Application of Exogenous Iron Alters the Microbial Community Structure and Reduces the Accumulation of Cadmium and Arsenic in Rice (Oryza sativa L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Sampling
2.3. Soil Chemical Analysis
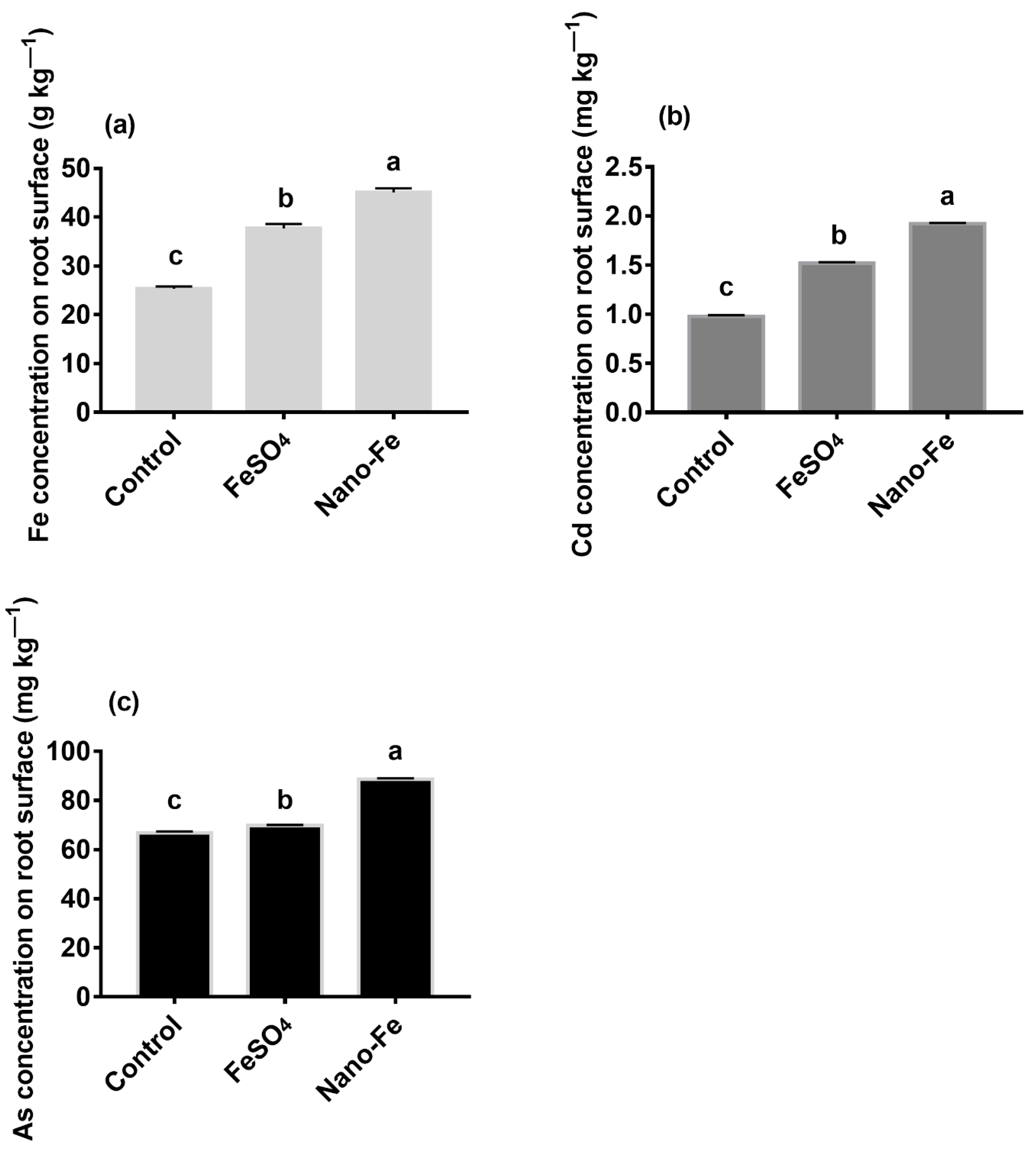
2.4. Analysis of Fe, Cd, and As on the Roots Surfaces
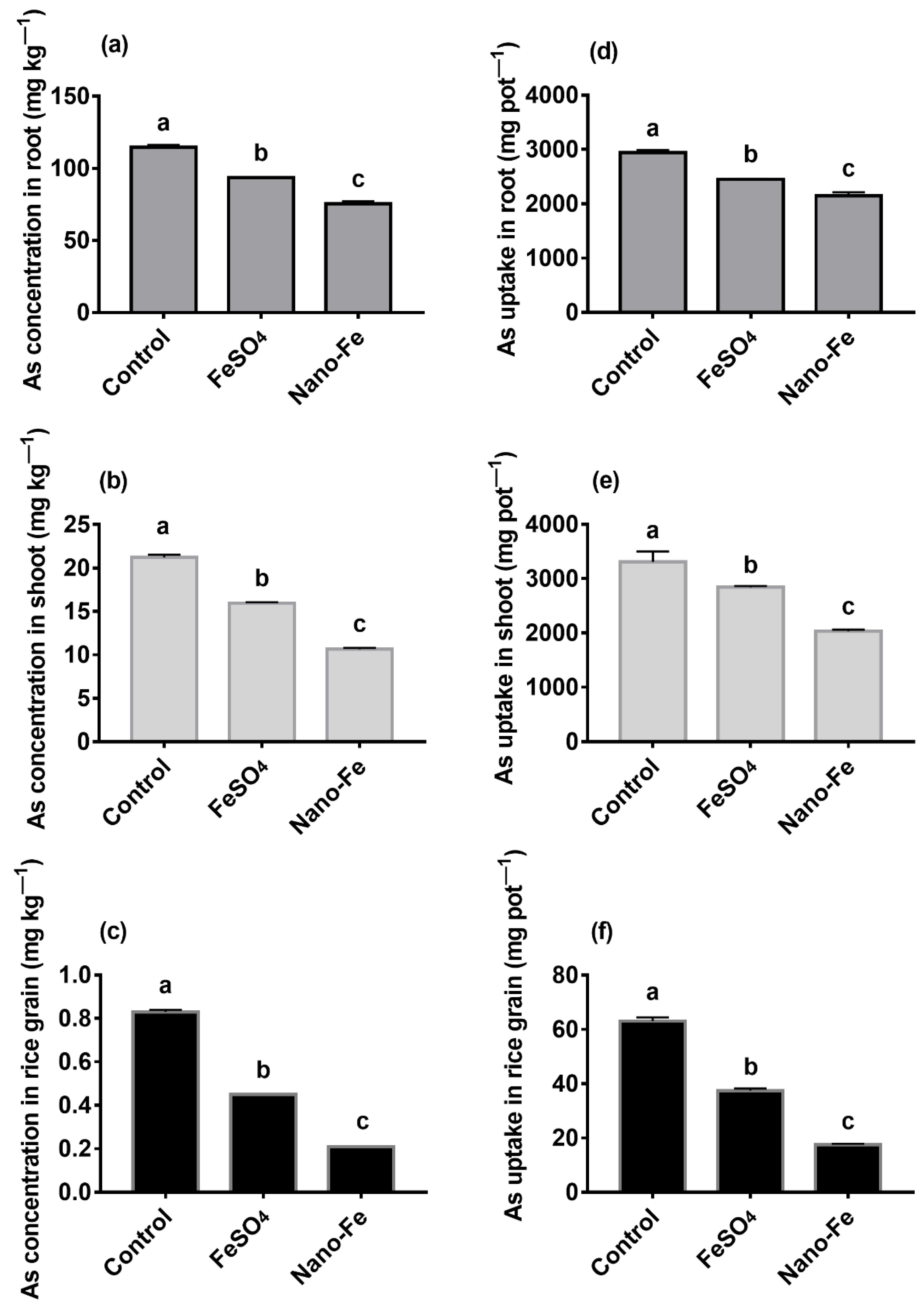
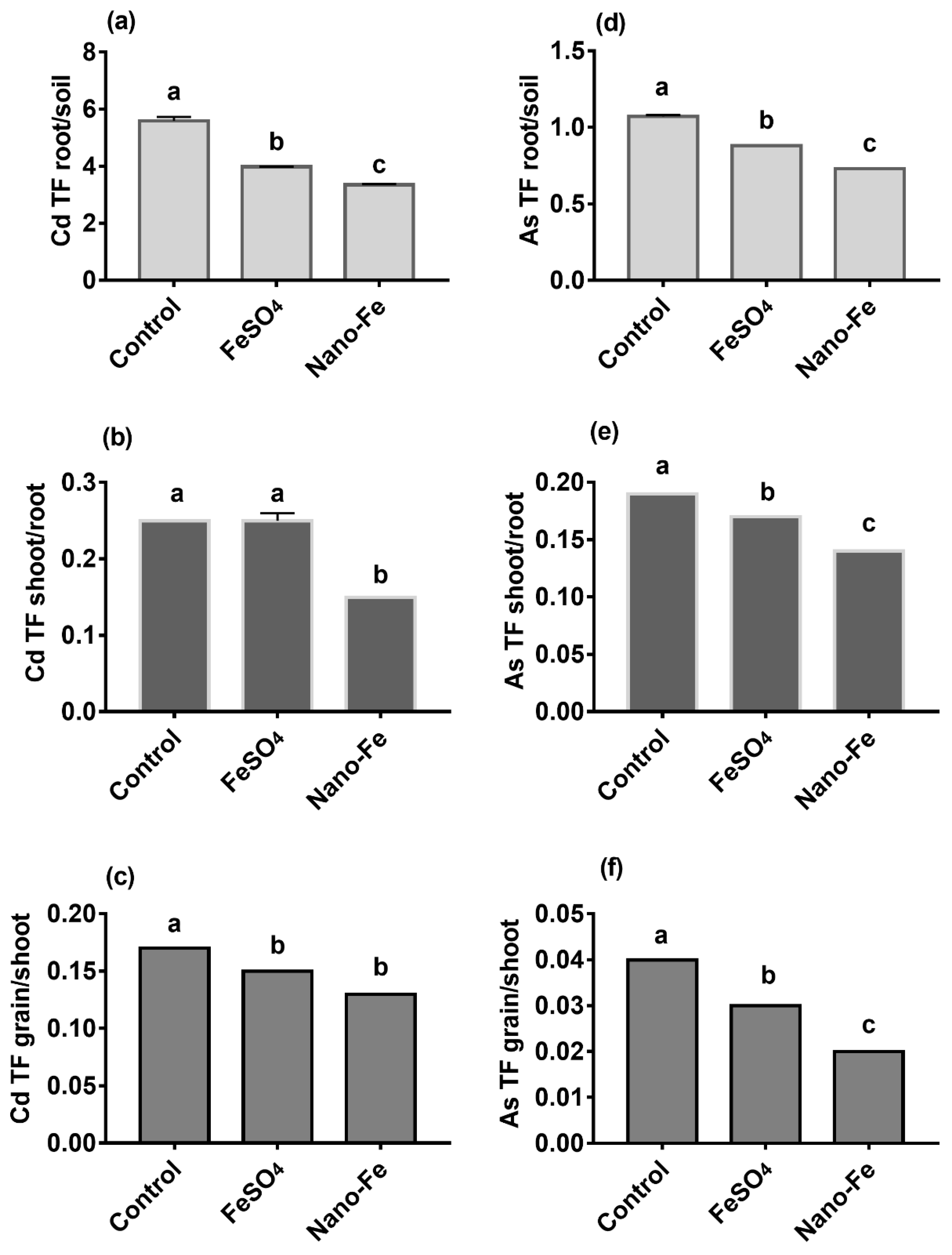
2.5. Analysis of Cd and As in the Roots, Shoots, and Grains
2.6. qPCR Amplification and High-Throughput Sequencing of the Soil Bacteria (16S rRNA)
2.7. Data Processing and Analysis
3. Results
3.1. Yield of Various Parts of Rice
3.2. Contents of Various Forms of Cd and As in the Soil
3.3. Concentrations of Fe, Cd, and As on the Roots Surfaces
3.4. Concentrations, Uptake, and TFs of Cd and As in Rice
3.5. Soil Microbial Community
3.6. Health Risk Assessment
4. Discussion
4.1. Speciation of Cd and As in Soil
4.2. Absorption of Cd and As in Rice
4.3. Health Risk Index
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Humans. Cadmium and Cadmium Compounds, Beryllium, Cadmium, Mercury, and Exposures in the Glass Manufacturing Industry; International Agency for Research on Cancer: Lyon, France, 1993. [Google Scholar]
- Humans. Arsenic and Arsenic Compounds, Arsenic, Metals, Fibres and Dusts; International Agency for Research on Cancer: Lyon, France, 2012. [Google Scholar]
- China, M. The Ministry of Land and Resources Report on the National Soil Contamination Survey; Ministry of Environmental Protection and Ministry of Land and Resources of the People’s Republic of China: Beijing, China, 2014. [Google Scholar]
- Abad-Valle, P.; Alvarez-Ayuso, E.; Murciego, A.; Munoz-Centeno, L.M.; Alonso-Rojo, P.; Villar-Alonso, P. Arsenic distribution in a pasture area impacted by past mining activities. Ecotoxicol. Environ. Saf. 2018, 147, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Health Risk Assessment of Dietary Cadmium Intake: Do Current Guidelines Indicate How Much is Safe? Environ. Health Perspect. 2017, 125, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, P. Arsenic and cadmium accumulation in rice and mitigation strategies. Plant Soil 2020, 446, 1–21. [Google Scholar] [CrossRef]
