Nanoplastics and Human Health: Hazard Identification and Biointerface
Abstract
:1. Introduction
2. Another Two Sources of Nanoplastics in the Environment
2.1. Nanoplastics from Tire Wear
2.2. Nanoplastics from Laundry Wastewater
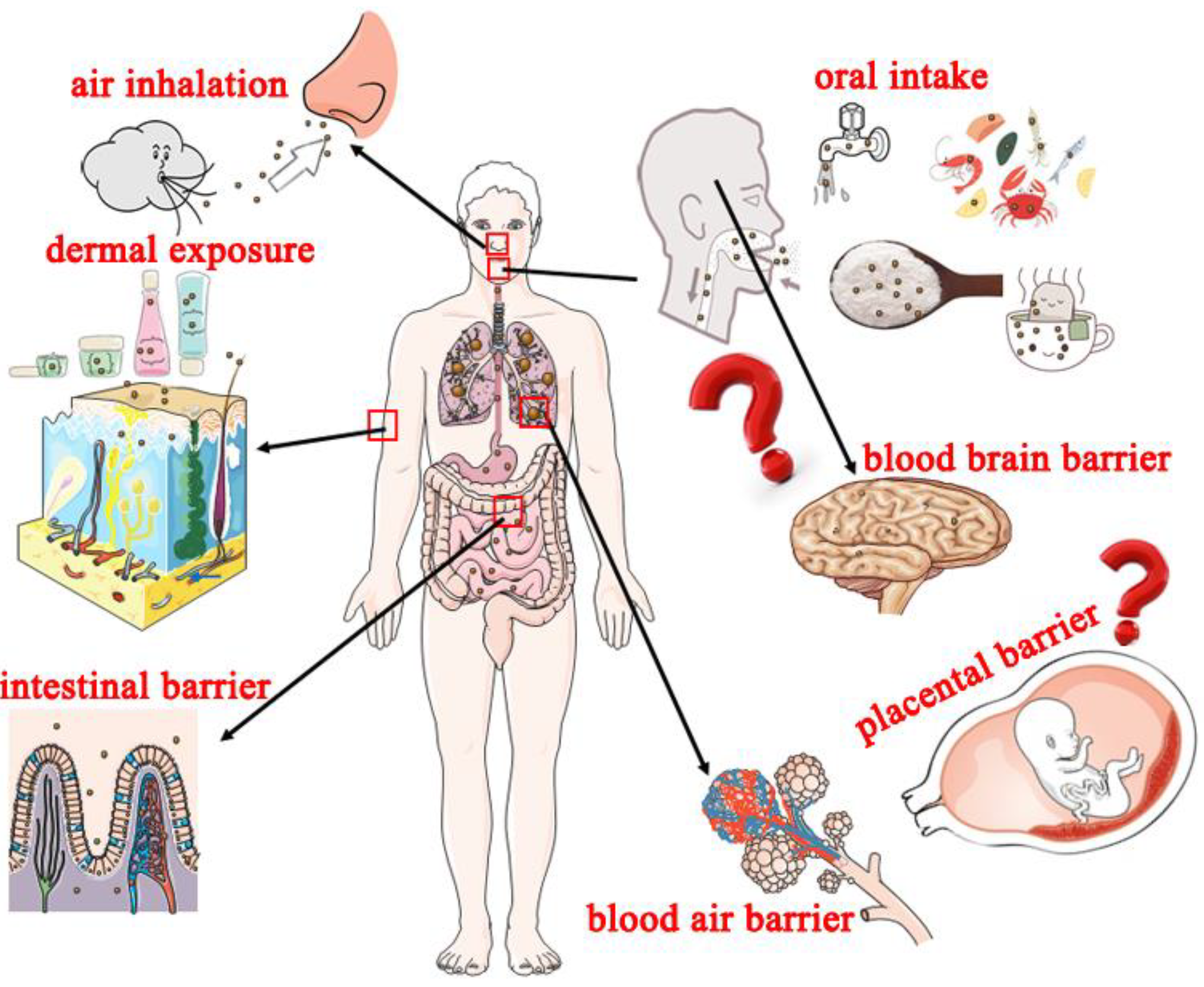
3. Potential Exposure Routes of Nanoplastics and Adverse Effects on Humans
3.1. Potential Exposure Routes of Nanoplastics to Humans
3.1.1. Oral Intake
3.1.2. Air Inhalation
3.1.3. Dermal Exposure
3.2. Potential Adverse Effects of Nanoplastics on Human
4. Behavior of Nanoplastics
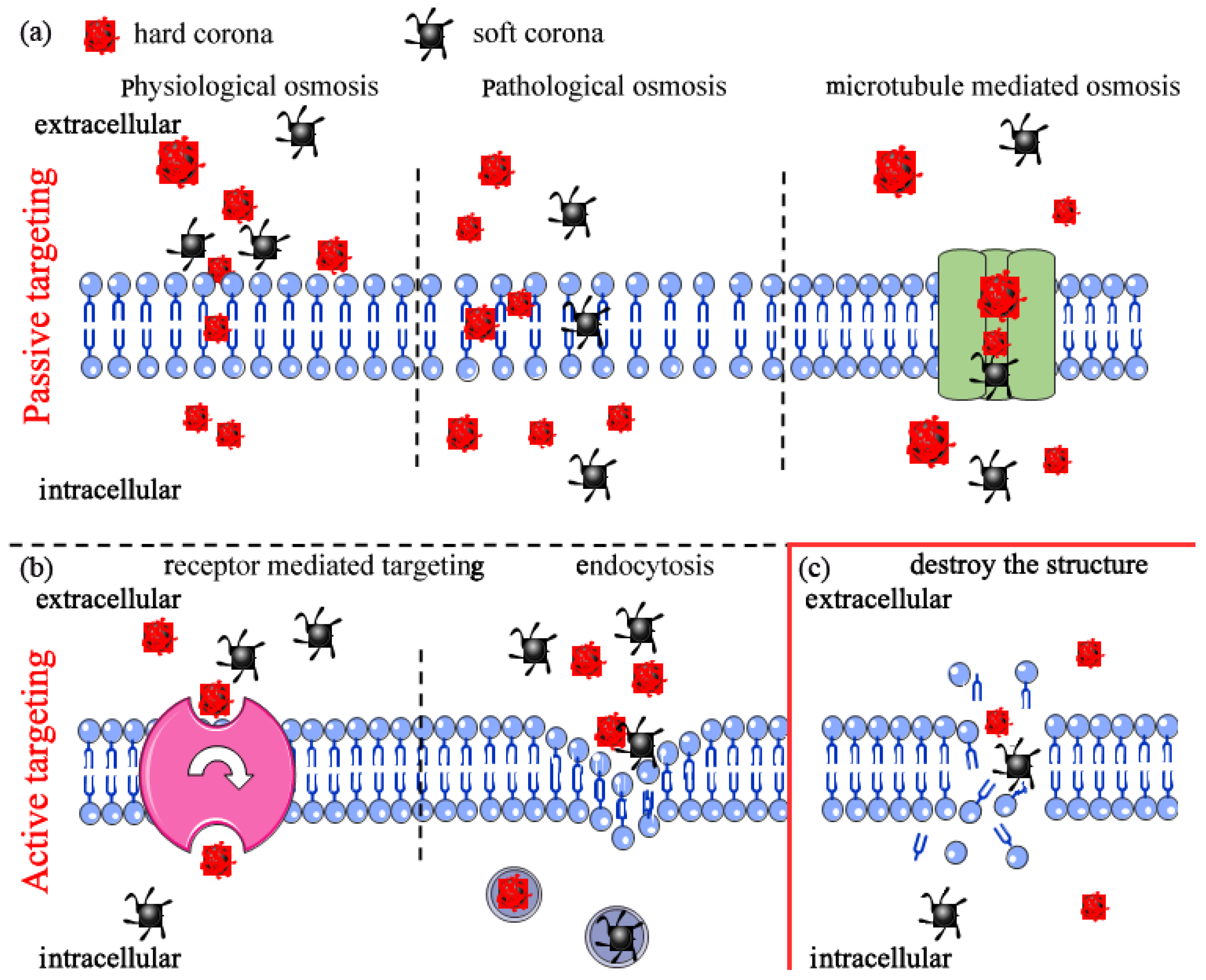
4.1. Interactions with Biological Media
4.2. Interactions with Cell Membrane
4.2.1. Cell Internalization
4.2.2. Nanoplastics Destroy Cell Membrane Structure Leading to Cell Death
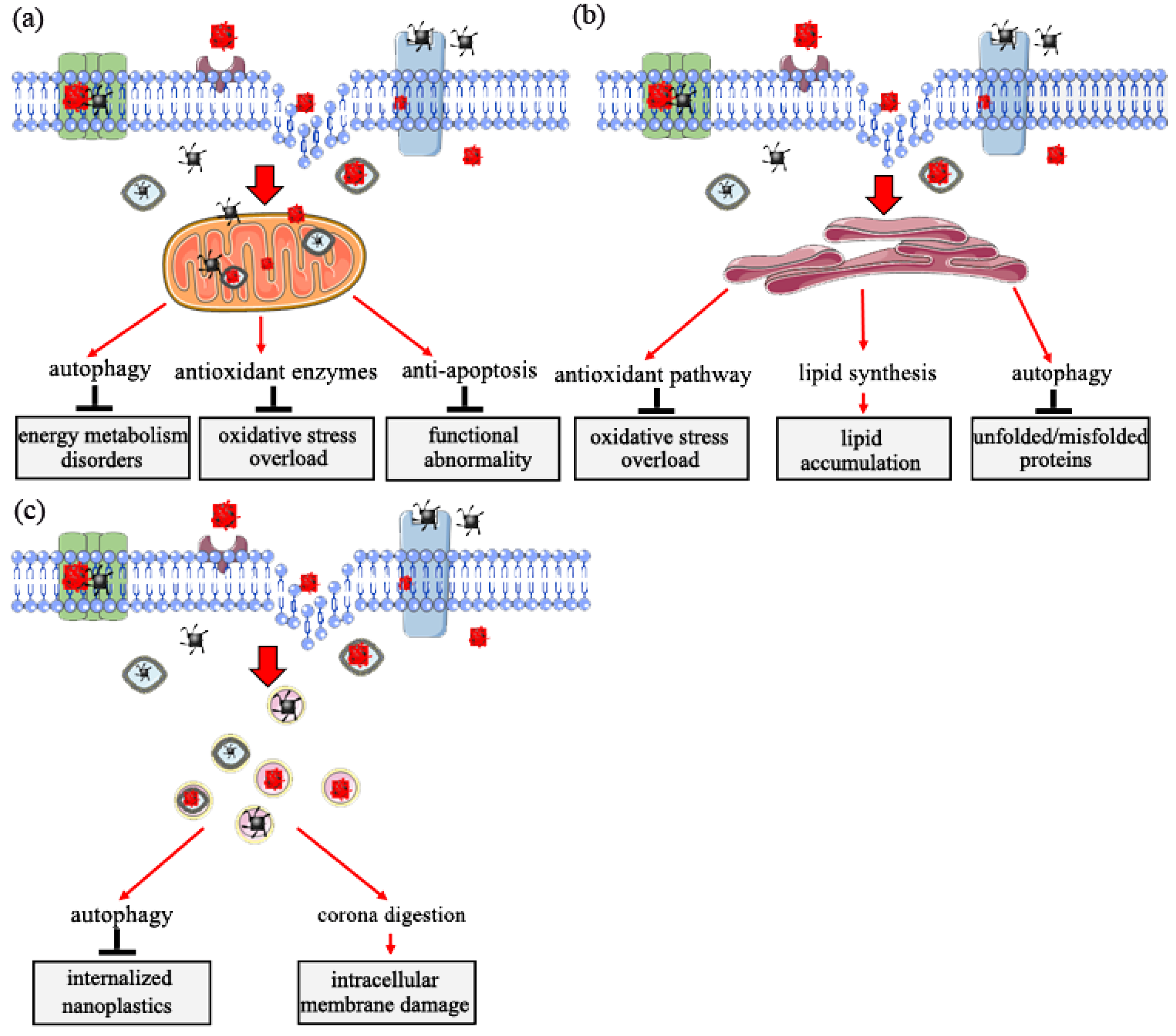
5. Target Organelle Toxicity Induced by Nanoplastics
5.1. Role of Mitochondria in Response to Nanoplastic Toxicity
5.2. Role of Endoplasmic Reticulum in Response to Nanoplastics Toxicity
5.3. Role of Lysosome in Response to Nanoplastics Toxicity
6. Challenges and Future Research
6.1. Detection of Nanoplastics in the Environment
6.2. Elimination or Reduction of Microplastic or Nanoplastic Pollution
6.2.1. Recycling
6.2.2. Substitute Materials
6.2.3. Degradation of Microplastic or Nanoplastic Pollutions
6.3. Comprehensive Analysis of Nanoplastics Toxicity
6.3.1. Toxicity of Aged Nanoplastics and Their Leachings
6.3.2. Toxicity of Nanoplastics at Environmentally Relevant Concentrations (ERC)
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lebordais, M.; Gutierrez-Villagomez, J.M.; Gigault, J.; Baudrimont, M.; Langlois, V.S. Molecular impacts of dietary exposure to nanoplastics combined with arsenic in Canadian oysters (Crassostrea virginica) and bioaccumulation comparison with Caribbean oysters (Isognomon alatus). Chemosphere 2021, 277, 130331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, S.H.; Luo, G.; Kang, Y.; Zhang, L.; Pan, Y.; Zhou, X.; Fan, L.; Liang, B.; Wang, A. The contamination of microplastics in China’s aquatic environment: Occurrence, detection and implications for ecological risk. Environ. Pollut. 2022, 296, 118737. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.J.; Le Goic, N.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef] [Green Version]
- Dawson, A.L.; Kawaguchi, S.; King, C.K.; Townsend, K.A.; King, R.; Huston, W.M.; Nash, S.M.B. Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Nat. Commun. 2018, 9, 1001. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [Green Version]
- Gigault, J.; El Hadri, H.; Nguyen, B.; Grassl, B.; Rowenczyk, L.; Tufenkji, N.; Feng, S.Y.; Wiesner, M. Nanoplastics are neither microplastics nor engineered nanoparticles. Nat. Nanotechnol. 2021, 16, 501–507. [Google Scholar] [CrossRef]
