Confining Fluorescent Probes in Nanochannels to Construct Reusable Nanosensors for Ion Current and Fluorescence Dual Gating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Preparation of the Nanotube-Shaped Alumina Nanochannels
2.4. Sequential Immobilization of APTES, FITC, and N2H4 on the Nanochannels
2.5. Ionic Current Measurements
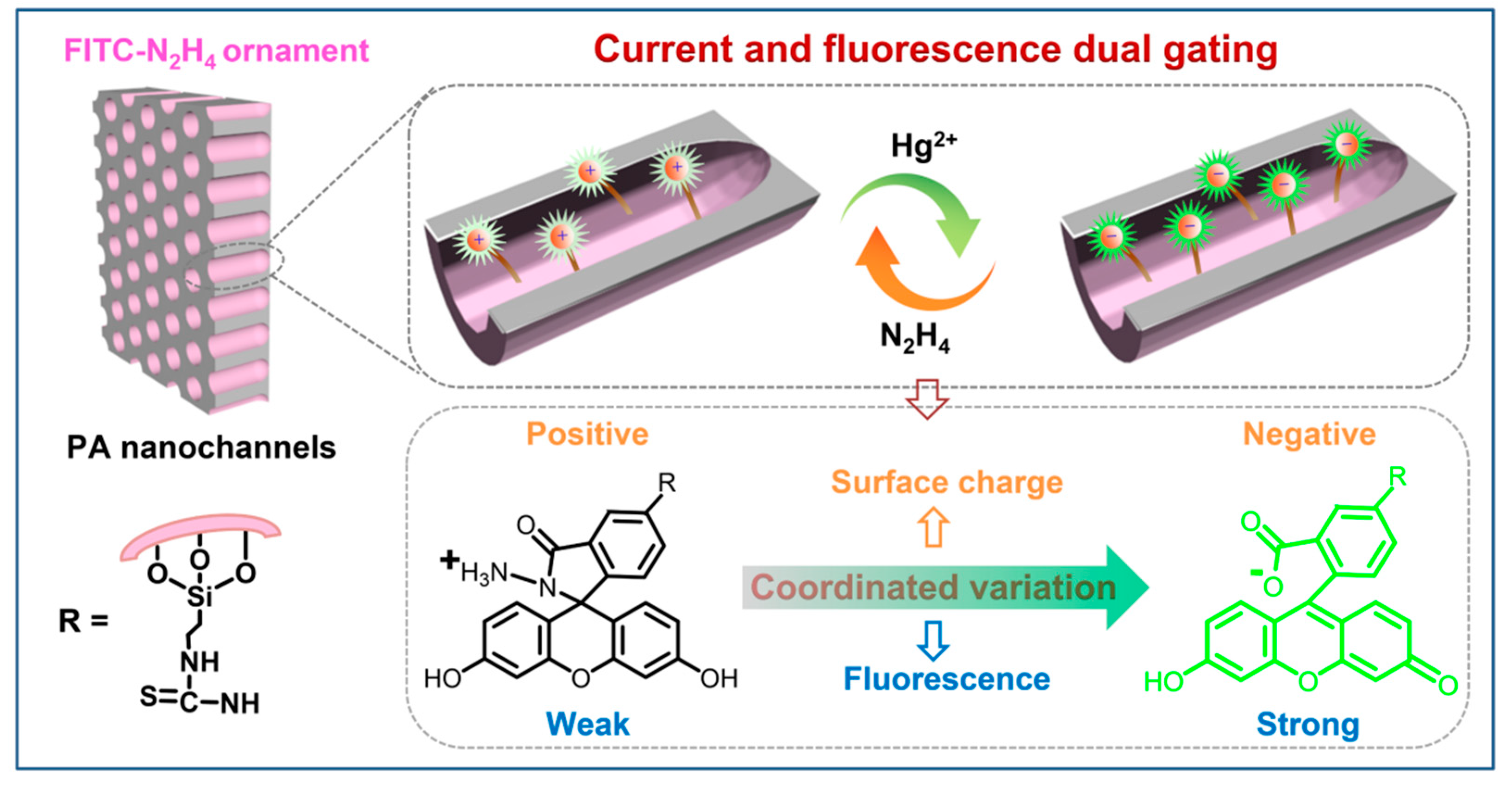
3. Results and Discussion
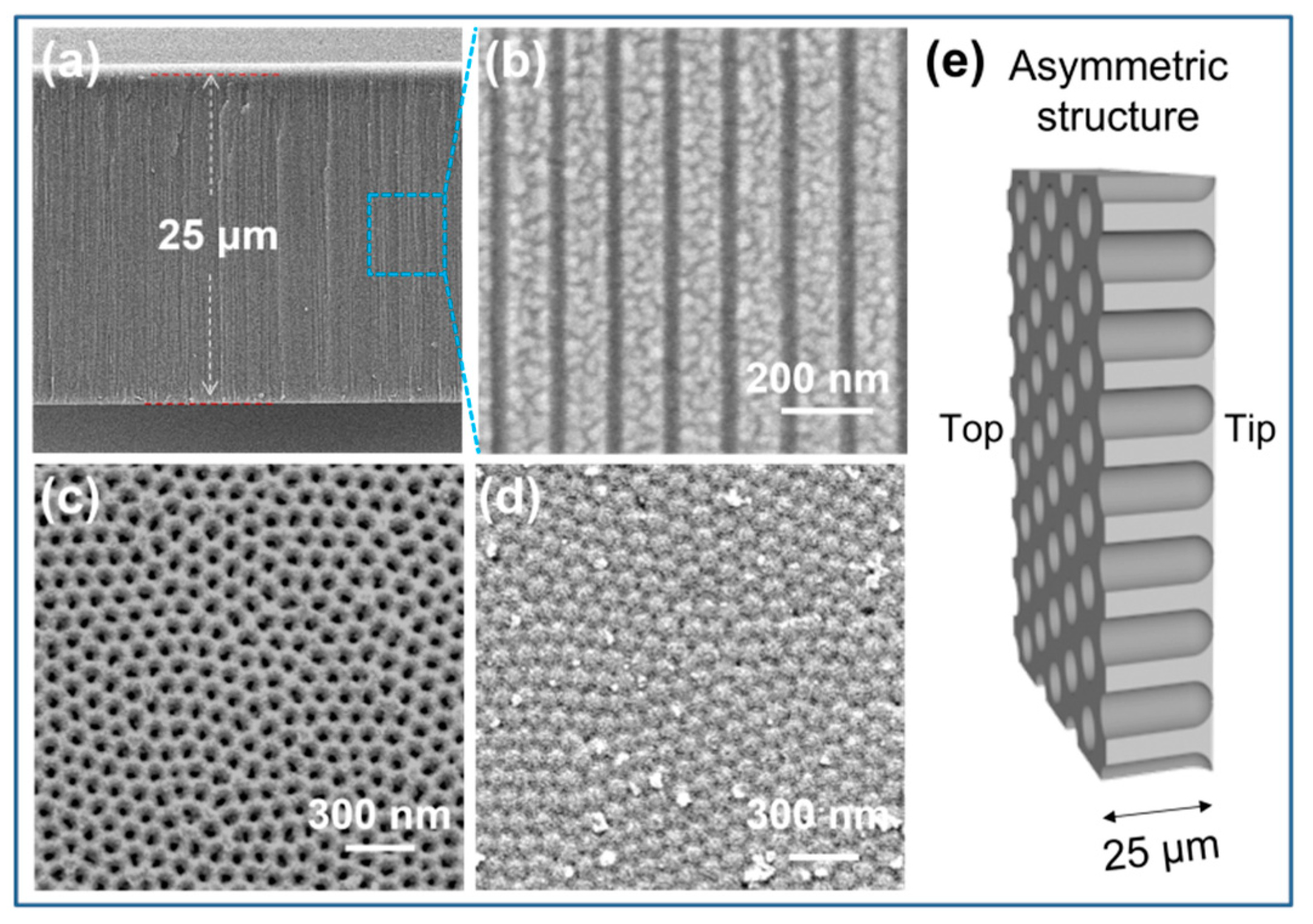
3.1. Characterization of the Nanochannels
3.2. Construction of the Functionalized Nanochannel Sensor
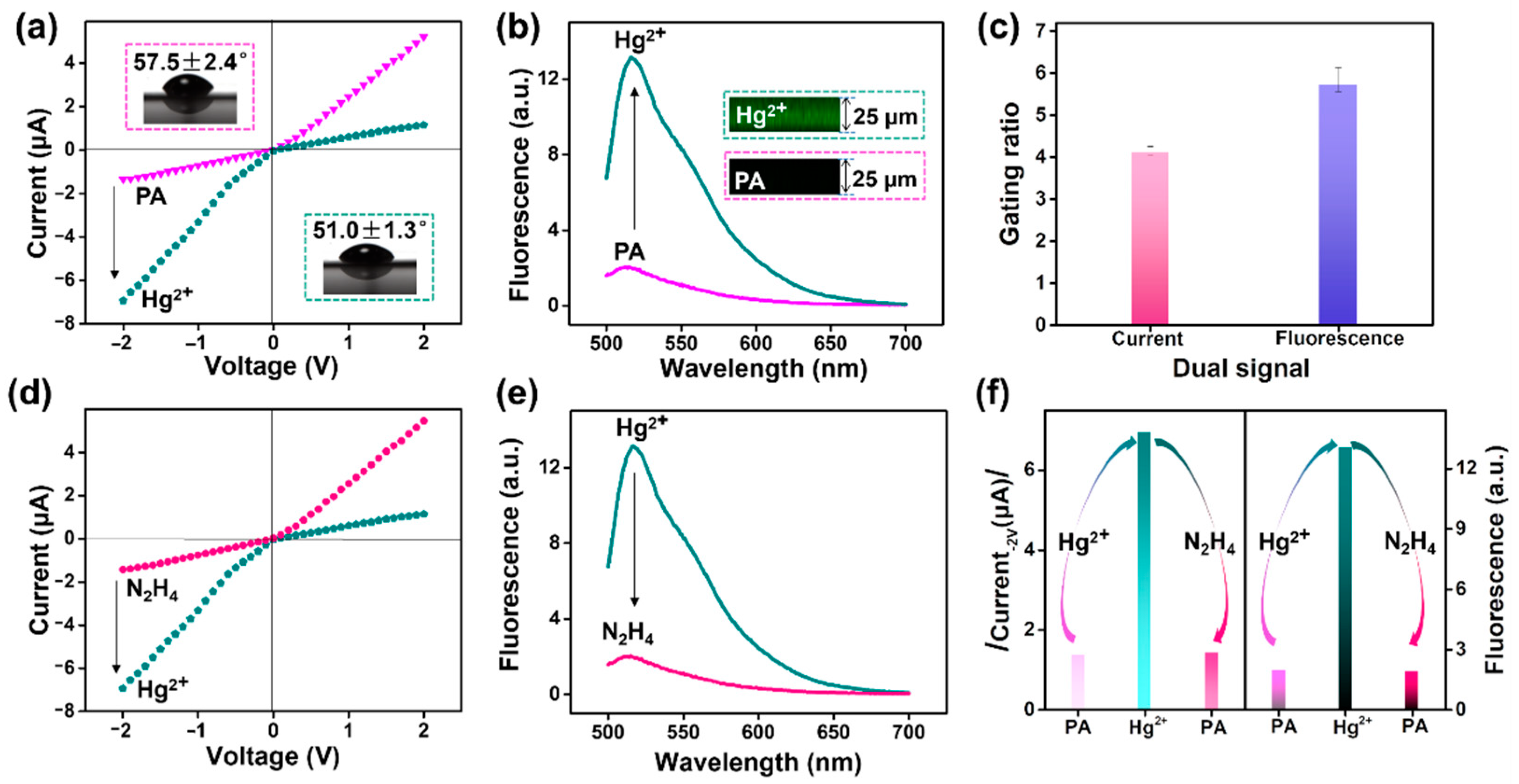
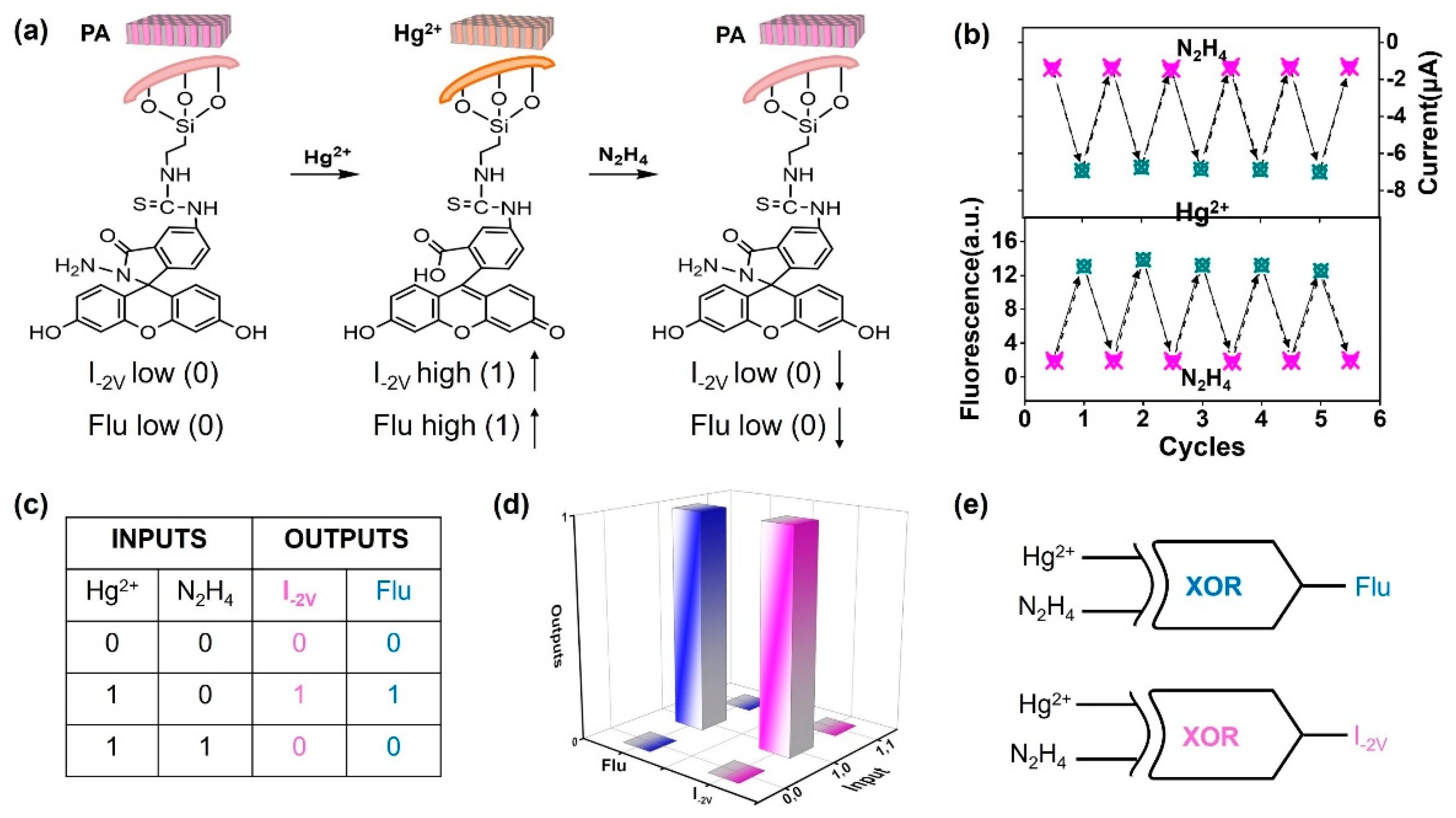
3.3. Current and Fluorescence Dual Gating and Renewability of the Nanosensor
3.4. Evaluation of Response Performance for Hg2+
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meng, Z.; Jiang, C.; Li, X.; Zhai, J. Calcein-modified multinanochannels on PET films for calcium-responsive nanogating. ACS Appl. Mater. Interfaces 2014, 6, 3794–3798. [Google Scholar] [CrossRef]
- Niu, B.; Xiao, K.; Huang, X.; Zhang, Z.; Kong, X.Y.; Wang, Z.; Wen, L.; Jiang, L. High-Sensitivity Detection of Iron(III) by Dopamine-Modified Funnel-Shaped Nanochannels. ACS Appl. Mater. Interfaces 2018, 10, 22632–22639. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, S.; Chen, X.; Xu, Y.; Zhang, S.; Ouyang, Q.; Yang, G.; Li, H. A highly selective and recyclable NO-responsive nanochannel based on a spiroring opening-closing reaction strategy. Nat. Commun. 2019, 10, 1323. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Sui, X.; Jiang, J.; Zhai, J.; Gao, L. Smooth Muscle Cell-Mimetic CO-Regulated Ion Nanochannels. Adv. Mater. 2016, 28, 10780–10785. [Google Scholar] [CrossRef]