- Pan, D.; Liu, C.; Yu, H.; Li, F. A paddy field study of arsenic and cadmium pollution control by using iron-modified biochar and silica sol together. Environ. Sci. Pollut. Res. 2019, 26, 24979–24987. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Liu, T.; Wang, X.; Li, F.; Lv, Y.; Cui, J.; Zeng, X.; Yuan, Y.; Liu, C. Simultaneous alleviation of cadmium and arsenic accumulation in rice by applying zero-valent iron and biochar to contaminated paddy soils. Chemosphere 2018, 195, 260–271. [Google Scholar] [CrossRef]
- Honma, T.; Ohba, H.; Kaneko-Kadokura, A.; Makino, T.; Nakamura, K.; Katou, H. Optimal soil Eh, pH, and water management for simultaneously minimizing arsenic and cadmium concentrations in rice grains. Environ. Sci. Technol. 2016, 50, 4178–4185. [Google Scholar] [CrossRef]
- Hussain, B.; Ashraf, M.N.; Shafeeq-ur-Rahman; Abbas, A.; Li, J.; Farooq, M. Cadmium stress in paddy fields: Effects of soil conditions and remediation strategies. Sci. Total Environ. 2021, 754, 142188. [Google Scholar] [CrossRef]
- Komárek, M.; Vaněk, A.; Ettler, V. Chemical stabilization of metals and arsenic in contaminated soils using oxides—A review. Environ. Pollut. 2013, 172, 9–22. [Google Scholar] [CrossRef]
- Zhang, J.; Martinoia, E.; Lee, Y. Vacuolar transporters for cadmium and arsenic in plants and their applications in phytoremediation and crop development. Plant Cell Physiol. 2018, 59, 1317–1325. [Google Scholar] [CrossRef]
- Cang, L.; Zhou, D.; Wang, Q.; Fan, G. Impact of electrokinetic-assisted phytoremediation of heavy metal contaminated soil on its physicochemical properties, enzymatic and microbial activities. Electrochim. Acta 2012, 86, 41–48. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; He, L.; Lu, K.; Sarmah, A.; Li, J.; Bolan, N.S.; Pei, J.; Huang, H. Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ. Sci. Pollut. Res. 2013, 20, 8472–8483. [Google Scholar] [CrossRef] [PubMed]
- Pawar, R.R.; Kim, M.; Kim, J.G.; Hong, S.M.; Sawant, S.Y.; Lee, S.M. Efficient removal of hazardous lead, cadmium, and arsenic from aqueous environment by iron oxide modified clay-activated carbon composite beads. Appl. Clay Sci. 2018, 162, 339–350. [Google Scholar] [CrossRef]
