NiFe2O4/Ketjen Black Composites as Efficient Membrane Separators to Suppress the Shuttle Effect for Long-Life Lithium-Sulfur Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of NiFe2O4 Nanoparticles
2.2. Preparation of NiFe2O4/KB-Modified Separator
2.3. Characterization of Materials
2.4. Electrochemical Characterization
2.5. Theoretical Calculation
3. Results
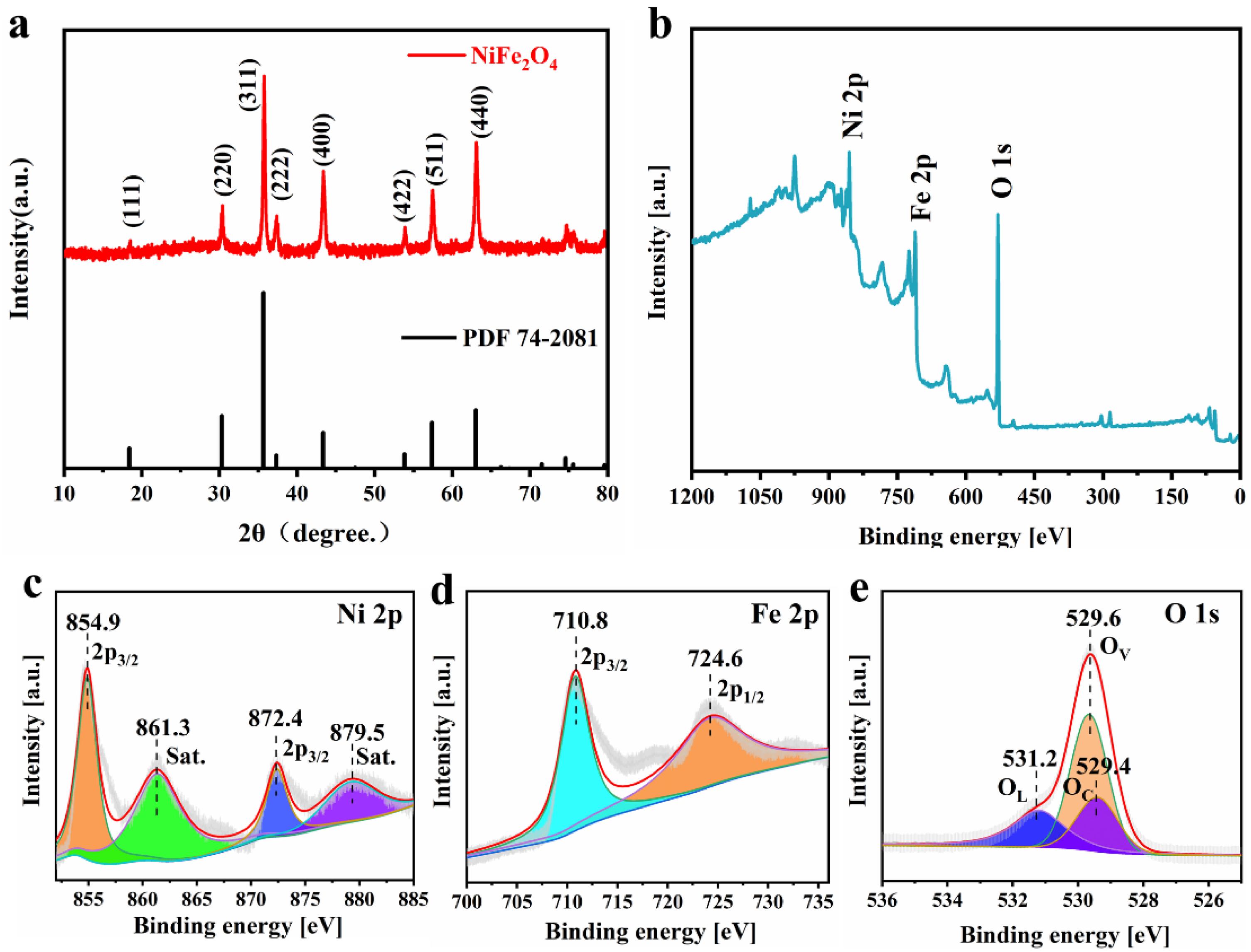
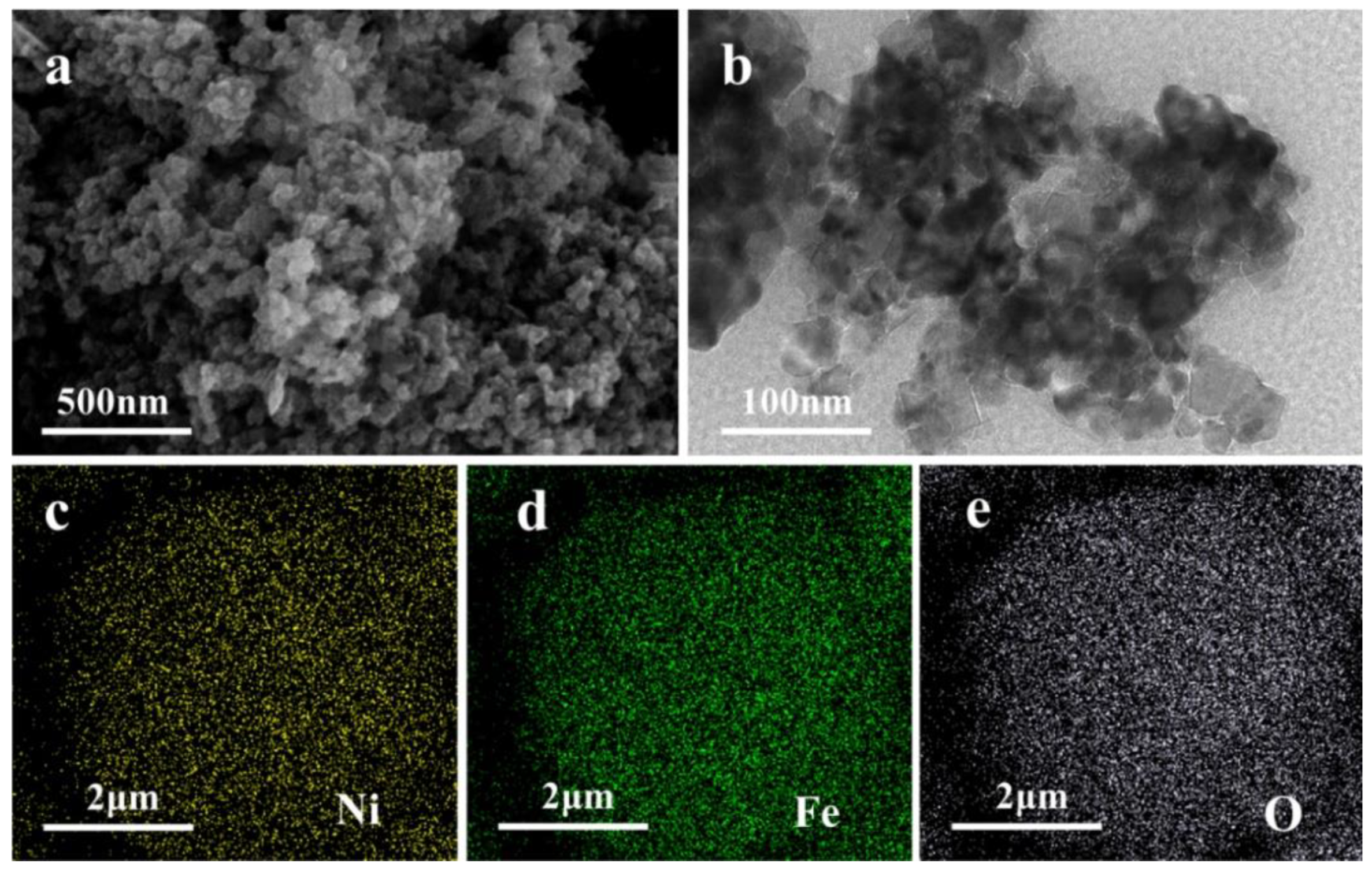
3.1. Characterization of Nano NiFe2O4 Materials
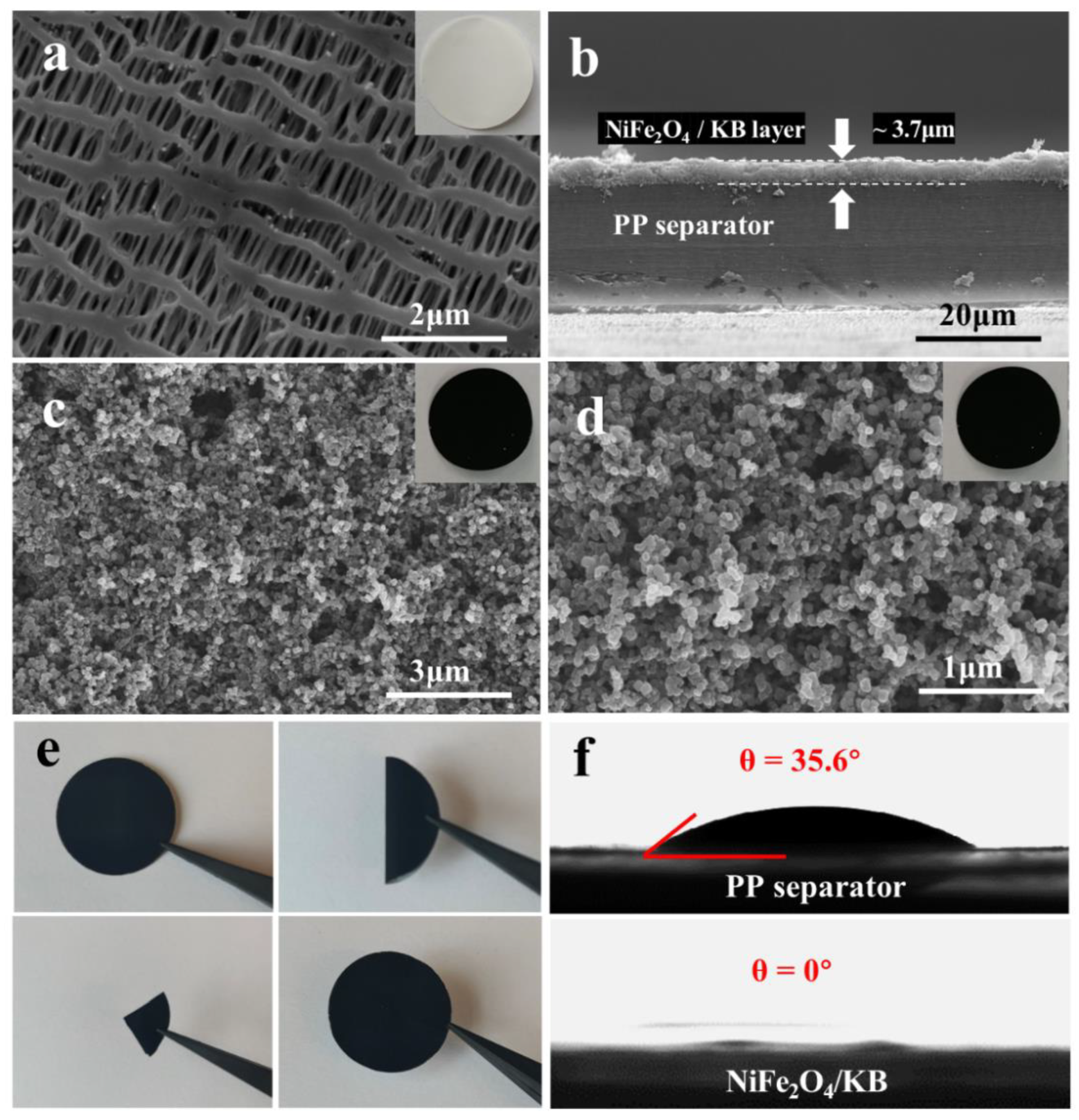
3.2. Morphology Analysis of NiFe2O4/KB-Modified Separator
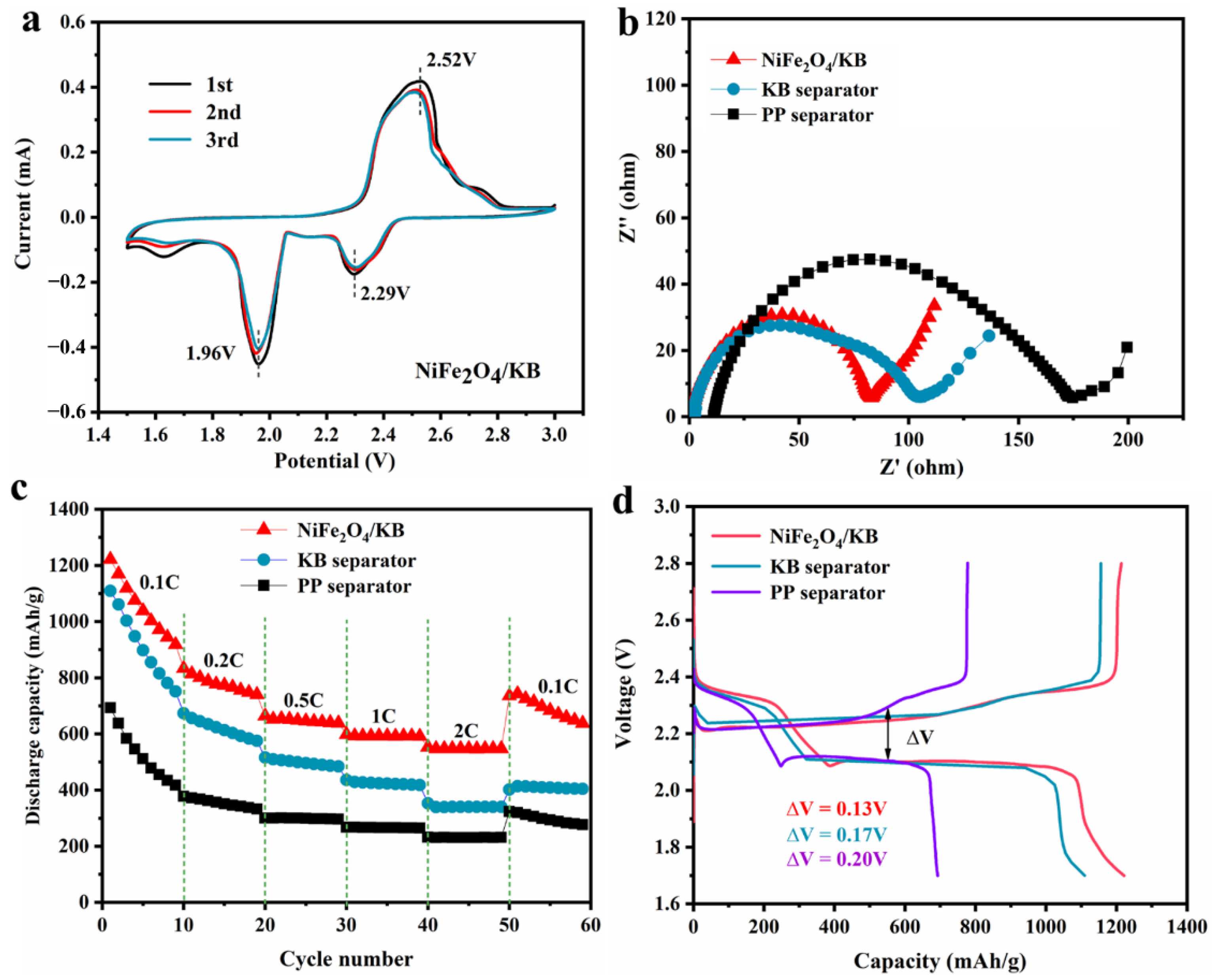
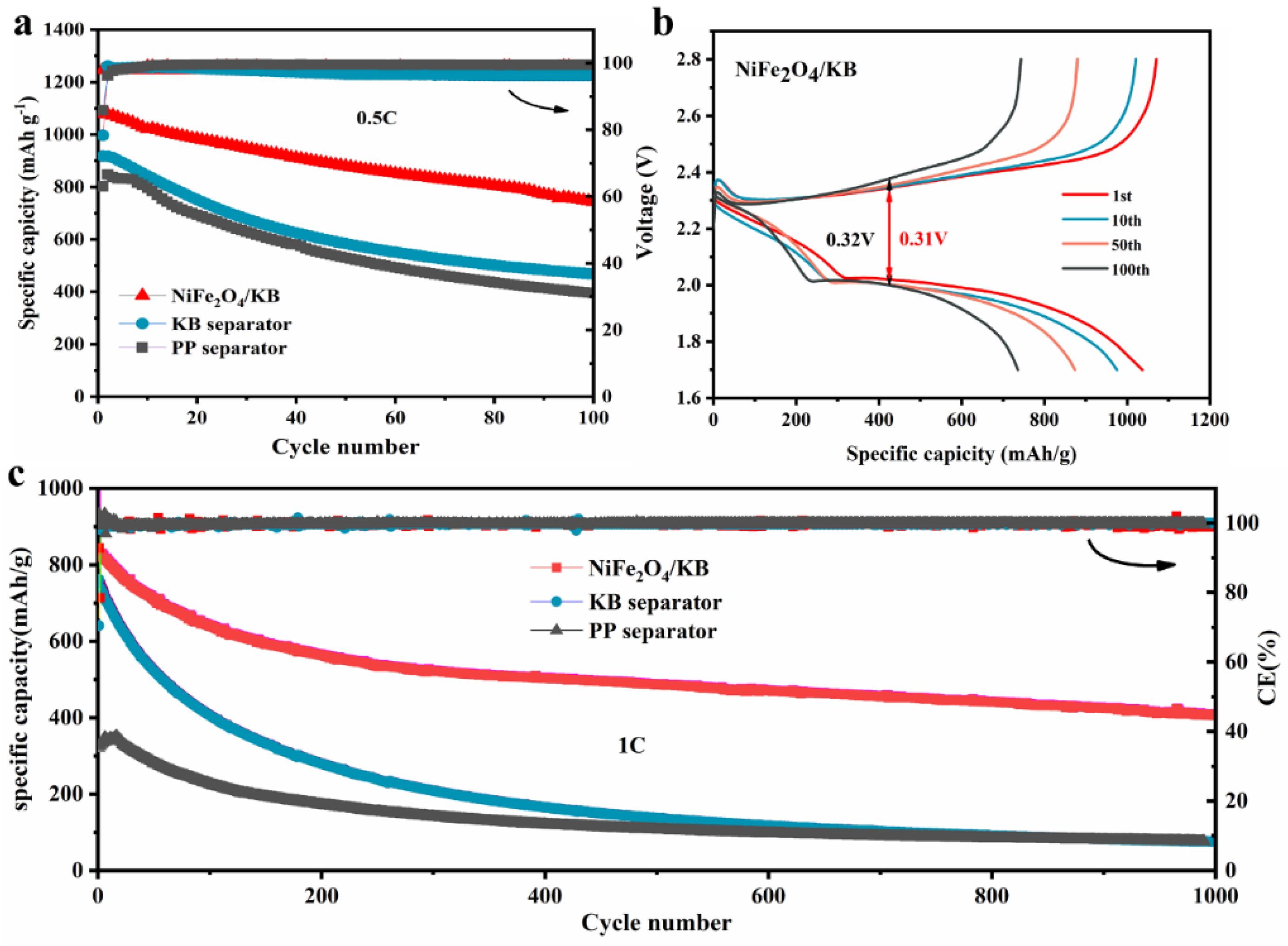
3.3. Electrochemical Analysis of NiFe2O4/KB-Modified Separator
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodenough, J.B. Energy Storage Materials: A Perspective. Energy Storage Mater. 2015, 1, 158–161. [Google Scholar] [CrossRef]
- Bao, W.; Liu, L.; Wang, C.; Choi, S.; Wang, D.; Wang, G. Facile Synthesis of Crumpled Nitrogen-Doped MXene Nanosheets as a New Sulfur Host for Lithium-Sulfur Batteries. Adv. Energy Mater. 2018, 8, 1702485. [Google Scholar] [CrossRef] [Green Version]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Zheng, G.; Cui, Y. Nanostructured Sulfur Cathodes. Chem. Soc. Rev. 2013, 42, 3018–3032. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-J.; Huang, J.-Q.; Cheng, X.-B.; Zhang, Q. Review on High-Loading and High-Energy Lithium-Sulfur Batteries. Adv. Energy Mater. 2017, 7, 1700260. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.M. Towards Greener and More Sustainable Batteries for Electrical Energy Storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef]
- Kim, H.M.; Hwang, J.Y.; Manthiram, A.; Sun, Y.K. High-Performance Lithium-Sulfur Batteries with a Self-Assembled Multiwall Carbon Nanotube Interlayer and a Robust Electrode-Electrolyte Interface. ACS Appl. Mater. Interfaces 2016, 8, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Han, P.; Manthiram, A. A Polysulfide-Trapping Interface for Electrochemically Stable Sulfur Cathode Development. ACS Appl. Mater. Interfaces 2016, 8, 4709–4717. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Manthiram, A. High-Performance Li-S Batteries with an Ultra-lightweight MWCNT-Coated Separator. J. Phys. Chem. Lett. 2014, 5, 1978–1983. [Google Scholar] [CrossRef]
- Chung, S.H.; Manthiram, A. A Polyethylene Glycol-Supported Microporous Carbon Coating as a Polysulfide Trap for Utilizing Pure Sulfur Cathodes in Lithium-Sulfur Batteries. Adv. Mater. 2014, 26, 7352–7357. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Hu, G.C.; Bi, H.L.; Xiang, H.F. A Trilayer Carbon Nanotube/AL2O3/Polypropylene Separator for Lithium-Sulfur Batteries. Ionics 2014, 21, 981–986. [Google Scholar] [CrossRef]
