Highly Selective Detection of Paraoxon in Food Based on the Platform of Cu Nanocluster/MnO2 Nanosheets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Preparation of Cu NCs
2.4. Preparation of MnO2 Nanosheets
2.5. Detection of BChE
2.6. Detection of OPs
3. Results
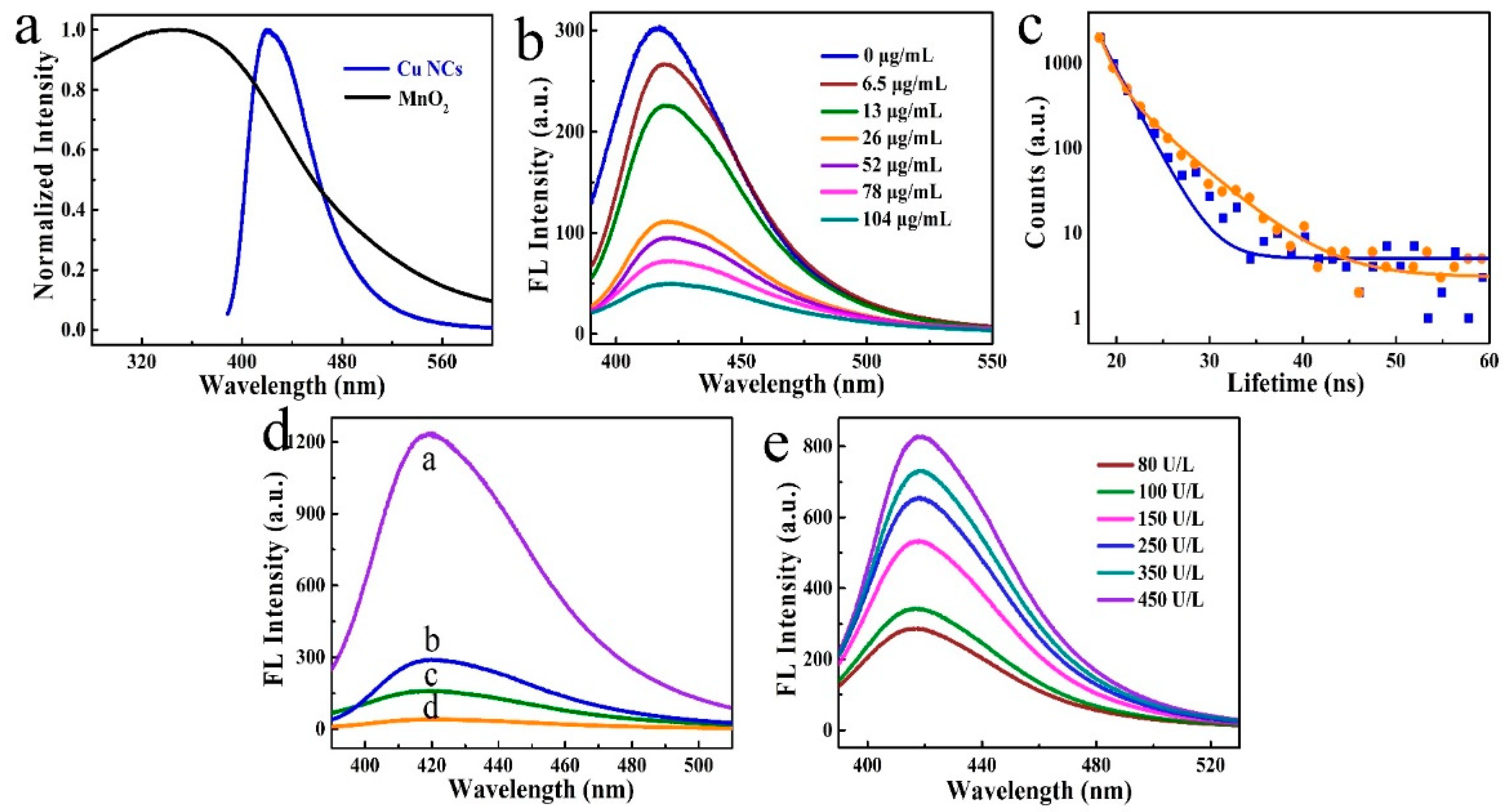
3.1. Characterization of Cu NCs and MnO2 Nanosheets
3.2. Modulation of Fluorescence Properties of Cu NCs by MnO2 Nanosheets
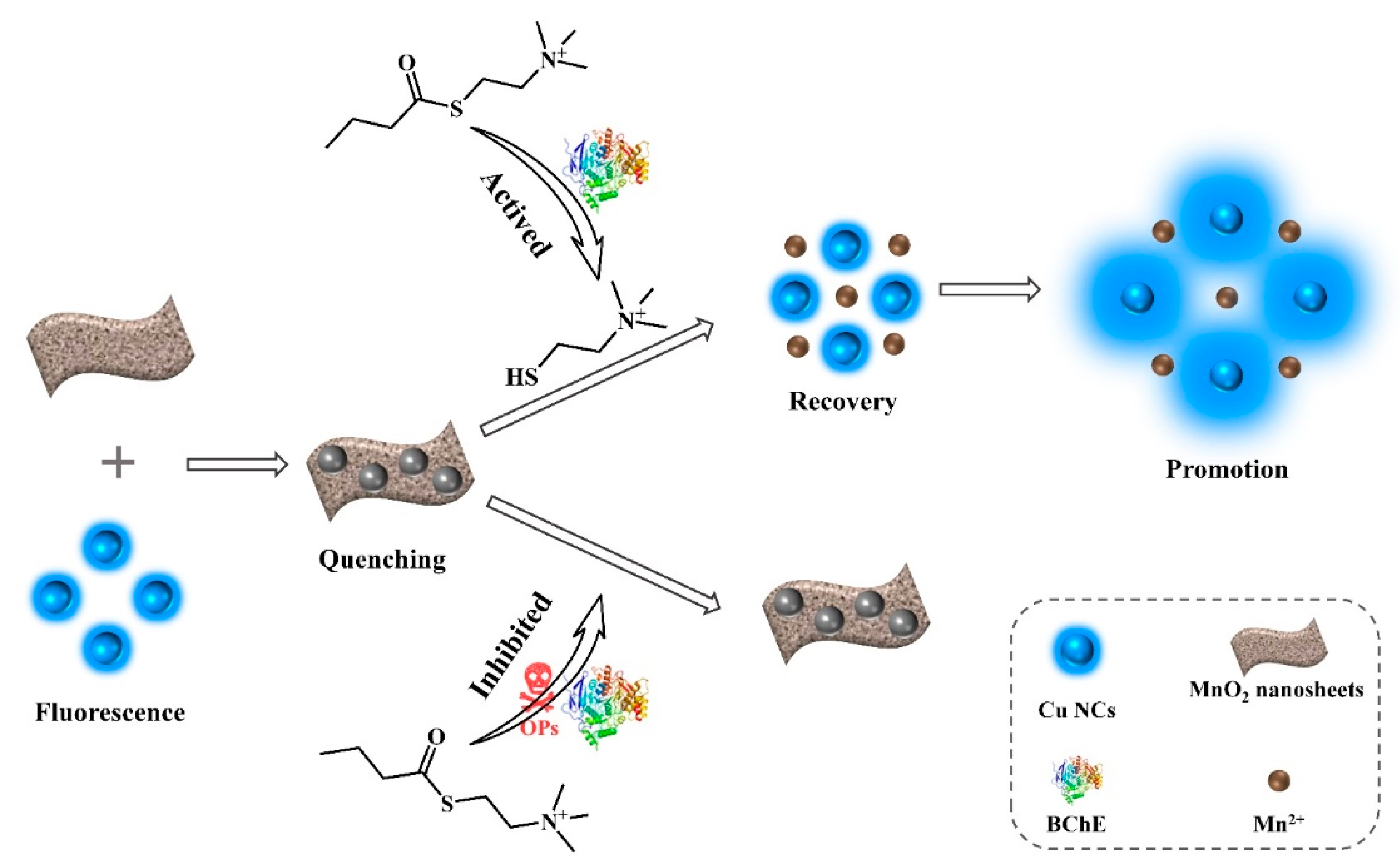
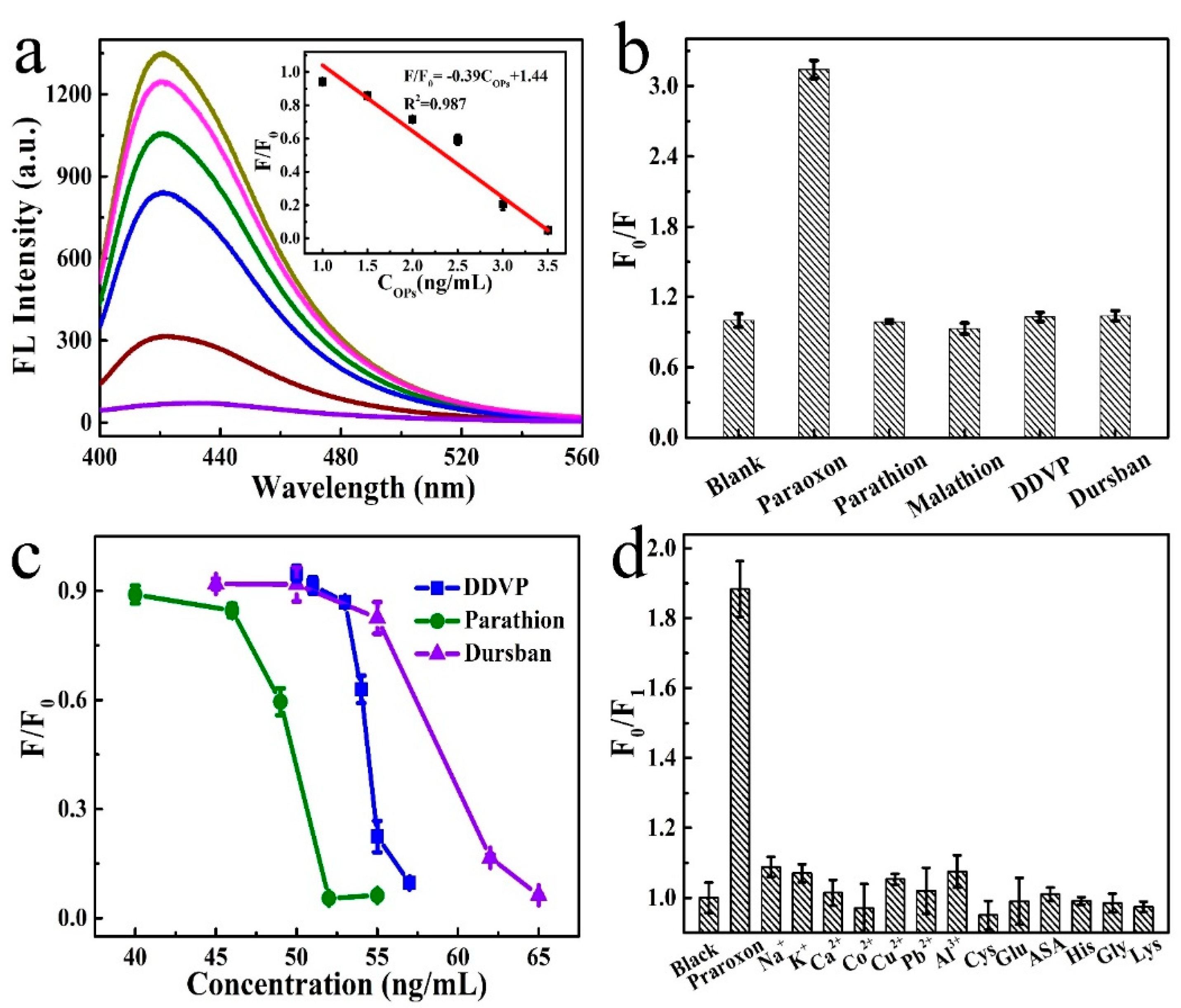
3.3. Feasibility and Mechanism of OPs Detection
3.4. Quantitative Detection of BChE and OPs
3.5. Selectivity and Practical Applications
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Long, E.Y.; Krupke, C.H. Non-cultivated plants present a season-long route of pesticide exposure for honey bees. Nat. Commun. 2016, 7, 11629. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Hassan, F.W.M.; Raoov, M.; Kamaruzaman, S.; Mohamed, A.H.; Ibrahim, W.N.W.; Hanapi, N.S.M.; Zain, N.N.M.; Yahaya, N.; Chen, D.D.Y. A rapid and efficient dispersive trehalose biosurfactant enhanced magnetic solid phase extraction for the sensitive determination of organophosphorus pesticides in cabbage (Brassica olearaceae var. capitate) samples by GC-FID. J. Food Compos. Anal. 2021, 102, 104057. [Google Scholar] [CrossRef]
- Gbadamosi, M.R.; Abdallah, M.A.-E.; Harrad, S. A critical review of human exposure to organophosphate esters with a focus on dietary intake. Sci. Total Environ. 2021, 771, 144752. [Google Scholar] [CrossRef] [PubMed]
- Lechenet, M.; Dessaint, F.; Py, G.; Makowski, D.; Munier-Jolain, N. Reducing pesticide use while preserving crop productivity and profitability on arable farms. Nat. Plants 2017, 3, 17008. [Google Scholar] [CrossRef]