- Wu, J.; Huang, D.; Liu, X.; Meng, J.; Tang, C.; Xu, J. Remediation of as (III) and Cd (II) co-contamination and its mechanism in aqueous systems by a novel calcium-based magnetic biochar. J. Hazard. Mater. 2018, 348, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Qiu, K.; Zou, X.; Lian, M.; Liu, P.; Niu, L.; Yu, L.; Li, X.; Zhang, Z. Mercapto propyltrimethoxysilane-and ferrous sulfate-modified nano-silica for immobilization of lead and cadmium as well as arsenic in heavy metal-contaminated soil. Environ. Pollut. 2020, 266, 115152. [Google Scholar] [CrossRef]
- Islam, M.S.; Magid, A.S.I.A.; Chen, Y.; Weng, L.; Ma, J.; Arafat, M.Y.; Khan, Z.H.; Li, Y. Effect of calcium and iron-enriched biochar on arsenic and cadmium accumulation from soil to rice paddy tissues. Sci. Total Environ. 2021, 785, 147163. [Google Scholar] [CrossRef]
- Yin, D.; Wang, X.; Peng, B.; Tan, C.; Ma, L. Effect of biochar and Fe-biochar on Cd and as mobility and transfer in soil-rice system. Chemosphere 2017, 186, 928–937. [Google Scholar] [CrossRef]
- Guha, T.; Barman, S.; Mukherjee, A.; Kundu, R. Nano-scale zero valent iron modulates Fe/Cd transporters and immobilizes soil Cd for production of Cd free rice. Chemosphere 2020, 260, 127533. [Google Scholar] [CrossRef]
- Lambrechts, T.; Gustot, Q.; Couder, E.; Houben, D.; Iserentant, A.; Lutts, S. Comparison of EDTA-enhanced phytoextraction and phytostabilisation strategies with Lolium perenne on a heavy metal contaminated soil. Chemosphere 2011, 85, 1290–1298. [Google Scholar] [CrossRef]
- Larios, R.; Fernández-Martínez, R.; LeHecho, I.; Rucandio, I. A methodological approach to evaluate arsenic speciation and bioaccumulation in different plant species from two highly polluted mining areas. Sci. Total Environ. 2012, 414, 600–607. [Google Scholar] [CrossRef]
- Sposito, G.; Lund, L.; Chang, A. Trace metal chemistry in arid-zone field soils amended with sewage sludge: I. Fractionation of Ni, Cu, Zn, Cd, and Pb in solid phases. Soil Sci. Soc. Am. J. 1982, 46, 260–264. [Google Scholar] [CrossRef]
- Wenzel, W.W.; Kirchbaumer, N.; Prohaska, T.; Stingeder, G.; Lombi, E.; Adriano, D.C. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal. Chim. Acta 2001, 436, 309–323. [Google Scholar] [CrossRef]
