Advanced Functionalized CeO2/Al2O3 Nanocomposite Sensor for Determination of Opioid Medication Tramadol Hydrochloride in Pharmaceutical Formulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instruments
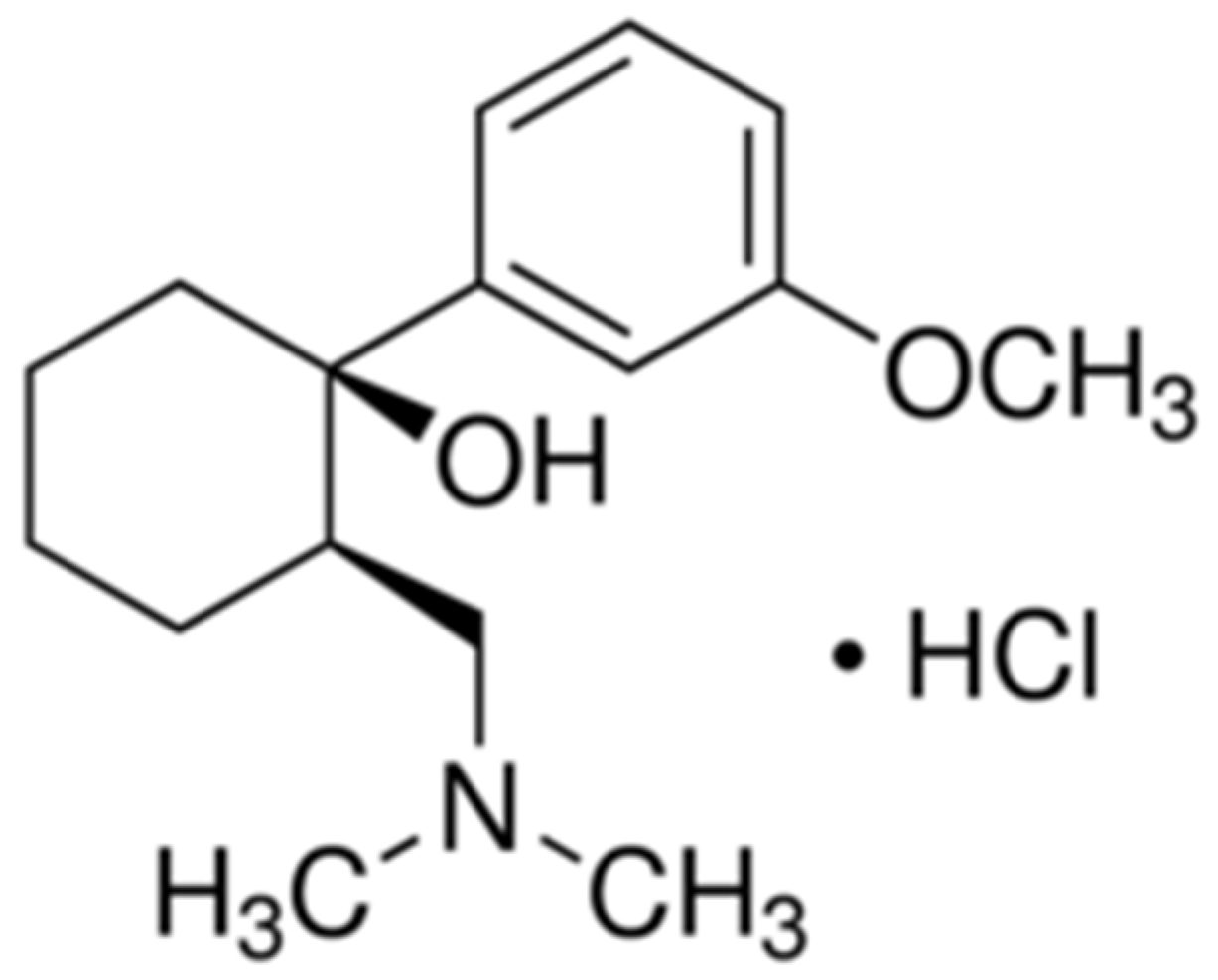
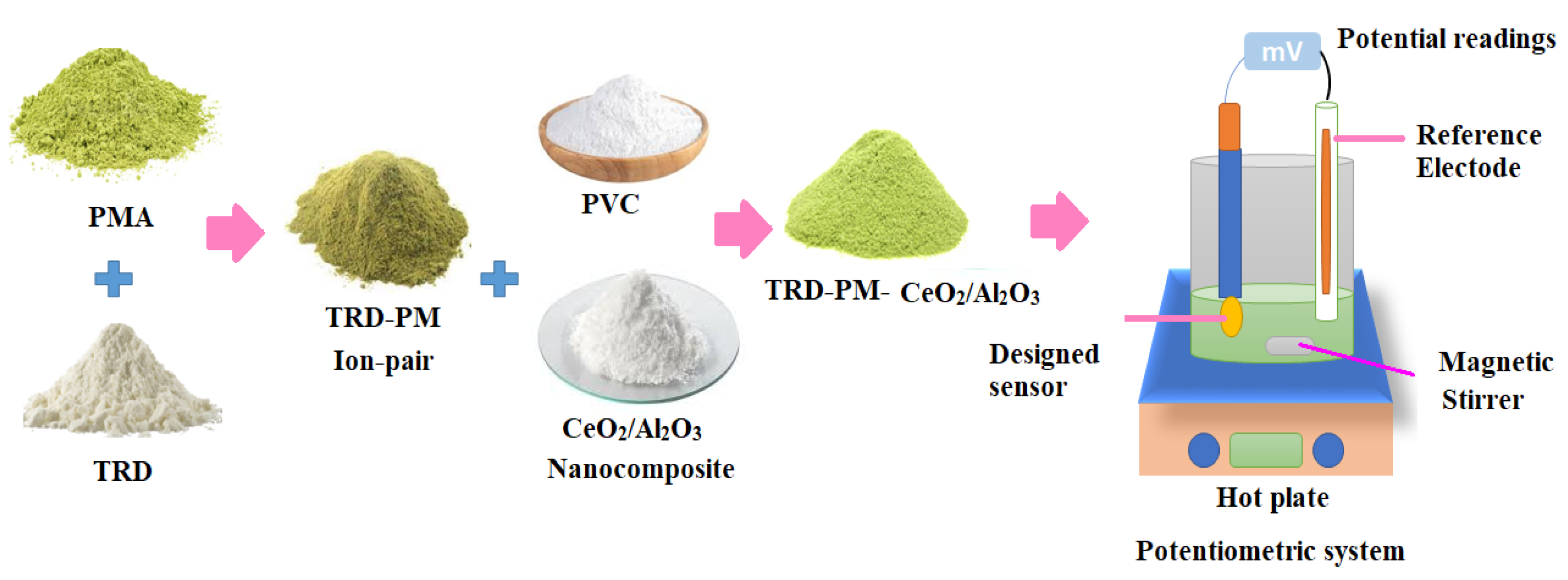
2.3. Preparation of TRD-PM Electroactive Material
2.4. Synthesis of CeO2 and Al2O3 Nanoparticles
2.5. Preparation of Polymeric TRD-PM-CeO2/Al2O3 Nanocomposite
2.6. Preparation of Standard TRD Solution
2.7. Sensor Design and Membrane Composition
2.8. Calibration Graph
2.9. Optimization of Analytical Conditions
2.10. Quantification of Tramadol Hydrochloride® Capsules
3. Results and Discussion
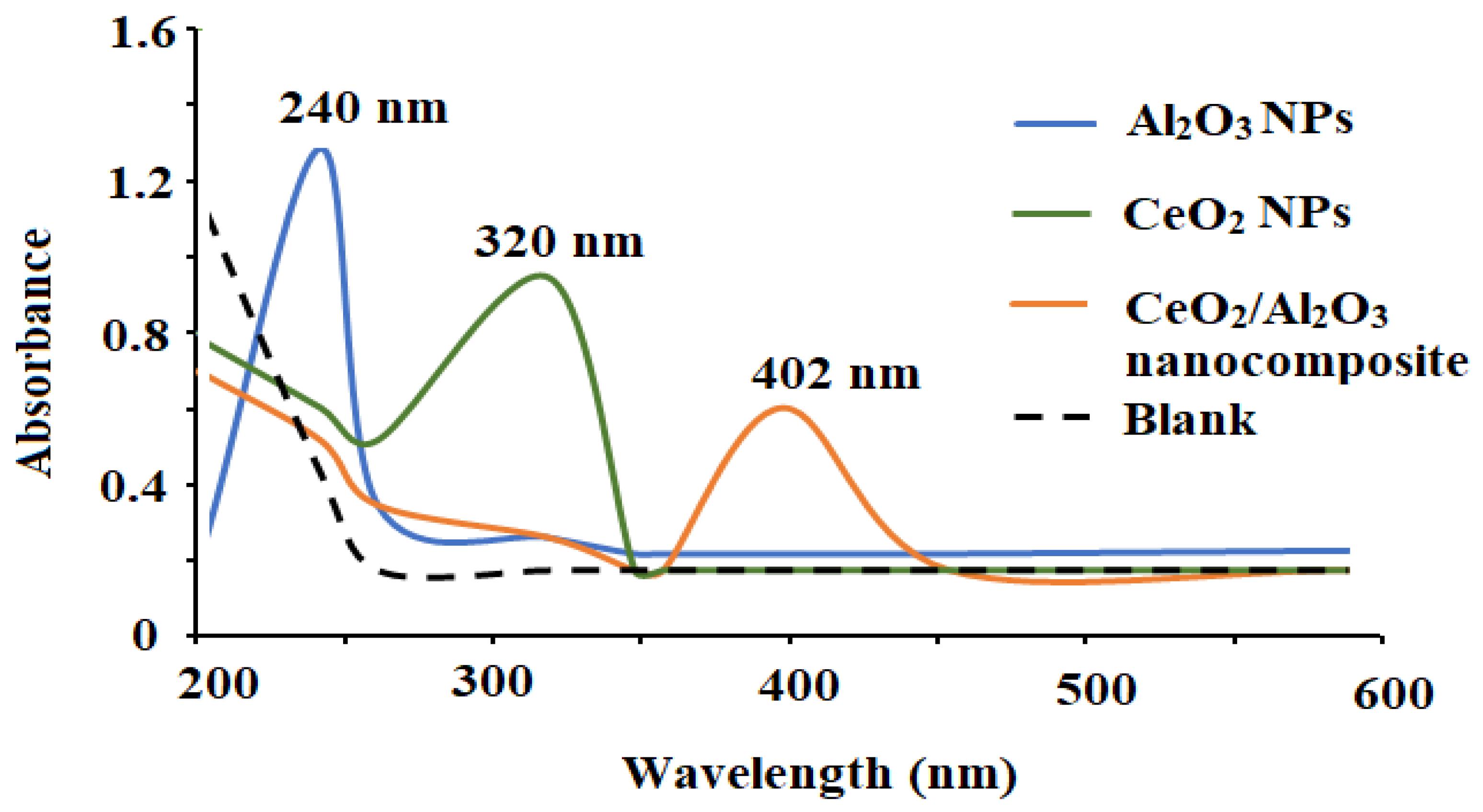
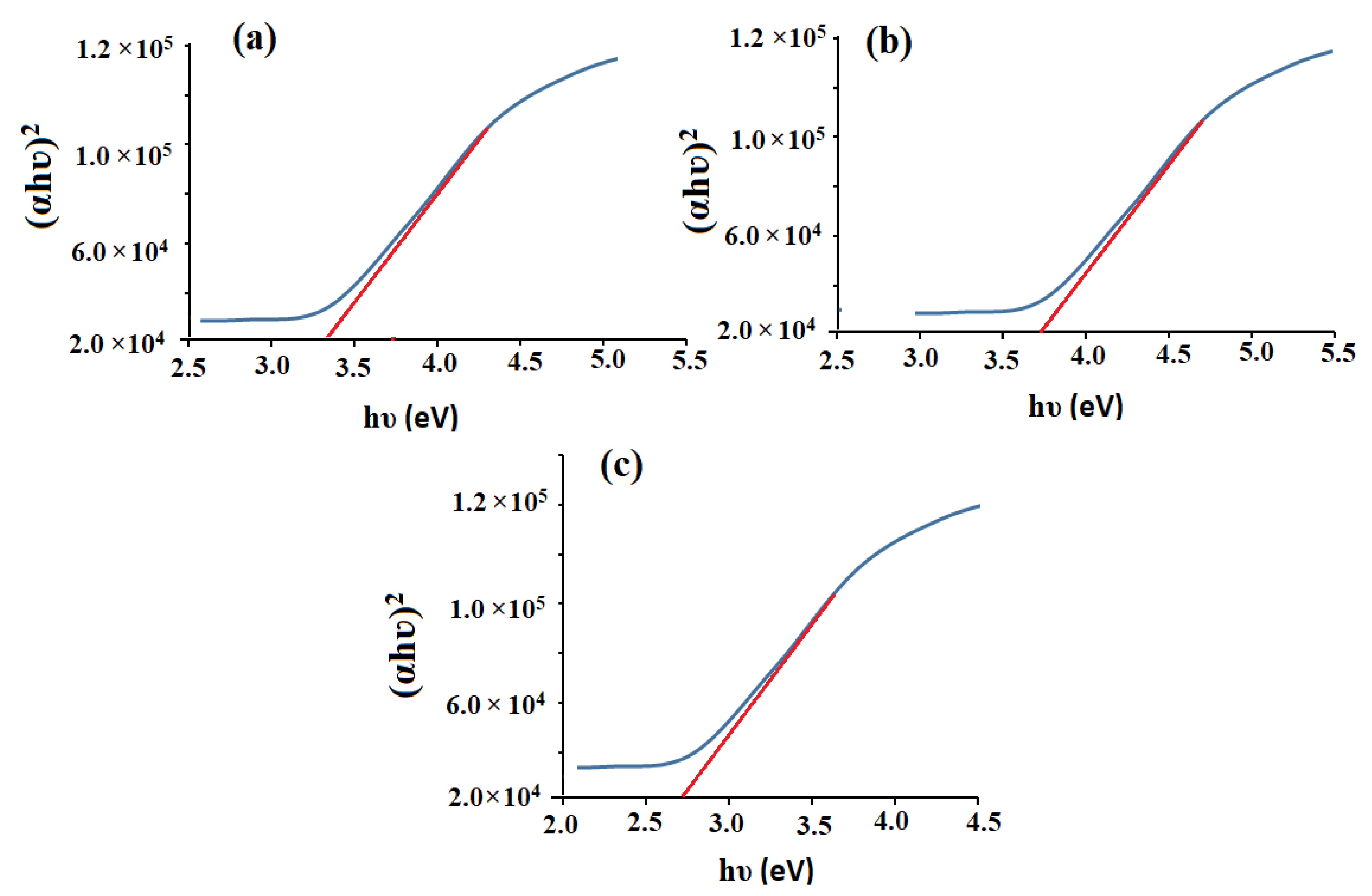
3.1. Characterization of CeO2/Al2O3 Nanocomposite
3.2. Performance Response of the Suggested Sensors
3.3. Quantification of TRD in Bulk Form
3.4. Validation of the Suggested Method
3.5. Estimation of TRD in Tramadol hydrochloride® Capsules
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hornyak, G.L.; Tibbals, H.F.; Dutta, J.; Moore, J.J. Introduction to Nanoscience and Nanotechnology; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar] [CrossRef]
- Omanovic-Miklicanin, E.; Badnjevic, A.; Kazlagic, A.; Hajlovac, M. Nanocomposites: A brief review. Health Technol. 2020, 10, 51–59. [Google Scholar] [CrossRef]
- Bet-Moushoul, E.; Mansourpanah, Y.; Farhadi, K.; Tabatabaei, M. TiO2 nanocomposite based polymeric membranes: A review on performance improvement for various applications in chemical engineering processes. Chem. Eng. J. 2016, 283, 29–46. [Google Scholar] [CrossRef]
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial bio-nanocomposites and their potential applications in food packaging. Food Control 2020, 112, 107086. [Google Scholar] [CrossRef]
- Ma, D.; Esmaeili, A.; Sbarufatti, C.; Giglio, M.; Manes, A. A method for determining the distribution of carbon nanotubes in nanocomposites by electric conductivity. Procedia Struct. Integr. 2022, 37, 105–114. [Google Scholar] [CrossRef]
- Stephanie, R.; Kim, M.W.; Kim, S.H.; Kim, J.K.; Park, C.Y.; Park, T.J. Recent advances of bimetallic nanomaterials and its nanocomposites for biosensing applications. TrAC Trends Anal. Chem. 2021, 135, 116159. [Google Scholar] [CrossRef]
- Dhatarwal, P.; Sengwa, R.J. Investigation on the optical properties of (PVP/PVA)/Al2O3 nanocomposite films for green disposable optoelectronics. Phys. B Condens. Matter 2021, 613, 412989. [Google Scholar] [CrossRef]
- Salauddin, M.; Rana, S.S.; Sharifuzzaman, M.; Rahman, M.T.; Park, C.; Cho, H.; Maharjan, P.; Bhatta, T.; Park, J.Y. A Novel MXene/Ecoflex Nanocomposite-coated Fabric as a highly negative and stable friction layer for high-output triboelectric nanogenerators. Adv. Energy Mater. 2021, 11, 2002832. [Google Scholar] [CrossRef]
- Choi, S.; Han, S.I.; Kim, D.; Hyeon, T.; Kim, D.H. High-performance stretchable conductive nanocomposites: Materials, processes, and device applications. Chem. Soci. Rev. 2019, 48, 1566–1595. [Google Scholar] [CrossRef]
