A Colorimetric Ag+ Probe for Food Real-Time Visual Monitoring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Synthesis of the Colorimetric Ag+ Probe
2.4. Preparation of Colorimetric Sensing Label
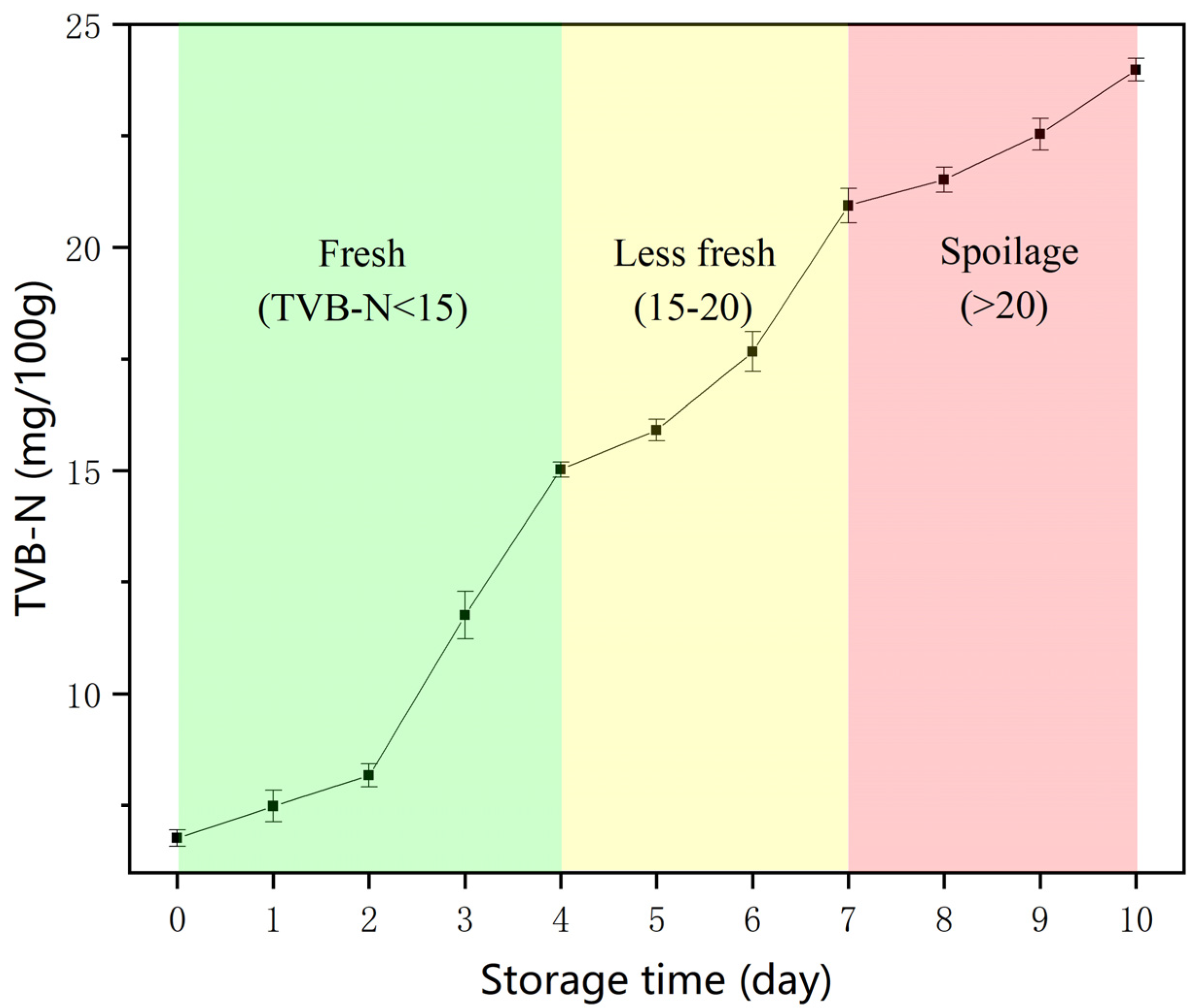
2.5. Detection of TVB-N
3. Results and Discussion
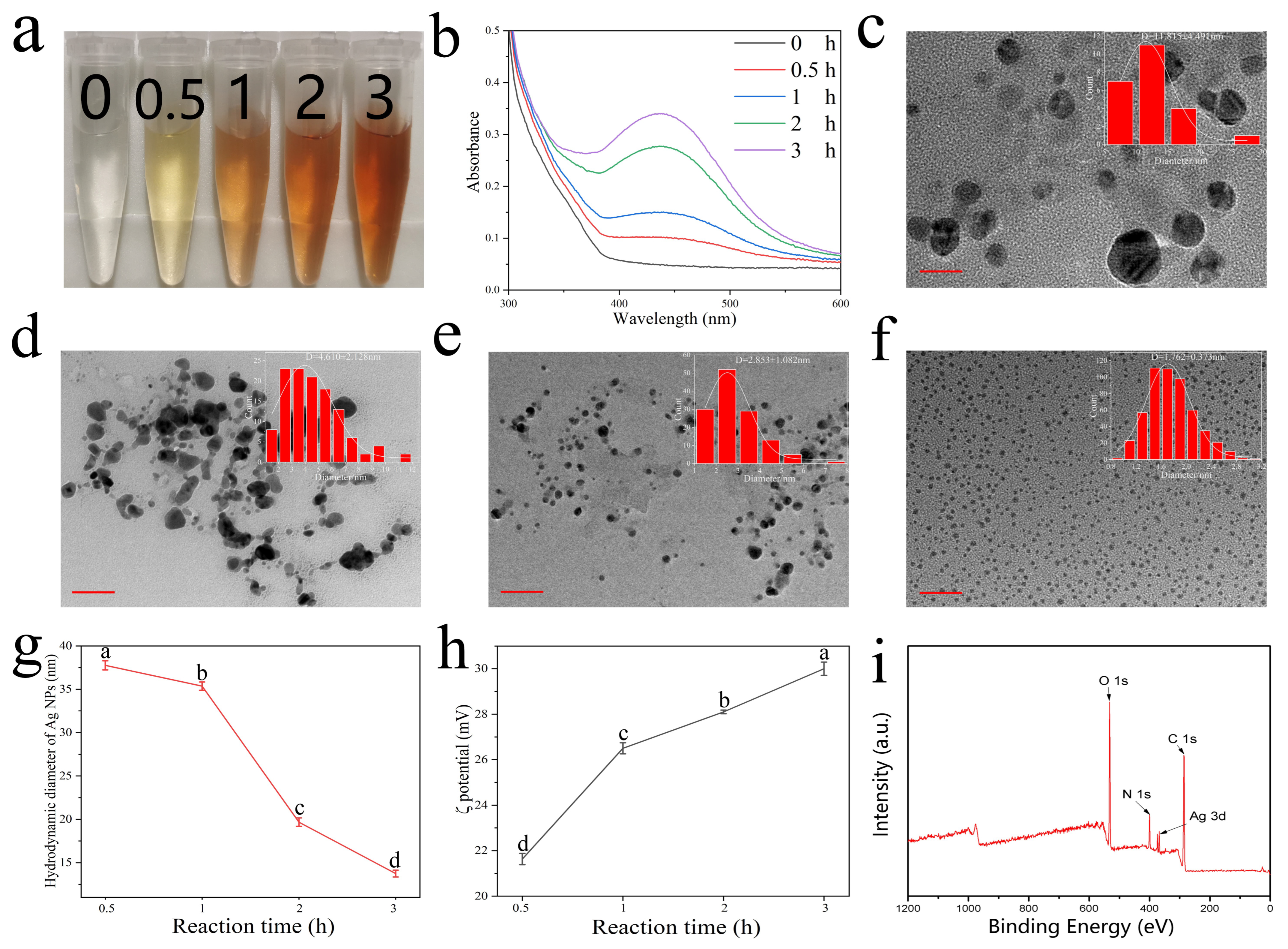
3.1. Preparation and Characterization of Colorimetric Ag+ Probe
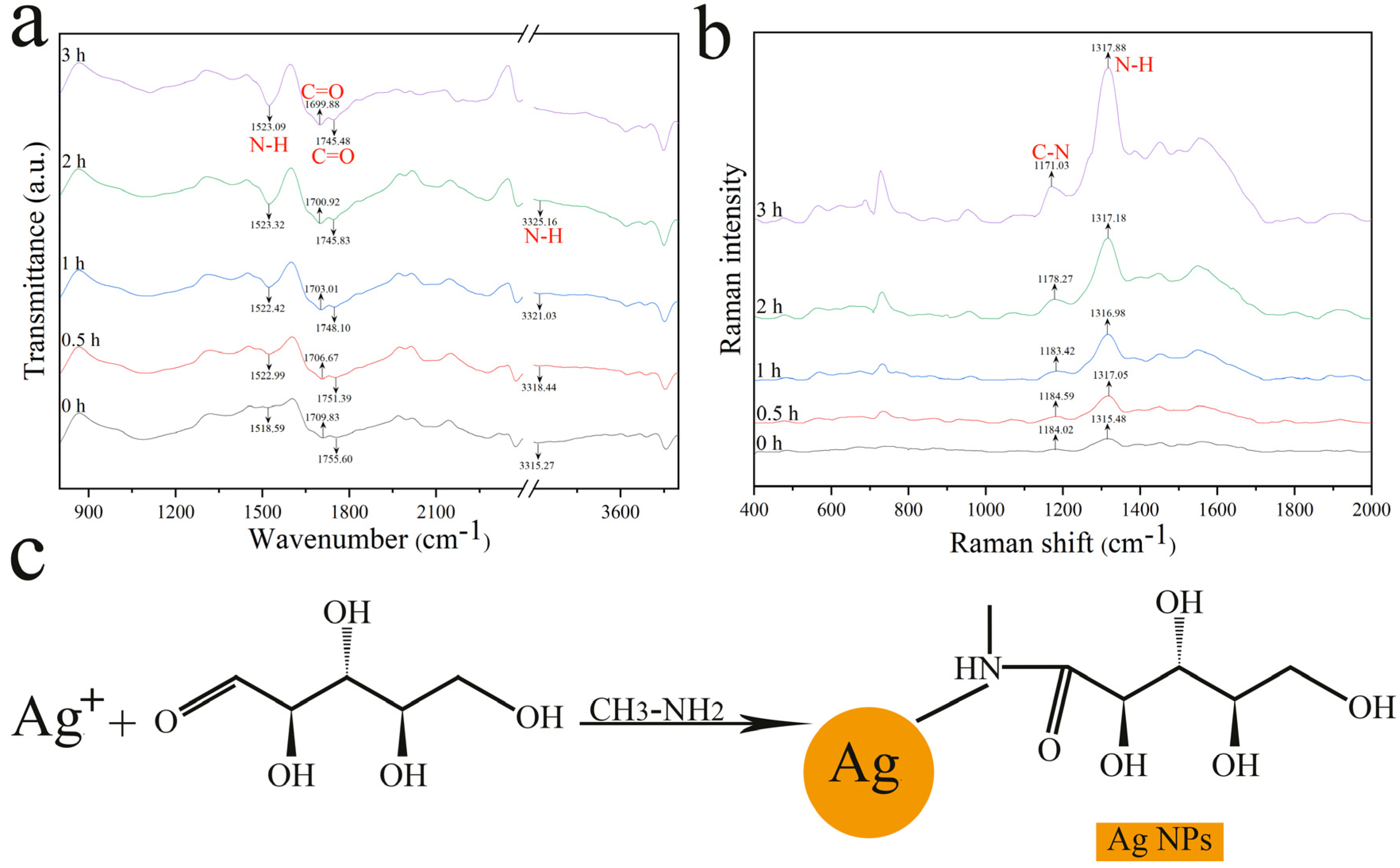
3.2. Sensing Mechanism Exploration
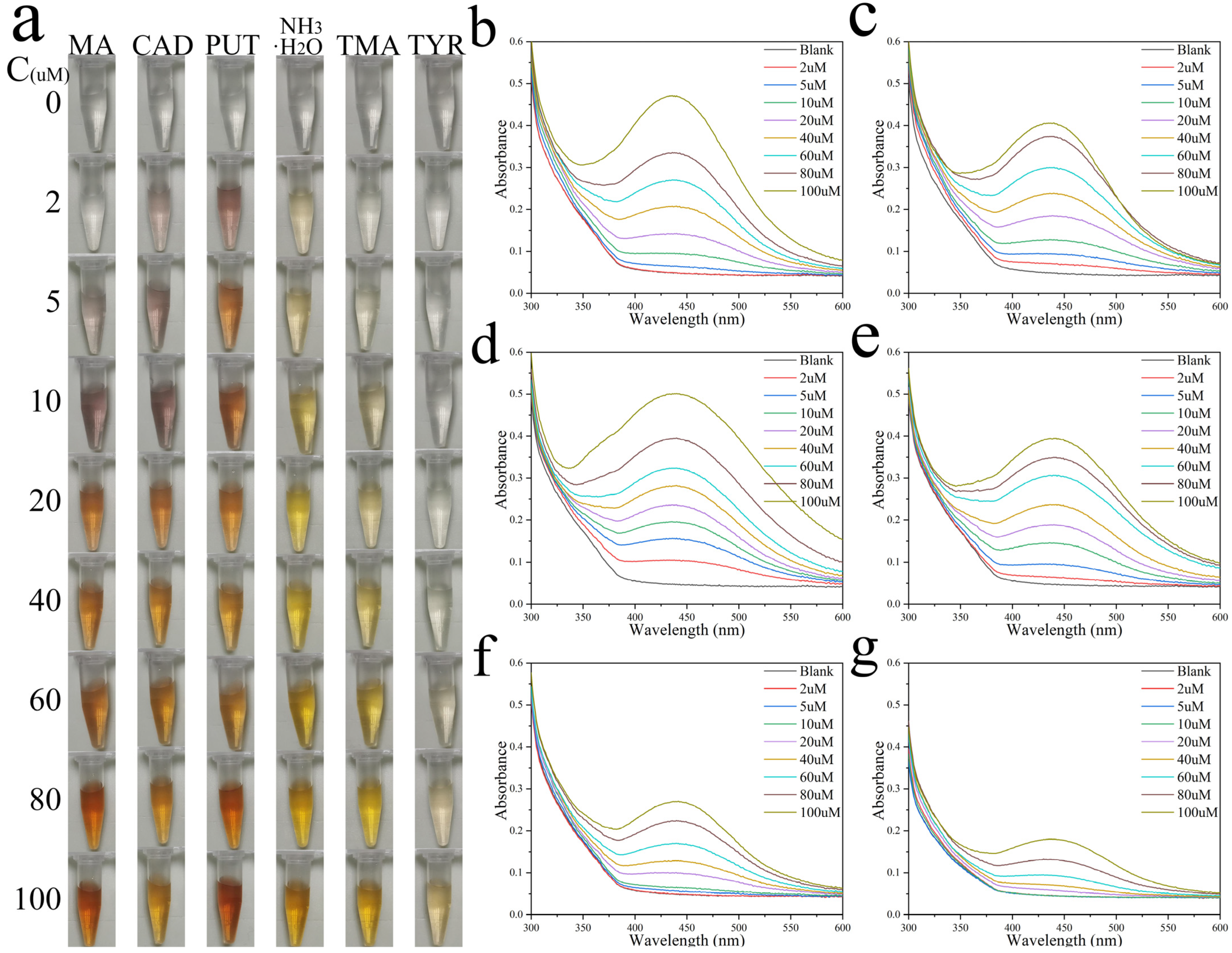
3.3. BAs-Response Behavior of Colorimetric Ag+ Probe
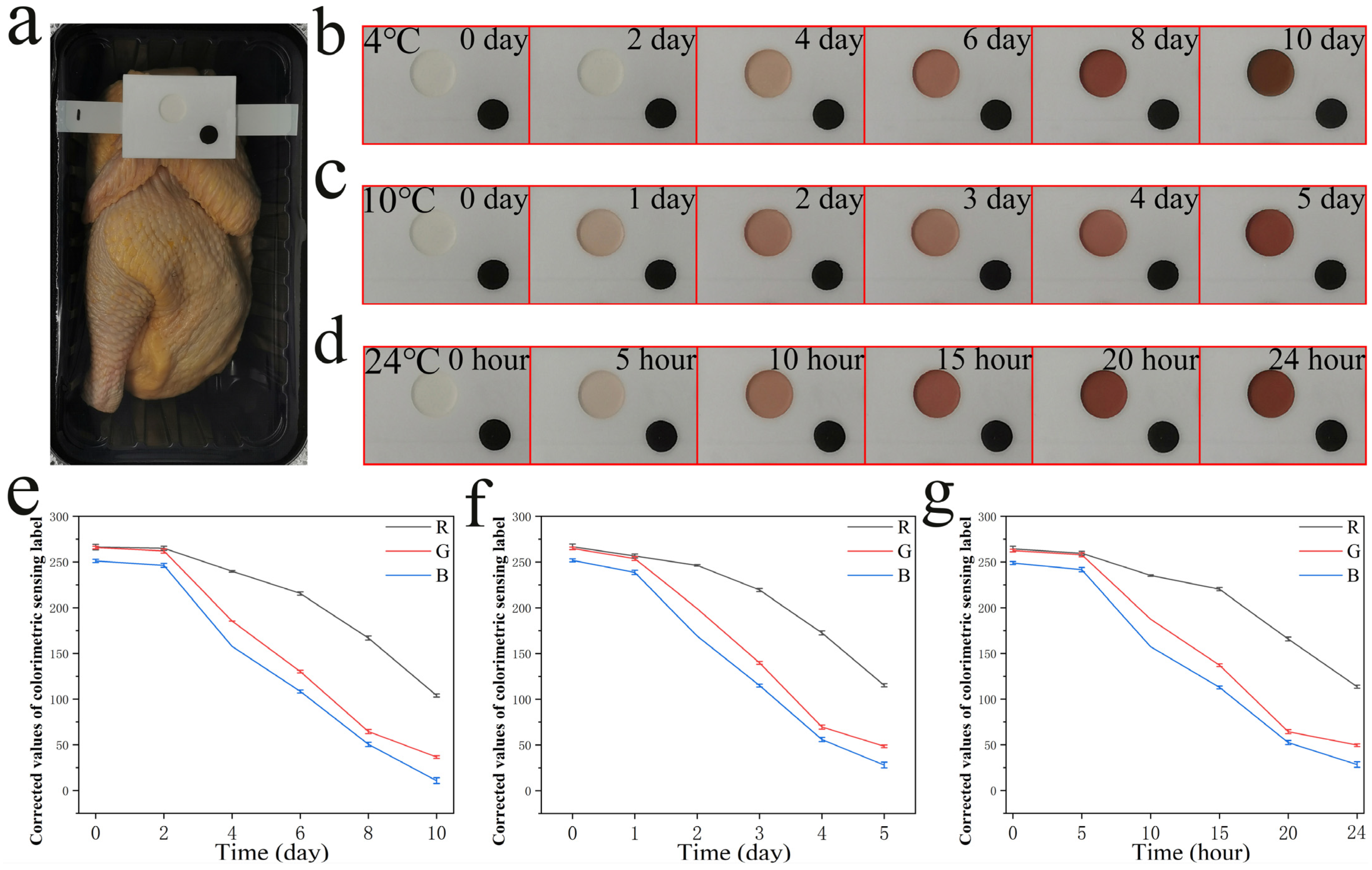
3.4. Immobilization of a Colorimetric Ag+ Probe in Colorimetric Tank as a Colorimetric Sensing Label
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hick, C.C.; Cohen, P.J.; Graham, N.A.J.; Nash, K.L.; Allison, E.H.; D’Lima, C.; Mills, D.J.; Roscher, M.; Thilsted, S.H.; Thorne-Lyman, A.L.; et al. Harnessing global fisheries to tackle micronutrient deficiencies. Nature 2019, 574, 95–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voytek, B.; Knight, R.T. Dynamic network communication as a unifying neural basis for cognition, development, aging, and disease. Biol. Psychiatry 2015, 77, 1089–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, S.; Liu, Y.; Zhuang, S.; Sun, X.; Li, Y.; Hong, H.; Lv, Y.; Luo, Y. Effect of ε-polylysine and ice storage on microbiota composition and quality of Pacific white shrimp (Litopenaeus vannamei) stored at 0 °C. Food Microbiol. 2019, 83, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Kim, T.I.; Jin, H.; Lee, U.; Bae, J.; Bouffard, J.; Kim, Y. Amine-Reactive Activated Esters of meso-CarboxyBODIPY: Fluorogenic Assays and Labeling of Amines, Amino Acids, and Proteins. J. Am. Chem. Soc. 2020, 142, 9231–9239. [Google Scholar] [CrossRef]