- Zhang, Z.; Lai, Y.; Zhang, Z.; Zhang, K.; Li, J. Al2O3-Coated Porous Separator for Enhanced Electrochemical Performance of Lithium Sulfur Batter-Ies. Electrochim. Acta 2014, 129, 55–61. [Google Scholar] [CrossRef]
- Wei, W.; Li, J.; Wang, Q.; Liu, D.; Niu, J.; Liu, P. Hierarchically Porous SnO2 Nanoparticle-Anchored Polypyrrole Nanotubes as a High-Efficient Sulfur/Polysulfide Trap for High-Performance Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2020, 12, 6362–6370. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Ou, X.; Yue, X.; Yang, Y.; Wang, Z.; Rooney, D.; Sun, K. A Simply Effective Double-Coating Cathode with MnO2 Nanosheets/Graphene as Functionalized Interlayer for High Performance Lithium-Sulfur Batteries. Electrochim. Acta 2016, 207, 198–206. [Google Scholar] [CrossRef]
- Wu, F.; Qian, J.; Chen, R.; Ye, Y.; Sun, Z.; Xing, Y.; Li, L. Light-Weight Functional Layer on a Separator as a Polysulfide Immobilizer to Enhance Cycling Stability for Lithium–Sulfur Batteries. J. Mater. Chem. A 2016, 4, 17033–17041. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, G.; Lai, Y.; Li, J.; Zhang, Z.; Chen, W. Nitrogen-Doped Porous Hollow Carbon Sphere-Decorated Separators for Advanced Lithium–Sulfur Batteries. J. Power Sources 2015, 300, 157–163. [Google Scholar] [CrossRef]
- Wu, Z.Z.; Wang, S.Y.; Wang, R.Y.; Liu, J.; Ye, S.H. Carbon Nanotubes as Effective Interlayer for High Performance Li-I2 Batteries: Long Cycle Life and Superior Rate Performance. J. Electrochem. Soc. 2018, 165, A1156–A1159. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, P.; Zhang, Y.; Wang, L.; Zhang, L.; Zhang, L.; Xu, L.; Liu, L. Biomass Based Nitrogen-Doped Structure-Tunable Versatile Porous Carbon Materials. J. Mater. Chem. A 2017, 5, 12958–12968. [Google Scholar] [CrossRef]
- Zhang, S.S. Heteroatom-Doped Carbons: Synthesis, Chemistry and Application in Lithium/Sulphur Batteries. Inorg. Chem. Front. 2015, 2, 1059–1069. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Y.; Ding, Y.; Peng, L.; Zhang, W.; Yu, G. A Conductive Molecular Framework Derived Li2S/N,P-Codoped Carbon Cathode for Advanced Lithium-Sulfur Batteries. Adv. Energy Mater. 2017, 7, 1602876. [Google Scholar] [CrossRef]
- Ramakrishnan, P.; Baek, S.-H.; Park, Y.; Kim, J.H. Nitrogen and Sulfur Co-doped Metal Monochalcogen Encapsulated Honeycomb Like Carbon Nanostructure as a High Performance Lithium-Ion Battery Anode Material. Carbon 2017, 115, 249–260. [Google Scholar] [CrossRef]