- Javanbakht, S.; Pooresmaeil, M.; Namazi, H. Green one-pot synthesis of carboxymethylcellulose/Zn-based metal-organic framework/graphene oxide bio-nanocomposite as a nanocarrier for drug delivery system. Carbohydr. Polym. 2019, 208, 294–301. [Google Scholar] [CrossRef]
- Barkhordari, S.; Alizadeh, A.; Yadollahi, M.; Namazi, H. One-pot synthesis of magnetic chitosan/iron oxide bio-nanocomposite hydrogel beads as drug delivery systems. Soft Mater. 2021, 19, 373–381. [Google Scholar] [CrossRef]
- Kalyani, T.; Sangili, A.; Nanda, A.; Prakash, S.; Kaushik, A.; Jana, S.K. Bio-nanocomposite based highly sensitive and label-free electrochemical immunosensor for endometriosis diagnostics application. Bioelectrochemistry 2021, 139, 107740. [Google Scholar] [CrossRef] [PubMed]
- Maleki, P.; Nemati, F.; Gholoobi, A.; Hashemzadeh, A.; Sabouri, Z.; Darroudi, M. Green facile synthesis of silver-doped cerium oxide nanoparticles and investigation of their cytotoxicity and antibacterial activity. Inorg. Chem. Commun. 2021, 131, 108762. [Google Scholar] [CrossRef]
- Song, G.; Cheng, N.; Zhang, J.; Huang, H.; Yuan, Y.; He, X.; Luo, Y.; Huang, K. Nanoscale cerium oxide: Synthesis, biocatalytic mechanism, and applications. Catalysts 2021, 11, 1123. [Google Scholar] [CrossRef]
- Kızılkonca, E.; Torlak, E.; Erim, F.B. Preparation and characterization of antibacterial nano cerium oxide/chitosan/hydroxyethylcellulose/polyethylene glycol composite films. Int. J. Biol. Macromol. 2021, 177, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tripathy, S.; Singh, O.K.; Singh, S.G. Cerium oxide nanofiber based electroanalytical sensor for TNF-α detection: Improved interfacial stability with Nafion. Bioelectrochemistry 2021, 138, 107725. [Google Scholar] [CrossRef] [PubMed]
- Prakash, V.; Peralta-Videa, J.; Tripathi, D.K.; Ma, X.; Sharma, S. Recent insights into the impact, fate and transport of cerium oxide nanoparticles in the plant-soil continuum. Ecotoxicol. Environ. Saf. 2021, 221, 112403. [Google Scholar] [CrossRef]
- Casals, G.; Perramon, M.; Casals, E.; Portoles, I.; Fernandez-Varo, G.; Morales-Ruiz, M.; Puntes, V.; Jimenez, W. Cerium oxide nanoparticles: A new therapeutic tool in liver diseases. Antioxidants 2021, 10, 660. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, N. Optical Properties of Aluminium Oxide Nanoparticles Synthesized by Leaf Extract of Ocimum Sanctum. J. Nanosci. Technol. 2019, 5, 817–819. [Google Scholar] [CrossRef]
- Channappagoudra, M. Influence of the aluminium oxide (Al2O3) nanoparticle additive with biodiesel on the modified diesel engine performance. Int. J. Ambient Energy 2021, 42, 1776–1784. [Google Scholar] [CrossRef]
- Menni, Y.; Chamkha, A.J.; Massarotti, N.; Ameur, H.; Kaid, N.; Bensafi, M. Hydrodynamic and thermal analysis of water, ethylene glycol and water-ethylene glycol as base fluids dispersed by aluminum oxide nano-sized solid particles. Int. J. Numer. Method. Heat Fluid Flow 2020, 30, 4349–4386. [Google Scholar] [CrossRef]
