Insight into the Growth Mechanism of Mixed Phase CZTS and the Photocatalytic Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemical and Reagents
2.2. Synthesis of CZTS
2.3. Characterizations
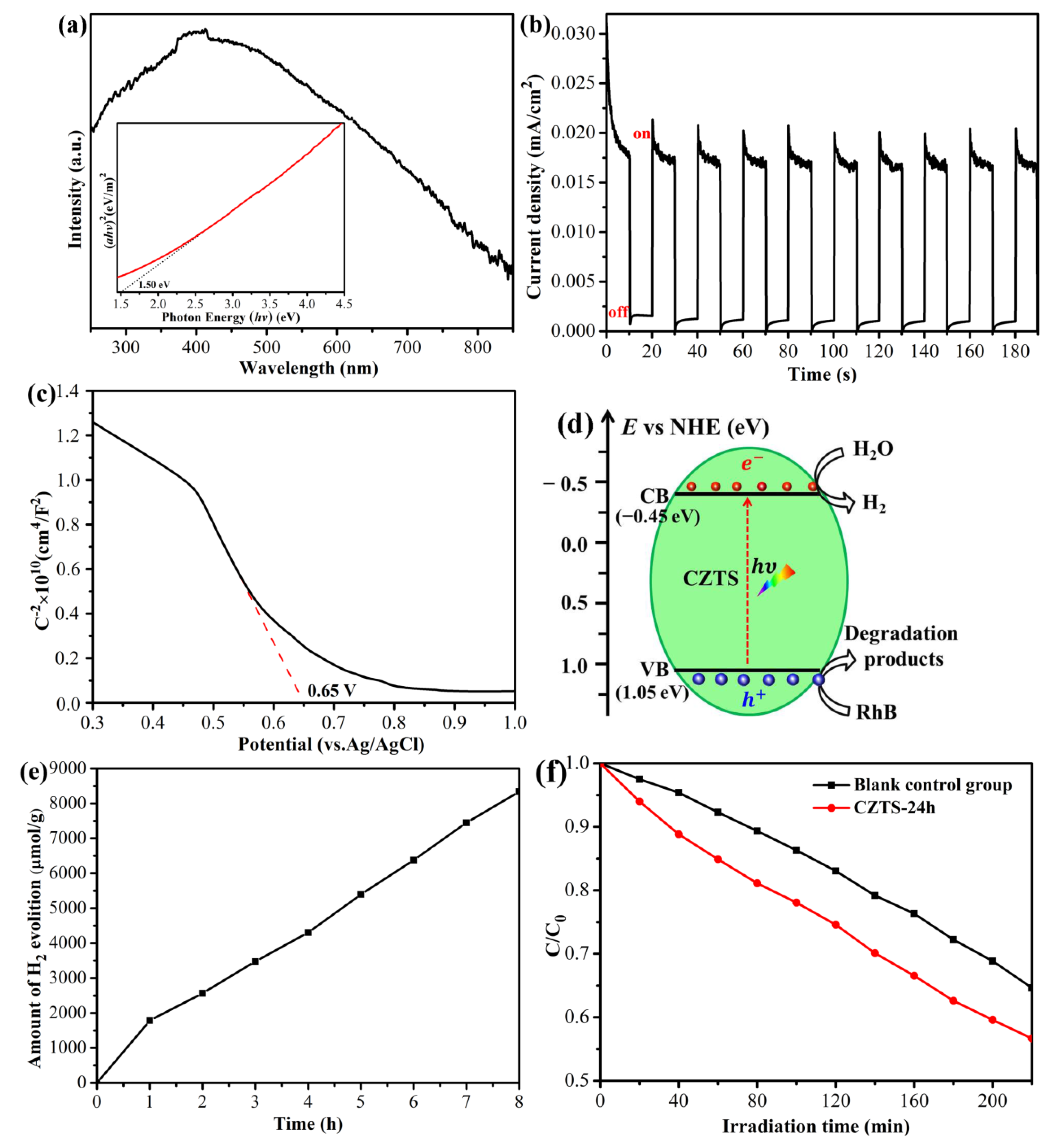
2.4. Photocatalytic Performance
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Zhou, W.H.; Guo, J.; Zhou, Y.L.; Hou, Z.L.; Jiao, J.; Zhou, Z.J.; Du, Z.L.; Wu, S.X. Synthesis of pure metastable wurtzite CZTS nanocrystals by facile one-pot method. J. Phys. Chem. C. 2012, 116, 26507–26516. [Google Scholar] [CrossRef]
- Mkawi, E.M.; Ibrahim, K.; Ali, M.K.M.; Farrukh, M.A.; Allam, N.K. Solvent solution-dependent properties of nonstoichiometric cubic Cu2ZnSnS4 nanoparticles. Chem. Phys. Lett. 2014, 608, 393–397. [Google Scholar] [CrossRef]
- Tian, Q.W.; Xu, X.F.; Han, L.B.; Tang, M.H.; Zou, R.J.; Chen, Z.G.; Yu, M.H.; Yang, J.M.; Hu, J.Q. Hydrophilic Cu2ZnSnS4 nanocrystals for printing flexible, low-cost and environmentally friendly solar cells. Crystengcomm 2012, 14, 3847–3850. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Zhou, W.H.; Li, M.; Du, Y.F.; Wu, S.X. Hierarchical Cu2ZnSnS4 particles for a low-cost solar cell: Morphology control and growth mechanism. J. Phys. Chem. C. 2011, 115, 19632–19639. [Google Scholar] [CrossRef]