- Zuo, P.; Hua, J.; He, M.; Zhang, H.; Qian, Z.; Ma, Y.; Du, C.; Cheng, X.; Gao, Y.; Yin, G. Facilitating the Redox Reaction of Polysulfides by an Electrocatalytic Layer-Modified Separator for Lithium–Sulfur Batteries. J. Mater. Chem. A 2017, 5, 10936–10945. [Google Scholar] [CrossRef]
- Balach, J.; Jaumann, T.; Muhlenhoff, S.; Eckert, J.; Giebeler, L. Enhanced Polysulphide Redox Reaction Using a RuO2 Nanoparticle-Decorated Mesoporous Carbon as Functional Separator Coating for Advanced Lithium-Sulphur Batteries. Chem. Commun. 2016, 52, 8134–8137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.X.; Zhao, T.S.; Liu, M.; Zeng, Y.K.; Jiang, H.R. A Self-Cleaning Li-S Battery Enabled by a Bifunctional Redox Mediator. J. Power Sources 2017, 361, 203–210. [Google Scholar] [CrossRef]
- Tashima, D.; Kishita, T.; Maeno, S.; Nagasawa, Y. Mesoporous Graphitized Ketjenblack as Conductive Nanofiller for Supercapacitors. Mater. Lett. 2013, 110, 105–107. [Google Scholar] [CrossRef]
- Tang, H.; Yao, S.; Shen, X.; Xi, X.; Xiao, K. Lithium-Sulfur Batteries with High Rate and Cycle Performance by using Multilayered Separators Coated with Ketjen Black. Energy Technol. 2017, 5, 623–628. [Google Scholar] [CrossRef]
- Rana, M.; Li, M.; Huang, X.; Luo, B.; Gentle, I.; Knibbe, R. Recent Advances in Separators to Mitigate Technical Challenges Associated with Re-Chargeable Lithium Sulfur Batteries. J. Mater. Chem. A 2019, 7, 6596–6615. [Google Scholar] [CrossRef]
- Park, C.K.; Park, S.B.; Lee, S.Y.; Lee, H.; Jang, H.; Cho, W.I. Electrochemical Performances of Lithium-air Cell with Carbon Materials. Bull. Korean Chem. Soc. 2010, 31, 3221–3224. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Qian, X.; Jin, L.; Yang, X.; Wang, S.; Shen, X.; Yao, S.; Rao, D.; Zhou, Y.; Xi, X. Separator Modified by Ketjen Black for Enhanced Electrochemical Performance of Lithium–Sulfur Batteries. RSC Adv. 2016, 6, 13680–13685. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Y.; Zhang, K.; Feng, X.; Li, S. Self-Assembled Flower-like NiFe2O4 Decorated on 2D Graphene Nanosheets Composite and Their Excellent Electrochemical Performance as Anode Materials for LIBs. J. Alloy. Compd. 2016, 686, 905–913. [Google Scholar] [CrossRef]
- Cherian, C.T.; Sundaramurthy, J.; Reddy, M.V.; Suresh Kumar, P.; Mani, K.; Pliszka, D.; Sow, C.H.; Ramakrishna, S.; Chowdari, B.V. Morphologically Robust NiFe2O4 Nanofibers as High Capacity LI-Ion Battery Anode Material. ACS Appl. Mater. Interfaces 2013, 5, 9957–9963. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.R.; Mitra, S. Nickel Ferrite as a Stable, High Capacity and High Rate Anode for Li-Ion Battery Applications. RSC Adv. 2013, 3, 25058–25064. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Li, S.; Li, Q.; Xu, J.; Liu, X.; Liu, C.; Xu, Y.; Liu, J.; Li, H.; et al. Optimization of NiFe2O4/rGO Composite Electrode for Lithium-Ion Batteries. Appl. Surf. Sci. 2017, 416, 308–317. [Google Scholar] [CrossRef] [Green Version]