- Hassan, M.; Faisal, A.; Bhatti, M.M. Interaction of aluminum oxide nanoparticles with flow of polyvinyl alcohol solutions base nanofluids over a wedge. Appl. Nanosci. 2018, 8, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Naik, M.C.; Bamane, S.R.; Pakhare, K.S.; Potdar, S.S.; Patil, U.M. Synthesis of CeO2-Al2O3 nanocomposite by chemical combustion method for NO2 gas-sensing application. J. Mater. Sci. Mater. Electron. 2021, 32, 19925–19937. [Google Scholar] [CrossRef]
- Krishnan, A.; Sha, M.A.; Basheer, R.; Riyas, A.H.; Shibli, S.M.A. Vacancy mediated improvement in electrocatalytic HER on Nano-CeO2-Al2O3 incorporated Ni-P electrode. Mater. Sci. Semicond. Process. 2020, 116, 105138. [Google Scholar] [CrossRef]
- Avramova, I.; Stefanov, P.; Nicolova, D.; Stoychev, D.; Marinova, T. Characterization of nanocomposite CeO2–Al2O3 coatings electrodeposited on stainless steel. Compos. Sci. Technol. 2005, 65, 1663–1667. [Google Scholar] [CrossRef]
- Yulizar, Y.; Juliyanto, S.; Apriandanu, D.O.B.; Surya, R.M. Novel sol-gel synthesis of CeO2 nanoparticles using Morinda citrifolia L. fruit extracts: Structural and optical analysis. J. Mol. Struct. 2021, 1231, 129904. [Google Scholar] [CrossRef]
- Tabesh, S.; Davar, F.; Loghman-Estarki, M.R. Preparation of γ-Al2O3 nanoparticles using modified sol-gel method and its use for the adsorption of lead and cadmium ions. J. Alloys Compd. 2018, 730, 441–449. [Google Scholar] [CrossRef]
- Al-Shawafi, W.M.; Salah, N.; Alshahrie, A.; Ahmed, Y.M.; Moselhy, S.S.; Hammad, A.H.; Hussain, M.A.; Memic, A. Size controlled ultrafine CeO2 nanoparticles produced by the microwave assisted route and their antimicrobial activity. J. Mater. Sci. Mater. Med. 2017, 28, 1–10. [Google Scholar] [CrossRef]
- Hasanpoor, M.; Fakhr Nabavi, H.; Aliofkhazraei, M. Microwave-assisted synthesis of alumina nanoparticles using some plants extracts. J. Nanostruct. 2017, 7, 40–46. [Google Scholar] [CrossRef]
- Wang, T.; Sun, D.C. Preparation and characterization of nanometer-scale powders ceria by electrochemical deposition method. Mater. Res. Bull. 2008, 43, 1754–1760. [Google Scholar] [CrossRef]
- Pathania, D.; Katwal, R.; Kaur, H. Enhanced photocatalytic activity of electrochemically synthesized aluminum oxide nanoparticles. Int. J. Miner. Metall. and Mater. 2016, 23, 358–371. [Google Scholar] [CrossRef]
- Sudha, V.; Murugadoss, G.; Thangamuthu, R. Structural and morphological tuning of Cu-based metal oxide nanoparticles by a facile chemical method and highly electrochemical sensing of sulphite. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Pu, H.; Sun, D.W. DNA functionalized metal and metal oxide nanoparticles: Principles and recent advances in food safety detection. Crit. Rev. Food Sci. Nutr. 2021, 61, 2277–2296. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Ryu, H.; Lei, Y. Metal oxide based non-enzymatic electrochemical sensors for glucose detection. Electrochim. Acta 2021, 370, 137744. [Google Scholar] [CrossRef]
- Pragathiswaran, C.; Thulasi, G.; Al-Ansari, M.M.; Al-Humaid, L.A.; Saravanan, M. Experimental investigation and electrochemical characterization of titanium coated nanocomposite materials for biomedical applications. J. Mol. Struct. 2021, 1231, 129932. [Google Scholar] [CrossRef]