- Gigault, J.; Halle, A.T.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Revel, M.; Chatel, A.; Mouneyrac, C. Micro(nano)plastics: A threat to human health? Curr. Opin. Environ. Sci. Health 2018, 1, 17–23. [Google Scholar] [CrossRef]
- Mintenig, S.M.; Loder, M.G.J.; Primpke, S.; Gerdts, G. Low numbers of microplastics detected in drinking water from ground water sources. Sci. Total Environ. 2019, 648, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Jemec, A.; Horvat, P.; Kunej, U.; Bele, M.; Krzan, A. Uptake and effects of microplastic textile fibers on freshwater crustacean Daphnia magna. Environ. Pollut. 2016, 219, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Dalela, M.; Shrivastav, T.G.; Kharbanda, S.; Singh, H. pH-Sensitive Biocompatible nanoparticles of paclitaxel-conjugated poly(styrene-co-maleic acid) for anticancer drug delivery in solid tumors of syngeneic mice. ACS Appl. Mater. Inter. 2015, 7, 26530–26548. [Google Scholar] [CrossRef]
- Wang, L.; Xie, X.J.; Cao, T.C.; Bosset, J.; Bakker, E. Surface-doped polystyrene microsensors containing lipophilic solvatochromic dye transducers. Chem. Eur. J. 2018, 24, 7921–7925. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Yousefi, N.; Tufenkji, N. Are there nanoplastics in your personal care products? Environ. Sci. Technol. Let. 2017, 4, 280–285. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.K.; Hong, S.H.; Eo, S.; Jang, M.; Han, G.M.; Isobe, A.; Shim, W.J. Horizontal and vertical distribution of microplastics in korean coastal waters. Environ. Sci. Technol. 2018, 52, 12188–12197. [Google Scholar] [CrossRef]
- Zhang, G.S.; Liu, Y.F. The distribution of microplastics in soil aggregate fractions in southwestern China. Sci. Total Environ. 2018, 642, 12–20. [Google Scholar] [CrossRef]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic pollution in table salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar] [CrossRef]
- Peng, L.; Fu, D.; Qi, H.; Lan, C.Q.; Yu, H.; Ge, C. Micro- and nano-plastics in marine environment: Source, distribution and threats—A review. Sci. Total Environ. 2020, 698, 134254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Su, J.; Xiong, X.; Wu, X.; Wu, C.; Liu, J. Microplastic pollution of lakeshore sediments from remote lakes in Tibet plateau, China. Environ. Pollut. 2016, 219, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.J.; Salvati, A.; Boya, P. Lysosome-dependent cell death and deregulated autophagy induced by amine-modified polystyrene nanoparticles. Open Biol. 2018, 8, 170271. [Google Scholar] [CrossRef] [Green Version]