- Zhao, X.P.; Zhou, Y.; Zhang, Q.W.; Yang, D.R.; Wang, C.; Xia, X.H. Nanochannel-Ion Channel Hybrid Device for Ultrasensitive Monitoring of Biomolecular Recognition Events. Anal. Chem. 2019, 91, 1185–1193. [Google Scholar] [CrossRef]
- Freedman, K.J.; Otto, L.M.; Ivanov, A.P.; Barik, A.; Oh, S.H.; Edel, J.B. Nanopore sensing at ultra-low concentrations using single-molecule dielectrophoretic trapping. Nat. Commun. 2016, 7, 10217. [Google Scholar] [CrossRef] [Green Version]
- Kong, Y.; Fan, X.; Zhang, M.; Hou, X.; Liu, Z.; Zhai, J.; Jiang, L. Nanofluidic diode based on branched alumina nanochannels with tunable ionic rectification. ACS Appl. Mater. Interfaces 2013, 5, 7931–7936. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, S.; Liu, Y.; Fan, X.; Zhang, M.; Zhai, J.; Jiang, L. Self-Assembled Porphyrin Nanofiber Membrane-Decorated Alumina Channels for Enhanced Photoelectric Response. ACS Nano 2018, 12, 11169–11177. [Google Scholar] [CrossRef]
- Xiao, K.; Chen, L.; Xie, G.; Li, P.; Kong, X.Y.; Wen, L.; Jiang, L. A bio-inspired dumbbell-shaped nanochannel with a controllable structure and ionic rectification. Nanoscale 2018, 10, 6850–6854. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, Y.; Hou, J.; Hou, X.; Hou, G.; Ou, R.; Wang, H.; Jiang, L. Bioinspired Smart Gate-Location-Controllable Single Nanochannels: Experiment and Theoretical Simulation. ACS Nano 2015, 9, 12264–12273. [Google Scholar] [CrossRef]
- Gao, P.; Ma, Q.; Ding, D.; Wang, D.; Lou, X.; Zhai, T.; Xia, F. Distinct functional elements for outer-surface anti-interference and inner-wall ion gating of nanochannels. Nat. Commun. 2018, 9, 4557. [Google Scholar] [CrossRef] [Green Version]
