Enhanced Optical Response of Zinc-Doped Tin Disulfide Layered Crystals Grown with the Chemical Vapor Transport Method
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, H.; Cui, X. An optical spectroscopic study on two-dimensional group-VI transition metal dichalcogenides. Chem. Soc. Rev. 2015, 44, 2629–2642. [Google Scholar] [CrossRef] [PubMed]
- Voiry, D.; Mohite, A.; Chhowalla, M. Phase engineering of transition metal dichalcogenides. Chem. Soc. Rev. 2015, 44, 2702–2712. [Google Scholar] [CrossRef] [PubMed]
- Schwierz, F.; Pezoldt, J.; Granzner, R. Two-dimensional materials and their prospects in transistor electronics. Nanoscale 2015, 7, 8261–8283. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Z.; Wang, Q.; Wang, F.; Yin, L.; Xu, K.; Huang, Y.; He, J. Synthesis, properties and applications of 2D non-graphene materials. Nanotechnology 2015, 26, 292001. [Google Scholar] [CrossRef]
- Kolobov, A.V.; Tominaga, J. Two-Dimensional Transition-Metal Dichalcogenides; Springer: Cham, Switzerland, 2016; pp. 29–77. [Google Scholar]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent development of two-dimensional transition metal dichalcogenides and their applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033. [Google Scholar] [CrossRef]
- Li, H.; Jia, X.; Zhang, Q.; Wang, X. Metallic transition-metal dichalcogenide nanocatalysts for energy conversion. Chem 2018, 4, 1510–1537. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Wilson, J.A.; Yoffe, A.D. The transition metal dichalcogenides discussion and interpretation of the observed optical, electrical and structural properties. Adv. Phys. 1969, 18, 193–335. [Google Scholar] [CrossRef]
- Friend, R.H.; Yoffe, A.D. Electronic properties of intercalation complexes of the transition metal dichalcogenides. Adv. Phys. 1987, 36, 1–94. [Google Scholar] [CrossRef]