- Qin, G.; Wu, X.; Wen, J.; Li, J.; Zeng, M. A Core-Shell NiFe2O4@SiO2 Structure as a High-Performance Anode Material for Lithium-Ion Batteries. Chem. Electro. Chem. 2019, 6, 911–916. [Google Scholar]
- Qu, L.; Hou, X.; Huang, X.; Liang, Q.; Ru, Q.; Wu, B.; Lam, K.-H. Self-Assembled Porous NiFe2O4 Floral Microspheres Inlaid on Ultrathin Flake Graphite as Anode Materials for Lithium Ion Batteries. ChemElectroChem 2017, 4, 3148–3155. [Google Scholar]
- Yu, H.; Fan, H.; Yadian, B.; Tan, H.; Liu, W.; Hng, H.H.; Huang, Y.; Yan, Q. General Approach for MOF-Derived Porous Spinel AFe2O4 Hollow Structures and Their Superior Lithium Storage Properties. ACS Appl. Mater. Interfaces 2015, 7, 26751–26757. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Ren, C.; Wang, G.; Li, G.; Jin, R. Oxygen-Vacancy-Abundant Ferrites on N-Doped Carbon Nanosheets as High-Performance Li-Ion Battery Anodes. Chemistry 2020, 26, 10575–10584. [Google Scholar] [CrossRef]
- Fan, Q.; Liu, W.; Weng, Z.; Sun, Y.; Wang, H. Ternary Hybrid Material for High-Performance Lithium-Sulfur Battery. J. Am. Chem. Soc. 2015, 137, 12946–12953. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, D.-H.; Zhou, Z.; Li, G.-R.; Liu, S.; Gao, X.-P. Sulfur/Nickel Ferrite Composite as Cathode with High-Volumetric-Capacity for Lithium-Sulfur Battery. Sci. China Mater. 2018, 62, 74–86. [Google Scholar] [CrossRef] [Green Version]
- Wei, K.; Zhao, Y.; Cui, Y.; Wang, J.; Cui, Y.; Zhu, R.; Zhuang, Q.; Xue, M. Lithium Phosphorous Oxynitride (LiPON) Coated NiFe2O4 Anode Material with Enhanced Electrochemical Performance for Lithium Ion Batteries. J. Alloy. Compd. 2018, 769, 110–119. [Google Scholar] [CrossRef]
- Zhang, Z.; Basu, S.; Zhu, P.; Zhang, H.; Shao, A.; Koratkar, N.; Yang, Z. Highly Sulfiphilic Ni-Fe Bimetallic Oxide Nanoparticles Anchored on Carbon Nanotubes Enable Effective Immobilization and Conversion of Polysulfides for Stable Lithium-Sulfur Batteries. Carbon 2019, 142, 32–39. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, N.; Li, Y.; Zhang, P.; Chen, Z.; Zhang, C.; Qiao, X.; Dai, Y.; Wang, Q.; Liu, S. Unique Flexible NiFe2O4@S/rGO-CNT Electrode via the Synergistic Adsorption/Electrocatalysis Effect toward High-Performance Lithium-Sulfur Batteries. J. Phys. Chem. Lett. 2019, 10, 6518–6524. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Fu, Z.; Qian, X.; Huang, B.; Li, F.; Wang, Y.; Shen, X. Catalytic Co-N-C Hollow Nanocages as Separator Coating Layer for Lithium Sulfur Batterys. Microporous Mesoporous Mater. 