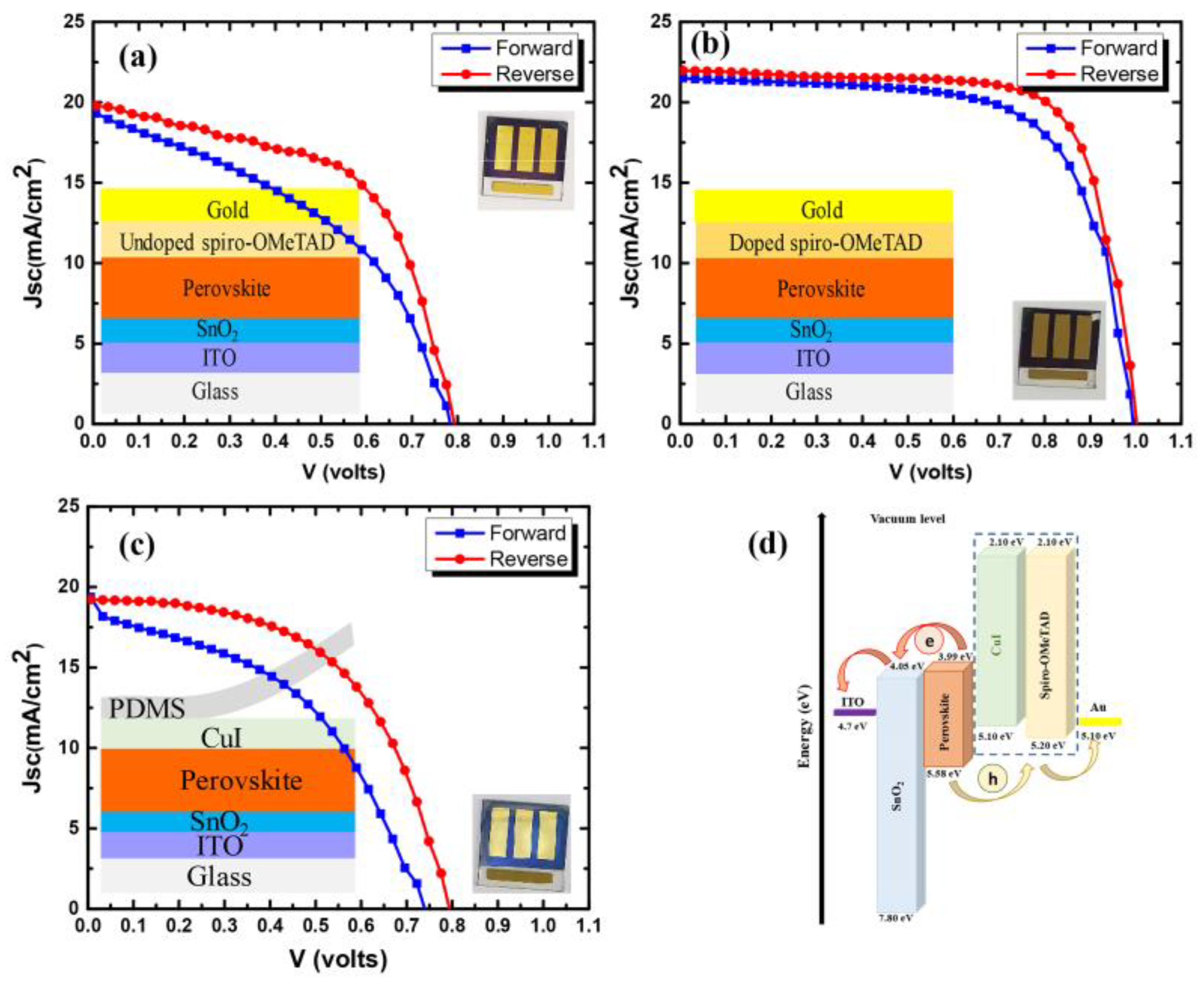
| Undoped spiro-OMeTAD | Forward Reverse | 779 796 | 19.5 19.9 | 42.6 55.5 | 6.5 8.8 | 0.26 |
| Doped spiro-OMeTAD | Forward Reverse | 994 1001 | 21.4 21.6 | 67.7 73.2 | 14.5 16.1 | 0.09 |
| Transfer-printed CuI | Forward Reverse | 742 795 | 18.8 19.3 | 44.1 53.8 | 6.3 8.3 | 0.24 |
| S. No. | Device Structure | CuI Deposition Method | VOC (V) | JSC (mA/cm2) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1. | TiO2/CH3NH3PbI3/CuI | Solution pumping | 0.55 | 17.8 | 0.62 | 6.0 | [32] |
| 2. | TiO2/CH3NH3PbI3/CuI3−x/CuI | Spray coating | 0.61 | 22.3 | 0.42 | 5.8 | [39] |
| 3. | TiO2/CH3NH3PbI3/CuI | Doctor blade | 0.78 | 16.7 | 0.57 | 7.5 | [43] |
| 4. | TiO2/CH3NH3PbI3/CuI | Gas–solid treatment | 0.73 | 32.7 | 0.31 | 7.4 | [51] |
| 5. | TiO2/CH3NH3PbI3/CuI/Cu | Thermal evaporation | 0.85 | 23.0 | 0.47 | 9.2 | [40] |
| 6. | TiO2/CH3NH3PbI3/CuI | Spin coating | 0.42 | 14.7 | 0.40 | 2.2 | [38] |
| 7. | TiO2/CH3NH3PbI3/CuI3−x/CuI | Powder pressing | 0.67 | 24.2 | 0.50 | 8.1 | [42] |
| 8. | TiO2/PVSK/CuI | Thermal evaporation | 0.83 | 15.6 | 0.62 | 8.1 | [41] |
| 9. | SnO2/PVSK/CuI | Transfer printing | 0.79 | 19.3 | 0.54 | 8.3 | Present |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, R.P.; Jung, H.-S.; Khang, D.-Y. Transfer-Printed Cuprous Iodide (CuI) Hole Transporting Layer for Low Temperature Processed Perovskite Solar Cells. Nanomaterials 2022, 12, 1467. https://doi.org/10.3390/nano12091467
Srivastava RP, Jung H-S, Khang D-Y. Transfer-Printed Cuprous Iodide (CuI) Hole Transporting Layer for Low Temperature Processed Perovskite Solar Cells. Nanomaterials. 2022; 12(9):1467. https://doi.org/10.3390/nano12091467
Chicago/Turabian StyleSrivastava, Ravi P., Hyun-Suh Jung, and Dahl-Young Khang. 2022. "Transfer-Printed Cuprous Iodide (CuI) Hole Transporting Layer for Low Temperature Processed Perovskite Solar Cells" Nanomaterials 12, no. 9: 1467. https://doi.org/10.3390/nano12091467
APA StyleSrivastava, R. P., Jung, H.-S., & Khang, D.-Y. (2022). Transfer-Printed Cuprous Iodide (CuI) Hole Transporting Layer for Low Temperature Processed Perovskite Solar Cells. Nanomaterials, 12(9), 1467. https://doi.org/10.3390/nano12091467