- Hua, R.; Hao, N.; Lu, J.; Qian, J.; Liu, Q.; Li, H.; Wang, K. A sensitive Potentiometric resolved ratiometric Photoelectrochemical aptasensor for Escherichia coli detection fabricated with non-metallic nanomaterials. Biosens. Bioelectron. 2018, 106, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Numan, A.; Somaily, H.H.; Dawsari, M.M.; Alqarni, M.H.S.; Alam, A.; Kumar, P. A novel, eco-friendly multi-walled carbon nanotubes functionalized copper metal-organic framework for ultrasensitive potentiometric detection of cadmium ions. J. Environ. Chem. Eng. 2021, 9, 106534. [Google Scholar] [CrossRef]
- Fazio, E.; Spadaro, S.; Corsaro, C.; Neri, G.; Leonardi, S.G.; Neri, F.; Lavanya, N.; Sekar, C.; Donato, N.; Neri, G. Metal-oxide based nanomaterials: Synthesis, characterization and their applications in electrical and electrochemical sensors. Sensors 2021, 21, 2494. [Google Scholar] [CrossRef]
- Khorshid, A.F. New analysis for itopride hydrochloride utilizing chemically modified carbon paste sensor in ganaton, garopride, bulk, human urine/plasma. Sens. Bio Sens. Res. 2022, 100479. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, C.; Wu, S.; Cheng, R.; Gao, J.; Yu, Y.; Zhou, W. Phosphomolybdic acid-decorated carbon nanotubes for low-power sensing of NH3 and NO2 at room temperature. ACS Appl. Nano Mater. 2021, 4, 1976–1984. [Google Scholar] [CrossRef]
- Vazzana, M.; Andreani, T.; Fangueiro, J.; Faggio, C.; Silva, C.; Santini, A.; Garcia, M.L.; Silva, A.M.; Souto, E.B. Tramadol hydrochloride: Pharmacokinetics, pharmacodynamics, adverse side effects, co-administration of drugs and new drug delivery systems. Biomed. Pharmacother. 2015, 70, 234–238. [Google Scholar] [CrossRef]
- Taghizade, M.; Ebrahimi, M.; Fooladi, E.; Yoosefian, M. Simultaneous spectrophotometric determination of the residual of ciprofloxacin, famotidine, and tramadol using magnetic solid phase extraction coupled with multivariate calibration methods. Microchem. J. 2021, 160, 105627. [Google Scholar] [CrossRef]
- Hamdy, M.M.; Abdel Moneim, M.M. HPLC-fluorescence detection for assay of tramadol binary mixtures with ibuprofen or chlorzoxazone in tablets and plasma: Analytical Eco-Scale and GAPI tools for green assessment. Acta Chromatogr. 2021, 34, 185–196. [Google Scholar] [CrossRef]
- Pereira, F.J.; Rodriguez-Cordero, A.; Lopez, R.; Robles, L.C.; Aller, A.J. Development and validation of an RP-HPLC-PDA method for determination of paracetamol, caffeine and tramadol hydrochloride in pharmaceutical formulations. Pharmaceuticals 2021, 14, 466. [Google Scholar] [CrossRef] [PubMed]
- Yoo, O.; Tang, E.K.Y.; Nguyen, M.N.; Salman, S.; Hua, A.J.; von Ungern Sternberg, B.S.; Lim, L.Y. HPLC-UV assay of tramadol and O-desmethyltramadol in human plasma containing other drugs potentially co-administered to participants in a paediatric population pharmacokinetic study. J. Chromatogr. B 2021, 1184, 122971. [Google Scholar] [CrossRef] [PubMed]
- Hojjati-Najafabadi, A.; Salmanpour, S.; Sen, F.; Asrami, P.N.; Mahdavian, M.; Khalilzadeh, M.A. A tramadol drug electrochemical sensor amplified by biosynthesized Au nanoparticle using mentha aquatic extract and ionic liquid. Top. Catal. 2021, 1–8. [Google Scholar] [CrossRef]
