Reaction Sintering of Machinable TiB2-BN-C Ceramics with In-Situ Formed h-BN Nanostructure
Abstract
1. Introduction
2. Experimental Procedure
2.1. Sample Sintering and Preparation
2.2. Material Characterization
3. Results and Discussion
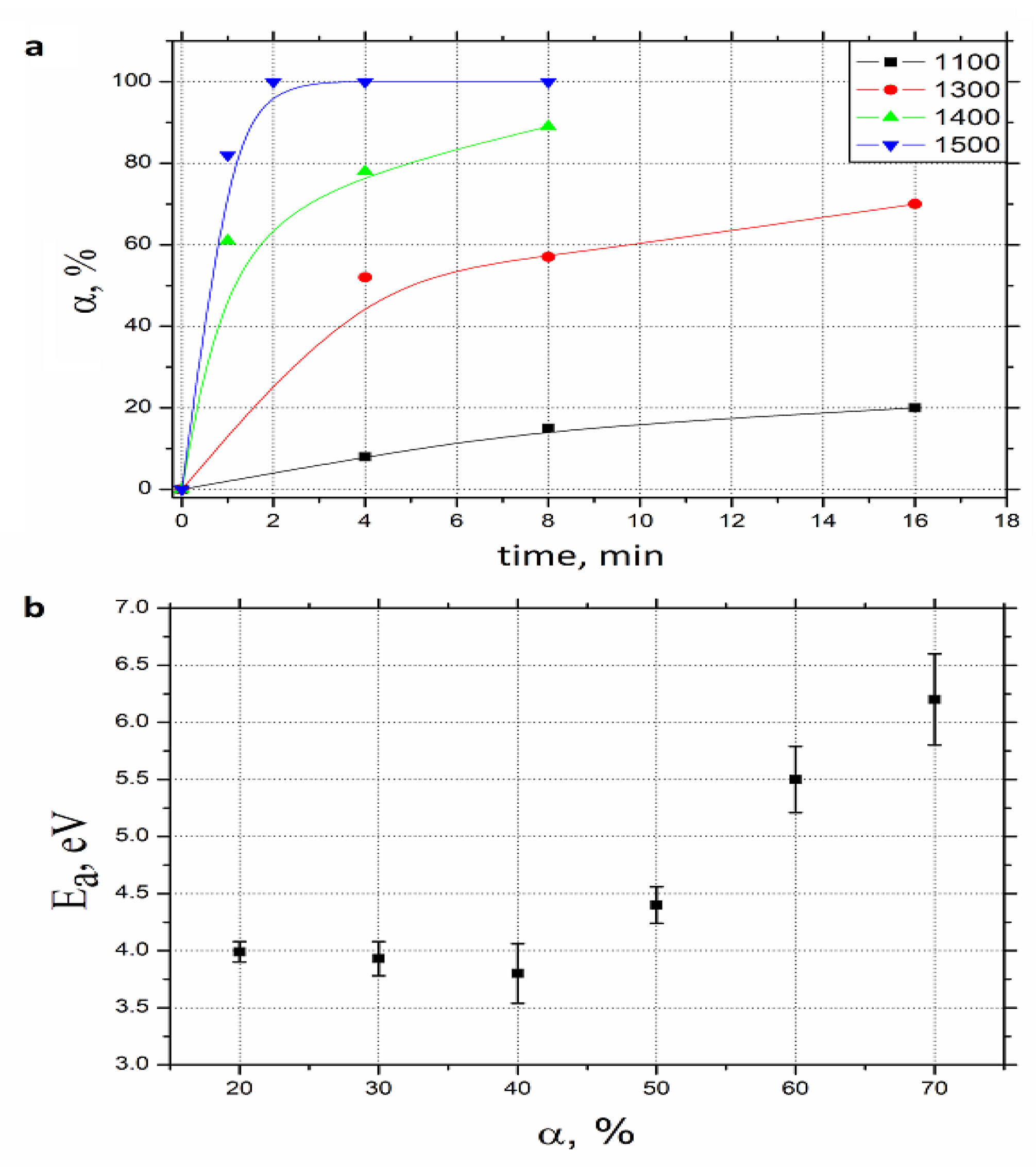
3.1. Densification and Reaction Kinetics
- Despite the fact that both mixtures were milled in the same way and the samples contained the same green body mass, at the beginning of the process the non-reactive sample was 10% higher (thicker, e.g., had 10% more volume) than the reactive one. It can be explained on the basis that the bulk density of the non-reactive composition (3.42 g/cm3) is 10% lower than that of the reactive one (3.72 g/cm3).
- The non-reactive charge started shrinking (e.g., densifying) after 600 °C while the reactive one remained almost unchanged up to 1250 °C. At 1250 °C, the green body expands and then begins to shrink at 1350 °C. The behavior of the non-reactive powder is most probably mediated by the powder densification helped by the lubrication provided by h-BN and graphite. The reactive sample growth corresponded to the TiB2 arrival (the degree of conversion raised from zero, Figure 3b) and could be caused by the reaction (2) dilatometry: the product density being lower than that of the precursors.
- The non-reactive sample densification proceeds at an almost constant rate over a broad span of time (more than 13 min) and sintering temperature (600 to 1900 °C). Even at 1900 °C and after holding at this temperature for 4 min the densification continues. While the reactive sample densification is almost complete within 5 min and in the interval of 1350–1900 °C.
- The density of sintered reactive sample is approximately 99% which is 8% higher than that of the non-reactive one.