- Taylor, G.J.; Crowder, A.A. Use of the DCB technique for extraction of hydrous iron oxides from roots of wetland plants. Botany 1983, 70, 1254–1257. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Hussain, A.; Ali, Q.; Shakoor, M.B.; Zia-Ur-Rehman, M.; Farid, M.; Asma, M. Effect of zinc-lysine on growth, yield and cadmium uptake in wheat (Triticum aestivum L.) and health risk assessment. Chemosphere 2017, 187, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sharma, R.K.; Agrawal, M.; Marshall, F.M. Health risk assessment of heavy metals via dietary intake of foodstuffs from the wastewater irrigated site of a dry tropical area of India. Food Chem. Toxicol. 2010, 48, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chai, L.; Yang, Z.; Yang, W. Simultaneous immobilization of lead, cadmium, and arsenic in combined contaminated soil with iron hydroxyl phosphate. J. Soils Sediments 2017, 17, 432–439. [Google Scholar] [CrossRef]
- Huang, G.; Ding, C.; Hu, Z.; Cui, C.; Zhang, T.; Wang, X. Topdressing iron fertilizer coupled with pre-immobilization in acidic paddy fields reduced cadmium uptake by rice (Oryza sativa L.). Sci. Total Environ. 2018, 636, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Muehe, E.M.; Adaktylou, I.J.; Obst, M.; Zeitvogel, F.; Behrens, S.; Planer-Friedrich, B.; Kraemer, U.; Kappler, A. Organic carbon and reducing conditions lead to cadmium immobilization by secondary Fe mineral formation in a pH-neutral soil. Environ. Sci. Technol. 2013, 47, 13430–13439. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, X.; Lu, Y.; Bai, L.; Su, S.; Wu, C. Dynamic arsenic aging processes and their mechanisms in nine types of Chinese soils. Chemosphere 2017, 187, 404–412. [Google Scholar] [CrossRef]
- Hou, Q.; Han, D.; Zhang, Y.; Han, M.; Huang, G.; Xiao, L. The bioaccessibility and fractionation of arsenic in anoxic soils as a function of stabilization using low-cost Fe/Al-based materials: A long-term experiment. Ecotoxicol. Environ. Saf. 2020, 191, 110210. [Google Scholar] [CrossRef]