- Fantke, P.; Gillespie, B.W.; Juraske, R.; Jolliet, O. Estimating Half-Lives for Pesticide Dissipation from Plants. Environ. Sci. Technol. 2014, 48, 8588–8602. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Chen, S.-W.; Zhang, B.; Tu, Q.; Wang, J.; Yuan, M.-S. Non-biological fluorescent chemosensors for pesticides detection. Talanta 2022, 240, 123200. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oladoye, P.O.; Olanrewaju, C.A.; Akinsola, G.O. Organophosphorus pesticides: Impacts, detection and removal strategies. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100655. [Google Scholar] [CrossRef]
- Ghorbani, M.; Mohammadi, P.; Keshavarzi, M.; Saghi, M.H.; Mohammadi, M.; Shams, A.; Aghamohammadhasan, M. Simultaneous determination of organophosphorus pesticides residues in vegetable, fruit juice, and milk samples with magnetic dispersive micro solid-phase extraction and chromatographic method; recruitment of simplex lattice mixture design for optimization of novel sorbent composites. Anal. Chim. Acta 2021, 1178, 338802. [Google Scholar] [CrossRef]
- Ferri, D.; Gaviña, P.; Costero, A.M.; Parra, M.; Vivancos, J.-L.; Martínez-Máñez, R. Detection and discrimination of organophosphorus pesticides in water by using a colorimetric probe array. Sens. Actuators B 2014, 202, 727–731. [Google Scholar] [CrossRef]
- Pundir, C.S.; Malik, A.; Preety. Bio-sensing of organophosphorus pesticides: A review. Biosens. Bioelectron. 2019, 140, 111348. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Jin, Y.; Wu, Y.; Liang, J. Simple and rapid colorimetric visualization of tetramethylthiuram disulfide (thiram) sensing based on anti-aggregation of gold nanoparticles. Food Chem. 2022, 384, 132223. [Google Scholar] [CrossRef] [PubMed]