- Saichanapan, J.; Promsuwan, K.; Saisahas, K.; Soleh, A.; Chang, K.H.; Abdullah, A.F.L.; Limbut, W. Voltammetric Determination of Tramadol Using a Hierarchical Graphene Oxide Nanoplatelets Modified Electrode. J. Electrochem. Soci. 2021, 168, d117512. [Google Scholar] [CrossRef]
- Borman, P.; Elder, D. Q2 (R1) validation of analytical procedures. ICH Qual. Guidel. 2017, 5, 127–166. [Google Scholar]
- Egorov, V.V.; Zdrachek, E.A.; Nazarov, V.A. Improved separate solution method for determination of low selectivity coefficients. Anal. Chem. 2014, 86, 3693–3696. [Google Scholar] [CrossRef]
- Kusmierek, E. A CeO2 semiconductor as a photocatalytic and photoelectrocatalytic material for the remediation of pollutants in industrial wastewater: A review. Catalysts 2020, 10, 1435. [Google Scholar] [CrossRef]
- Abdollahifar, M.; Zamani, R.M.; Beiygie, E.; Nekouei, H. Synthesis of micro-mesopores flowerlike γ-Al2O3 nano-architectures. J. Serb. Chem. Soci. 2014, 79, 1007–1017. [Google Scholar] [CrossRef]
- Jana, J.; Ganguly, M.; Pal, T. Enlightening surface plasmon resonance effect of metal nanoparticles for practical spectroscopic application. RSC Adv. 2016, 6, 86174–86211. [Google Scholar] [CrossRef]
- Reddy, K.P.; Choi, H.; Kim, D.; Choi, M.; Ryoo, R.; Park, J.Y. The facet effect of ceria nanoparticles on platinum dispersion and catalytic activity of methanol partial oxidation. Chem. Commun. 2021, 57, 7382–7385. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Zhang, T.; Yang, D.; Qiu, F.; Rong, J.; Xu, J.; Fang, J. The synthesis of hierarchical porous Al2O3/acrylic resin composites as durable, efficient and recyclable absorbents for oil/water separation. Chem. Eng. J. 2017, 309, 522–531. [Google Scholar] [CrossRef]
- Chelliah, M.; Rayappan, J.B.B.; Krishnan, U.M. Synthesis and characterization of cerium oxide nanoparticles by hydroxide mediated approach. J. Appl. Sci. 2012, 12, 1734–1737. [Google Scholar] [CrossRef] [Green Version]
- Fathi, M.H.; Hanifi, A. Evaluation and characterization of nanostructure hydroxyapatite powder prepared by simple sol-gel method. Mater. Lett. 2007, 61, 3978–3983. [Google Scholar] [CrossRef]
- Singh, R.D.; Koli, P.B.; Jagdale, B.S.; Patil, A.V. Effect of firing temperature on structural and electrical parameters of synthesized CeO2 thick films. SN Appl. Sci. 2019, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pugachevskii, M.A.; Chibisov, A.N.; Kuzmenko, A.P.; Fedorov, A.S. Theoretical and experimental studies of structural defects in CeO2 nanoparticles. Solid State Phenom. 2020, 312, 68–73. [Google Scholar] [CrossRef]
- Sun, C.; Xue, D. Size-dependent oxygen storage ability of nano-sized ceria. Phys. Chem. Chem. Phys. 2013, 15, 14414–14419. [Google Scholar] [CrossRef]
- Young, M.J.; Bedford, N.M.; Yanguas-Gil, A.; Letourneau, S.; Coile, M.; Mandia, D.J.; Aoun, B.; Cavanagh, A.S.; George, S.M.; Elam, J.W. Probing the atomic-scale structure of amorphous aluminum oxide grown by atomic layer deposition. ACS Appl. Mater. Inter. 2020, 12, 22804–22814. [Google Scholar] [CrossRef]