- Zhang, Q.; Pei, L.; Liu, C.; Han, M.; Wang, W. Impact of redox condition on fractionation and bioaccessibility of arsenic in arsenic-contaminated soils remediated by iron amendments: A long-term experiment. Geofluids 2018, 2018, 5243018. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Feng, Y.; Ni, J.; Sun, Y.; Xue, L.; Feng, Y.; Yu, Y.; Lin, X.; Yang, L. Different responses of soil microbial metabolic activity to silver and iron oxide nanoparticles. Chemosphere 2016, 147, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; He, F.; Su, B.; Sun, M.; Owens, G.; Chen, Z. The stabilizing mechanism of cadmium in contaminated soil using green synthesized iron oxide nanoparticles under long-term incubation. J. Hazard. Mater. 2019, 379, 120832. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Nasir, M.; Lv, J.; Dai, Y.; Gao, J. Understanding the variation of microbial community in heavy metals contaminated soil using high throughput sequencing. Ecotoxicol. Environ. Saf. 2017, 144, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, S.; Chen, S.; Meng, N.; Li, X.; Zheng, H.; Zhao, C.; Wang, D. Manipulation of the rhizosphere bacterial community by biofertilizers is associated with mitigation of cadmium phytotoxicity. Sci. Total Environ. 2019, 649, 413–421. [Google Scholar] [CrossRef]
- Price, A.; Macey, M.C.; Miot, J.; Olsson-Francis, K. Draft genome sequences of the nitrate-dependent iron-oxidizing proteobacteria Acidovorax sp. strain BoFeN1 and Paracoccus pantotrophus strain KS1. Microbiol. Resour. Announc. 2018, 7, e01050-18. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Han, Y.; Chen, Y.; Zhu, L.; Ma, L. Arsenic transformation and plant growth promotion characteristics of As-resistant endophytic bacteria from As-hyperaccumulator Pteris vittata. Chemosphere 2016, 144, 1233–1240. [Google Scholar] [CrossRef]
- Zhu, L.; Guan, D.; Luo, J.; Rathinasabapathi, B.; Ma, L. Characterization of arsenic-resistant endophytic bacteria from hyperaccumulators Pteris vittata and Pteris multifida. Chemosphere 2014, 113, 9–16. [Google Scholar] [CrossRef]
- Rashid, M.I.; Shahzad, T.; Shahid, M.; Imran, M.; Dhavamani, J.; Ismail, I.M.I.; Basahi, J.M.; Almeelbi, T. Toxicity of iron oxide nanoparticles to grass litter decomposition in a sandy soil. Sci. Rep. 2017, 7, 41965. [Google Scholar] [CrossRef]