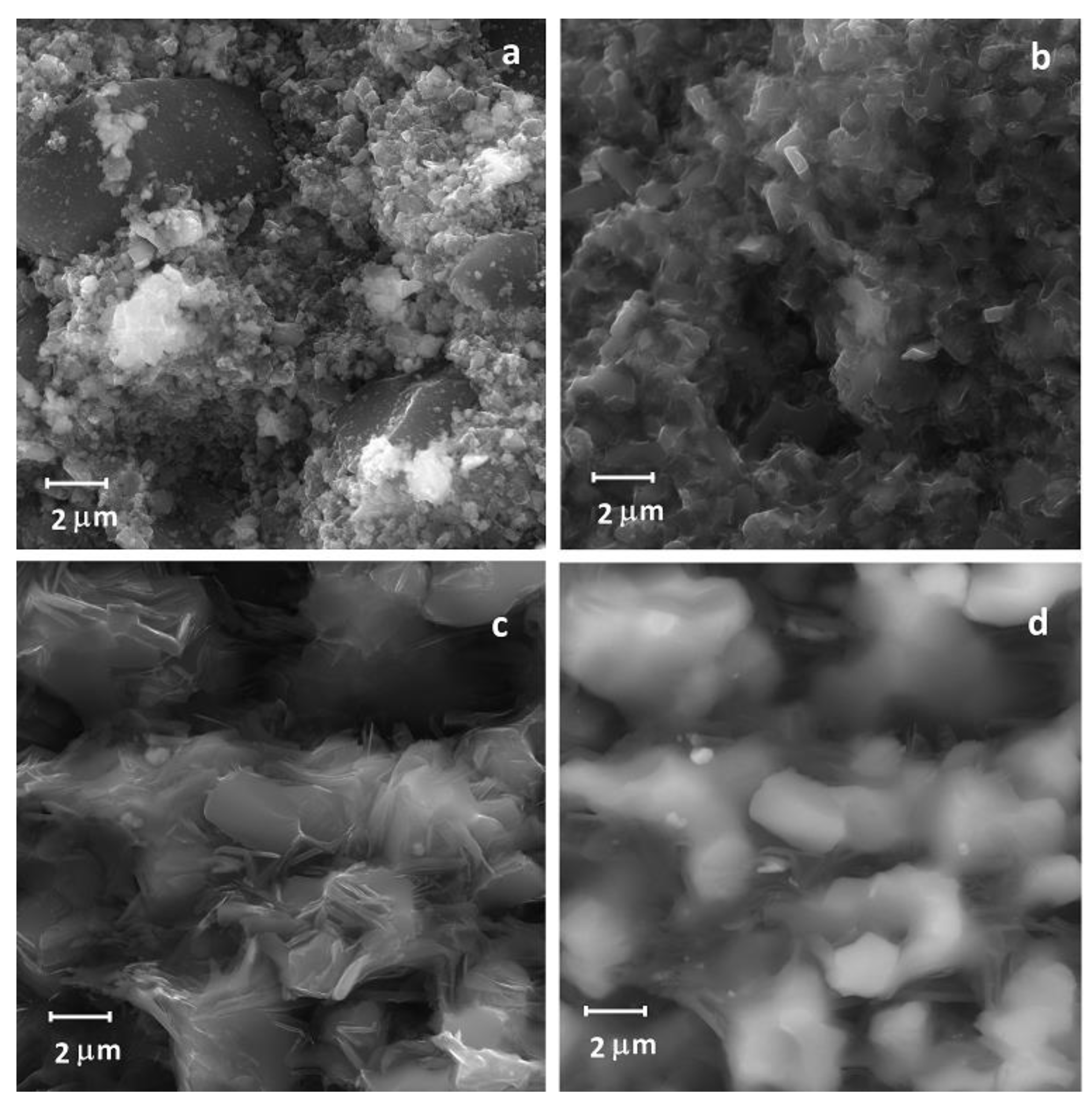
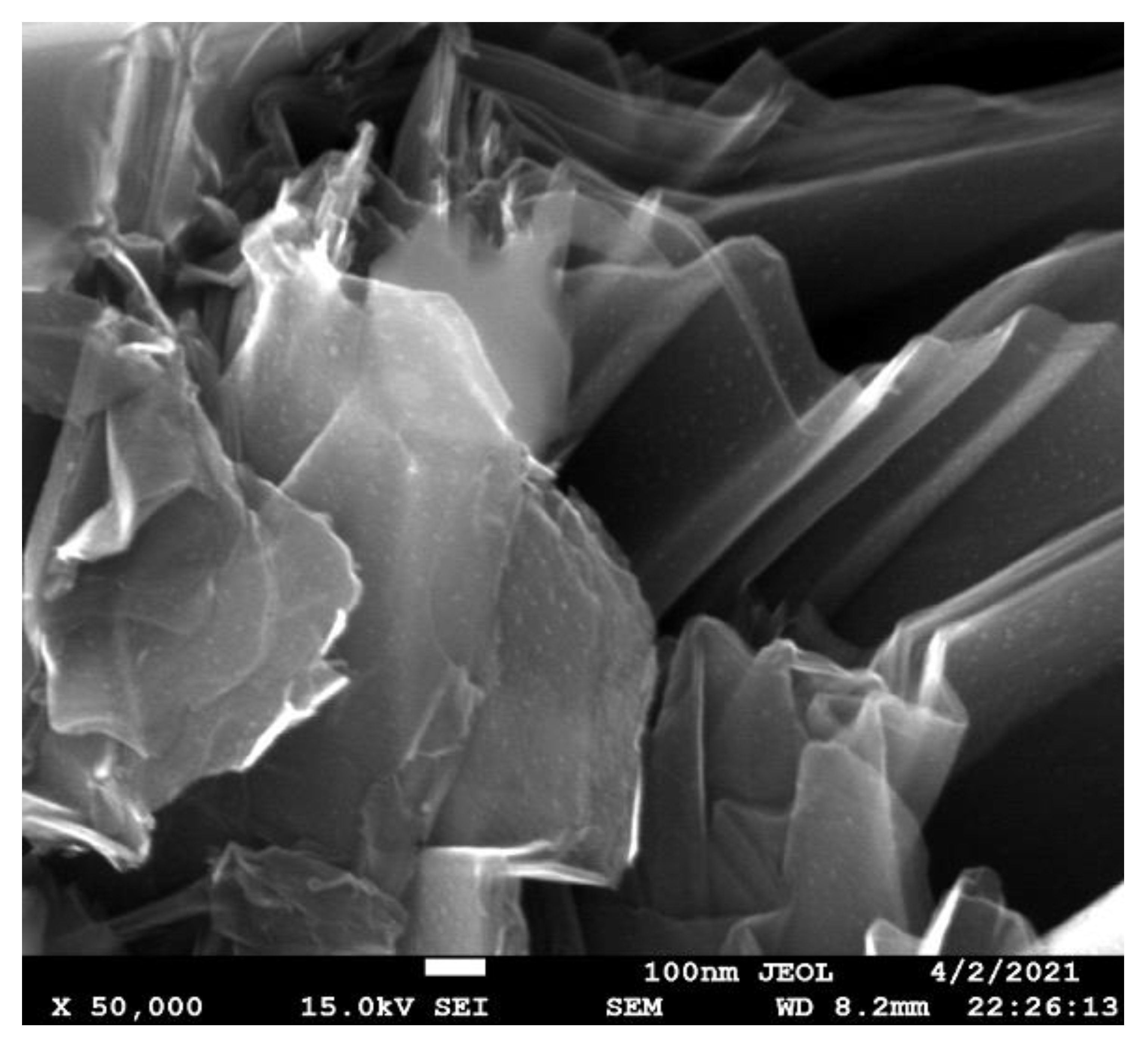
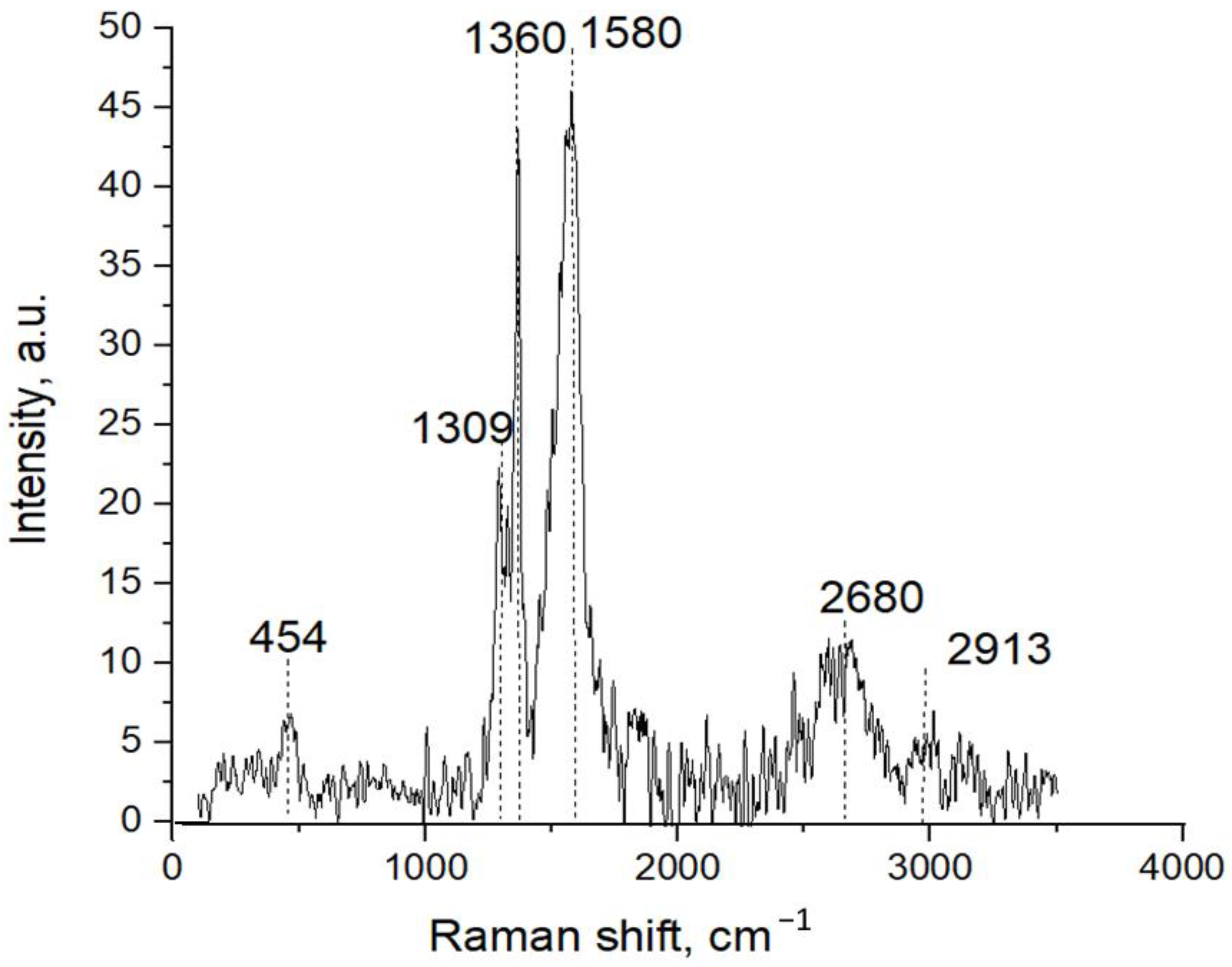
3.2. Atomic Structure Formation
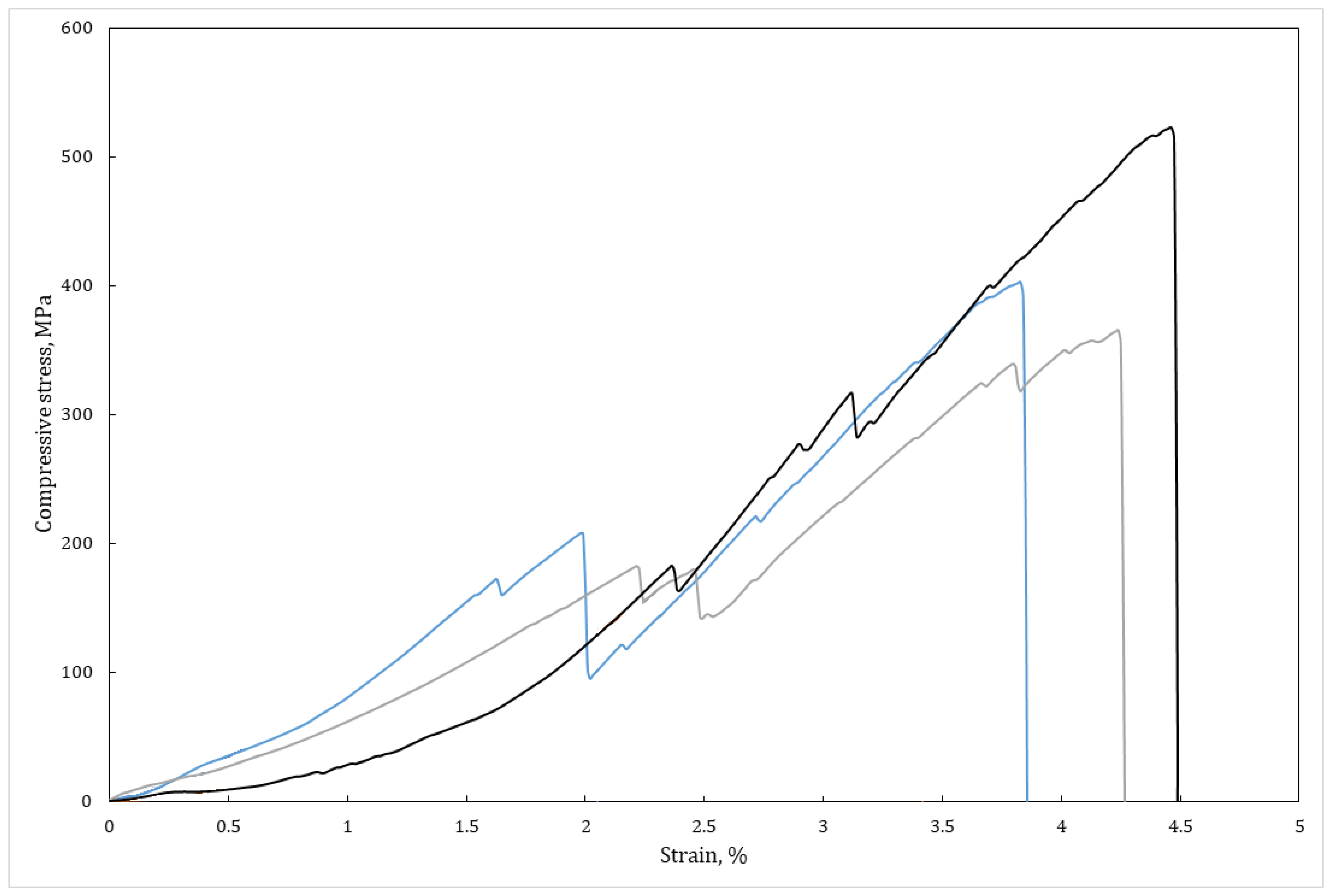
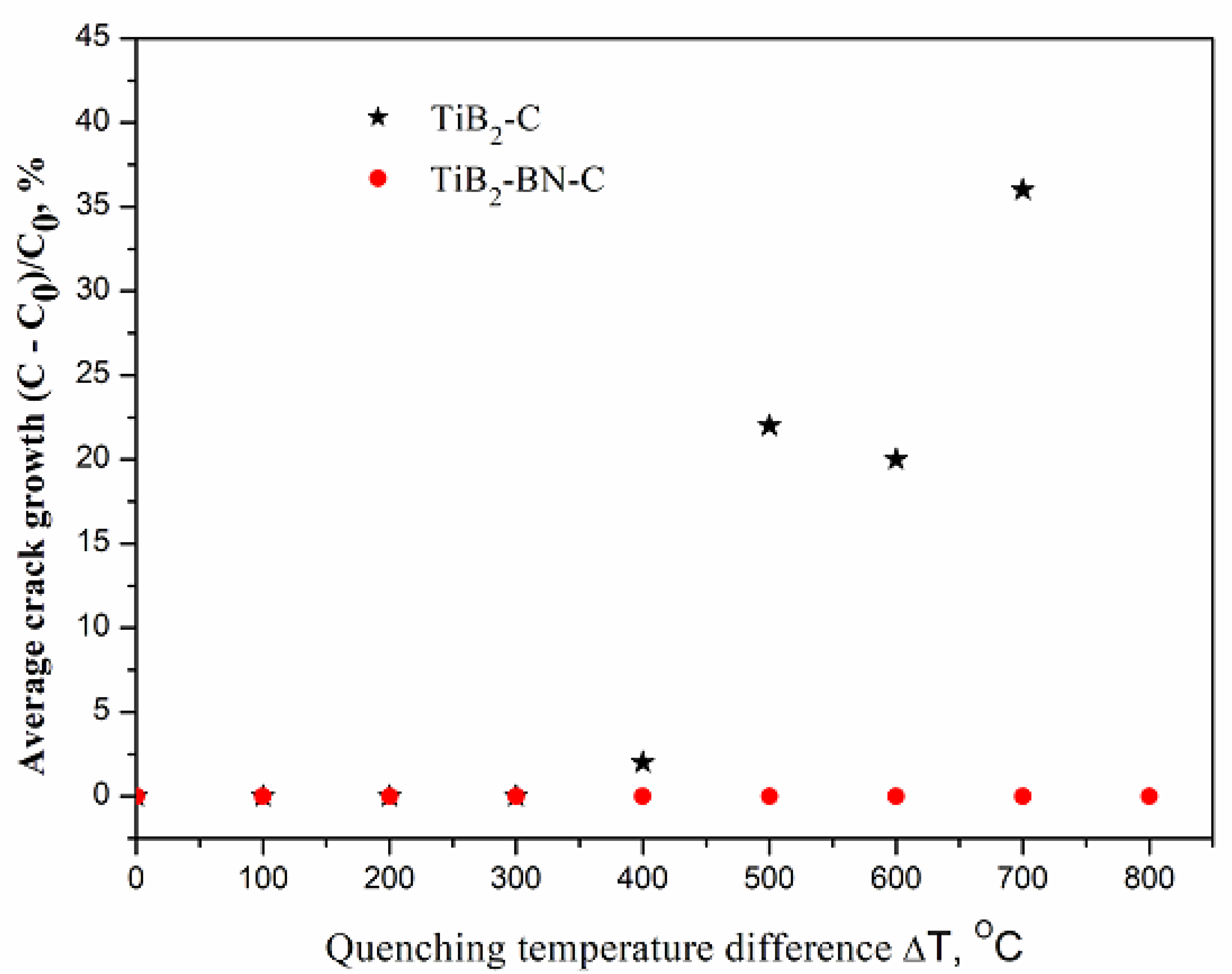
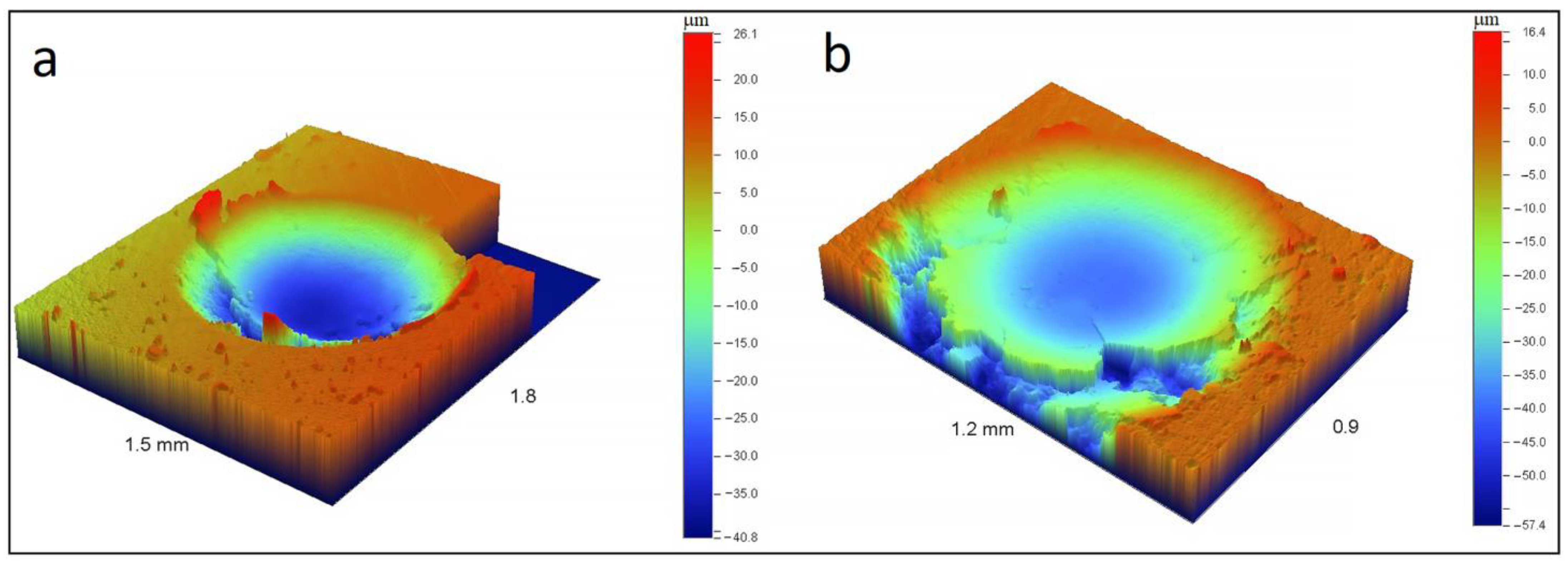
3.3. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fahrenholtz, W.G.; Hilmas, G.E. Ultra-high temperature ceramics: Materials for extreme environments. Scr. Mater. 2017, 129, 94–99. [Google Scholar] [CrossRef]
- Padture, N.P. Advanced structural ceramics in aerospace propulsion. Nat. Mater. 2016, 15, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Sani, E.; Mercatelli, L.; Sans, J.-L.; Silvestroni, L.; Sciti, D. Porous and dense hafnium and zirconium ultra-high temperature ceramics for solar receivers. Opt. Mater. 2013, 36, 163–168. [Google Scholar] [CrossRef]
- Sani, E.; Meucci, M.; Mercatelli, L.; Balbo, A.; Musa, C.; Licheri, R.; Orrù, R.; Cao, G. Titanium diboride ceramics for solar thermal absorbers. Sol. Energy Mater. Sol. Cells 2017, 169, 313–319. [Google Scholar] [CrossRef]
- Shabalin, I.L. Ultra-High. Temperature Materials, I.; Springer: Amsterdam, The Netherlands, 2014; p. 794. [Google Scholar]
- Bannister, M.K.; Swain, M.V. Thermal Shock of a Titanium Di-boride Based Composite. Ceram. Int. 1990, 16, 77–83. [Google Scholar] [CrossRef]