- Wik, A.; Dave, G. Occurrence and effects of tire wear particles in the environment—A critical review and an initial risk assessment. Environ. Pollut. 2009, 157, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kreider, M.L.; Panko, J.M.; McAtee, B.L.; Sweet, L.I.; Finley, B.L. Physical and chemical characterization of tire-related particles: Comparison of particles generated using different methodologies. Sci. Total Environ. 2010, 408, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.M. Traffic generated non-exhaust particulate emissions from concrete pavement: A mass and particle size study for two-wheelers and small cars. Atmos. Environ. 2009, 43, 5691–5697. [Google Scholar]
- Dahl, A.; Gharibi, A.; Swietlicki, E.; Gudmundsson, A.; Bohgard, M.; Ljungman, A.; Blomqvist, G.; Gustafsson, M. Traffic-generated emissions of ultrafine particles from pavement-tire interface. Atmos. Environ. 2006, 40, 1314–1323. [Google Scholar] [CrossRef]
- Mathissen, M.; Scheer, V.; Vogt, R.; Benter, T. Investigation on the potential generation of ultrafine particles from the tire–road interface. Atmos. Environ. 2011, 45, 6172–6179. [Google Scholar] [CrossRef]
- Leads, R.R.; Weinstein, J.E. Occurrence of tire wear particles and other microplastics within the tributaries of the Charleston Harbor Estuary, South Carolina, USA. Mar. Pollut. Bull. 2019, 145, 569–582. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.; Rui, T.; Tufenkji, N. Plastic teabags release billions of microparticles and nanoparticles into tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef] [PubMed]
- Carney Almroth, B.M.; Åström, L.; Roslund, S.; Petersson, H.; Johansson, M.; Persson, N.K. Quantifying shedding of synthetic fibers from textiles; a source of microplastics released into the environment. Environ. Sci. Pollut. Res. Int. 2018, 25, 1191–1199. [Google Scholar] [CrossRef] [Green Version]
- Sillanpää, M.; Sainio, P. Release of polyester and cotton fibers from textiles in machine washings. Environ. Sci. Pollut. Res. Int. 2017, 24, 19313–19321. [Google Scholar] [CrossRef] [PubMed]
- Lenz, R.; Enders, K.; Nielsen, T.G. Microplastic exposure studies should be environmentally realistic. Proc. Natl. Acad. Sci. USA 2016, 113, E4121–E4122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galloway, T. Micro- and Nano-Plastics and Human Health; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and nanoplastics in aquatic environments: Aggregation, deposition, and enhanced contaminant transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef] [PubMed]
- Al-Sid-Cheikh, M.; Rowland, S.J.; Stevenson, K.; Rouleau, C.; Henry, T.B.; Thompson, R.C. Uptake, whole-body distribution, and depuration of nanoplastics by the Scallop Pecten maximus at environmentally realistic concentrations. Environ. Sci. Technol. 2018, 52, 14480–14486. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; He, Q.; Liu, C.; Huangfu, X. Are micro- or nanoplastics leached from drinking water distribution systems? Environ. Sci. Technol. 2019, 53, 9339–9340. [Google Scholar] [CrossRef] [Green Version]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human consumption of microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. [Google Scholar] [CrossRef] [Green Version]