- Khan, S.; Akhtar, N.; Rehman, S.U.; Shujah, S.; Rha, E.S.; Jamil, M. Biosynthesized iron oxide nanoparticles (Fe3O4 NPs) mitigate arsenic toxicity in rice seedlings. Toxics 2020, 9, 2. [Google Scholar] [CrossRef]
- Bidi, H.; Fallah, H.; Niknejad, Y.; Tari, D.B. Iron oxide nanoparticles alleviate arsenic phytotoxicity in rice by improving iron uptake, oxidative stress tolerance and diminishing arsenic accumulation. Plant Physiol. Biochem. 2021, 163, 348–357. [Google Scholar] [CrossRef]
- Maisch, M.; Lueder, U.; Kappler, A.; Schmidt, C. From plant to paddy—how rice root iron plaque can affect the paddy field iron cycling. Soil Syst. 2020, 4, 28. [Google Scholar] [CrossRef]
- Chen, C.; Dixon, J.B.; Turner, F.T. Iron coatings on rice roots: Morphology and models of development. Soil Sci. Soc. Am. J. 1980, 44, 1113–1119. [Google Scholar] [CrossRef]
- Sebastian, A.; Prasad, M. Iron plaque decreases cadmium accumulation in Oryza sativa L. and serves as a source of iron. Plant Biol. 2016, 18, 1008–1015. [Google Scholar]
- Martínez-Fernández, D.; Komárek, M. Comparative effects of nanoscale zero-valent iron (nZVI) and Fe2O3 nanoparticles on root hydraulic conductivity of Solanum lycopersicum L. Environ. Exp. Bot. 2016, 131, 128–136. [Google Scholar] [CrossRef]
- Yu, H.; Wang, X.; Li, F.; Li, B.; Liu, C.; Wang, Q.; Lei, J. Arsenic mobility and bioavailability in paddy soil under iron compound amendments at different growth stages of rice. Environ. Pollut. 2017, 224, 136–147. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, S.; Rizwan, M.; ur Rehman, M.Z.; Qayyum, M.F.; Wang, H.; Rinklebe, J. Responses of wheat (Triticum aestivum) plants grown in a Cd contaminated soil to the application of iron oxide nanoparticles. Ecotoxicol. Environ. Saf. 2019, 173, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.; Fahad, S.; Aamer, M.; Hassan, M.U.; Tahir, M.M.; Wu, Z. Role of genetic factors in regulating cadmium uptake, transport and accumulation mechanisms and quantitative trait loci mapping in rice: A review. Appl. Ecol. Environ. Res. 2020, 18, 4005–4023. [Google Scholar] [CrossRef]