- Chen, X.; Wang, D.; Li, J.; Xu, T.; Lai, K.; Ding, Q.; Lin, H.; Sun, L.; Lin, M. A spectroscopic approach to detect and quantify phosmet residues in Oolong tea by surface-enhanced Raman scattering and silver nanoparticle substrate. Food Chem. 2020, 312, 126016. [Google Scholar] [CrossRef] [PubMed]
- Raja, I.S.; Vedhanayagam, M.; Preeth, D.R.; Kim, C.; Lee, J.H.; Han, D.W. Development of Two-Dimensional Nanomaterials Based Electrochemical Biosensors on Enhancing the Analysis of Food Toxicants. Int. J. Mol. Sci. 2021, 22, 3277. [Google Scholar] [CrossRef]
- Guo, L.; Wang, T.; Wu, Z.; Wang, J.; Wang, M.; Cui, Z.; Ji, S.; Cai, J.; Xu, C.; Chen, X. Portable Food-Freshness Prediction Platform Based on Colorimetric Barcode Combinatorics and Deep Convolutional Neural Networks. Adv. Mater. 2020, 32, 2004805. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Y.; Feng, L. Specific detection and discrimination of dithiocarbamates using CTAB-encapsulated fluorescent copper nanoclusters. Talanta 2020, 210, 120627. [Google Scholar] [CrossRef]
- Bagheri, E.; Abnous, K.; Alibolandi, M.; Ramezani, M.; Taghdisi, S.M. Triple-helix molecular switch-based aptasensors and DNA sensors. Biosens Bioelectron. Biosens. Bioelectron. 2018, 111, 1–9. [Google Scholar] [CrossRef]
- Dassary, S.S.R.; Singh, A.K.; Lee, K.S.; Yu, H.; Ray, P.C. A miniaturized fiber-optic fluorescence analyzer for detection of picric-acid explosive from commercial and environmental samples. Sens. Actuator B 2018, 255, 1646–1654. [Google Scholar] [CrossRef]
- Sheini, A.; Khajehsharifi, H.; Shahbazy, M.; Kompany-Zareh, M. A chemosensor array for the colorimetric identification of some carboxylic acids in human urine samples. Sens. Actuators B 2017, 242, 288–298. [Google Scholar] [CrossRef]