- Jain, U.; Saxena, K.; Hooda, V.; Balayan, S.; Singh, A.P.; Tikadar, M.; Chauhan, N. Emerging vistas on pesticides detection based on electrochemical biosensors–An update. Food Chem. 2022, 371, 131126. [Google Scholar] [CrossRef] [PubMed]
- Zairov, R.; Zairov, R.; Dovzhenko, A.; Podyachev, S.; Sudakova, S.; Masliy, A.; Syakaev, V.; Gimazetdinova, G.; Nizameev, I.; Lapaev, D.; et al. Rational design of efficient nanosensor for glyphosate and temperature out of terbium complexes with 1,3-diketone calix[4]arenes. Sens. Actuators B 2022, 350, 130845. [Google Scholar] [CrossRef]
- Du, Y.; Sheng, H.; Astruc, D.; Zhu, M. Atomically Precise Noble Metal Nanoclusters as Efficient Catalysts: A Bridge between Structure and Properties. Chem. Rev. 2020, 120, 526–622. [Google Scholar] [CrossRef]
- Chen, T.; Lin, H.; Cao, Y.; Yao, Q.; Xie, J. Interactions of Metal Nanoclusters with Light: Fundamentals and Applications. Adv. Mater. 2021, 2103918. [Google Scholar] [CrossRef]
- Zhang, M.-M.; Dong, X.-Y.; Wang, Y.-J.; Zang, S.-Q.; Mak, T.C.W. Recent progress in functional atom-precise coinage metal clusters protected by alkynyl ligands. Coord. Chem. Rev. 2022, 453, 214315. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Zhou, M.; Liu, X.; Li, H.; Wang, H.; Shen, Y.; Zhu, M.; Jin, R. Ultrabright Au@Cu14 nanoclusters: 71.3% phosphorescence quantum yield in non-degassed solution at room temperature. Sci. Adv. 2021, 7, eabd2091. [Google Scholar] [CrossRef]
- Wu, Z.; Yao, Q.; Zang, S.-q.; Xie, J. Aggregation-induced emission in luminescent metal nanoclusters. Natl. Sci. Rev. 2020, 8, nwaa208. [Google Scholar] [CrossRef]