- Ma, G.J.; Minegishi, T.; Yokoyama, D.; Kubota, J.; Domen, K. Photoelectrochemical hydrogen production on Cu2ZnSnS4/Mo-mesh thin-film electrodes prepared by electroplating. Chem. Phys. Lett. 2011, 501, 619–622. [Google Scholar] [CrossRef]
- Zhu, L.M.; Tao, J.; Tao, H.J.; Chen, S.L.; Shen, Y.Z.; Xu, A.C.; Jiang, J.J.; Pan, L. In-situ growth of Cu2ZnSnS4 nanosheets on TiO2 nanowires for enhanced photoelectrochemical performance. J. Alloy. Compd. 2015, 649, 704–711. [Google Scholar] [CrossRef]
- Li, B.J.; Yin, P.F.; Zhou, Y.Z.; Gao, Z.M.; Ling, T.; Du, X.W. Single crystalline Cu2ZnSnS4 nanosheet arrays for efficient photochemical hydrogen generation. Rsc. Adv. 2015, 5, 2543–2549. [Google Scholar] [CrossRef]
- Mahalakshmi, V.; Venugopal, D.; Ramachandran, K.; Ramesh, R. Synthesis of 2D-CZTS nanoplate as photocathode material for efficient PEC water splitting. J. Mater. Sci. Mater. Electron. 2021, 33, 8493–8503. [Google Scholar] [CrossRef]
- Patro, B.; Vijaylakshmi, S.; Reddy, R.K.; Sharma, P. Rapid microwave-assisted solvothermal synthesis of Cu2ZnSnS4 (CZTS) nanocrystals for low-cost thin film photovoltaic: Investigation of synthesis parameters and morphology control. J. Mater. Sci. Mater. Electron. 2018, 29, 3370–3380. [Google Scholar] [CrossRef]