- Irber, D.M.; Poggiali, F.; Kong, F.; Kieschnick, M.; Lühmann, T.; Kwiatkowski, D.; Meijer, J.; Du, J.; Shi, F.; Reinhard, F. Robust all-optical single-shot readout of nitrogen-vacancy centers in diamond. Nature communications. Nat. Commun. 2021, 12, 532. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef] [PubMed]
- Ratchford, D.C. Plasmon-Induced Charge Transfer: Challenges and Outlook. ACS Nano 2019, 13, 13610–13614. [Google Scholar] [CrossRef]
- Lavel, B.S.; Nambiar, M.; Shapter, J.G. Electrochemical Detection of Copper Using a Gly-Gly-His Modified Carbon Nanotube Biosensor. Silicon 2011, 3, 163–171. [Google Scholar]
- Wong, S.; Zhao, J.; Cao, C.; Wong, C.K.; Kuchel, R.P.; Luca, S.D.; Hook, J.M.; Garvey, C.J.; Smith, S.; Ho, J.; et al. Just add sugar for carbohydrate induced self-assembly of curcumin. Nat. Commun. 2019, 10, 582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jumeaux, C.; Kim, E.; Howes, P.D.; Kim, H.; Chandrawati, R.; Stevens, M.M. Detection of microRNA biomarkers via inhibition of DNA-mediated liposome fusion. Nanoscale Adv. 2019, 1, 532–536. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Sikdar, D.; He, Q.; Kho, D.; Kucernak, A.R.; Kornyshev, A.A.; Edel, J.B. Self-assembling two-dimensional nanophotonic arrays for reflectivity-based sensing. Chem. Sci. 2020, 11, 9563–9570. [Google Scholar] [CrossRef]
- Wang, T.; Can, I.; Zhang, S.; He, J.; Sun, P.; Liu, F.; Lu, G. Self-Assembly Template Driven 3D Inverse Opal Microspheres Functionalized with Catalyst Nanoparticles Enabling a Highly Efficient Chemical Sensing Platform. ACS Appl. Mater. Interfaces 2018, 10, 5835–5844. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, D.; Cao, X.; Gu, H.; Deng, W. Self-Assembled Microgels Arrays for Electrostatic Concentration and Surface-Enhanced Raman Spectroscopy Detection of Charged Pesticides in Seawater. Anal. Chem. 2019, 91, 11192–11199. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H.; Chen, J.; Shahzad, S.A.; Yu, C. Controlled self-assembly of small molecule probes and the related applications in bioanalysis. Biosens. Bioelectron. 2016, 76, 38–53. [Google Scholar] [CrossRef]