- Talbot, D.; Queiros Campos, J.; Checa-Fernandez, B.L.; Marins, J.A.; Lomenech, C.; Hurel, C.; Godeau, G.D.; Raboisson-Michel, M.; Verger-Dubois, G.; Obeid, L.; et al. Adsorption of organic dyes on magnetic iron oxide nanoparticles. Part I: Mechanisms and adsorption-induced nanoparticle agglomeration. ACS Omega 2021, 6, 19086–19098. [Google Scholar] [CrossRef]
- Keskin, B.; Zeytuncu-Gokoglu, B.; Koyuncu, I. Polymer inclusion membrane applications for transport of metal ions: A critical review. Chemosphere 2021, 279, 130604. [Google Scholar] [CrossRef] [PubMed]
- Santha, N.I.; Sebastian, M.T.; Mohanan, P.; Alford, N.M.; Sarma, K.; Pullar, R.C.; Kamba, S.; Pashkin, A.; Samukhina, P.; Petzelt, J. Effect of doping on the Dielectric properties of Cerium Oxide in the Microwave and Far-Infrared Frequency Range. J. Am. Ceram. Soci. 2004, 87, 1233–1237. [Google Scholar] [CrossRef]
- Acharya, J.; Wilt, J.; Liu, B.; Wu, J. Probing the dielectric properties of ultrathin Al/Al2O3/Al trilayers fabricated using in situ sputtering and atomic layer deposition. ACS Appl. Mater. Interf. 2018, 10, 3112–3120. [Google Scholar] [CrossRef] [PubMed]
- Isa, I.M.; Sohaimi, N.M.; Hashim, N.; Kamari, A.; Mohamed, A.; Ahmad, M.; Ghani, S.A. Determination of salicylate ion by potentiometric membrane electrode based on zinc aluminium layered double hydroxides-4 (2, 4-dichlorophenoxy) butyrate nanocomposites. Int. J. Electrochem. Sci. 2013, 8, 2112–2121. [Google Scholar]
- Grady, T.; Cadogan, A.; McKittrick, T.; Harris, S.J.; Diamond, D.; McKervey, M.A. Sodium-selective electrodes based on triester monoacid derivatives of p-tert-butylcalix [4] arene. Comparison with tetraester calix [4] arene ionophores. Anal. Chim. Acta 1992, 336, 1–12. [Google Scholar] [CrossRef]
- Bakker, E. Evaluation of Egorov’s improved separate solution method for determination of low selectivity coefficients by numerical simulation. Anal. Chem. 2014, 86, 8021–8024. [Google Scholar] [CrossRef]
- Shawish, H.M.A.; Saadeh, S.M.; Al-Dalou, A.R.; Ghalwa, N.A.; Abou Assi, A.A. Optimization of tramadol–PVC membrane electrodes using miscellaneous plasticizers and ion-pair complexes. Mater. Sci. Eng. C 2011, 31, 300–306. [Google Scholar] [CrossRef]
- De Winter, J.C. Using the Student’s t-test with extremely small sample sizes. Pract. Assess. Res. Eval. 2013, 18, 10. [Google Scholar] [CrossRef]
- Tawade, B.V.; Apata, I.E.; Singh, M.; Das, P.; Pradhan, N.; Al-Enizi, A.M.; Karim, A.; Raghavan, D. Recent developments in the synthesis of chemically modified nanomaterials for use in dielectric and electronics applications. Nanotechnol. 2021, 32, 142004. [Google Scholar] [CrossRef]
- Mohaisen, A.; Hamad, Z. Fabrication and characterization of polymer blend doped with metal carbide nanoparticles for humidity sensors. J. Nanostruct. 2019, 9, 340–348. [Google Scholar] [CrossRef]
- Shrivastava, S.; Jadon, N.; Jain, R. Next-generation polymer nanocomposite-based electrochemical sensors and biosensors: A review. TrAC Trend. Anal. Chem. 2016, 82, 55–67. [Google Scholar] [CrossRef]