- Chen, Y.B.; Xia, H.Y.; Zhang, W.S.; Zheng, W.Y.; Feng, X.Y.; Jiang, J.G. Template synthesis of porous hierarchical Cu2ZnSnS4 nanostructures for photoelectrochemical water splitting. Int. J. Hydrog. Energy 2021, 46, 2862–2870. [Google Scholar] [CrossRef]
- Sheebha, I.; Venugopal, V.; James, J.; Maheskumar, V.; Sakunthala, A.; Vidhya, B. Comparative studies on hierarchical flower like Cu2XSnS4[X = Zn, Ni, Mn & Co] quaternary semiconductor for electrocatalytic and photocatalytic applications. Int. J. Hydrog. Energy 2020, 45, 8139–8150. [Google Scholar]
- Chen, S.; Walsh, A.; Luo, Y.; Yang, J.-H.; Gong, X.G.; Wei, S.-H. Wurtzite-derived polytypes of kesterite and stannite quaternary chalcogenide semiconductors. Phys. Rev. B. 2010, 82, 195203. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Zhuang, Z.; Peng, Q.; Li, Y. Wurtzite Cu2ZnSnS4 nanocrystals: A novel quaternary semiconductor. Chem. Commun. 2011, 47, 3141–3143. [Google Scholar] [CrossRef] [PubMed]
- Wadhene, R.; Lamouchi, A.; Ben Assaker, I.; Ben Naceur, J.; Martínez-Huerta, M.V.; Chtourou, R. Electrodeposition of Cu2ZnSnS4 thin films onto TiO2 nanorods for photocatalytic application: Effect of deposition time. Inorg. Chem. Commun. 2020, 122, 108298. [Google Scholar] [CrossRef]