- Winkler, A.; Santo, N.; Ortenzi, M.A.; Bolzoni, E.; Bacchetta, R.; Tremolada, P. Does mechanical stress cause microplastic release from plastic water bottles? Water Res. 2019, 166, 115082. [Google Scholar] [CrossRef]
- Hoogenboom, L.A.P.; Hoogenboom, L.A.P.; Hoogenboom, L.A.P. Statement: Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016, 14, e04501. [Google Scholar]
- Yee, S.L.; Hii, L.W.; Looi, C.K.; Lim, W.M.; Leong, C.O. Impact of microplastics and nanoplastics on human health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef]
- Hong, S.H.; Shim, W.J.; Hong, L. Methods of analysing chemicals associated with microplastics: A review. Anal. Methods 2017, 9, 1361–1368. [Google Scholar] [CrossRef]
- Kole, P.J.; Lhr, A.J.; Belleghem, F.G.A.J.V.; Ragas, A.M.J. Wear and tear of tyres in the global environment: Size distribution, emission, pathways and health effects. In Proceedings of the SETAC Europe 29th Annual Meeting, Helsinki, Finland, 26–30 May 2019. [Google Scholar]
- Vogt, A.; Combadiere, B.; Hadam, S.; Stieler, K.M.; Lademann, J.; Schaefer, H.; Autran, B.; Sterry, W.; Blume-Peytavi, U. 40 nm, but not 750 or 1,500 nm, nanoparticles enter epidermal CD1a+ cells after transcutaneous application on human skin. J. Investig. Dermatol. 2006, 126, 1316–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, M.; Stracke, F.; Hansen, S.; Schaefer, U.F. Nanoparticles and their interactions with the dermal barrier. Dermatoendocrinology 2009, 1, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Pitt, J.A.; Trevisan, R.; Massarsky, A.; Kozal, J.S.; Levin, E.D.; Di Giulio, R.T. Maternal transfer of nanoplastics to offspring in zebrafish (Danio rerio): A case study with nanopolystyrene. Sci. Total Environ. 2018, 643, 324–334. [Google Scholar] [CrossRef]
- Lehner, R.; Petri-Fink, A.; Rothen-Rutishauser, B. Nanoplastic impact on human health—A 3D intestinal model to study the interaction with nanoplastic particles. In Proceedings of the International Conference on Microplastic Pollution in the Mediterranean Sea; Springer Water: Capri, Italy, 2017. [Google Scholar]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of various microplastics in human stool: A prospective case series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Tagesson, C.; Sjödahl, R. Passage of molecules through the wall of the gastrointestinal tract. Scand. J. Gastroenterol. 1978, 13, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Gehr, P.; Bachofen, M.; Weibel, E.R. The normal human lung: Ultrastructure and morphometric estimation of diffusion capacity. Resp. Physiol. 1978, 32, 121–140. [Google Scholar] [CrossRef]