- Fang, R.; Zhang, H.; Yang, L.; Wang, H.; Tian, Y.; Zhang, X.; Jiang, L. Supramolecular Self-Assembly Induced Adjustable Multiple Gating States of Nanofluidic Diodes. J. Am. Chem. Soc. 2016, 138, 16372–16379. [Google Scholar] [CrossRef]
- Wu, K.; Kong, X.Y.; Xiao, K.; Wei, Y.; Zhu, C.; Zhou, R.; Si, M.; Wang, J.; Zhang, Y.; Wen, L. Engineered Smart Gating Nanochannels for High Performance in Formaldehyde Detection and Removal. Adv. Funct. Mater. 2019, 29, 1807953. [Google Scholar] [CrossRef]
- Guo, W.; Hong, F.; Liu, N.; Huang, J.; Wang, B.; Duan, R.; Lou, X.; Xia, F. Target-specific 3D DNA gatekeepers for biomimetic nanopores. Adv. Mater. 2015, 27, 2090–2095. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, X.P.; Liu, F.F.; Chen, Y.; Xia, X.H.; Li, J. Dendrimer-Au Nanoparticle Network Covered Alumina Membrane for Ion Rectification and Enhanced Bioanalysis. Nano Lett. 2020, 20, 1846–1854. [Google Scholar] [CrossRef]
- Xu, Y.; Sui, X.; Guan, S.; Zhai, J.; Gao, L. Olfactory sensory neuron-mimetic CO2 activated nanofluidic diode with fast response rate. Adv. Mater. 2015, 27, 1851–1855. [Google Scholar] [CrossRef]
- Xie, G.; Xiao, K.; Zhang, Z.; Kong, X.Y.; Liu, Q.; Li, P.; Wen, L.; Jiang, L. A Bioinspired Switchable and Tunable Carbonate-Activated Nanofluidic Diode Based on a Single Nanochannel. Angew. Chem. Int. Ed. Engl. 2015, 54, 13664–13668. [Google Scholar] [CrossRef]
- Perez-Mitta, G.; Peinetti, A.S.; Cortez, M.L.; Toimil-Molares, M.E.; Trautmann, C.; Azzaroni, O. Highly Sensitive Biosensing with Solid-State Nanopores Displaying Enzymatically Reconfigurable Rectification Properties. Nano Lett. 2018, 18, 3303–3310. [Google Scholar] [CrossRef]