2021, 316, 110927. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Reddy, M.V.; Zaghib, K.; Armand, M.; Ramakrishna, S. Growth Mechanism of Micro/Nano Metal Dendrites and Cumulative Strategies for Countering Its Impacts in Metal Ion Batteries: A Review. Nanomaterials 2021, 11, 2476. [Google Scholar] [CrossRef]
- Xue, W.; Shi, Z.; Suo, L.; Wang, C.; Wang, Z.; Wang, H.; So, K.P.; Maurano, A.; Yu, D.; Chen, Y.; et al. Intercalation-Conversion Hybrid Cathodes Enabling Li–S Full-Cell Architectures with Jointly Superior Gravimetric and Volumetric Energy Densities. Nat. Energy 2019, 4, 374–382. [Google Scholar] [CrossRef]
- Yao, W.; Zheng, W.; Xu, J.; Tian, C.; Han, K.; Sun, W.; Xiao, S. ZnS-SnS@NC Heterostructure as Robust Lithiophilicity and Sulfiphilicity Mediator toward High-Rate and Long-Life Lithium-Sulfur Batteries. ACS Nano. 2021, 15, 7114–7130. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Permien, S.; Rana, J.; Krengel, M.; Sun, F.; Schumacher, G.; Bensch, W.; Banhart, J. MNFO Investigation of Electronic and Local Structural Changes during Lithium Uptake and Release of Nano-Crystalline NiFe2O4 by X-ray Absorption Spectroscopy. J. Power Sources 2017, 342, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Lima, D.R.; Jiang, N.; Liu, X.; Wang, J.; Vulcani, V.A.S.; Martins, A.; Machado, D.S.; Landers, R.; Camargo, P.H.C.; Pancotti, A. Employing Calcination as a Facile Strategy to Reduce the Cytotoxicity in CoFe2O4 and NiFe2O4 Nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 39830–39838. [Google Scholar] [CrossRef] [PubMed]
- Duraia, E.-S.M.; Adebiyi, B.M.; Beall, G.W. An Approach to Nickel Ferrite Synthesis. J. Mater. Sci. Mater. Electron. 2019, 30, 8286–8290. [Google Scholar] [CrossRef]
- Athika, M.; Devi, V.S.; Elumalai, P. Cauliflower-Like Hierarchical Porous Nickel/Nickel Ferrite/Carbon Composite as Superior Bifunctional Catalyst for Lithium-Air Battery. ChemistrySelect 2020, 5, 3529–3538. [Google Scholar] [CrossRef]
- Salah, L.M. Spectroscopic Studies of the Effect of Addition of Y3+on Structural Characteristics of Ni-Zn Ferrites. Phys. Status Solidi 2006, 203, 271–281. [Google Scholar] [CrossRef]
- Soam, A.; Kumar, R.; Sahoo, P.K.; Mahender, C.; Kumar, B.; Arya, N.; Singh, M.; Parida, S.; Dusane, R.O. Synthesis of Nickel Ferrite Nanoparticles Supported on Graphene Nanosheets as Composite Electrodes for High Performance Supercapacitor. ChemistrySelect 2019, 4, 9952–9958. [Google Scholar] [CrossRef]