- Frindt, R.F.; Yoffe, A.D. Physical properties of layer structures: Optical properties and photoconductivity of thin crystals of molybdenum disulphide. Proc. R. Soc. Lond. A 1963, 273, 69–83. [Google Scholar]
- Frindt, R.F. Single crystals of MoS2 several molecular layers thick. J. Appl. Phys. 1966, 37, 1928–1929. [Google Scholar] [CrossRef]
- Joensen, P.; Frindt, R.F.; Morrison, S.R. Single-layer MoS2. Mat. Res. Bull. 1986, 21, 457–461. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; He, Q.; Khalil, A.; Liu, D.; Xiang, T.; Wu, X.; Song, L. Gram-scale aqueous synthesis of stable few-layered 1T-MoS2: Applications for visible-light-driven photocatalytic hydrogen evolution. Small 2015, 11, 5556–5564. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Gong, C.; Wangyang, P.; Chu, J.; Hu, K.; Li, C.; Wang, X.; Du, X.; Zhai, T.; Li, Y.; et al. 2D group IVB transition metal dichalcogenides. Adv. Funct. Mater. 2018, 28, 1803305. [Google Scholar] [CrossRef]
- Samadi, M.; Sarikhani, N.; Zirak, M.; Zhang, H.; Zhang, H.-L.; Moshfegh, A.Z. Group 6 transition metal dichalcogenide nanomaterials: Synthesis, applications and future perspectives. Nanoscale Horiz. 2018, 3, 90–204. [Google Scholar] [CrossRef]
- Mak, K.F.; Shan, J. Photonics and optoelectronics of 2D semiconductor transition metal dichalcogenides. Nat. Photonics 2016, 10, 216–2226. [Google Scholar] [CrossRef]
- Guenter, J.R.; Oswald, H.R. Neue polytype form von zinn(IV)-sulfid. Naturwissenschaften 1968, 55, 177. [Google Scholar] [CrossRef]
- Mitchell, R.S.; Fujiki, Y.; Ishizawa, Y. Structural polytypism of SnS2. Nature 1974, 247, 537–538. [Google Scholar] [CrossRef]
- Ramsdell, L.S. Studies on silicon carbide. Am. Mineral. 1947, 32, 64–82. [Google Scholar]