- Cheng, T.; Li, W.; Fang, D. Thermal Shock Resistance of Ultra-High-Temperature Ceramics. AIAA J. 2013, 51, 840–848. [Google Scholar] [CrossRef]
- Shabalin, I.L.; Tomkinson, D.M.; Shabalin, L.I. High-temperature hot-pressing of titanium carbide–graphite hetero-modulus ceramics. J. Eur. Ceram. Soc. 2007, 27, 2171–2181. [Google Scholar] [CrossRef]
- Gao, L.; Jin, X.; Li, J.; Li, Y.; Sun, J. BN/Si3N4 nanocomposite with high strength and good machinability. Mater. Sci. Eng. A 2006, 415, 145–148. [Google Scholar] [CrossRef]
- Gömze, L.A.; Gömze, L.N. Hetero-modulus alumina matrix nanoceramics and CMCs with extreme dynamic strength. IOP Conf. Ser. Mater. Sci. Eng. 2011, 18, 082001. [Google Scholar] [CrossRef]
- Popov, O.; Vishnyakov, V. Fracture toughness in some hetero-modulus composite carbides: Carbon inclusions and voids. Adv. Appl. Ceram. 2017, 116, 61–70. [Google Scholar] [CrossRef]
- Popov, O.; Avramenko, T.; Vishnyakov, V. Thermal conductivity and thermal shock resistance of TiB2-based UHTCs enhanced by graphite platelets. Mater. Today Commun. 2020, 26, 101756. [Google Scholar] [CrossRef]
- Yuan, B.; Wang, G. Preparation and properties of Si3N4/BN ceramic composites. Procedia Eng. 2012, 27, 1292–1298. [Google Scholar] [CrossRef][Green Version]
- Jiang, T.; Jin, Z.; Yang, J.; Qiao, G. Mechanical property and R-curve behavior of the B4C/BN ceramics composites. Mater. Sci. Eng. A 2008, 494, 203–216. [Google Scholar] [CrossRef]