- Shinde, A.; Kumar, K. Mechanisms of arsenic transport, accumulation, and distribution in rice. In Arsenic Toxicity: Challenges and Solutions; Springer: Singapore, 2021; pp. 279–300. [Google Scholar]
- Liu, Y.; Zhang, C.; Zhao, Y.; Sun, S.; Liu, Z. Effects of growing seasons and genotypes on the accumulation of cadmium and mineral nutrients in rice grown in cadmium contaminated soil. Sci. Total Environ. 2017, 579, 1282–1288. [Google Scholar] [CrossRef]
- Takahashi, R.; Bashir, K.; Ishimaru, Y.; Nishizawa, N.K.; Nakanishi, H. The role of heavy-metal ATPases, HMAs, in zinc and cadmium transport in rice. Plant Signal. Behav. 2012, 7, 1605–1607. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, W.; Huang, S.; Xu, P.; Cao, Z.; Chen, M.; Lin, X. The potential role of plasma membrane proteins in response to Zn stress in rice roots based on iTRAQ and PRM under low Cd condition. J. Hazard. Mater. 2022, 429, 128324. [Google Scholar] [CrossRef]
- Zhiguo, E.; Li, T.; Chen, C.; Wang, L. Genome-wide survey and expression analysis of P1B-ATPases in rice, maize and sorghum. Rice Sci. 2018, 25, 208–217. [Google Scholar] [CrossRef]
- Sharafi, K.; Yunesian, M.; Nodehi, R.N.; Mahvi, A.H.; Pirsaheb, M. A systematic literature review for some toxic metals in widely consumed rice types (domestic and imported) in Iran: Human health risk assessment, uncertainty and sensitivity analysis. Ecotoxicol. Environ. Saf. 2019, 176, 64–75. [Google Scholar] [CrossRef] [PubMed]


| Treatments | Root Yield (g) | Shoot Yield (g) | Grain Yield (g) |
|---|---|---|---|
| Control | 25.66 ± 0.14c | 156.07 ± 8.61b | 76.33 ± 0.93b |
| FeSO4 | 26.18 ± 0.03bc | 178.20 ± 0.88a | 83.33 ± 0.79a |
| Nano-Fe | 28.40 ± 0.45a | 191.00 ± 0.43a | 83.46 ± 0.22a |
| Treatments | Exchangeable Cd (μg·kg−1) | Organic Bound Cd (μg·kg−1) | Inorganic Bound Cd (μg·kg−1) | Residual Cd (μg·kg−1) |
|---|---|---|---|---|
| Control | 6.75 ± 0.06a | 19.27 ± 0.06a | 1119.67 ± 0.98a | 583.33 ± 5.44c |
| FeSO4 | 6.13 ± 0.01b | 13.36 ± 0.18b | 1076.67 ± 10.89b | 633.33 ± 7.20b |
| Nano-Fe | 3.31 ± 0.09c | 6.79 ± 0.26c | 965.67 ± 14.03c | 753.33 ± 7.20a |
| Treatments | Non-Specifically Sorbed As (mg·kg−1) | Specifically Sorbed As (mg·kg−1) | Amorphous Iron Oxide As (mg·kg−1) | Fe/Al Hydrated Oxide As (mg·kg−1) | Residual As (mg·kg−1) |