- Shawish, H.M.A.; Al-Dalou, A.R.; Ghalwa, N.A.; Assi, A.A.A. Potentiometric sensor for determination of tramadol hydrochloride in pharmaceutical preparations and biological fluids. Pharm. Anal. Acta 2010, 1, 1000103. Available online: https://scholar.alaqsa.edu.ps/id/eprint/571 (accessed on 20 March 2022). [CrossRef] [Green Version]
- Shawish, H.M.A.; Ghalwa, N.A.; Al-Dalou, A.R.; Zaggout, F.R.; Saadeh, S.M.; Abou Assi, A.A. Effect of plasticizers and ion-exchangers on the detection limit of tramadol-PVC membrane electrodes. Eur. J. Anal. Chem. 2011, 6, 70–83. [Google Scholar]
- Zare, E.N.; Makvandi, P.; Ashtari, B.; Rossi, F.; Motahari, A.; Perale, G. Progress in conductive polyaniline-based nanocomposites for biomedical applications: A review. J. Med. Chem. 2019, 63, 1–22. [Google Scholar] [CrossRef]
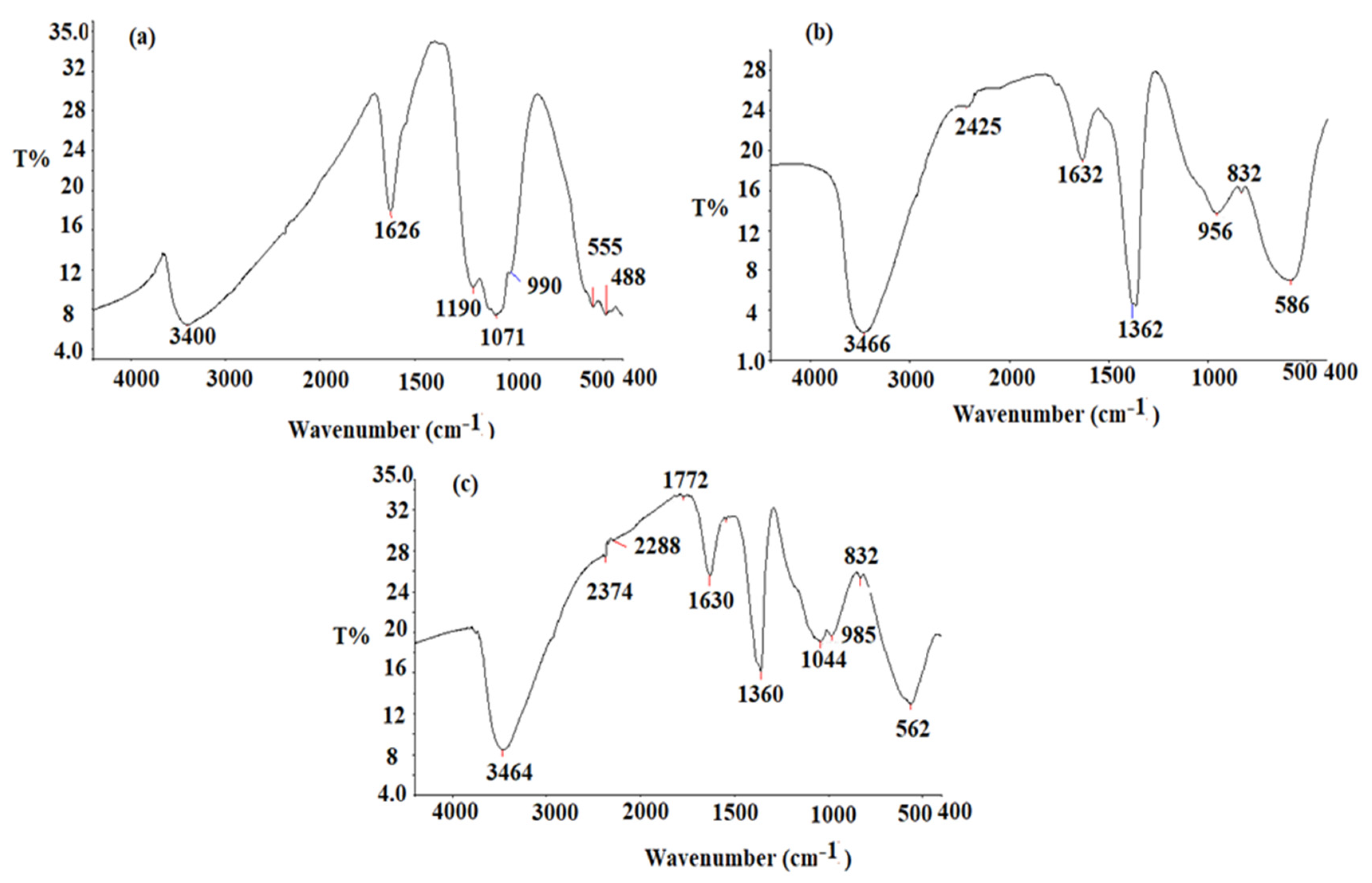
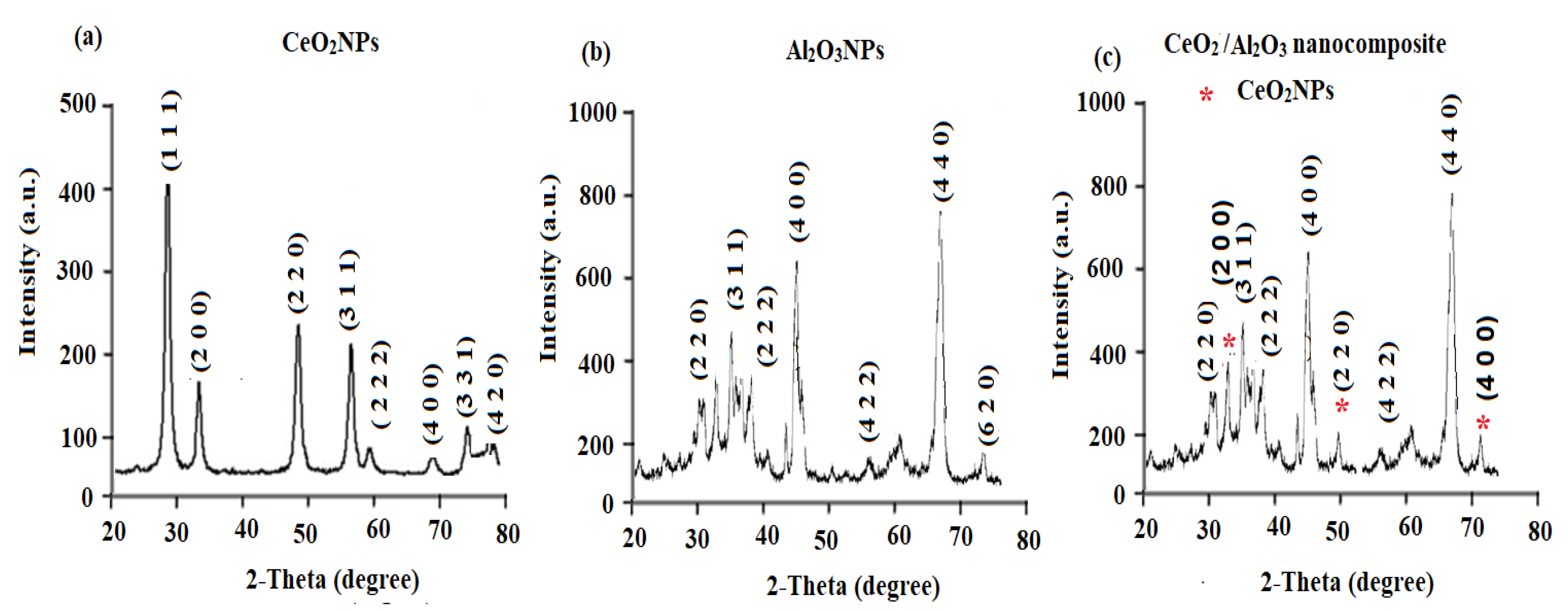
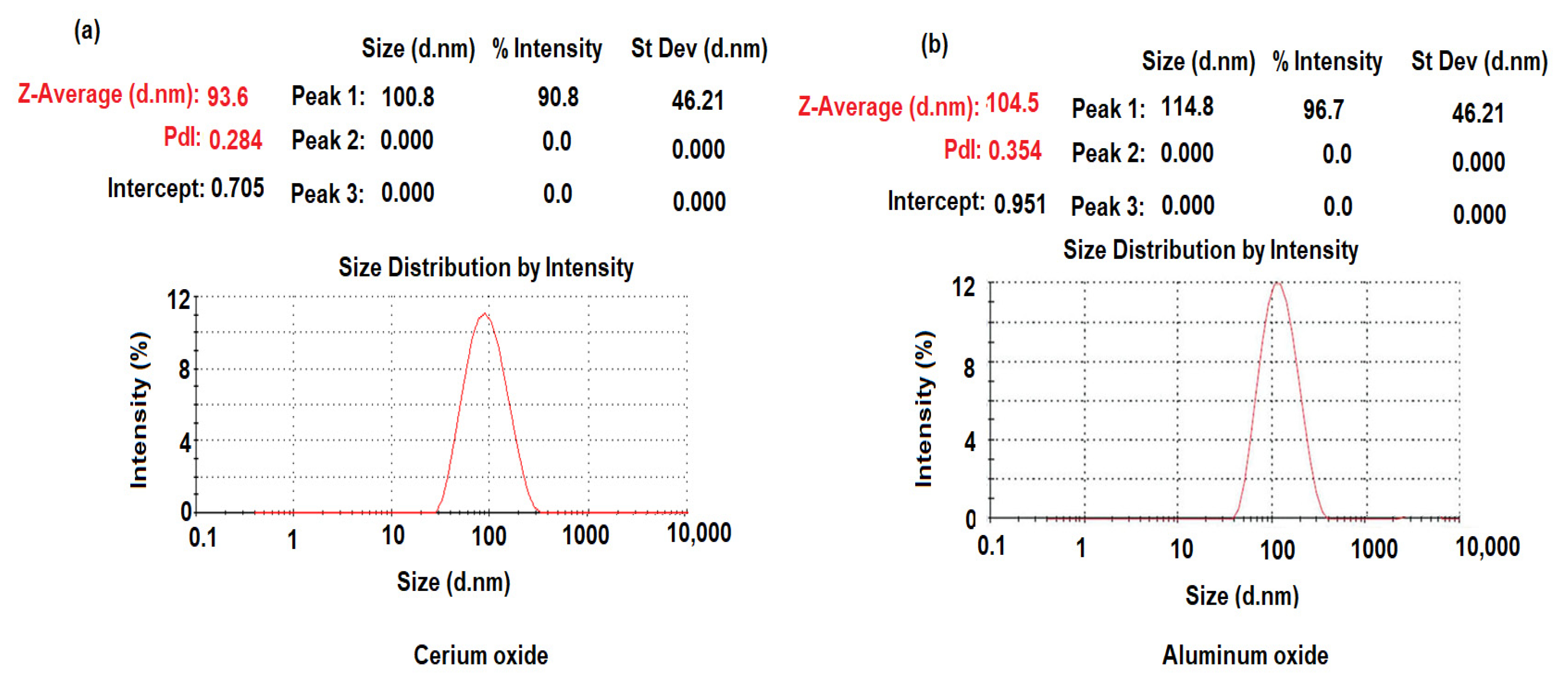
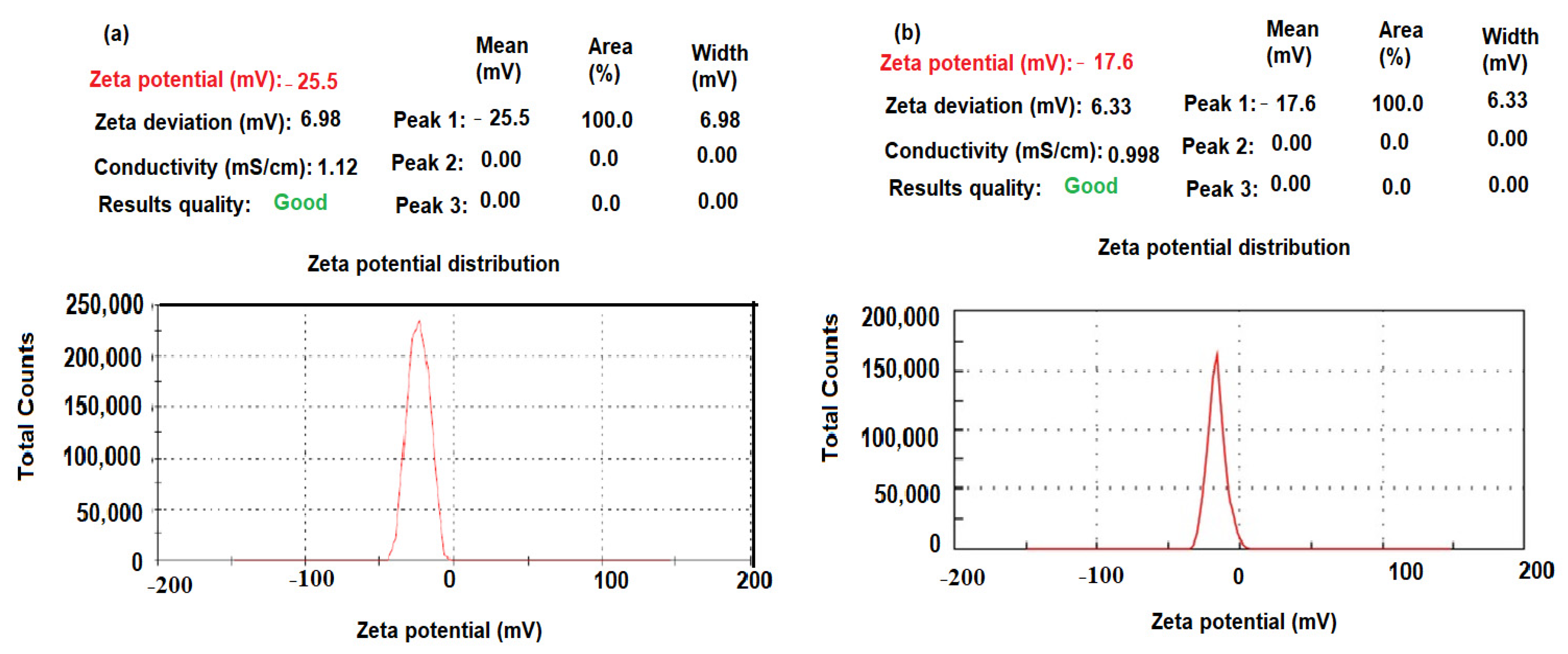


| Parameter | TRD-PM Coated Wire Sensor | Functionalized TRD-PM-CeO2/Al2O3 Coated Wire Sensor |
|---|---|---|
| Linearity (mol L−1) | 1.0 × 10−6–1.0 × 10−2 | 1.0 × 10−10–1.0 × 10−2 |
| Least square equation | EmV = (52.143) log [TRD] + 431.45 | EmV = (57.567 ± 0.2) log [TRD] + 676.29 |
| Correlation coefficient (r) | 0.9997 | 0.9998 |
| Slope | 52.143 | 57.567 |
| Lower limit of detection | 5.0 × 10−7 | 5.0 × 10−11 |
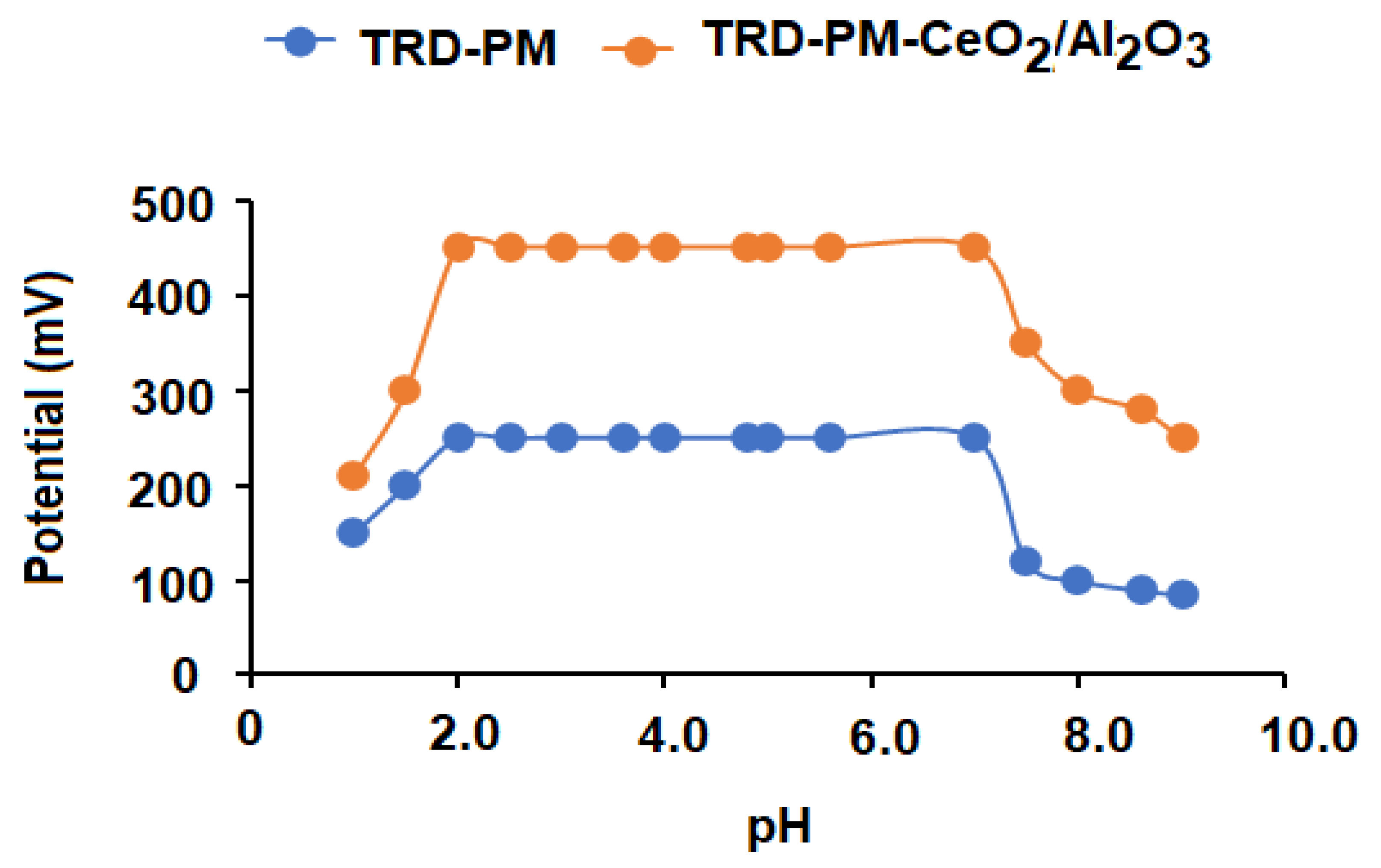
| pH | 2–7 | 2–7 |
| Optimum temperature °C | 25 | 25 |
| Response time (s) | 60 | 35 |
| Life time (day) | 20 | 35 |
| Accuracy | 98.56 ± 0.8 | 99.85 ± 0.2 |
| Robustness | 98.36 ± 0.5 | 99.66 ± 0.3 |
| Ruggedness | 98.83 ± 0.7 | 99.72 ± 0.4 |
| Foreign Substance | TRD-PM Coated Wire Sensor | Functionalized TRD-PM-CeO2/Al2O3 Coated Wire Sensor |
|---|---|---|
| Na+ | 2.38 × 10−4 | 1.12 × 10−6 |
| K+ | 1.56 × 10−3 | 1.89 × 10−5 |
| Ag+ | 5.07 × 10−4 | 4.25 × 10−5 |
| Mg2+ | 2.16 × 10−6 | 3.63 × 10−7 |
| Ca2+ | 5.70 × 10−4 | 7.51 × 10−6 |
| Zn2+ | 1.84 × 10−3 | 1.02 × 10−8 |
| Lactose | 4.47 × 10−3 | 6.35 × 10−4 |
| Fructose | 3.86 × 10−3 | 1.89 × 10−6 |
| Starch | 2.74 × 10−4 | 5.89 × 10−5 |
| Histidine | 5.49 × 10−3 | 3.74 × 10−4 |
| Glycine | 1.29 × 10−5 | 1.11 × 10−6 |
| Lysin | 2.34 × 10−3 | 2.39 × 10−7 |
| Tryptophan | 1.23 × 10−3 | 1.57 × 10−6 |
| TRD-PM Coated Wire Sensor | Functionalized TRD-PM-CeO2/Al2O3 Coated Wire Sensor | |||
|---|---|---|---|---|
| Taken Sample -log [TRD] mol L−1 | % Recovery | Taken Sample -log [TRD] mol L−1 | % Recovery | |
| Statistical analysis | 6 | 99.7 | 10 | 99.9 |
| 5.3 | 98.9 | 9 | 100 | |
| 5 | 99.6 | 8 | 99.8 | |
| 4 | 98.8 | 6 | 99.6 | |
| 3.3 | 98.8 | 5 | 99.5 | |
| 3 | 97.3 | 4 | 99.9 | |
| 2.3 | 97.8 | 3 | 99.8 | |
| 2 | 99.5 | 2 | 100 | |
| Mean ± SD | 98.80 ± 0.9 | 99.81 ± 0.2 | ||
| n | 8 | 8 | ||
| Variance | 0.81 | 0.04 | ||
| %RED | 0.91 | 0.2 | ||
| %Error * | 0.32 | 0.07 | ||
| TRD-PM Coated Wire Sensor | Functionalized TRD-PM-CeO2/Al2O3 Coated Wire Sensor | |||
|---|---|---|---|---|
| Taken Sample -log [TRD] mol L−1 | % Recovery | Taken Sample -log [TRD] mol L−1 | % Recovery | |
| Statistical analysis | 6 | 98.9 | 10 | 100 |
| 5.3 | 98.5 | 9 | 99.8 | |
| 5 | 99.6 | 8 | 99.7 | |
| 4.3 | 97.3 | 6 | 100 | |