- Shabalin, I.L.; Wang, Y.; Krynkin, A.V.; Umnova, O.V.; Vishnyakov, V.M.; Shabalin, L.I.; Churkin, V.K. Physicomechanical properties of ultrahigh temperature heteromodulus ceramics based on group 4 transition metal carbides. Adv. Appl. Ceram. 2010, 109, 405–416. [Google Scholar] [CrossRef]
- Kovalčíková, A.; Tatarko, P.; Sedlák, R.; Medved, D.; Chlup, Z.; Múdra, E.; Dusza, J. Mechanical and tribological properties of TiB2-SiC and TiB2-SiC-GNPs ceramic composites. J. Eur. Ceram. Soc. 2020, 40, 4860–4871. [Google Scholar] [CrossRef]
- Kusunose, T.; Sekino, T.; Choa, Y.H.; Niihara, K. Machinability of Silicon Nitride/Boron Nitride Nanocomposites. J. Am. Ceram. Soc. 2002, 85, 2689–2695. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Pan, W.; Wang, X.; Song, L.; Zhong, Z.; Wu, S. Fabrication and characterization of B4C-based ceramic composites with different mass fractions of hexagonal boron nitride. Ceram. Int. 2015, 41, 27–36. [Google Scholar] [CrossRef]
- BSong, B.; Yang, W.; Liu, X.; Chen, H.; Akhlaghi, M. Microstructural characterization of TiB2–SiC–BN ceramics prepared by hot pressing. Ceram. Int. 2021, 47, 29174–29182. [Google Scholar]
- Kusunose, T. Fabrication of Boron Nitride Dispersed Nanocomposites by Chemical Processing and Their Mechanical Properties. J. Ceram. Soc. Jpn. 2006, 114, 167–173. [Google Scholar] [CrossRef]