- Mitchell, R.S. Polytypism of cadmium iodide and its relationship to screw dislocations: I. Cadmium iodide polytypes. Z. Für Krist. 1956, 108, 296–315. [Google Scholar] [CrossRef]
- Shibata, T.; Muranushi, Y.; Miura, T.; Kishi, T. Electrical characterization of 2H-SnS2 single crystals synthesized by the low temperature chemical vapor transport method. J. Phys. Chem. Solids 1991, 52, 551–553. [Google Scholar] [CrossRef]
- Yuan, H.T.; Toh, M.; Morimoto, K.; Tan, W.; Wei, F.; Shimotani, H.; Kloc, C.; Iwasa, Y. Liquid-gated electric-double-layer transistor on layered metal dichalcogenide, SnS2. Appl. Phys. Lett. 2011, 98, 012102. [Google Scholar] [CrossRef]
- De, D.; Manongdo, J.; See, S.; Zhang, V.; Guloy, A.; Peng, H. High on/off ratio field effect transistors based on exfoliated crystalline SnS2 nano-membranes. Nanotechnology 2013, 24, 025202. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.-H.; Lee, M.-J.; Heo, H.; Sung, J.H.; Kim, K.; Hwang, H.; Jo, M.-H. Deterministic two-dimensional polymorphism growth of hexagonal n-type SnS2 and orthorhombic p-type SnS crystals. Nano Lett. 2015, 15, 3703–3708. [Google Scholar] [CrossRef]
- Camassel, J.; Schlüter, M.; Kohn, S.; Voitchovsky, J.P.; Shen, Y.R.; Cohen, M.L. Wavelength modulation spectra and electronic band structure of SnS2, and SnSe. Phys. Stat. Sol. 1976, 76, 303–314. [Google Scholar] [CrossRef]
- George, J.; Joseph, K.S. Absorption edge measurements in tin disulphide thin films. J. Phys. D Appl. Phys. 1982, 15, 1109–1116. [Google Scholar] [CrossRef]
- Shibata, T.; Kambe, N.; Muranushi, Y.; Miura, T.; Kishi, T. Optical characterisation of single crystal 2H-SnS2 synthesised by the chemical vapour transport method at low temperatures. J. Phys. D Appl. Phys. 1990, 23, 719–723. [Google Scholar] [CrossRef]
- Lokhande, C.D. A chemical method for tin disulfide thin film deposition. J. Phys. D Appl. Phys. 1990, 23, 1703–1705. [Google Scholar] [CrossRef]