- Nativo, P.; Prior, L.A.; Brust, M. Uptake and Intracellular Fate of Surface-Modified Gold Nanoparticles. ACS Nano 2008, 2, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Estevez, M.B.; Raffaelli, S.; Mitchell, S.G.; Faccio, R.; Alborés, S. Biofilm Eradication Using Biogenic Silver Nanoparticles. Molecules 2020, 25, 2023. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Soliman, M.G.; Sun, X.; Pelaz, B.; Feliu, N.; Parak, W.J.; Liu, S. How Entanglement of Different Physicochemical Properties Complicates the Prediction of in Vitro and in Vivo Interactions of Gold Nanoparticles. ACS Nano 2018, 12, 10104–10113. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhao, J.; Chen, Y.; Cheng, B.; Yu, Z.; Zhao, Y.; Yan, X.; Tong, Z.; Jin, S. Preparation and characterization of chitosan physical hydrogels with enhanced mechanical and antibacterial properties. Carbohydr. Polym. 2017, 157, 1383–1392. [Google Scholar] [CrossRef]
- Truta, L.A.A.N.A.; MoreiraM, F.T.C.; Sales, M.G.F. A dye-sensitized solar cell acting as the electrical reading box of an immunosensor: Application to CEA determination. Biosens. Bioelectron. 2018, 107, 94–102. [Google Scholar] [CrossRef]
- Kumar, A.; Bhardwaj, N.K.; Singh, S.P. Polyacrylamide stabilized alkenyl succinic anhydride emulsion as sizing agent for various cellulosic pulps and fillers. Carbohydr. Polym. 2020, 236, 116069. [Google Scholar] [CrossRef]
- Dong, L.; Mai, Y.; Liu, Q.; Zhang, W.; Yang, J. Mechanism and Improved Dissolution of Glycyrrhetinic Acid Solid Dispersion by Alkalizers. Pharmaceutics 2020, 12, 82. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Xu, Z.; Zhang, L.; Jiao, N. Copper-Catalyzed Aerobic Oxidative Coupling of Aryl Acetaldehydes with Anilines Leading to α-Ketoamides. Angew. Chem. Int. Ed. 2011, 50, 11088–11092. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, M.; Wang, X.; Li, S.; Liu, Y.; Yang, G. Influence of reaction media on synthesis of dialdehyde cellulose/GO composites and their adsorption performances on heavy metals. Carbohydr. Polym. 2020, 232, 115781. [Google Scholar] [CrossRef]
- Baniukevic, J.; Boyaci, I.H.; Bozkurt, A.G.; Tamer, U.; Ramanavicius, A.; Ramanaviciene, A. Magnetic gold nanoparticles in SERS-based sandwich immunoassay for antigen detection by well oriented antibodies. Biosens. Bioelectron. 2013, 43, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Sivashanmugan, K.; Liao, J.-D.; Shao, P.-L.; Liu, B.H.; Tseng, T.-Y.; Chang, C.-U. Intense Raman scattering on hybrid Au/Ag nanoplatforms for the distinction of MMP-9-digested collagen type-I fiber detection. Biosens. Bioelectron. 2015, 72, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Y.; Deng, R.; Yang, L.; Yu, S.; Xu, S.; Xu, W. Fe3O4@Graphene Oxide@Ag Particles for Surface Magnet Solid-Phase Extraction Surface-Enhanced Raman Scattering (SMSPE-SERS): From Sample Pretreatment to Detection All-in-One. ACS Appl. Mater. Interfaces 2016, 8, 14160–14168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Z.; Du, J.; Yao, X.; Zheng, X.; Lei, R.; Liu, J.; Hu, H.; Li, H. Interaction of Taxol with intravenous immunoglobulin: An inhibition of Taxol from crystallizing in aqueous solution. Int. Immunopharmacol. 2008, 8, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, M.S.; Lee, W.-H.; Cho, B.-K. Determination of the total volatile basic nitrogen (TVB-N) content in pork meat using hyperspectral fluorescence imaging. Sens. Actuators B 2018, 259, 532–539. [Google Scholar] [CrossRef]