- Yang, C.S.; Chang, C.H.; Tsai, P.J.; Chen, W.Y.; Tseng, F.G.; Lo, L.W. Nanoparticle-based in vivo investigation on blood-brain barrier permeability following ischemia and reperfusion. Anal. Chem. 2004, 76, 4465–4471. [Google Scholar] [CrossRef]
- Mattsson, K.; Johnson, E.V.; Malmendal, A.; Linse, S.; Hansson, L.A.; Cedervall, T. Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain. Sci. Rep. 2017, 7, 11452. [Google Scholar] [CrossRef]
- Grafmueller, S.; Manser, P.; Diener, L.; Diener, P.A.; Maeder-Althaus, X.; Maurizi, L.; Jochum, W.; Krug, H.F.; Buerki-Thurnherr, T.; von Mandach, U.; et al. Bidirectional transfer study of polystyrene nanoparticles across the placental barrier in an ex vivo human placental perfusion model. Environ. Health Perspect. 2015, 123, 1280–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francia, V.; Yang, K.; Deville, S.; Reker-Smit, C.; Nelissen, I.; Salvati, A. Corona composition can affect the mechanisms cells use to internalize nanoparticles. ACS Nano 2019, 13, 11107–11121. [Google Scholar] [CrossRef] [PubMed]
- Dzuricky, M.; Xiong, S.; Weber, P.; Chilkoti, A. Avidity and cell uptake of integrin-targeting polypeptide micelles is strongly shape-dependent. Nano Lett. 2019, 19, 6124–6132. [Google Scholar] [CrossRef] [PubMed]
- Kihara, S.; Ghosh, S.; McDougall, D.R.; Whitten, A.E.; Mata, J.P.; Köper, I.; McGillivray, D.J. Structure of soft and hard protein corona around polystyrene nanoplastics-particle size and protein types. Biointerphases 2020, 15, 051002. [Google Scholar] [CrossRef] [PubMed]
- Kihara, S.; van der Heijden, N.J.; Seal, C.K.; Mata, J.P.; Whitten, A.E.; Köper, I.; McGillivray, D.J. Soft and Hard interactions between polystyrene nanoplastics and human serum albumin protein corona. Bioconjugate Chem. 2019, 30, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Baimanov, D.; Cai, R.; Chen, C. Understanding the chemical nature of nanoparticle-protein interactions. Bioconjug. Chem. 2019, 30, 1923–1937. [Google Scholar] [CrossRef]
- Xu, M.; Halimu, G.; Zhang, Q.; Song, Y.; Fu, X.; Li, Y.; Li, Y.; Zhang, H. Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci. Total Environ. 2019, 694, 133794. [Google Scholar] [CrossRef]
- Lee, W.S.; Cho, H.J.; Kim, E.; Huh, Y.H.; Kim, H.J.; Kim, B.; Kang, T.; Lee, J.S.; Jeong, J. Bioaccumulation of polystyrene nanoplastics and their effect on the toxicity of Au ions in zebrafish embryos. Nanoscale 2019, 11, 3173–3185. [Google Scholar] [CrossRef]
- Bhushan, B.; Khanadeev, V.; Khlebtsov, B.; Khlebtsov, N.; Gopinath, P. Impact of albumin based approaches in nanomedicine: Imaging, targeting and drug delivery. Adv. Colloid Interfac. 2017, 246, 13–39. [Google Scholar] [CrossRef]
- Montel, L.; Pinon, L.; Fattaccioli, J. A Multiparametric and high-throughput assay to quantify the influence of target size on phagocytosis. Biophys. J. 2019, 117, 408–419. [Google Scholar] [CrossRef]