- Su, Z.H.; Sun, K.W.; Han, Z.L.; Cui, H.T.; Liu, F.Y.; Lai, Y.Q.; Li, J.; Hao, X.J.; Liu, Y.X.; Green, M.A. Fabrication of Cu2ZnSnS4 solar cells with 5.1% efficiency via thermal decomposition and reaction using a non-toxic sol-gel route. J. Mater. Chem. A. 2014, 2, 500–509. [Google Scholar] [CrossRef]
- Jayasree, Y.; Kumar, Y.B.K.; Babu, G.S.; Bhaskar, P.U. Growth of Cu2ZnSnS4 thin films by hybrid chemical approach. Phys. B-Condens. Matter. 2021, 618, 413199. [Google Scholar] [CrossRef]
- Wang, X.Z.; Wang, Z.; Jiang, X.S.; Zhang, M.; Wang, Y.F.; Lv, J.G.; He, G.; Sun, Z.Q. In-situ deposition and growth of Cu2ZnSnS4 nanocrystals on TiO2 nanorod arrays for enhanced photoelectrochemical performance. J. Electrochem. Soc. 2017, 164, H863–H871. [Google Scholar] [CrossRef]
- Gao, Y.; Long, F.; Wang, J.L.; Zhang, J.; Mo, S.Y.; Zou, Z.G. Understanding the growth mechanism of wurtzite Cu2ZnSnS4 nanocrystals and the photodegradation properties. Mater. Design 2017, 123, 24–31. [Google Scholar] [CrossRef]
- Pal, M.; Mathews, N.R.; Paraguay-Delgado, F.; Mathew, X. Phase controlled solvothermal synthesis of Cu2ZnSnS4, Cu2ZnSn(S,Se)4 and Cu2ZnSnSe4 nanocrystals: The effect of se and s sources on phase purity. Mater. Chem. Phys. 2015, 166, 201–206. [Google Scholar] [CrossRef]