- Rukchon, C.; Nopwinyuwong, A.; Trevanich, S.; Jinkarn, T.; Suppakul, P. Development of a food spoilage indicator for monitoring freshness of skinless chicken breast. Talanta 2014, 130, 547–554. [Google Scholar] [CrossRef]
- Lin, H.; Jang, M.; Suslick, K.S. Preoxidation for Colorimetric Sensor Array Detection of Vocs. J. Am. Chem. Soc. 2011, 133, 16786–16789. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Fang, M.; LaGasse, M.K.; Askim, J.R.; Suslick, K.S. Colorimetric Recognition of Aldehydes and Ketones. Angew. Chem. Int. Ed. 2017, 56, 9860–9863. [Google Scholar] [CrossRef]
- Koo, C.-K.; Samain, F.; Dai, N.; Kool, E.T. DNA Poly fluorophores as Highly Diverse Chemosensors of Toxic Gases. Chem. Sci. 2011, 2, 1910–1917. [Google Scholar] [CrossRef]
- Jia, M.-Y.; Wu, Q.-S.; Li, H.; Zhang, Y.; Guan, Y.-F.; Feng, L. The calibration of cellphone camera-based colorimetric sensor array and its application in the determination of glucose in urine. Biosens. Bioelectron. 2015, 74, 1029–1037. [Google Scholar] [CrossRef]
- Yuan, Z.; Bariya, M.; Fahad, H.M.; Wu, J.; Han, R.; Gupta, N.; Javey, A. Trace-Level, Multi-Gas Detection for Food Quality Assessment Based on Decorated Silicon Transistor Arrays. Adv. Mater. 2020, 32, 1908385. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Ping, C.; Wen, C.; Kun, Y.; Lijia, P.; Yi, S.; Guihua, Y. Highly Sensitive, Printable Nanostructured Conductive Polymer Wireless Sensor for Food Spoilage Detection. Nano Lett. 2018, 18, 4570–4575. [Google Scholar]
- Liu, S.F.; Petty, A.R.; Sazama, G.T.; Swager, T.M. Single-Walled Carbon Nanotube/Metalloporphyrin Composites for the Chemiresistive Detection of Amines and Meat Spoilage. Angew. Chem. Int. Ed. 2015, 54, 6554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Z.; Lv, T.; Zhu, K.; Li, Y.; Wang, L.; Gooding, J.J.; Liu, G.; Liu, B. Paper-Based Ratiometric Fluorescence Analytical Devices towards Point-of-Care Testing of Human Serum Albumin. Angew. Chem. Int. Ed. 2020, 59, 3131–3136. [Google Scholar] [CrossRef]