- Huang, Y.; Sutter, E.; Sadowski, J.T.; Cotlet, M.; Monti, O.L.A.; Racke, D.A.; Neupane, M.R.; Wickramaratne, D.; Lake, R.K.; Parkinson, B.A.; et al. Tin disulfide–an emerging layered metal dichalcogenide semiconductor: Materials properties and device characteristics. ACS Nano 2014, 8, 10743–10755. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, Q.; Gan, L.; Li, H.; Zhai, T. Large-size growth of ultrathin SnS2 nanosheets and high performance for phototransistors. Adv. Funct. Mater. 2016, 26, 4405–4413. [Google Scholar] [CrossRef]
- Burton, L.A.; Whittles, T.J.; Hesp, D.; Linhart, W.M.; Skelton, J.M.; Hou, B.; Webster, R.F.; O’Dowd, G.; Reece, C.; Cherns, D.; et al. Electronic and optical properties of single crystal SnS2: An earth-abundant disulfide photocatalyst. J. Mater. Chem. A 2016, 4, 1312–1318. [Google Scholar] [CrossRef]
- Lin, D.-Y.; Hsu, H.-P.; Tsai, C.-F.; Wang, C.-W.; Shih, Y.-T. Temperature dependent excitonic transition energy and enhanced electron-phonon coupling in layered ternary SnS2−xSex Semiconductors with fully tunable stoichiometry. Molecules 2021, 26, 2184. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.R.; Liu, X.M. Nonlinear optical response and applications of tin disulfide in the near- and mid-infrared. Appl. Phys. Lett. 2017, 110, 171106. [Google Scholar] [CrossRef]
- Wang, H.; Yu, L.; Lee, Y.H.; Shi, Y.; Hsu, A.; Chin, M.L.; Li, L.J.; Dubey, M.; Kong, J.; Palacios, T. Integrated circuits based on bilayer MoS2 transistors. Nano Lett. 2012, 12, 4674–4680. [Google Scholar] [CrossRef]
- Song, H.S.; Li, S.L.; Gao, L.; Xu, Y.; Ueno, K.; Tang, J.; Cheng, Y.B.; Tsukagoshi, K. High-performance top-gated monolayer SnS2 field effect transistors and their integrated logic circuits. Nanoscale 2013, 5, 9666–9670. [Google Scholar] [CrossRef]