- Xiao, Z.; Yang, Z.; Wang, L.; Nie, H.; Zhong, M.; Lai, Q.; Xu, X.; Zhang, L.; Huang, S. A Lightweight TiO2/Graphene Interlayer, Applied as a Highly Effective Polysulfide Absorbent for Fast, Long-Life Lithium-Sulfur Batteries. Adv. Mater. 2015, 27, 2891–2898. [Google Scholar] [CrossRef]
- Seh, Z.W.; Sun, Y.; Zhang, Q.; Cui, Y. Designing High-Energy Lithium-Sulfur Batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef]
- Park, G.D.; Lee, J.; Piao, Y.; Kang, Y.C. Mesoporous Graphitic Carbon-TiO2 Composite Microspheres Produced by a Pilot-Scale Spray-Drying Process as an Efficient Sulfur Host Material for Li-S Batteries. Chem. Eng. J. 2018, 335, 600–611. [Google Scholar] [CrossRef]
- Zeng, P.; Huang, L.; Zhang, X.; Zhang, R.; Wu, L.; Chen, Y. Long-Life and High-Areal-Capacity Lithium-Sulfur Batteries Realized by a Honeycomb-like N, P Dual-Doped Carbon Modified Separator. Chem. Eng. J. 2018, 349, 327–337. [Google Scholar] [CrossRef]
- Ghazi, Z.A.; He, X.; Khattak, A.M.; Khan, N.A.; Liang, B.; Iqbal, A.; Wang, J.; Sin, H.; Li, L.; Tang, Z. MoS2 /Celgard Separator as Efficient Polysulfide Barrier for Long-Life Lithium-Sulfur Batteries. Adv. Mater. 2017, 29, 1606817. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, G.D.; Choi, J.H.; Kang, Y.C. Structural Combination of Polar Hollow Microspheres and Hierarchical N-Doped Carbon Nanotubes for High-Performance Li-S Batteries. Nanoscale 2020, 12, 2142–2153. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, X.; Chen, C.; Wang, Y.; Li, Q.; Wu, Z.; Zhong, B.; Chen, Y. Alleviating the Shuttle Effect via Bifunctional MnFe2O4/AB Modified Separator for High Performance Lithium Sulfur Battery. Electrochim. Acta 2020, 354, 136704. [Google Scholar] [CrossRef]
- Zhang, S. Improved Cyclability of Liquid Electrolyte Lithium/Sulfur Batteries by Optimizing Electrolyte/Sulfur Ratio. Energies 2012, 5, 5190–5197. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Y.; Zhang, Y.; Luo, D.; Liu, J.; Wang, T.; Gao, W.; Li, H.; Wang, X. A New Defect-Rich and Ultrathin ZnCo Layered Double Hydroxide/Carbon Nanotubes Architecture to Facilitate Catalytic Conversion of Polysulfides for High-Performance Li-S Batteries. Chem. Eng. J. 2021, 417, 129248. [Google Scholar] [CrossRef]
- Gu, X.; Tong, C.-J.; Lai, C.; Qiu, J.; Huang, X.; Yang, W.; Wen, B.; Liu, L.-M.; Hou, Y.; Zhang, S. A porous nitrogen and phosphorous dual doped graphene blocking layer for high performance Li–S batteries. J. Mater. Chem. A 2015, 3, 16670–16678. [Google Scholar] [CrossRef] [Green Version]
- Raja, M.; Suriyakumar, S.; Angulakshmi, N.; Manuel Stephan, A. High performance multi-functional trilayer membranes as permselective separators for lithium–sulfur batteries. Inorg. Chem. Front. 2017, 4, 1013–1021. [Google Scholar] [CrossRef]