- Zhao, Y.; Araki, S.; Wu, J.; Teramoto, T.; Chang, Y.; Nakano, M.; Abdelfattah, A.S.; Fujiwara, M.; Ishihara, T.; Nagai, T.; et al. An expanded palette of genetically encoded Ca2+ indicators. Science 2011, 333, 1888–1891. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.; Wei, B.; Jin, Z.; Tian, Y. Facile Method for Fluorescent Labeling of Starch Nanocrystal. ACS Sustain. Chem. Eng. 2017, 5, 3751–3761. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Amr, E.G.E.; El-Naby, H.A.; Al-Omar, M.A.; Kamel, A.H.; Khalifa, N.M. Potentiometric PVC-Membrane-Based Sensor for Dimethylamine Assessment Using A Molecularly Imprinted Polymer as A Sensory Recognition Element. Polymers 2019, 11, 1695. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Misra, T.N. Manganese phthalocyanine for the detection of fish freshness by its trimethylamine emission. Sens. Actuators B 1997, 41, 199–202. [Google Scholar] [CrossRef]
- Hernández-Jover, T.; Zquierdo-Pulido, M.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Ion-pair high-performance liquid chromatographic determination of biogenic amines in meat and meat products. J. Agric. Food Chem. 1996, 44, 3097. [Google Scholar] [CrossRef]
- Wang, Q.H.; Fang, G.Z.; Liu, Y.Y.; Zhang, D.D.; Liu, J.M.; Wang, S. Fluorescent sensing probe for the sensitive detection of histamine based on molecular imprinting ionic liquid-modified quantum dots. Food Anal. Methods 2017, 10, 2585–2592. [Google Scholar] [CrossRef]
- Gao, F.; Grant, E.; Lu, X. Determination of histamine in canned tuna by molecularly imprinted polymers-surface enhanced Raman spectroscopy. Anal. Chim. Acta. 2015, 901, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, J.; Wei, W.; Wu, Y.; Li, T. Synthesis, characterization and application of TiO2/Ag recyclable SERS substrates. RSC Adv. 2017, 7, 26704–26709. [Google Scholar] [CrossRef] [Green Version]
