Impact of Nano- and Micro-Sized Chromium(III) Particles on Cytotoxicity and Gene Expression Profiles Related to Genomic Stability in Human Keratinocytes and Alveolar Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Physicochemical Characterization of Cr2O3 Particles
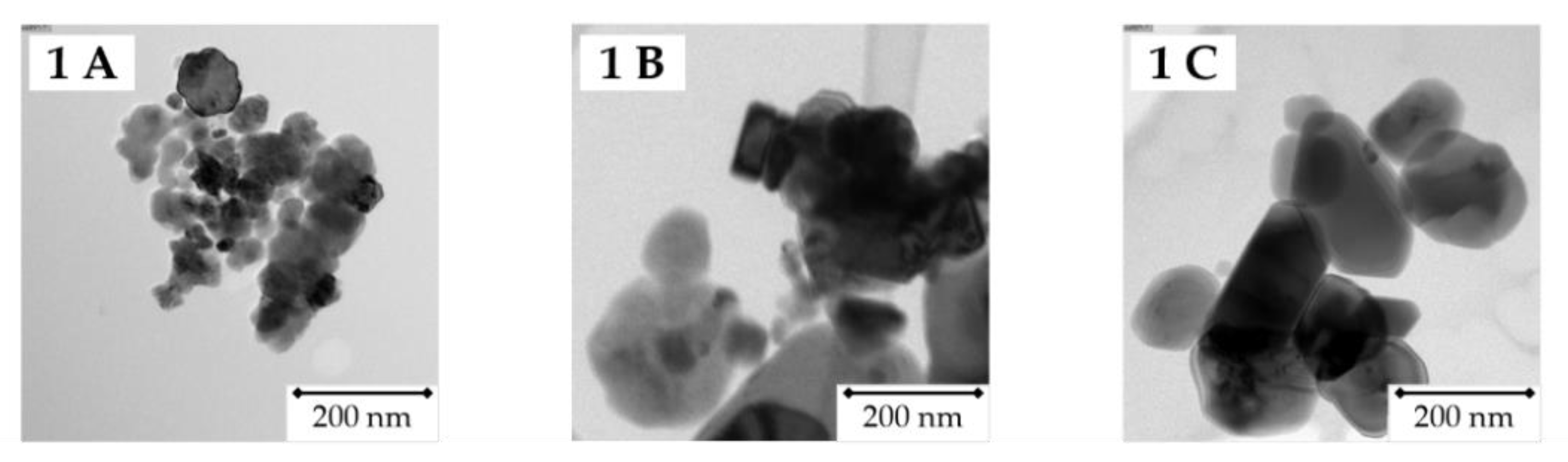
2.2.1. TEM
2.2.2. Hydrodynamic Size and Polydispersity Index (PDI)
2.2.3. XPS
2.2.4. Solubility Measurement/Oxidation State
2.3. Cell Culture Experiments
2.3.1. Cr2O3 Particle Suspensions and CrCl3 as Well as K2Cr2O7 Incubation Dilutions
2.3.2. Cell Culture and Incubation
2.3.3. Cytotoxicity Assay
2.3.4. Gene Expression Analyses
2.3.5. Statistics
3. Results
3.1. Particle Characteristics
3.2. Cytotoxicity
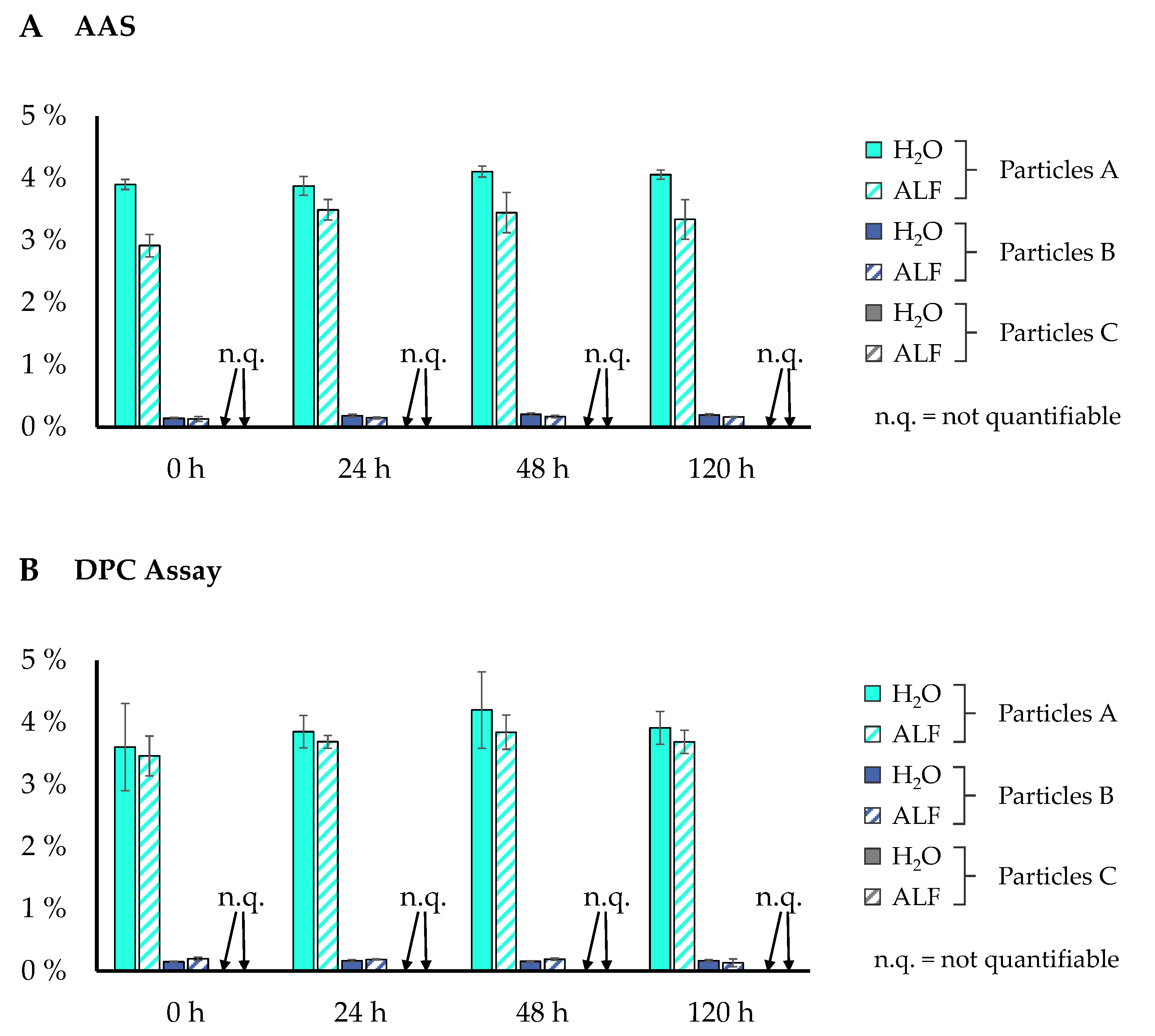
3.3. Release of Soluble Chromium from Cr2O3 Particles
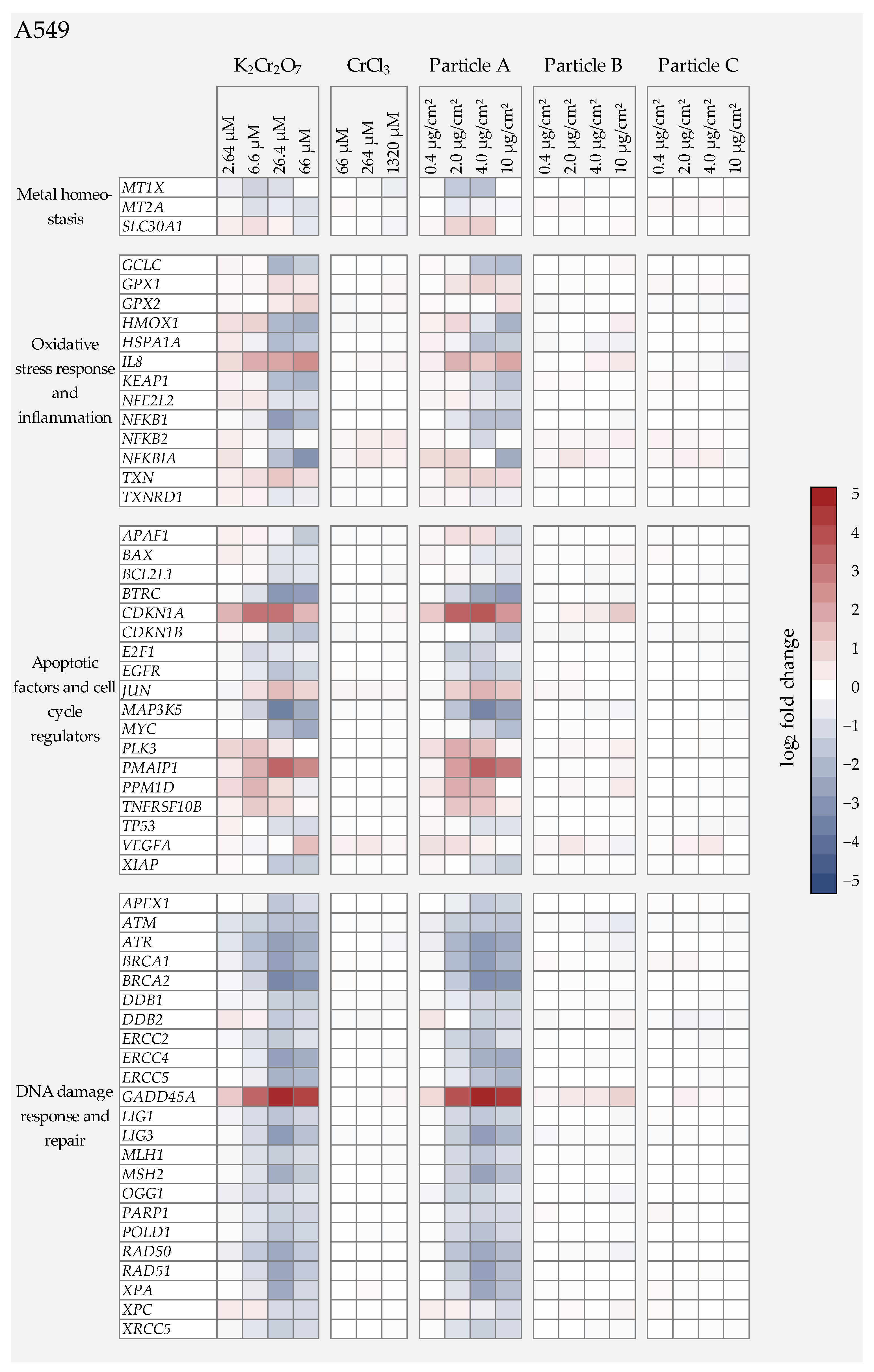
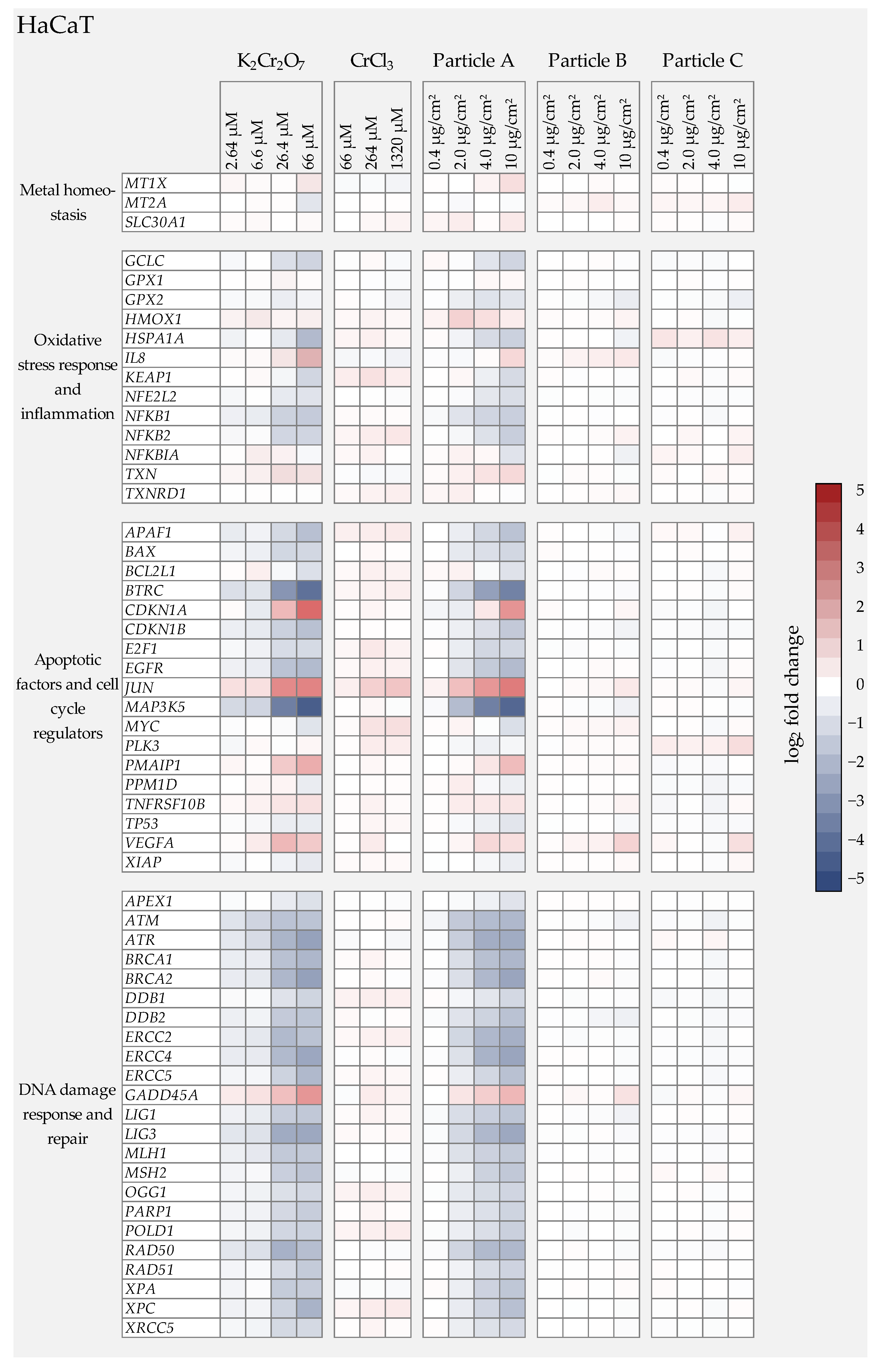
3.4. Gene Expression Analysis
3.4.1. Water Soluble Cr(VI) Treatment
3.4.2. Water Soluble Cr(III) Treatment