- John, D.; Jenkins, G.M. Hot-working and strengthening in metal carbide-graphite composites. J. Mater. Sci. 1986, 21, 2941–2958. [Google Scholar] [CrossRef]
- Akarsu, A.; Gökçe, H.; Boyraz, T.; Ertuğ, B.; Addemir, A.; Öveçoğlu, M. The Characterization of The Mechanical And Thermal Properties of Hot-Pressed Hexagonal Boron Nitride-Titanium Diboride Composites. In Proceedings of the Scientific Works of 3rd International Symposium on SiAlONs and Non-Oxides, Nevşehir, Turkey, 1–4 June 2010. [Google Scholar]
- Eichler, J.; Lesniak, C. Boron nitride (BN) and BN composites for high-temperature applications. J. Eur. Ceram. Soc. 2008, 28, 1105–1109. [Google Scholar] [CrossRef]
- Ping, H.; Weimin, W.; Yanling, D. Thermal Shock Resistance and Erosion Resistance of TiB2 Multiphase Ceramic Composites. J. Wuhan Univ. Technol. 2006, 21, 117–120. [Google Scholar] [CrossRef]
- MSalari, M.A.; Muğlu, G.M.; Rezaei, M.; Kumar, M.S.; Pulikkalparambil, H.; Siengchin, S. In-situ synthesis of TiN and TiB2 compounds during reactive spark plasma sintering of BN–Ti composites. Synth. Sinter. 2021, 1, 48–53. [Google Scholar]
- Zou, J.; Liu, J.; Zhao, J.; Zhang, G.J.; Huang, S.; Qian, B.; Vleugels, J.; Biest, O.V.; Shen, J.Z. A top-down ap-proach to densify ZrB2–SiC–BN composites with deeper homogeneity and improved reliability. Chem. Eng. J. 2014, 249, 93–101. [Google Scholar] [CrossRef]