- Deng, H.-H.; Shi, X.-Q.; Balasubramanian, P.; Huang, K.-Y.; Xu, Y.-Y.; Huang, Z.-N.; Peng, H.-P.; Chen, W. 6-Aza-2-Thio-Thymine Stabilized Gold Nanoclusters as Photoluminescent Probe for Protein Detection. Nanomaterials 2020, 10, 281. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Liu, J.; Gao, Y.; Liu, H.; Li, T.; Zou, H.; Wang, Z.; Zhang, K.; Wang, Y.; Zhang, H.; et al. Assembly-Induced Enhancement of Cu Nanoclusters Luminescence with Mechanochromic Property. J. Am. Chem. Soc. 2015, 137, 12906–12913. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shi, Y.-E.; Yang, X.; Xiong, Y.; Li, Y.; Chen, B.; Lai, W.-F.; Rogach, A.L. Water-Soluble Biocompatible Copolymer Hypromellose Grafted Chitosan Able to Load Exogenous Agents and Copper Nanoclusters with Aggregation-Induced Emission. Adv. Funct. Mater. 2018, 28, 1802848. [Google Scholar] [CrossRef]
- Kolay, S.; Bain, D.; Maity, S.; Devi, A.; Patra, A.; Antoine, R. Self-Assembled Metal Nanoclusters: Driving Forces and Structural Correlation with Optical Properties. Nanomaterials 2022, 12, 544. [Google Scholar] [CrossRef] [PubMed]
- Babu Busi, K.; Palanivel, M.; Kanta Ghosh, K.; Basu Ball, W.; Gulyás, B.; Padmanabhan, P.; Chakrabortty, S. The Multifarious Applications of Copper Nanoclusters in Biosensing and Bioimaging and Their Translational Role in Early Disease Detection. Nanomaterials 2022, 12, 301. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, H.; Hu, T.; Su, X. A novel fluorimetric sensing platform for highly sensitive detection of organophosphorus pesticides by using egg white-encapsulated gold nanoclusters. Biosens. Bioelectron. 2017, 91, 232–237. [Google Scholar] [CrossRef]
- Wang, M.; Liu, L.; Xie, X.; Zhou, X.; Lin, Z.; Su, X. Single-atom iron containing nanozyme with peroxidase-like activity and copper nanoclusters based ratio fluorescent strategy for acetylcholinesterase activity sensing. Sens. Actuators B 2020, 313, 128023. [Google Scholar] [CrossRef]