- Lu, L.; Zhou, L.; Chen, J.; Yan, F.; Liu, J.; Dong, X.; Xi, F.; Chen, P. Nanochannel-Confined Graphene Quantum Dots for Ultrasensitive Electrochemical Analysis of Complex Samples. ACS Nano 2018, 12, 12673–12681. [Google Scholar] [CrossRef]
- Liu, J.; Fu, B.; Zhang, Z. Ionic Current Rectification Triggered Photoelectrochemical Chiral Sensing Platform for Recognition of Amino Acid Enantiomers on Self-Standing Nanochannel Arrays. Anal. Chem. 2020, 92, 8670–8674. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, S.; Du, C.; Fu, Y.; Xiao, K.; Zhang, X.; Chen, J. Highly Selective and Sensitive microRNA-210 Assay Based on Dual-Signaling Electrochemical and Photocurrent-Polarity-Switching Strategies. Anal. Chem. 2021, 93, 14272–14279. [Google Scholar] [CrossRef]
- Xu, X.; Hou, R.; Gao, P.; Miao, M.; Lou, X.; Liu, B.; Xia, F. Highly Robust Nanopore-Based Dual-Signal-Output Ion Detection System for Achieving Three Successive Calibration Curves. Anal. Chem. 2016, 88, 2386–2391. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, W.; Gao, P.; Li, H.; Feng, G.; Zhao, Z.; Lou, X. Coordination of the electrical and optical signals revealing nanochannels with an ‘onion-like’ gating mechanism and its sensing application. NPG Asia Mater. 2016, 8, e234. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.; Guo, W.; Xia, F.; Nie, F.Q.; Dong, H.; Tian, Y.; Wen, L.; Wang, L.; Cao, L.; Yang, Y.; et al. A Biomimetic Potassium Responsive Nanochannel: G-Quadruplex DNA Conformational Switching in a Synthetic Nanopore. J. Am. Chem. Soc. 2009, 131, 7800–7805. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Z.; Wen, L.; Ma, J.; Zhang, Y.; Liu, W.; Zhai, J.; Jiang, L. A biomimetic mercury(II)-gated single nanochannel. Chem. Commun. 2013, 49, 10679–10681. [Google Scholar] [CrossRef]