- Derakhshandeh, M.R.; Fazili, A.; Golenji, R.B.; Alipour, F.; Eshraghi, M.J.; Nikzad, L. Fabrication of (TixZr1−x)B2-(ZrxTi1−x)N composites by reactive spark plasma sintering of ZrB2-TiN. J. Alloys Compd. 2021, 887, 161403. [Google Scholar] [CrossRef]
- Namini, A.S.; Delbari, S.A.; Oh, Y.; Asl, M.S.; Van Le, Q.; Cha, J.H.; Lee, S.-H.; Jang, H.W.; Han, H.N.; Shokouhimehr, M. Role of TiCN addition on the characteristics of reactive spark plasma sintered ZrB2-based novel composites. J. Alloys Compd. 2021, 875, 159901. [Google Scholar] [CrossRef]
- Namini, A.S.; Delbari, S.A.; Asl, M.S.; Van Le, Q.; Shokouhimehr, M. Characterization of reactive spark plasma sintered (Zr,Ti)B2–ZrC–SiC composites. J. Taiwan Inst. Chem. Eng. 2021, 119, 187–195. [Google Scholar] [CrossRef]
- Popov, O.; Chornobuk, S.; Vishnyakov, V. Structure formation of TiB2-TiC-B4C-C hetero-modulus ceramics via reaction hot pressing. Int. J. Refract. Met. Hard Mater. 2017, 64, 106–112. [Google Scholar] [CrossRef]
- Popov, O.; Vishnyakov, V.; Fleming, L.; Podgurskiy, M.; Blunt, L. Reaction sintering of biocompatible Al2O3-hBN ceramics. ACS Omega 2022, 7, 2205–2209. [Google Scholar] [CrossRef] [PubMed]
- Popov, O.; Vishnyakov, V.; Poperenko, L.; Yurgelevych, I.; Avramenko, T.; Ovcharenko, A. Reactively sintered TiB2-based heteromodulus UHT ceramics with in-situ formed graphene for machinable concentrated solar light absorbers. Ceram. Int. 2022, in press. [Google Scholar] [CrossRef]