- Dos Santos, T.; Varela, J.; Lynch, I.; Salvati, A.; Dawson, K.A. Quantitative assessment of the comparative nanoparticle-uptake efficiency of a range of cell lines. Small 2011, 7, 3341–3349. [Google Scholar] [CrossRef] [Green Version]
- Hollóczki, O.; Gehrke, S. Can nanoplastics alter cell membranes? ChemPhysChem 2020, 21, 9–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, J.T.; Rösslein, M.; Song, N.W.; Toman, B.; Kinsner-Ovaskainen, A.; Maniratanachote, R.; Salit, M.L.; Petersen, E.J.; Sequeira, F.; Romsos, E.L.; et al. Toward achieving harmonization in a nano-cytotoxicity assay measurement through an interlaboratory comparison study. Altex 2017, 34, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Leroueil, P.R.; Janus, E.K.; Peters, J.L.; Kober, M.M.; Islam, M.T.; Orr, B.G.; Baker, J.R., Jr.; Banaszak Holl, M.M. Interaction of polycationic polymers with supported lipid bilayers and cells: Nanoscale hole formation and enhanced membrane permeability. Bioconjugate Chem. 2006, 17, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Qiu, Y.; Kong, Y.; Wang, D. Amino modification enhances reproductive toxicity of nanopolystyrene on gonad development and reproductive capacity in nematode Caenorhabditis elegans. Environ. Pollut. 2019, 254, 112978. [Google Scholar] [CrossRef] [PubMed]
- Pinsino, A.; Bergami, E.; Della Torre, C.; Vannuccini, M.L.; Addis, P.; Secci, M.; Dawson, K.A.; Matranga, V.; Corsi, I. Amino-modified polystyrene nanoparticles affect signalling pathways of the sea urchin (Paracentrotus lividus) embryos. Nanotoxicology 2017, 11, 201–209. [Google Scholar] [CrossRef]
- Lim, S.L.; Ng, C.T.; Zou, L.; Lu, Y.; Chen, J.; Bay, B.H.; Shen, H.M.; Ong, C.N. Targeted metabolomics reveals differential biological effects of nanoplastics and nanoZnO in human lung cells. Nanotoxicology 2019, 13, 1117–1132. [Google Scholar] [CrossRef]
- Qu, M.; Liu, Y.; Xu, K.; Wang, D. Activation of p38 MAPK signaling-mediated endoplasmic reticulum unfolded protein response by nanopolystyrene particles. Adv. Biosys. 2019, 3, e1800325. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef]
- Reinholz, J.; Diesler, C.; Schöttler, S.; Kokkinopoulou, M.; Ritz, S.; Landfester, K.; Mailänder, V. Protein machineries defining pathways of nanocarrier exocytosis and transcytosis. Acta Biomater. 2018, 71, 432–443. [Google Scholar] [CrossRef]
- Armstrong, J.S. Mitochondrial medicine: Pharmacological targeting of mitochondria in disease. Brit. J. Pharmacol. 2007, 151, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Q.; Zhao, Y.; Bai, Y.; Chen, P.; Xia, T.; Wang, D. Response of microRNAs to in vitro treatment with graphene oxide. ACS Nano 2014, 8, 2100–2110. [Google Scholar] [CrossRef]
- Bergami, E.; Emerenciano, A.K.; Gonzalez-Aravena, M.; Cardenas, C.A.; Hernandez, P.; Silva, J.; Corsi, I. Polystyrene nanoparticles affect the innate immune system of the Antarctic sea urchin Sterechinus neumayeri. Polar Biol. 2019, 42, 743–757. [Google Scholar] [CrossRef]