- Yang, L.; Li, G.; Jiang, X.; Zhang, T.; Lin, H.; Lee, J.Y. Balancing the chemisorption and charge transport properties of the interlayer in lithium–sulfur batteries. J. Mater. Chem. A 2017, 5, 12506–12512. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Sun, L.; Zhao, Y.; Tan, T.; Zhang, Y. A novel CuS/graphene-coated separator for suppressing the shuttle effect of lithium/sulfur batteries. Appl. Surf. Sci. 2019, 466, 309–319. [Google Scholar] [CrossRef]
- Shan, L.; Yurong, C.; Jing, Y.; Feixia, R.; Jun, W.; Babu, S.; Xin, Y.; Junkuo, G.; Juming, Y. Entrapment of polysulfides by a Ketjen Black & mesoporous TiO2 modified glass fiber separator for high performance lithium-sulfur batteries. J. Alloy. Compd. 2019, 779, 412–419. [Google Scholar]
- Zhu, F.; Liu, J.; Zhao, H.; Li, J.; Li, Q.; Xi, Y.; Liu, M.; Wang, C. Preparation and Performance of Porous Polyetherimide/Al2O3 Separator for Enhanced Lithium-Sulfur Batteries. ChemElectroChem 2019, 6, 2883–2890. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, X.; Zhang, S.; Liang, G.; Kang, F.; Chen, G.; Li, B. Fe3O4-Decorated Porous Graphene Interlayer for High-Performance Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 26264–26273. [Google Scholar] [CrossRef]
- Li, Q.; Liu, M.; Qin, X.; Wu, J.; Han, W.; Liang, G.; Zhou, D.; He, Y.-B.; Li, B.; Kang, F. Cyclized-polyacrylonitrile modified carbon nanofiber interlayers enabling strong trapping of polysulfides in lithium–sulfur batteries. J. Mater. Chem. A 2016, 4, 12973–12980. [Google Scholar] [CrossRef]
- Fan, C.-Y.; Liu, S.-Y.; Li, H.-H.; Shi, Y.-H.; Wang, H.-C.; Wang, H.-F.; Sun, H.-Z.; Wu, X.-L.; Zhang, J.-P. Synergistic mediation of sulfur conversion in lithium–sulfur batteries by a Gerber tree-like interlayer with multiple components. J. Mater. Chem. A 2017, 5, 11255–11262. [Google Scholar] [CrossRef]
- Lai, Y.; Wang, P.; Qin, F.; Xu, M.; Li, J.; Zhang, K.; Zhang, Z. A carbon nanofiber@mesoporous δ-MnO2 nanosheet-coated separator for high-performance lithium-sulfur batteries. Energy Storage Mater. 2017, 9, 179–187. [Google Scholar] [CrossRef]
- Bizuneh, G.G.; Fan, J.; Sun, C.; Xiangfei, Y.; Xue, F.; Deng, D.; Lei, J.; Lin, X.; Jia, Y.; Yang, J.; et al. LaLiO2-Based Multi-Functional Interlayer for Enhanced Performance of Li-S Batteries. J. Electrochem. Soc. 2019, 166, A68–A73. [Google Scholar] [CrossRef]
- Ali, S.; Waqas, M.; Jing, X.; Chen, N.; Chen, D.; Xiong, J.; He, W. Carbon-Tungsten Disulfide Composite Bilayer Separator for High-Performance Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 39417–39421. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.; Zhang, L.; Deng, Y.; Xu, H.; Qin, X.; Chen, G. CoS-interposed and Ketjen black-embedded carbon nanofiber framework as a separator modulation for high performance Li-S batteries. Chem. Eng. J. 2019, 369, 77–86. [Google Scholar] [CrossRef]