- Mali, S.S.; Kim, H.; Shim, C.S.; Patil, P.S.; Hong, C.K. Polyvinylpyrrolidone (PVP) assisted single-step synthesis of kesterite Cu2ZnSnS4 nanoparticles by solvothermal process. Phys. Status Solidi-Rapid Res. Lett. 2013, 7, 1050–1054. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Zhou, W.H.; Du, Y.F.; Li, M.; Wu, S.X. Sphere-like kesterite Cu2ZnSnS4 nanoparticles synthesized by a facile solvothermal method. Mater. Lett. 2011, 65, 1535–1537. [Google Scholar] [CrossRef]
- Luo, Q.; Zeng, Y.Q.; Chen, L.W.; Ma, C.Q. Controllable synthesis of wurtzite cu2znsns4 nanocrystals by hot-injection approach and growth mechanism studies. Chem. Asian J. 2014, 9, 2309–2316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cao, M.; Gao, W.S.; Yang, J.; Shen, J.S.; Huang, J.; Sun, Y.; Wang, L.J.; Shen, Y. Phase-selective and photoactivity investigation of solvothermal synthesized Cu2ZnSnS4 nanoparticles. Mater. Des. 2016, 91, 37–45. [Google Scholar] [CrossRef]
- Li, Z.; Lui, A.L.K.; Lam, K.H.; Xi, L.; Lam, Y.M. Phase-selective synthesis of Cu2ZnSnS4 nanocrystals using different sulfur precursors. Inorg. Chem. 2014, 53, 10874–10880. [Google Scholar] [CrossRef]
- Tan, J.M.R.; Lee, Y.H.; Pedireddy, S.; Baikie, T.; Ling, X.Y.; Wong, L.H. Understanding the synthetic pathway of a single-phase quarternary semiconductor using surface-enhanced raman scattering: A case of wurtzite Cu2ZnSnS4 nanoparticles. J. Am. Chem. Soc. 2014, 136, 6684–6692. [Google Scholar] [CrossRef]