- Popov, O.; Vishnyakov, V.; Chornobuk, S.; Totsky, I.; Plyushchay, I. Mechanisms of TiB2 and graphite nucleation during TiC-B4C high temperature interaction. Ceram. Int. 2019, in press. [Google Scholar] [CrossRef]
- Chornobuk, S.V.; Popov, A.Y.; Makara, V.A. Structure and mechanical properties of reaction-sintered ceramic composite materials based on titanium and hafnium diborides. J. Superhard Mater. 2009, 31, 86–88. [Google Scholar] [CrossRef]
- Evans, J.S.O.; Evans, I.R. Structure Analysis from Powder Diffraction Data: Rietveld Refinement in Excel. J. Chem. Educ. 2021, 98, 495–505. [Google Scholar] [CrossRef]
- Niihara, K.; Morena, R.; Hasselman, D.P.H. Evaluation of K1c of brittle solids by the indentation method with low crack-to-indent ratios. J. Mater. Sci. Lett. 1982, 1, 13–16. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Y.; Fang, G.; Han, J. Research on thermal shock resistance of ZrB2–SiC–AlN ceramics using an indentation-quench method. J. Alloys Compd. 2010, 493, 695–698. [Google Scholar] [CrossRef]
- Popov, O.; Klepko, A.; Lutsak, E. The influence of high pressure on TiC-B4C reaction kinetics. Int. J. Refract. Met. Hard Mater. 2018, 75, 234–237. [Google Scholar] [CrossRef]
- NIST. Chemistry WebBook. Available online: http://webbook.nist.gov/chemistry/ (accessed on 1 September 2021).