- Shi, L.; Jia, F.; Wang, L.; Jalalah, M.; Al-Assiri, M.S.; Gao, T.; Harraz, F.A.; Li, G. Fabrication of an artificial ionic gate inspired by mercury-resistant bacteria for simple and sensitive detection of mercury ion. Sens. Actuators B Chem. 2021, 326, 128976. [Google Scholar] [CrossRef]
- Nolan, E.M.; Lippard, S.J. Tools and Tactics for the Optical Detection of Mercuric Ion. Chem. Rev. 2008, 108, 3443–3480. [Google Scholar] [CrossRef]
- Liu, T.; Dong, J.X.; Liu, S.G.; Li, N.; Lin, S.M.; Fan, Y.Z.; Lei, J.L.; Luo, H.Q.; Li, N.B. Carbon quantum dots prepared with polyethyleneimine as both reducing agent and stabilizer for synthesis of Ag/CQDs composite for Hg(2+) ions detection. J. Hazard. Mater. 2017, 322, 430–436. [Google Scholar] [CrossRef]
- Monisha; Shrivas, K.; Kant, T.; Patel, S.; Devi, R.; Dahariya, N.S.; Pervez, S.; Deb, M.K.; Rai, M.K.; Rai, J. Inkjet-printed paper-based colorimetric sensor coupled with smartphone for determination of mercury (Hg(2+)). J. Hazard. Mater. 2021, 414, 125440. [Google Scholar] [CrossRef]
- Chaudhary, A.; Dwivedi, C.; Chawla, M.; Gupta, A.; Nandi, C.K. Lysine and dithiothreitol promoted ultrasensitive optical and colorimetric detection of mercury using anisotropic gold nanoparticles. J. Mater. Chem. C 2015, 3, 6962–6965. [Google Scholar] [CrossRef]
- Chen, G.H.; Chen, W.Y.; Yen, Y.C.; Wang, C.W.; Chang, H.T.; Chen, C.F. Detection of mercury(II) ions using colorimetric gold nanoparticles on paper-based analytical devices. Anal. Chem. 2014, 86, 6843–6849. [Google Scholar] [CrossRef]
- Kempahanumakkagari, S.; Malingappa, P.; Ambikapathi, G.; Kuramkote Shivanna, D. 2,7-Dichlorofluorescein Hydrazide as a New Fluorescent Probe for Mercury Quantification: Application to Industrial Effluents and Polluted Water Samples. J. Spectrosc. 2013, 2013, 276981. [Google Scholar] [CrossRef]