- Domingo, G.; Itoga, R.S.; Kannewurf, C.R. Fundamental optical absorption in SnS2 and SnSe2. Phys. Rev. 1966, 143, 536–542. [Google Scholar] [CrossRef]
- Fong, C.Y.; Cohen, M.L. Electronic energy-band structure of SnS2 and SnSe2. Phys. Rev. B 1972, 5, 3095–3101. [Google Scholar] [CrossRef]
- Williams, R.H.; Murray, R.B.; Govan, D.W.; Thomast, J.M.; Evans, E.L. Band structure and photoemission studies of SnS2 and SnSe2: I. Experimental. J. Phys. C Solid States Phys. 1973, 6, 3631–3642. [Google Scholar] [CrossRef]
- Fong, C.Y.; Cohen, M.L. Electronic charge densities for two layer semiconductors-SnS2 and SnSe2. J. Phys. C Solid State Phys. 1974, 7, 107–112. [Google Scholar] [CrossRef][Green Version]
- Smith, A.J.; Meek, P.E.; Liang, W.Y. Raman scattering studies of SnS2 and SnSe2. J. Phys. C Solid State Phys. 1977, 10, 1321–1333. [Google Scholar] [CrossRef]
- Parkinson, B.A. Dye sensitization of van der Waals surfaces of tin disulfide photoanodes. Langmuir 1988, 4, 967–976. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, H.; Gao, S.; Sun, Z.; Liu, Q.; Liu, Q.; Lei, F.; Yao, T.; He, J.; Wei, S.; et al. Freestanding tin disulfide single-layers realizing efficient visible-light water splitting. Angew. Chem. 2012, 51, 8727–8731. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.M.; Oleynik, I.I. Layer-dependent properties of SnS2 and SnSe2 novel two-dimensional materials. Phys. Rev. B 2016, 94, 125443. [Google Scholar] [CrossRef]
- Zhou, J.; Xue, K.; Liu, Y.; Liang, T.; Zhang, P.; Zhang, X.; Zhang, W.; Dai, Z. Highly sensitive NO2 response and abnormal P-N sensing transition with ultrathin Mo-doped SnS2 nanosheets. Chem. Eng. J. 2021, 420, 127572. [Google Scholar] [CrossRef]
- Bouzid, H.; Rodan, S.; Singh, K.; Jin, Y.; Jiang, J.; Yoon, D.; Song, H.Y.; Lee, Y.H. Enhanced magnetic moment with cobalt dopant in SnS2 semiconductor. APL Mater. 2021, 9, 051106. [Google Scholar] [CrossRef]