- Schmidt, H.; Borchardt, G. Self-diffusion of boron in TiB2. J. Appl. Phys. 2003, 93, 907–911. [Google Scholar] [CrossRef]
- Mar, R.; Bedford, R. Sublimation of boron. High Temp. Sci. 1976, 8, 365–376. [Google Scholar]
- Golberg, D.; Bando, Y.; Huang, Y.; Terao, T.; Mitome, M.; Tang, C.; Zhi, C. Boron Nitride Nanotubes and Nanosheets. ACS Nano 2010, 4, 2979–2993. [Google Scholar] [CrossRef] [PubMed]
- Bergman, L.; Nemanich, R.J. Raman spectroscopy characterization of hard, wide-bandgap semiconductors: Diamond, GaN, GaAlN, AlN, BN. Annu. Rev. Mater. Sci. 1996, 26, 551–579. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman spectra of carbon-based materials (from graphite to carbon black) and of some silicone composites. C-J. Carbon Res. 2015, 1, 77–94. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman spectroscopy as a tool for the analysis of carbon-based materials (highly oriented pyrolitic graphite, multilayer graphene and multiwall carbon nanotubes) and of some of their elastomeric composites. Vib. Spectrosc. 2014, 74, 57–63. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Saito, R.; Hofmann, M.; Dresselhaus, G.; Jorio, A.; Dresselhaus, M.S. Raman scattering of graphene and carbon nanotubes. Adv. Phys. 2011, 60, 413–550. [Google Scholar] [CrossRef]
- Casari, C.S.; Bassi, A.L.; Baserga, A.; Ravagnan, L.; Piseri, P.; Lenardi, C.; Tommasini, M.; Milani, A.; Fazzi, D.; Bottani, C.E.; et al. Low-frequency modes in the Raman spectrum of sp-sp2 nanostructured carbon. Phys. Rev. B 2008, 77, 195444. [Google Scholar] [CrossRef]
- Tian, S.; Xing, W.; Wang, W.; FU, Z.; Wang, H.; Zhang, J. Influence of particle size of TiB2 on densification and properties of BN-TiB2 composite ceramics. J. Chin. Ceram. Soc. 2001, 39, 1757–1762. [Google Scholar]
- Kitiwan, M.; Ito, A.; Goto, T. Spark plasma sintering of TiN–TiB2–hBN composites and their properties. Ceram. Int. 2015, 41, 4498–4503. [Google Scholar] [CrossRef]
- Li, W.; Li, D.; Cheng, T.; Fang, D. Temperature-damage-dependent thermal shock resistance model for ultra-high temperature ceramics. Eng. Fract. Mech. 2012, 82, 9–16. [Google Scholar] [CrossRef]
- Lu, T.; Fleck, N. The thermal shock resistance of solids. Acta Mater. 1998, 46, 4755–4768. [Google Scholar] [CrossRef]




| Title | TiN, wt.% | B4C, wt.% | TiB2, wt.% | BN, wt.% | C, wt.% |
|---|---|---|---|---|---|
| Reactive | 60.4 | 39.6 | - | - | - |
| Nonreactive | - | - | 71.1 | 20.3 | 8.6 |
| Composition, vol.% | Ref. | Sintering Route | ρ/ρth, % | HV, GPa | K1C, MPa·m1/2 | σflex, MPa | σcomp, MPa |
|---|---|---|---|---|---|---|---|
| TiB2-33BN-13C | This work | RHP, 30 MPa, 1900 °C, 4 min | 99 | 1.6 | 5.3 | 420 | |
| TiB2-31C | [12] | RHP, 30MPa, 1800 °C, 8 min | 100 | 4.3 | 5 | ||
| TiC-41C | [15] | HP, 8 MPa, 2700 °C, 30 min | 95.7 | 0.8 | 5.5 | 50 | 180 |
| TiC-33C | [15] | HP, 8 MPa, 2700 °C, 30 min | 99.7 | 1.5 | 5.5 | 120 | 350 |
| TiB2-60BN | [49] | HP, 30 MPa, 1850 °C, 90 min | 83.9 | 85 | |||
| TiB2-50BN | [22] | HP, 30 MPa, 1900 °C, 60 min | 71 | 1.2 | |||
| TiB2-21TiN-30BN | [50] | SPS, 100 MPa, 1700 °C, 5 min | 96 | 8 | <3.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popov, O.; Shtansky, D.V.; Vishnyakov, V.; Klepko, O.; Polishchuk, S.; Kutzhanov, M.K.; Permyakova, E.S.; Teselko, P. Reaction Sintering of Machinable TiB2-BN-C Ceramics with In-Situ Formed h-BN Nanostructure. Nanomaterials 2022, 12, 1379. https://doi.org/10.3390/nano12081379
Popov O, Shtansky DV, Vishnyakov V, Klepko O, Polishchuk S, Kutzhanov MK, Permyakova ES, Teselko P. Reaction Sintering of Machinable TiB2-BN-C Ceramics with In-Situ Formed h-BN Nanostructure. Nanomaterials. 2022; 12(8):1379. https://doi.org/10.3390/nano12081379
Chicago/Turabian StylePopov, Oleksii, Dmitry V. Shtansky, Vladimir Vishnyakov, Oleksandra Klepko, Sergey Polishchuk, Magzhan K. Kutzhanov, Elizaveta S. Permyakova, and Petro Teselko. 2022. "Reaction Sintering of Machinable TiB2-BN-C Ceramics with In-Situ Formed h-BN Nanostructure" Nanomaterials 12, no. 8: 1379. https://doi.org/10.3390/nano12081379
APA StylePopov, O., Shtansky, D. V., Vishnyakov, V., Klepko, O., Polishchuk, S., Kutzhanov, M. K., Permyakova, E. S., & Teselko, P. (2022). Reaction Sintering of Machinable TiB2-BN-C Ceramics with In-Situ Formed h-BN Nanostructure. Nanomaterials, 12(8), 1379. https://doi.org/10.3390/nano12081379