- Regulacio, M.D.; Ye, C.; Lim, S.H.; Bosman, M.; Ye, E.Y.; Chen, S.Y.; Xu, Q.H.; Han, M.Y. Colloidal nanocrystals of wurtzite-type Cu2ZnSnS4: Facile noninjection synthesis and formation mechanism. Chem. Eur. J. 2012, 18, 3127–3131. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Lu, G.X.; Wu, Y.Q.; Dong, J.L.; Wang, C.W. TiO2 protection layer and well-matched interfaces enhance the stability of Cu2ZnSnS4/CdS/TiO2 for visible light driven water splitting. Catal. Sci. Technol. 2021, 11, 5505–5517. [Google Scholar] [CrossRef]
- Ahmad, R.; Saddiqi, N.U.; Wu, M.J.; Prato, M.; Spiecker, E.; Peukert, W.; Distaso, M. Phase evolution of Cu2ZnSnS4 (CZTS) nanoparticles from in situ formed binary sulphides under solvothermal conditions. Crystengcomm 2021, 23, 7944–7954. [Google Scholar] [CrossRef]
- Ahmad, R.; Saddiqi, N.U.; Wu, M.J.; Prato, M.; Spiecker, E.; Peukert, W.; Distaso, M. Effect of the counteranion on the formation pathway of Cu2ZnSnS4 (CZTS) nanoparticles under solvothermal conditions. Inorg. Chem. 2020, 59, 1973–1984. [Google Scholar] [CrossRef]
- Long, F.; Chi, S.S.; He, J.Y.; Wang, J.L.; Wu, X.L.; Mo, S.Y.; Zou, Z.G. Synthesis of hexagonal wurtzite Cu2ZnSnS4 prisms by an ultrasound-assisted microwave solvothermal method. J. Solid State Chem. 2015, 229, 228–234. [Google Scholar] [CrossRef]