- Satsoura, D.; Leber, B.; Andrews, D.W.; Fradin, C. Circumvention of fluorophore photobleaching in fluorescence fluctuation experiments: A beam scanning approach. Chemphyschem 2007, 8, 834–848. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Gao, Y.; Qi, R.; Han, L. One-pot synthesis of a recyclable ratiometric fluorescent probe based on MOFs for turn-on sensing of Mg2+ ions and bioimaging in live cells. New J. Chem. 2019, 43, 18377–18383. [Google Scholar] [CrossRef]
- Wang, H.; Hou, S.; Wang, Q.; Wang, Z.; Fan, X.; Zhai, J. Dual-response for Hg(2+) and Ag(+) ions based on biomimetic funnel-shaped alumina nanochannels. J. Mater. Chem. B 2015, 3, 1699–1705. [Google Scholar] [CrossRef]
- Mo, R.; Yuan, Q.; Yan, X.; Su, T.; Feng, Y.; Lv, L.; Zhou, C.; Hong, P.; Sun, S.; Li, C. A Mercury Ion Electrochemical Sensor Based on Porous Anodized Alumina Membrane Nanochannels Modified with DNA. J. Electrochem. Soc. 2018, 165, H750–H755. [Google Scholar] [CrossRef]
- Xu, S.; Chen, L.; Li, J.; Guan, Y.; Lu, H. Novel Hg2+-imprinted polymers based on thymine-Hg2+-thymine interaction for highly selective preconcentration of Hg2+ in water samples. J. Hazard. Mater. 2012, 237–238, 347–354. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, Y.; Fan, X.; Zhai, J.; Jiang, L. N3/Al2O3 composite nanochannels: Photoelectric and photoelectric-and-pH cooperatively controlled ion gating. J. Mater. Chem. A 2017, 5, 19220–19226. [Google Scholar] [CrossRef]