- Tan, L.; Li, X.; Wang, Z.; Guo, H.; Wang, J. Lightweight Reduced Graphene Oxide@MoS2 Interlayer as Polysulfide Barrier for High-Performance Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 3707–3713. [Google Scholar] [CrossRef]
- Chung, S.-H.; Han, P.; Singhal, R.; Kalra, V.; Manthiram, A. Electrochemically Stable Rechargeable Lithium-Sulfur Batteries with a Microporous Carbon Nanofiber Filter for Polysulfide. Adv. Energy Mater. 2015, 5, 1500738. [Google Scholar] [CrossRef]
- Yin, F.; Ren, J.; Zhang, Y.; Tan, T.; Chen, Z. A PPy/ZnO functional interlayer to enhance electrochemical performance of lithium/sulfur batteries. Nanoscale Res. Lett. 2018, 13, 307. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Shen, L.; Lei, D.; Wang, L.; Ye, H.; Li, B.; Kang, F.; He, Y.-B. An ultrathin and continuous Li4Ti5O12 coated carbon nanofiber interlayer for high rate lithium sulfur battery. J. Energy Chem. 2019, 31, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Shi, N.; Xi, B.; Feng, Z.; Wu, F.; Wei, D.; Liu, J.; Xiong, S. Insight into different-microstructured ZnO/graphene-functionalized separators affecting the performance of lithium–sulfur batteries. J. Mater. Chem. A 2019, 7, 4009–4018. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Dong, L.; Liu, S.; Zhao, S.; Han, K.; Zhang, W.; Pan, K.; Zhang, L. NiFe2O4/Ketjen Black Composites as Efficient Membrane Separators to Suppress the Shuttle Effect for Long-Life Lithium-Sulfur Batteries. Nanomaterials 2022, 12, 1347. https://doi.org/10.3390/nano12081347
Jiang W, Dong L, Liu S, Zhao S, Han K, Zhang W, Pan K, Zhang L. NiFe2O4/Ketjen Black Composites as Efficient Membrane Separators to Suppress the Shuttle Effect for Long-Life Lithium-Sulfur Batteries. Nanomaterials. 2022; 12(8):1347. https://doi.org/10.3390/nano12081347
Chicago/Turabian StyleJiang, Wen, Lingling Dong, Shuanghui Liu, Shuangshuang Zhao, Kairu Han, Weimin Zhang, Kefeng Pan, and Lipeng Zhang. 2022. "NiFe2O4/Ketjen Black Composites as Efficient Membrane Separators to Suppress the Shuttle Effect for Long-Life Lithium-Sulfur Batteries" Nanomaterials 12, no. 8: 1347. https://doi.org/10.3390/nano12081347
APA StyleJiang, W., Dong, L., Liu, S., Zhao, S., Han, K., Zhang, W., Pan, K., & Zhang, L. (2022). NiFe2O4/Ketjen Black Composites as Efficient Membrane Separators to Suppress the Shuttle Effect for Long-Life Lithium-Sulfur Batteries. Nanomaterials, 12(8), 1347. https://doi.org/10.3390/nano12081347