- Xue, Y.; Cheng, Z.; Luo, M.; Hu, H.; Xia, C. Synthesis of Copper Nanocluster and Its Application in Pollutant Analysis. Biosensors 2021, 11, 424. [Google Scholar] [CrossRef]
- Qian, S.; Wang, Z.; Zuo, Z.; Wang, X.; Wang, Q.; Yuan, X. Engineering luminescent metal nanoclusters for sensing applications. Coord. Chem. Rev. 2022, 451, 214268. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, B.; Susha, A.S.; Wang, W.; Reckmeier, C.J.; Chen, R.; Zhong, H.; Rogach, A.L. All-Copper Nanocluster Based Down-Conversion White Light-Emitting Devices. Adv. Sci. 2016, 3, 1600182. [Google Scholar] [CrossRef]
- Li, T.; Wang, Z.; Jiang, D.; Wang, H.; Lai, W.-F.; Lv, Y.; Zhai, Y. A FRET biosensor based on MnO2 nanosphere/copper nanocluster complex: From photoluminescence quenching to recovery and magnification. Sens. Actuators B 2019, 290, 535–543. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, H.; Li, T.; Liu, J.; Yin, J.; Mohammed, O.F.; Bakr, O.M.; Liu, Y.; Yang, B.; Zhang, H. Contribution of Metal Defects in the Assembly Induced Emission of Cu Nanoclusters. J. Am. Chem. Soc. 2017, 139, 4318–4321. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Ren, Y.; Bick, M.; Dudek, A.; Hong-Wang Waworuntu, E.; Tang, J.; Chen, J.; Chang, B. Highly fluorescent copper nanoclusters for sensing and bioimaging. Biosens. Bioelectron. 2020, 154, 112078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Meng, H.; Tian, Y.; Yang, R.; Du, D.; Li, Z.; Qu, L.; Lin, Y. Recent advances in functionalized MnO2 nanosheets for biosensing and biomedicine applications. Nanoscale Horiz. 2019, 4, 321–338. [Google Scholar] [CrossRef]