- Zhang, D.; Ren, Y.; Fan, X.; Zhai, J.; Jiang, L. Photoassisted salt-concentration-biased electricity generation using cation-selective porphyrin-based nanochannels membrane. Nano Energy 2020, 76, 105086. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Q.; Fan, X.; Zhang, M.; Zhai, J.; Jiang, L. An Effective Dark-Vis-UV Ternary Biomimetic Switching Based on N3/Spiropyran-Modified Nanochannels. Adv. Mater. 2018, 30, e1804862. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, D.; Wu, G.; Yang, N.; Wu, A. Modifying Fe3O4 microspheres with rhodamine hydrazide for selective detection and removal of Hg2+ ion in water. J. Hazard. Mater. 2013, 244–245, 621–627. [Google Scholar] [CrossRef]
- Fechler, N.; Fellinger, T.-P.; Antonietti, M. One-pot synthesis of nitrogen–sulfur-co-doped carbons with tunable composition using a simple isothiocyanate ionic liquid. J. Mater. Chem. A 2013, 1, 14097–14102. [Google Scholar] [CrossRef] [Green Version]
- Sui, X.; Zhang, Z.; Zhang, Z.; Wang, Z.; Li, C.; Yuan, H.; Gao, L.; Wen, L.; Fan, X.; Yang, L.; et al. Biomimetic Nanofluidic Diode Composed of Dual Amphoteric Channels Maintains Rectification Direction over a Wide pH Range. Angew. Chem. Int. Ed. Engl. 2016, 55, 13056–13060. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tu, L.; Ma, X.; Chen, H.; Fan, Y.; Zhou, Q.; Sun, Y. Engineering a Smart Nanofluidic Sensor for High-Performance Peroxynitrite Sensing through a Spirocyclic Ring Open/Close Reaction Strategy. ACS Sens. 2021, 6, 808–814. [Google Scholar] [CrossRef]
- Pandurangappa, M.; Kumar, K.S. Micellar mediated trace level quantification through the ring opening process. Anal. Methods 2011, 3, 715–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Li, C.Y.; Li, Y.F.; Li, Z.; Xu, F. Rhodamine-based chemodosimeter for fluorescent determination of Hg(2+) in 100% aqueous solution and in living cells. Anal. Chim. Acta 2016, 934, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Credi, A.; Balzani, V.; Langford, S.J.; Stoddart, J.F. Logic Operations at the Molecular Level. An XOR Gate Based on a Molecular Machine. J. Am. Chem. Soc. 1997, 119, 2679–2681. [Google Scholar] [CrossRef]