- Bouzid, H.; Sahoo, R.; Yun, S.J.; Singh, K.; Jin, Y.; Jiang, J.; Yoon, D.; Song, H.Y.; Kim, G.; Choi, W.; et al. Multiple magnetic phases in van der Waals Mn-doped SnS2 semiconductor. Adv. Funct. Mater. 2021, 31, 2102560. [Google Scholar] [CrossRef]
- Ali, A.; Zhang, J.-M.; Muhammad, I.; Shahid, I.; Ahmad, I.; Rehman, M.U.; Ahmad, I.; Kabir, F. First-principles investigation on electronic structure, magnetic states and optical properties of Mn-doped SnS2 monolayer via strain engineering. Physica E 2021, 134, 114842. [Google Scholar] [CrossRef]
- Fan, C.; Liu, Z.; Yuan, S.; Meng, X.; An, X.; Jing, Y.; Sun, C.; Zhang, Y. Enhanced photodetection performance of photodetectors based on indium-doped tin disulfide few layers. ACS Appl. Mater. Interfaces 2021, 13, 35889–35896. [Google Scholar] [CrossRef]
- Meng, X.; Fan, C.; An, X.; Yuan, S.; Jing, Y.; Liu, Z.; Sun, C.; Zhang, Y.; Zhang, Z.; Wang, M.; et al. Aluminum doping effects on photoresponse characteristics of hydrothermal tin disulfide nanosheets. Cryst. Eng. Comm. 2021, 23, 4694–4699. [Google Scholar] [CrossRef]
- Lin, L.; Chen, Y.; Tao, H.; Yao, L.; Huang, J.; Zhu, L.; Lou, M.; Chen, R.; Yan, L.; Zhang, Z. Ferromagnetism and optical properties of SnS2 doped with two impurities: First-principles calculations. Phys. Chem. Chem. Phys. 2021, 23, 6574–6582. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Y.; Zhou, X.; Jin, X.; Li, B.; Liu, J.; Chen, G. Cu doped SnS2 nanostructure induced sulfur vacancy towards boosted photocatalytic hydrogen evolution. Chem. Eng. J. 2021, 407, 127180. [Google Scholar] [CrossRef]
- Setayeshmehr, M.; Haghighi, M.; Mirabbaszadeh, K. Binder-free 3D flower-like alkali doped- SnS2 electrodes for high-performance supercapacitors. Electrochim. Acta 2021, 376, 137987. [Google Scholar] [CrossRef]