|---|---|---|---|---|---|
| Control | 0.25 ± 0.00a | 12.09 ± 0.05a | 49.61 ± 0.24a | 28.38 ± 0.14c | 16.61 ± 0.14b |
| FeSO4 | 0.26 ± 0.01a | 9.49 ± 0.14b | 48.83 ± 0.57ab | 30.96 ± 0.25b | 17.40 ± 0.62ab |
| Nano-Fe | 0.25 ± 0.01a | 8.2 ± 0.13c | 48.18 ± 0.31b | 32.09 ± 0.36a | 18.21 ± 0.12a |
| Treatments | Shannon’s Index | Simpson’s Index |
|---|---|---|
| Control | 6.4452 ± 0.0058c | 0.00536 ± 0.00004a |
| FeSO4 | 6.4920 ± 0.0078b | 0.00518 ± 0.00002b |
| Nano-Fe | 6.6208 ± 0.0124a | 0.00416 ± 0.00013c |
| Treatments | Cd | As | HRI Cd-As | ||
|---|---|---|---|---|---|
| DIM | HRI | DIM | HRI | ||
| Control | 0.00019 | 0.19 | 0.00040 | 1.34 | 1.53 |
| FeSO4 | 0.00012 | 0.12 | 0.00022 | 0.73 | 0.85 |
| Nano-Fe | 0.00006 | 0.06 | 0.00010 | 0.34 | 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Li, J.; Zhan, X.; Wang, X.; He, B.; Cao, F.; Liao, C.; Yu, Y.; Zhang, Z.; Zhang, J.; et al. Application of Exogenous Iron Alters the Microbial Community Structure and Reduces the Accumulation of Cadmium and Arsenic in Rice (Oryza sativa L.). Nanomaterials 2022, 12, 1311. https://doi.org/10.3390/nano12081311
Li T, Li J, Zhan X, Wang X, He B, Cao F, Liao C, Yu Y, Zhang Z, Zhang J, et al. Application of Exogenous Iron Alters the Microbial Community Structure and Reduces the Accumulation of Cadmium and Arsenic in Rice (Oryza sativa L.). Nanomaterials. 2022; 12(8):1311. https://doi.org/10.3390/nano12081311
Chicago/Turabian StyleLi, Tingting, Jiayuan Li, Xin Zhan, Xueli Wang, Bing He, Feishu Cao, Changjun Liao, Yuefeng Yu, Zengyu Zhang, Junhui Zhang, and et al. 2022. "Application of Exogenous Iron Alters the Microbial Community Structure and Reduces the Accumulation of Cadmium and Arsenic in Rice (Oryza sativa L.)" Nanomaterials 12, no. 8: 1311. https://doi.org/10.3390/nano12081311
APA StyleLi, T., Li, J., Zhan, X., Wang, X., He, B., Cao, F., Liao, C., Yu, Y., Zhang, Z., Zhang, J., Li, B., Chen, J., Li, H., Zhu, Z., Wei, Y., & Hu, J. (2022). Application of Exogenous Iron Alters the Microbial Community Structure and Reduces the Accumulation of Cadmium and Arsenic in Rice (Oryza sativa L.). Nanomaterials, 12(8), 1311. https://doi.org/10.3390/nano12081311