- Privman, l.; Zhou, J.; Halámek, J.; Katz, E. Realization and Properties of Biochemical-Computing Biocatalytic XOR Gate Based on Signal Change. J. Phys. Chem. B 2010, 114, 13601–13608. [Google Scholar] [CrossRef] [Green Version]
- Glionna, C.; Kumar, V.; Le Saux, G.; Pramanik, B.; Wagner, N.; Cohen-Luria, R.; Ashkenasy, G.; Ashkenasy, N. Dynamic Surface Layer Coiled Coil Proteins Processing Analog-to-Digital Information. J. Am. Chem. Soc. 2021, 143, 17441–17451. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Wang, C.; Wu, C.; Zhang, X. Confining Fluorescent Probes in Nanochannels to Construct Reusable Nanosensors for Ion Current and Fluorescence Dual Gating. Nanomaterials 2022, 12, 1468. https://doi.org/10.3390/nano12091468
Zhang D, Wang C, Wu C, Zhang X. Confining Fluorescent Probes in Nanochannels to Construct Reusable Nanosensors for Ion Current and Fluorescence Dual Gating. Nanomaterials. 2022; 12(9):1468. https://doi.org/10.3390/nano12091468
Chicago/Turabian StyleZhang, Dan, Chunfei Wang, Changfeng Wu, and Xuanjun Zhang. 2022. "Confining Fluorescent Probes in Nanochannels to Construct Reusable Nanosensors for Ion Current and Fluorescence Dual Gating" Nanomaterials 12, no. 9: 1468. https://doi.org/10.3390/nano12091468
APA StyleZhang, D., Wang, C., Wu, C., & Zhang, X. (2022). Confining Fluorescent Probes in Nanochannels to Construct Reusable Nanosensors for Ion Current and Fluorescence Dual Gating. Nanomaterials, 12(9), 1468. https://doi.org/10.3390/nano12091468