- Yang, X.; Makita, Y.; Liu, Z.-h.; Sakane, K.; Ooi, K. Structural Characterization of Self-Assembled MnO2 Nanosheets from Birnessite Manganese Oxide Single Crystals. Chem. Mater. 2004, 16, 5581–5588. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, C.; Wang, H.; Xiong, Y.; Yang, X.; Shi, Y.-e.; Rogach, A.L. Two-Step Oxidation Synthesis of Sulfur with a Red Aggregation-Induced Emission. Angew. Chem. Int. Ed. 2020, 59, 9997–10002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-P.; Zhao, C.-X.; Shu, Y.; Wang, J.-H. Gold Nanoclusters/Iron Oxyhydroxide Platform for Ultrasensitive Detection of Butyrylcholinesterase. Anal. Chem. 2019, 91, 15866–15872. [Google Scholar] [CrossRef] [PubMed]
- Bala, R.; Dhingra, S.; Kumar, M.; Bansal, K.; Mittal, S.; Sharma, R.K.; Wangoo, N. Detection of organophosphorus pesticide–Malathion in environmental samples using peptide and aptamer based nanoprobes. Chem. Eng. J. 2017, 311, 111–116. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Y.; Sheng, Z.; Li, T.; Li, X. A colorimetric detection method of pesticide acetamiprid by fine-tuning aptamer length. Anal. Biochem. 2016, 513, 87–92. [Google Scholar] [CrossRef]

| Detecting Materials Used | Linear Range (U/L) | LOD (U/L) | Reference |
|---|---|---|---|
| MnO2-NSs -PDA | 50–450 | 40.65 | Microchem. J., 2021, 166, 106. |
| IPAN | 25,000–31,000 | 11.7 | Anal. Chem., 2020, 92, 13405. |
| 3D printing device | 1000–12,000 | 100 | Sens. Actuators B., 2018, 258, 1015. |
| CDs-OPD | 1000–25,000 | 400 | Biosens. Bioelectron., 2022, 196, 113691 |
| 3D ER-PS | 80–550 | 30.3 | Anal. Chem., 2019, 20, 12874. |
| Cu NCs-MnO2 NS | 80–450 | 27.5 | This work |
| Detecting Materials Used | Linear Range (ng/mL) | LOD (ng/mL) | Reference |
|---|---|---|---|
| [Fe(CN)6]3− | 0–30 | 5 | Anal. Chim. Acta, 2019, 1060, 97. |
| COFML-DHTA | 0.57–300 | 0.19 | Anal. Methods, 2021, 13,5727. |