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell. Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marciniak, S.J. Endoplasmic reticulum stress in lung disease. Eur. Respir. Rev. 2017, 26, 170018. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Kaufman, R.J. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 2016, 529, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Bio. 2007, 8, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shao, H.; Wu, Q.; Wang, D. Lipid metabolic response to polystyrene particles in nematode Caenorhabditis elegans. Environ. Pollut. 2020, 256, 113439. [Google Scholar] [CrossRef]
- Song, W.; Popp, L.; Yang, J.; Kumar, A.; Gangoli, V.S.; Segatori, L. The autophagic response to polystyrene nanoparticles is mediated by transcription factor EB and depends on surface charge. J. Nanobiotechnol. 2015, 13, 87. [Google Scholar] [CrossRef] [Green Version]
- Saftig, P.; Haas, A. Turn up the lysosome. Nat. Cell Biol. 2016, 18, 1025–1027. [Google Scholar] [CrossRef]
- Fröhlich, E.; Meindl, C.; Roblegg, E.; Ebner, B.; Absenger, M.; Pieber, T.R. Action of polystyrene nanoparticles of different sizes on lysosomal function and integrity. Part. Fibre Toxicol. 2012, 9, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Yu, L.; Monopoli, M.P.; Sandin, P.; Mahon, E.; Salvati, A.; Dawson, K.A. The biomolecular corona is retained during nanoparticle uptake and protects the cells from the damage induced by cationic nanoparticles until degraded in the lysosomes. Nanomedicine 2013, 9, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.; Loeschner, K. Detection of nanoplastics in food by asymmetric flow field-flow fractionation coupled to multi-angle light scattering: Possibilities, challenges and analytical limitations. Anal. Bioanal. Chem. 2018, 410, 5603–5615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagné, F. Detection of polystyrene nanoplastics in biological tissues with a fluorescent molecular rotor probe. J. Xenobiot. 2019, 9, 8147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catarino, A.I.; Frutos, A.; Henry, T.B. Use of fluorescent-labelled nanoplastics (NPs) to demonstrate NP absorption is inconclusive without adequate controls. Sci. Total Environ. 2019, 670, 915–920. [Google Scholar] [CrossRef]
- Gillibert, R.; Balakrishnan, G.; Deshoules, Q.; Tardivel, M.; Magazzù, A.; Donato, M.G.; Maragò, O.M.; Lamy de La Chapelle, M.; Colas, F.; Lagarde, F.; et al. Raman tweezers for small microplastics and nanoplastics identification in seawater. Environ. Sci. Technol. 2019, 53, 9003–9013. [Google Scholar] [CrossRef]
- ScSchür, C.; Rist, S.; Baun, A.; Mayer, P.; Hartmann, N.B.; Wagner, M. When fluorescence is not a particle: The tissue translocation of microplastics in Daphnia magna seems an artifact. Environ. Toxicol. Chem. 2019, 38, 1495–1503. [Google Scholar] [CrossRef]