| 4 | 97.6 | 5 | 99.6 | |
| 3.3 | 98.7 | 4 | 99.8 | |
| 3 | 99.1 | 3 | 99.7 | |
| 2.3 | 98.5 | 2.3 | 99.9 | |
| 2 | 99.3 | 2 | 100 | |
| Mean ± SD | 98.56 ± 0.8 | 99.85 ± 0.2 | ||
| n | 9 | 9 | ||
| Variance | 0.64 | 0.04 | ||
| %RED | 0.81 | 0.2 | ||
| %Error * | 0.28 | 0.07 | ||
| Functionalized TRD-PM-CeO2/Al2O3 Coated Wire Sensor | ||||||
|---|---|---|---|---|---|---|
| Intra-Day Assay | Inter-Day Assay | |||||
| Sample | Found | % | Sample | Found | % | |
| Statistical analysis | -log [TRD] | -log [TRD] | Recovery | -log [TRD] | -log [TRD] | Recovery |
| mol L−1 | mol L−1 | mol L−1 | mol L−1 | |||
| 10 | 9.99 | 99.9 | 10 | 10.02 | 100.2 | |
| 8 | 7.97 | 99.6 | 8 | 7.99 | 99.9 | |
| 2 | 2 | 100 | 2 | 1.99 | 99.5 | |
| Mean ± SD | 99.83 ± 0.2 | 99.86 ± 0.4 | ||||
| n | 3 | 3 | ||||
| Variance | 0.04 | 0.16 | ||||
| %RED | 0.2 | 0.4 | ||||
| %Error * | 0.12 | 0.23 | ||||
| TRD-PM Coated Wire Sensor | Functionalized TRD-PM-CeO2/Al2O3 Coated Wire Sensor | ||||
|---|---|---|---|---|---|
| Sample -log [TRD] | Recovery | Sample -log [TRD] | % Recovery | ||
| Statistical analysis | mol L−1 | mol L−1 | Reported Method [68] | ||
| 6 | 98.9 | 10 | 99.6 | ||
| 5.3 | 99.8 | 8 | 99.7 | ||
| 5 | 98.7 | 6 | 99.9 | ||
| 4 | 97.9 | 4 | 98.6 | ||
| 3 | 97.8 | 3 | 99.9 | ||
| 2 | 99.5 | 2 | 100 | ||
| Mean ± SD | 98.77 ± 0.8 | 99.63 ± 0.5 | |||
| n | 6 | 6 | 99.25 ± 0.6 | ||
| Variance | 0.64 | 0.25 | 6 | ||
| %RED | 0.81 | 0.5 | 0.36 | ||
| %Error * | 0.33 | 0.2 | 0.6 | ||
| t-test | 1.176 (2.228) * | 1.216 (2.228) * | 0.24 | ||
| F-test | 1.77 (5.05) * | 1.44 (5.05) * | |||
| No. | Ion-Pair Complex | Linear Concentration Range (mol L−1) | LOD (mol L−1) | Reference |
|---|---|---|---|---|
| 1. | TRD-Phosphomolybdic acid | 2.0 × 10−6–1.0 × 10−1 | 1.3 × 10−5 | [73] |
| 2. | TRD-Phosphotunegstic acid | 9.0 × 10−6–1.0 × 10−1 | 6.2 × 10−6 | [69] |
| 3. | TRD-Silico-tungstic acid | 2.0 × 10−6–1.0 × 10−1 | 4.0 × 10−6 | [74] |
| 4. | TRD-Phosphomolybdic acid | 1.0 × 10−10–1.0 × 10−2 | 5.0 × 10−11 | Current study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alterary, S.S.; El-Tohamy, M.F. Advanced Functionalized CeO2/Al2O3 Nanocomposite Sensor for Determination of Opioid Medication Tramadol Hydrochloride in Pharmaceutical Formulations. Nanomaterials 2022, 12, 1373. https://doi.org/10.3390/nano12081373
Alterary SS, El-Tohamy MF. Advanced Functionalized CeO2/Al2O3 Nanocomposite Sensor for Determination of Opioid Medication Tramadol Hydrochloride in Pharmaceutical Formulations. Nanomaterials. 2022; 12(8):1373. https://doi.org/10.3390/nano12081373
Chicago/Turabian StyleAlterary, Seham S., and Maha F. El-Tohamy. 2022. "Advanced Functionalized CeO2/Al2O3 Nanocomposite Sensor for Determination of Opioid Medication Tramadol Hydrochloride in Pharmaceutical Formulations" Nanomaterials 12, no. 8: 1373. https://doi.org/10.3390/nano12081373
APA StyleAlterary, S. S., & El-Tohamy, M. F. (2022). Advanced Functionalized CeO2/Al2O3 Nanocomposite Sensor for Determination of Opioid Medication Tramadol Hydrochloride in Pharmaceutical Formulations. Nanomaterials, 12(8), 1373. https://doi.org/10.3390/nano12081373