- Stojanovi, Z.S.; Mehmeti, E.; Kalcher, K.; Guzsvány, V.; Stankovi, D.M. SWCNT-modified carbon paste electrode as an electrochemical sensor for histamine determination in alcoholic beverages. Food Anal. Methods 2016, 9, 2701–2710. [Google Scholar] [CrossRef]
- Amudha, J.; Noel, N.; Swaminathan, S.; Uma Maheswari, K.; John Bosco Balaguru, R. Chemometrics on Ceria-Polyaniline Modified Glassy Carbon Bioelectrode for Accurate Detection of Histamine in Fish. J. Comput. Theor. Nanosci. 2015, 12, 1911–1918. [Google Scholar]
- Huynh, K.; Pham, X.; Hahm, E.; An, J.; Kim, H.; Jo, A.; Seong, B.; Kim, Y.; Son, B.S.; Kim, J.; et al. Facile Histamine Detection by Surface-Enhanced Raman Scattering Using SiO2@Au@Ag Alloy Nanoparticles. Int. J. Mol. Sci. 2020, 21, 4048. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Ye, Y.; Qiu, Y.; Liu, J.; Huang, L.; Liang, B.; Chen, B. A Fluorescent Metal–Organic Framework for Food Real-Time Visual Monitoring. Adv. Mater. 2020, 33, 2008020. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Qi, J.; Li, Z.; Tian, H.; Xu, X. A Colorimetric Ag+ Probe for Food Real-Time Visual Monitoring. Nanomaterials 2022, 12, 1389. https://doi.org/10.3390/nano12091389
Yu J, Qi J, Li Z, Tian H, Xu X. A Colorimetric Ag+ Probe for Food Real-Time Visual Monitoring. Nanomaterials. 2022; 12(9):1389. https://doi.org/10.3390/nano12091389
Chicago/Turabian StyleYu, Jiahang, Jun Qi, Zhen Li, Huixin Tian, and Xinglian Xu. 2022. "A Colorimetric Ag+ Probe for Food Real-Time Visual Monitoring" Nanomaterials 12, no. 9: 1389. https://doi.org/10.3390/nano12091389
APA StyleYu, J., Qi, J., Li, Z., Tian, H., & Xu, X. (2022). A Colorimetric Ag+ Probe for Food Real-Time Visual Monitoring. Nanomaterials, 12(9), 1389. https://doi.org/10.3390/nano12091389