- Andrady, A.L. Plastics and the Environment; John Wiley Sons: Hoboken, NJ, USA, 2003; Volume 51, pp. 23–30. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Mazzanti, V.; Pariante, R.; Bonanno, A.; Ballesteros, O.D.; Mollica, F.; Filippone, G. Reinforcing mechanisms of natural fibers in green composites: Role of fibers morphology in a PLA/hemp model system. Compos. Sci. Technol. 2019, 180, 51–59. [Google Scholar] [CrossRef]
- Sepe, R.; Bollino, F.; Boccarusso, L.; Caputo, F. Influence of chemical treatments on mechanical properties of hemp fiber reinforced composites. Compos. Part B Eng. 2017, 133, 210–217. [Google Scholar] [CrossRef]
- Jian, K.; Li, Z.; Xiaoguang, D.; Hongqi, S.; Zhimin, A. Degradation of cosmetic microplastics via functionalized carbon nanosprings. Matter 2019, 1, 745–758. [Google Scholar]
- Day, M.; Cooney, J.D.; Klein, C.; Fox, J. Thermal Degradation of Automotive Plastics: A Possible Recycling Opportunity; ACS Publications: Washington, DC, USA, 1996. [Google Scholar]
- Ding, Q.; Liu, K.; Xu, K.; Sun, R.; Zhang, J.; Yin, L.; Pu, Y. Further understanding of degradation pathways of microcystin-lr by an indigenous Sphingopyxis sp. in environmentally relevant pollution concentrations. Toxins 2018, 10, 536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, H.; Xiang, Y.; He, D.; Li, Y.; Zhao, Y.; Wang, S.; Pan, X. Leaching behavior of fluorescent additives from microplastics and the toxicity of leachate to Chlorella vulgaris. Sci. Total Environ. 2019, 678, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Kong, Y.; Yuan, Y.; Wang, D. Neuronal damage induced by nanopolystyrene particles in nematode Caenorhabditis elegans. Environ. Sci. Nano 2019, 6, 2591–2601. [Google Scholar] [CrossRef]
- Tallec, K.; Huvet, A.; Di Poi, C.; Gonzalez-Fernandez, C.; Lambert, C.; Petton, B.; Le Goic, N.; Berchel, M.; Soudant, P.; Paul-Pont, I. Nanoplastics impaired oyster free living stages, gametes and embryos. Environ. Pollut. 2018, 242, 1226–1235. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, H.; Liu, X.; Qu, M. Nanoplastics and Human Health: Hazard Identification and Biointerface. Nanomaterials 2022, 12, 1298. https://doi.org/10.3390/nano12081298
Lai H, Liu X, Qu M. Nanoplastics and Human Health: Hazard Identification and Biointerface. Nanomaterials. 2022; 12(8):1298. https://doi.org/10.3390/nano12081298
Chicago/Turabian StyleLai, Hanpeng, Xing Liu, and Man Qu. 2022. "Nanoplastics and Human Health: Hazard Identification and Biointerface" Nanomaterials 12, no. 8: 1298. https://doi.org/10.3390/nano12081298
APA StyleLai, H., Liu, X., & Qu, M. (2022). Nanoplastics and Human Health: Hazard Identification and Biointerface. Nanomaterials, 12(8), 1298. https://doi.org/10.3390/nano12081298