- Raza, A.; Shen, H.; Haidry, A.A.; Shahzad, M.K.; Liu, R.; Cui, S. In-situ synthesis of mesoporous TiO2-Cu2ZnSnS4 heterostructured nanocomposite for enhanced photocatalytic degradation. Appl. Surf. Sci. 2020, 505, 144540. [Google Scholar] [CrossRef]
- Vargas-Rueda, J.A.; Alonso, A.R.; Melendez-Zamudio, M.; Melendez-Lira, M. Chemical stability diagrams as a powerful tool to the synthesis of Cu2SnS3 thin films by chemical bath deposition. Mater. Chem. Phys. 2021, 265, 124478. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, J.; Yang, Y.; Long, L.; Yang, L.; Yan, L.; Kong, W.; Liu, F.; Lv, F.; Liu, J. Fully-depleted dual P–N heterojunction with type-II band alignment and matched build-in electric field for high-efficient photocatalytic hydrogen production. Int. J. Hydrog. Energy 2021, 46, 36069–36079. [Google Scholar] [CrossRef]
- Wang, J.J.; Lin, S.; Tian, N.; Ma, T.Y.; Zhang, Y.H.; Huang, H.W. Nanostructured metal sulfides: Classification, modification strategy, and solar-driven CO2 reduction application. Adv. Funct. Mater. 2021, 31, 1–39. [Google Scholar]

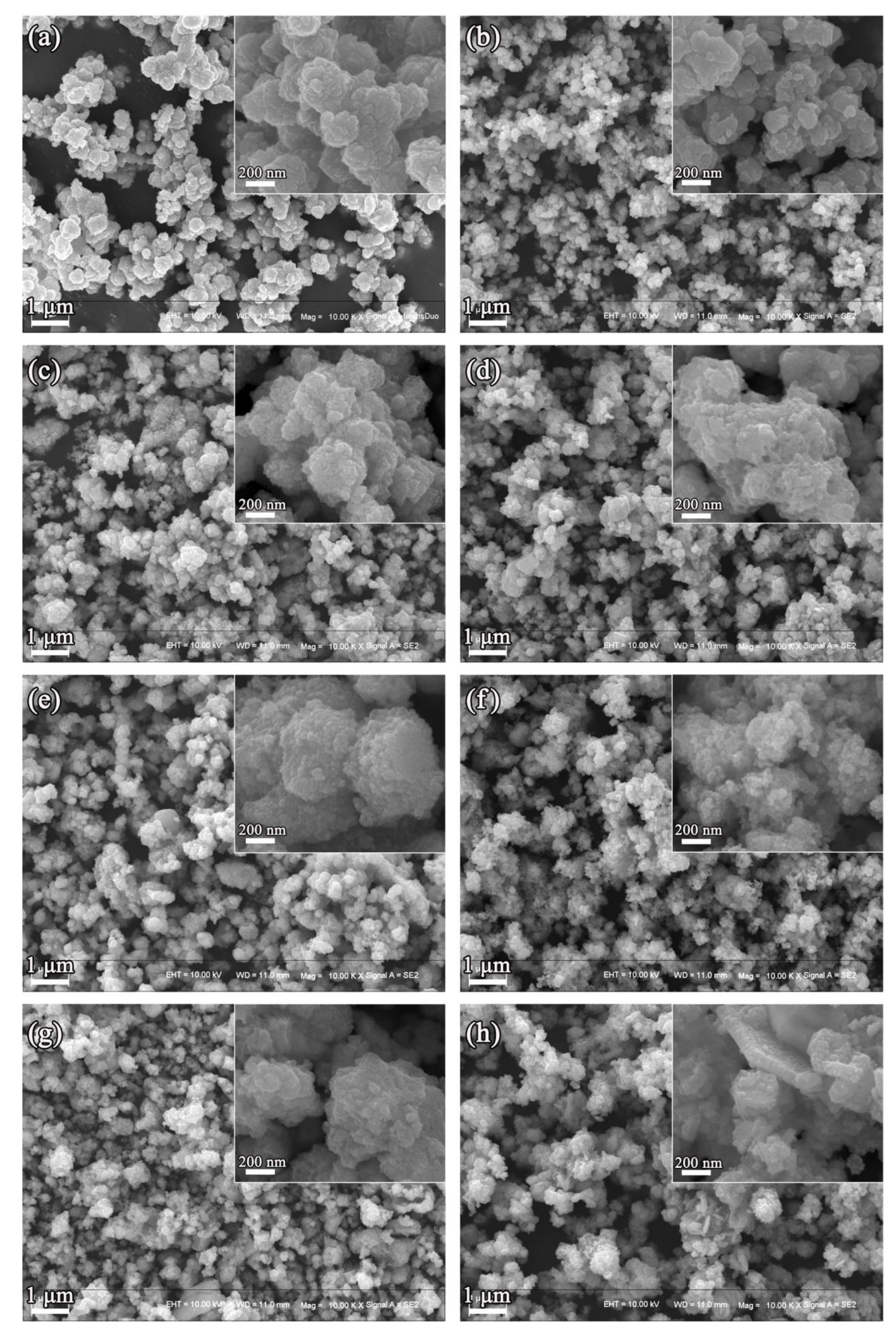
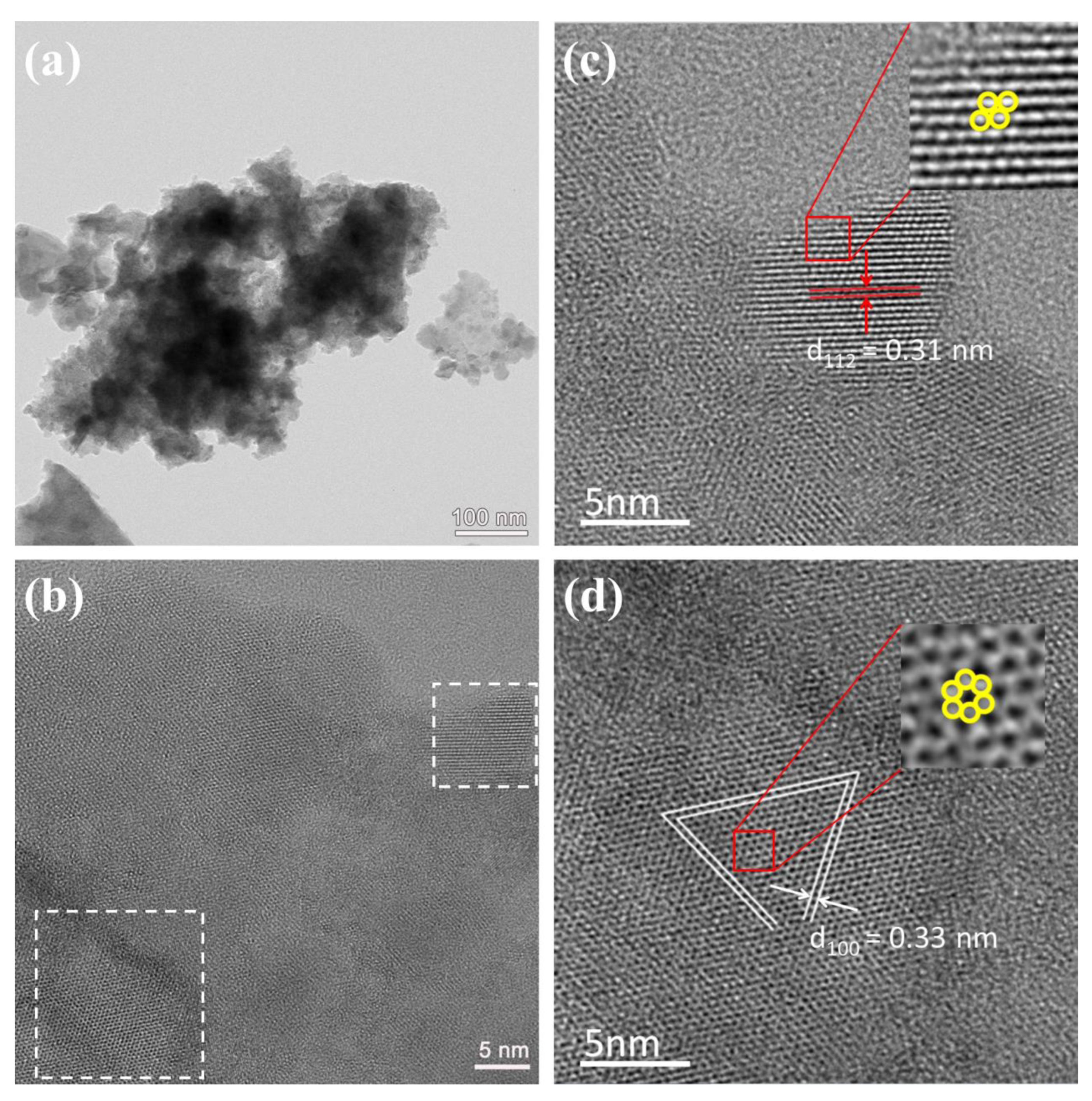
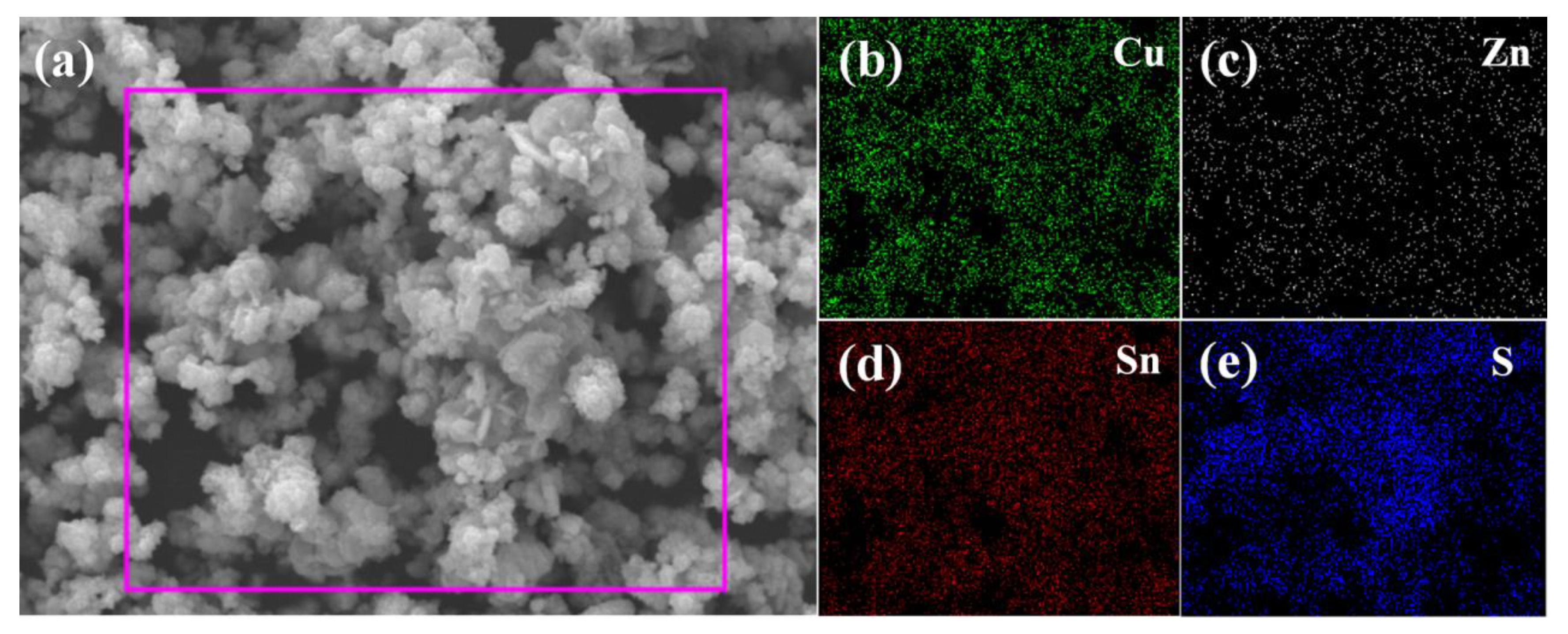
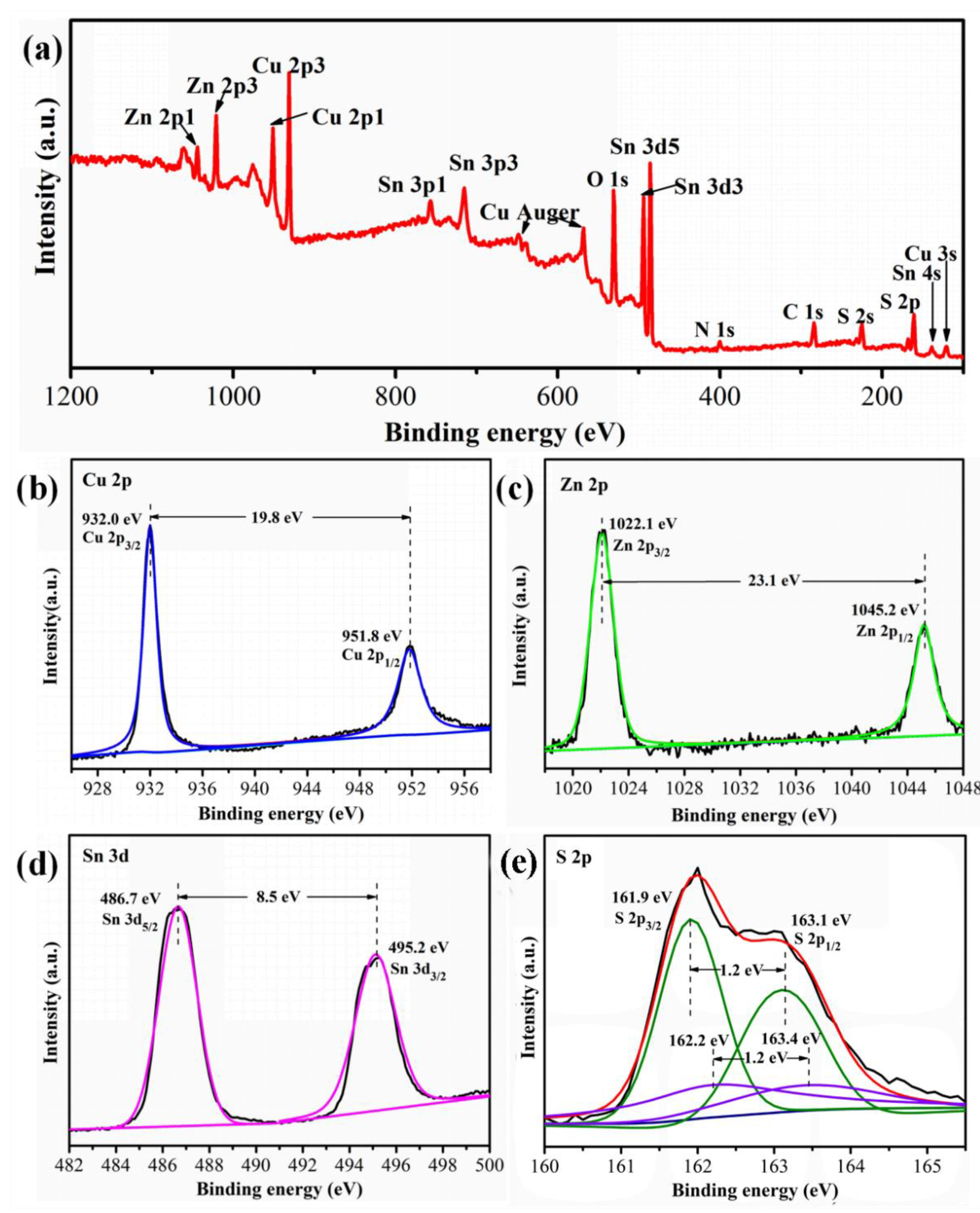
| Sample | Composition Ratio | |||||
|---|---|---|---|---|---|---|
| Cu | Zn | Sn | S | Cu/(Zn + Sn) | Zn/Sn | |