| AuNPs-MoS2-rGO/PI | 5–150 | 1.4 | Bioelectrochemistry, 2020, 131, 107392. |
| DTNB-CDs | 1–1000 | 0.4 | Sens. Actuators B., 2018, 260, 563. |
| Su-TPE/PrS | 0–384 | 5.28 | J. Mol. Liq., 2021, 333, 115980. |
| MnO2 NS-Cu NCs | 1.0–3.5 | 0.22 | This work |
| Sample | Spiked (ng/mL) | Detection (ng/mL) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Apple | 10 | 9.9 | 99.0 | 0.383 |
| 20 | 21.3 | 106.5 | 0.396 | |
| 30 | 29.3 | 97.6 | 0.750 | |
| Cowpeas | 10 | 10.1 | 101.0 | 0.175 |
| 20 | 19.7 | 98.5 | 0.647 | |
| 30 | 28.3 | 94.3 | 0.575 | |
| Pakchoi | 10 | 9.5 | 95.0 | 0.360 |
| 20 | 19.6 | 98.0 | 0.354 | |
| 30 | 32.4 | 108.0 | 0.377 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhang, P.; Miao, Y.; Li, C.; Shi, Y.-e.; Liu, J.; Lv, Y.-k.; Wang, Z. Highly Selective Detection of Paraoxon in Food Based on the Platform of Cu Nanocluster/MnO2 Nanosheets. Nanomaterials 2022, 12, 1429. https://doi.org/10.3390/nano12091429
Liu S, Zhang P, Miao Y, Li C, Shi Y-e, Liu J, Lv Y-k, Wang Z. Highly Selective Detection of Paraoxon in Food Based on the Platform of Cu Nanocluster/MnO2 Nanosheets. Nanomaterials. 2022; 12(9):1429. https://doi.org/10.3390/nano12091429
Chicago/Turabian StyleLiu, Shuo, Peng Zhang, Yuming Miao, Chenmin Li, Yu-e Shi, Jinhua Liu, Yun-kai Lv, and Zhenguang Wang. 2022. "Highly Selective Detection of Paraoxon in Food Based on the Platform of Cu Nanocluster/MnO2 Nanosheets" Nanomaterials 12, no. 9: 1429. https://doi.org/10.3390/nano12091429
APA StyleLiu, S., Zhang, P., Miao, Y., Li, C., Shi, Y.-e., Liu, J., Lv, Y.-k., & Wang, Z. (2022). Highly Selective Detection of Paraoxon in Food Based on the Platform of Cu Nanocluster/MnO2 Nanosheets. Nanomaterials, 12(9), 1429. https://doi.org/10.3390/nano12091429