- Kumar, G.M.; Ilanchezhiyan, P.; Cho, H.D.; Yuldashev, S.; Jeon, H.C.; Kim, D.Y.; Kang, T.W. Effective modulation of optical and photoelectrical properties of SnS2 hexagonal nanoflakes via Zn incorporation. Nanomaterials 2019, 9, 924. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction; Addison-Wesley: Reading, MA, USA, 1956; p. 459. [Google Scholar]
- Palosz, B.; Salje, E. Lattice parameters and spontaneous strain in AX2 polytypes: Cdl2, Pbl2, SnS2 and SnSe2. J. Appl. Cryst. 1989, 22, 622–623. [Google Scholar] [CrossRef]
- Palosz, B.; Steurer, W.; Schulz, H. Refinement of SnS2 polytypes 2H, 4H and 18R. Acta Cryst. 1990, B46, 449–455. [Google Scholar] [CrossRef]
- Bhattacharya, P. Semiconductor Optoelectronic Devices, 2nd ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 1997; p. 127. [Google Scholar]
- Peyghambarian, N.; Koch, S.W.; Mysyrowicz, A. Introduction to Semiconductor Optics; Prentice-Hall: Englewood Cliffs, NJ, USA, 1993; p. 132. [Google Scholar]
- Fox, M. Optical Properties of Solids; Oxford University Press: New York, NY, USA, 2001; p. 64. [Google Scholar]
- Tauc, J.; Grigorvici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Ryvkin, S.M. Photoelectric Effects in Semiconductors; Consultants Bureau: New York, NY, USA, 1964; p. 38. [Google Scholar]
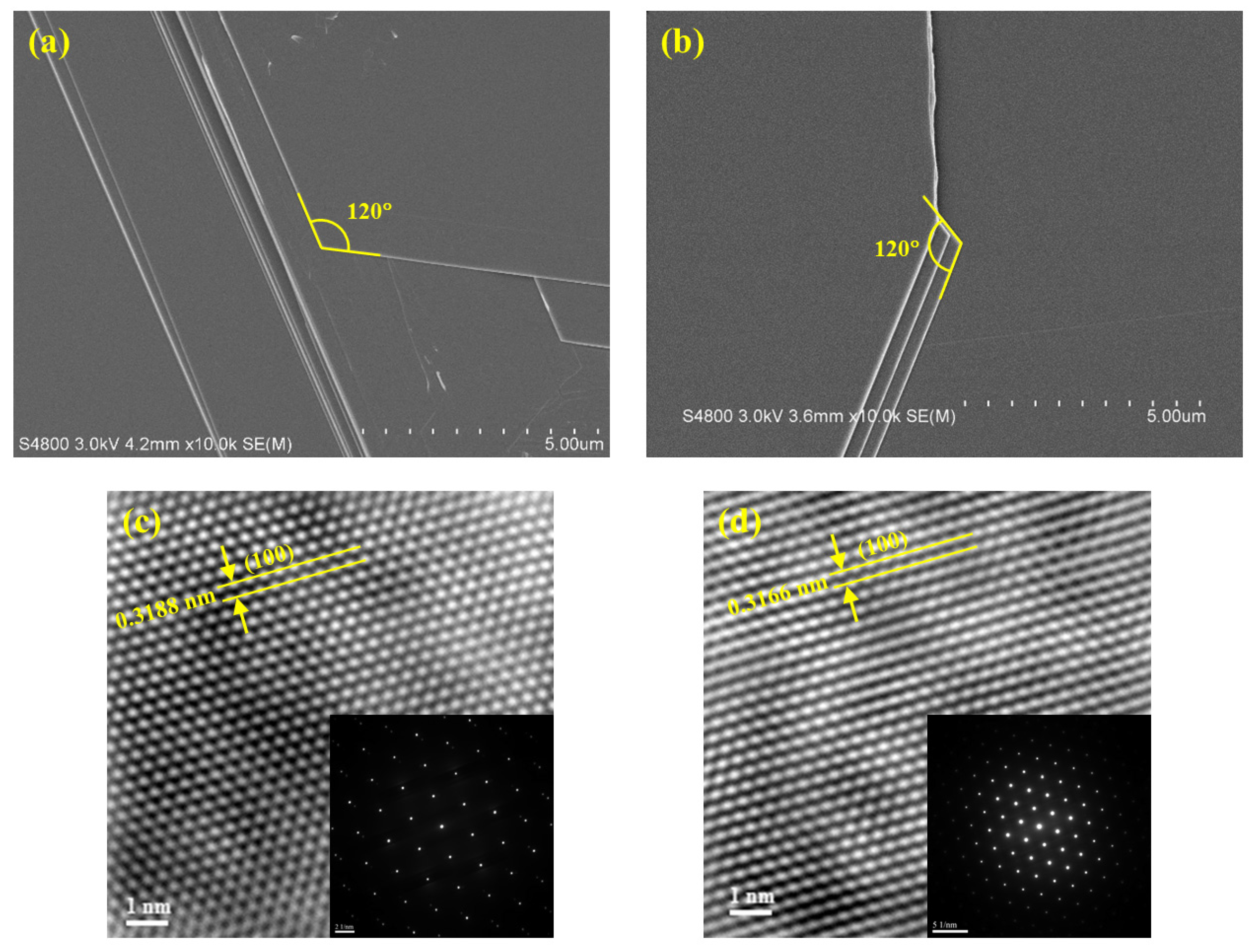
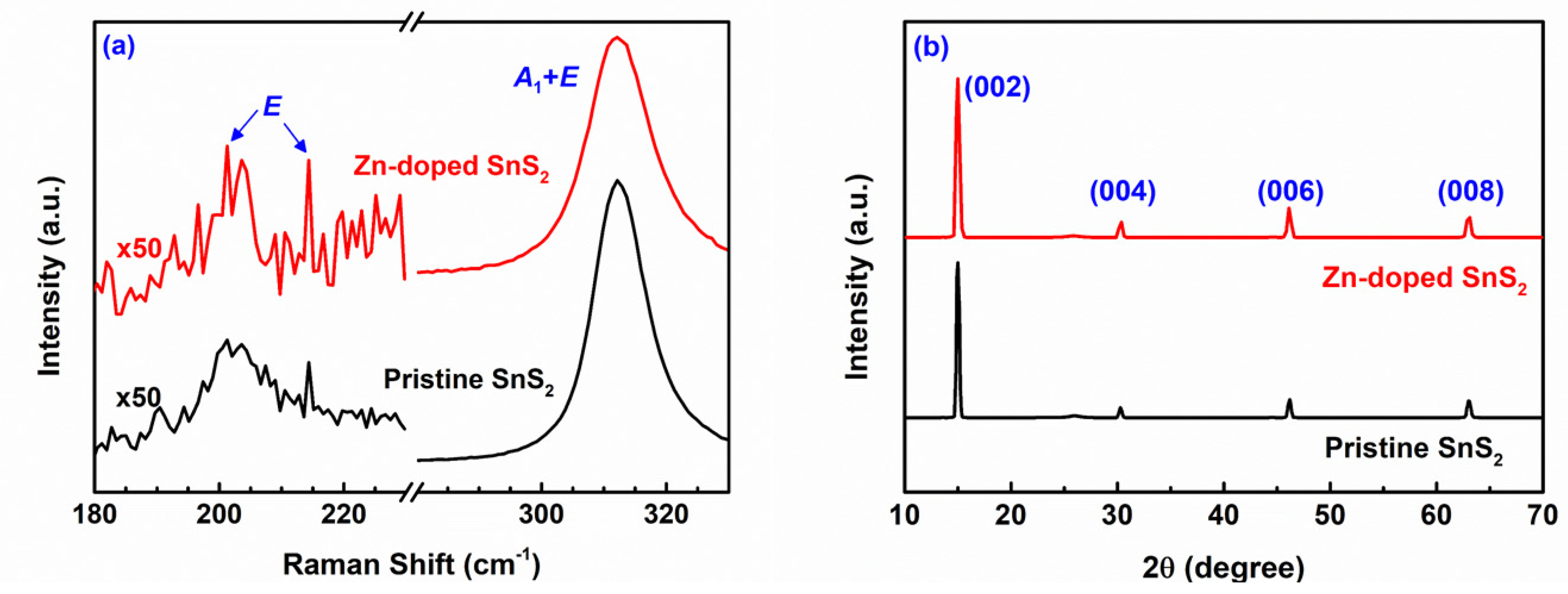
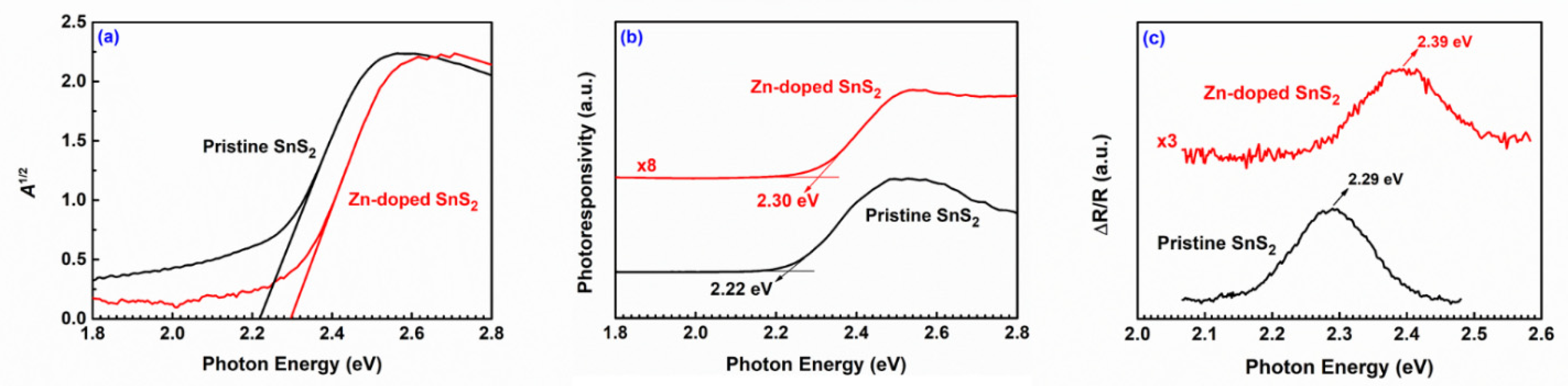
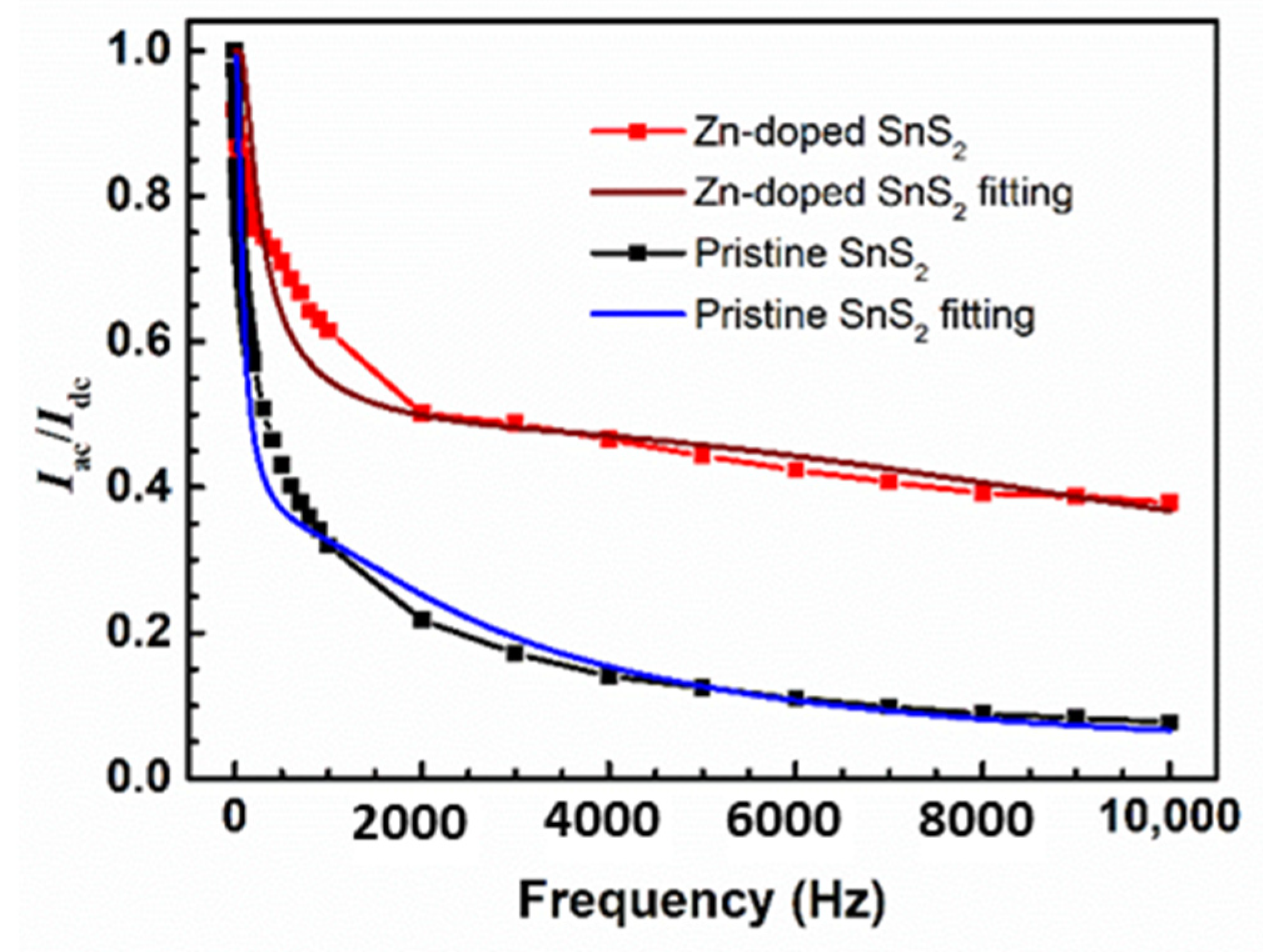
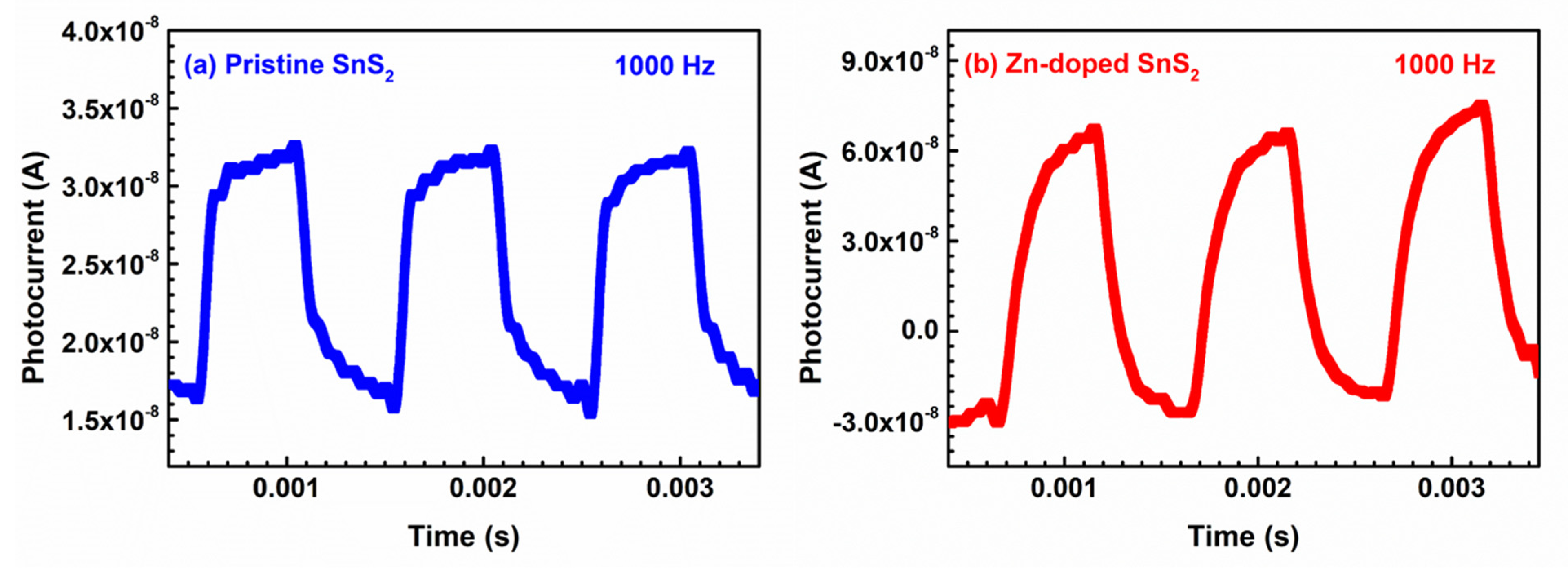
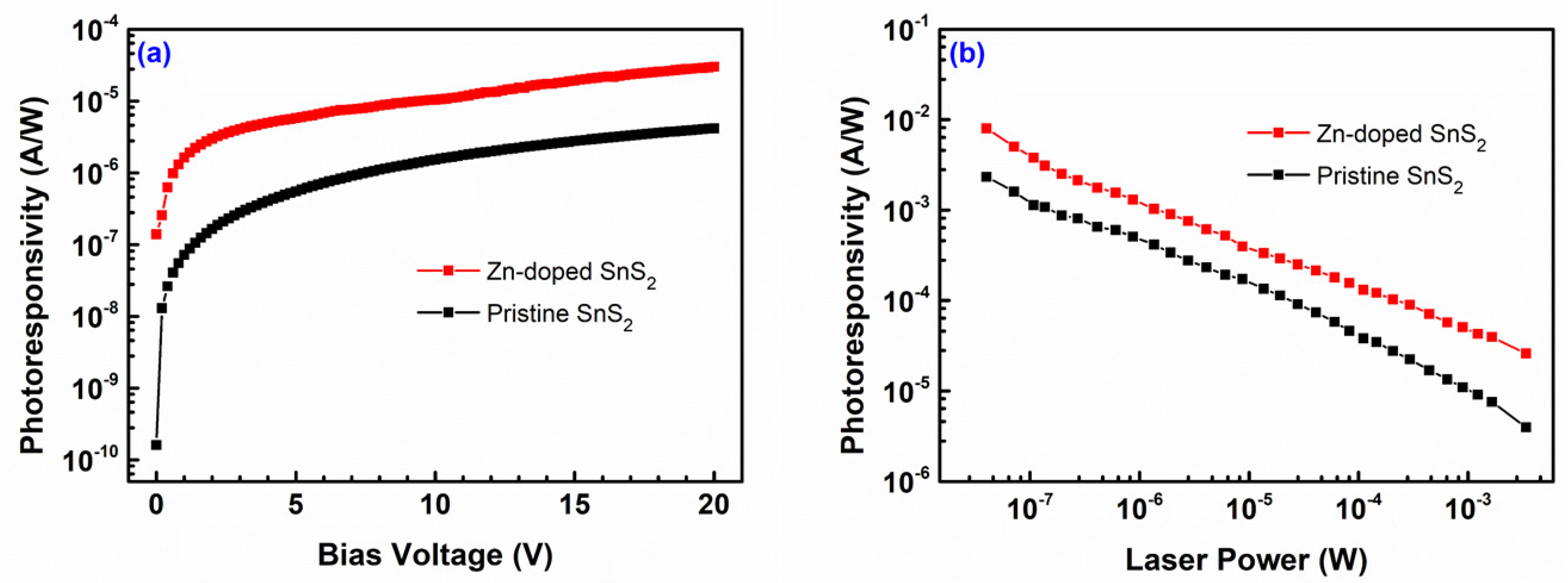
| Specimen | d100 (Å) | a (Å) | c (Å) | References |
|---|---|---|---|---|
| 2H SnS2 | 3.6470 | 5.8990 | [58,59] | |
| 4H SnS2 | 3.6470 | 11.811 | [58,59] | |
| Pristine SnS2 | 3.1880 | 3.6812 | 11.812 | This work |
| Zn-doped SnS2 | 3.1658 | 3.6556 | 11.812 | This work |