| CZTS-0.5 h | 66.33 | 0 | 0 | 33.67 | —— | —— |
| CZTS-1 h | 40.72 | 13.44 | 3.38 | 42.47 | 2.42 | 3.97 |
| CZTS-2 h | 42.94 | 8.04 | 7.22 | 41.81 | 2.81 | 1.11 |
| CZTS-5 h | 36.87 | 13.61 | 11.53 | 37.98 | 1.46 | 1.18 |
| CZTS-12 h | 31.96 | 12.24 | 10.97 | 44.83 | 1.37 | 1.11 |
| CZTS-24 h | 26.21 | 14.47 | 11.05 | 48.28 | 1.03 | 1.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Ding, Y.; Zhang, J.; Liang, N.; Long, L.; Liu, J. Insight into the Growth Mechanism of Mixed Phase CZTS and the Photocatalytic Performance. Nanomaterials 2022, 12, 1439. https://doi.org/10.3390/nano12091439
Yang Y, Ding Y, Zhang J, Liang N, Long L, Liu J. Insight into the Growth Mechanism of Mixed Phase CZTS and the Photocatalytic Performance. Nanomaterials. 2022; 12(9):1439. https://doi.org/10.3390/nano12091439
Chicago/Turabian StyleYang, Ying, Yaya Ding, Jingyu Zhang, Nina Liang, Lizhen Long, and Jun Liu. 2022. "Insight into the Growth Mechanism of Mixed Phase CZTS and the Photocatalytic Performance" Nanomaterials 12, no. 9: 1439. https://doi.org/10.3390/nano12091439
APA StyleYang, Y., Ding, Y., Zhang, J., Liang, N., Long, L., & Liu, J. (2022). Insight into the Growth Mechanism of Mixed Phase CZTS and the Photocatalytic Performance. Nanomaterials, 12(9), 1439. https://doi.org/10.3390/nano12091439