| Specimen | c1 | τ1 (ms) | c2 | τ2 (ms) |
|---|---|---|---|---|
| Pristine SnS2 | 0.70 | 4.96 | 0.30 | 0.119 |
| Zn-doped SnS2 | 0.55 | 1.40 | 0.45 | 0.023 |
| Frequency (Hz) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 100 | 500 | 1000 | |||||
| Specimen | trise (ms) | tfall (ms) | trise (ms) | tfall (ms) | trise (ms) | tfall (ms) | trise (ms) | tfall (ms) |
| Pristine SnS2 | 0.96 | 2.03 | 0.88 | 1.12 | 0.83 | 0.98 | 0.81 | 0.94 |
| Zn-doped SnS2 | 0.31 | 0.25 | 0.45 | 0.23 | 0.22 | 0.21 | 0.21 | 0.19 |
| Frequency (Hz) | ||||
|---|---|---|---|---|
| 1 | 100 | 500 | 1000 | |
| Specimen | Current Amplitude (μA) | |||
| Pristine SnS2 | 0.030 | 0.028 | 0.025 | 0.023 |
| Zn-doped SnS2 | 0.110 | 0.100 | 0.090 | 0.080 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, Y.-T.; Lin, D.-Y.; Li, Y.-C.; Tseng, B.-C.; Hwang, S.-B. Enhanced Optical Response of Zinc-Doped Tin Disulfide Layered Crystals Grown with the Chemical Vapor Transport Method. Nanomaterials 2022, 12, 1442. https://doi.org/10.3390/nano12091442
Shih Y-T, Lin D-Y, Li Y-C, Tseng B-C, Hwang S-B. Enhanced Optical Response of Zinc-Doped Tin Disulfide Layered Crystals Grown with the Chemical Vapor Transport Method. Nanomaterials. 2022; 12(9):1442. https://doi.org/10.3390/nano12091442
Chicago/Turabian StyleShih, Yu-Tai, Der-Yuh Lin, Yu-Cheng Li, Bo-Chang Tseng, and Sheng-Beng Hwang. 2022. "Enhanced Optical Response of Zinc-Doped Tin Disulfide Layered Crystals Grown with the Chemical Vapor Transport Method" Nanomaterials 12, no. 9: 1442. https://doi.org/10.3390/nano12091442
APA StyleShih, Y.-T., Lin, D.-Y., Li, Y.-C., Tseng, B.-C., & Hwang, S.-B. (2022). Enhanced Optical Response of Zinc-Doped Tin Disulfide Layered Crystals Grown with the Chemical Vapor Transport Method. Nanomaterials, 12(9), 1442. https://doi.org/10.3390/nano12091442