3.4.3. Cr(III) Oxide Particle Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer. Chromium, Nickel and Welding-IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization: Lyon, France, 1990; Volume 49. [Google Scholar]
- Wilbur, S.A.H.; Fay, M.; Yu, D.; Tencza, B.; Ingerman, L.; Klotzbach, J.; James, S. Toxicological Profile for Chromium; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2012. [Google Scholar]
- International Agency for Research on Cancer. A Review of Human Carcinogens-IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization: Lyon, France, 2012; Volume 100 F. [Google Scholar]
- Wetterhahn, K.E.; Hamilton, J.W. Molecular basis of hexavalent chromium carcinogenicity: Effect on gene expression. Sci. Total Environ. 1989, 86, 113–129. [Google Scholar] [CrossRef]
- Zhitkovich, A. Chromium in drinking water: Sources, metabolism, and cancer risks. Chem. Res. Toxicol. 2011, 24, 1617–1629. [Google Scholar] [CrossRef]
- Wise, S.S.; Aboueissa, A.E.; Martino, J.; Wise, J.P., Sr. Hexavalent Chromium-Induced Chromosome Instability Drives Permanent and Heritable Numerical and Structural Changes and a DNA Repair-Deficient Phenotype. Cancer Res. 2018, 78, 4203–4214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, J.M.; Antholine, W.E.; Myers, C.R. The intracellular redox stress caused by hexavalent chromium is selective for proteins that have key roles in cell survival and thiol redox control. Toxicology 2011, 281, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Hartwig, A. Metal interaction with redox regulation: An integrating concept in metal carcinogenesis? Free Radic. Biol. Med. 2013, 55, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.Y.; Murphy, A.; Sun, H.; Costa, M. Molecular and epigenetic mechanisms of Cr(VI)-induced carcinogenesis. Toxicol. Appl. Pharmacol. 2019, 377, 114636. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Nishio, K.; Endoh, S.; Kato, H.; Fujita, K.; Miyauchi, A.; Nakamura, A.; Kinugasa, S.; Yamamoto, K.; Niki, E.; et al. Chromium(III) oxide nanoparticles induced remarkable oxidative stress and apoptosis on culture cells. Environ. Toxicol. 2011, 28, 61–75. [Google Scholar] [CrossRef]
- Fischer, B.M.; Neumann, D.; Piberger, A.L.; Risnes, S.F.; Koberle, B.; Hartwig, A. Use of high-throughput RT-qPCR to assess modulations of gene expression profiles related to genomic stability and interactions by cadmium. Arch. Toxicol. 2016, 90, 2745–2761. [Google Scholar] [CrossRef] [Green Version]
- Semisch, A.; Ohle, J.; Witt, B.; Hartwig, A. Cytotoxicity and genotoxicity of nano—and microparticulate copper oxide: Role of solubility and intracellular bioavailability. Part. Fibre Toxicol. 2014, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- Bustin, S.A. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 2000, 25, 169–193. [Google Scholar] [CrossRef] [Green Version]
- Pfaffl, M.W.; Hageleit, M. Validities of mRNA quantification using recombinant RNA and recombinant DNA external calibration curves in real-time RT-PCR. Biotechnol. Lett. 2001, 23, 275–282. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Brown, C.; Mycroft, J.R.; Davidson, R.D.; McIntyre, N.S. X-ray photoelectron spectroscopy studies of chromium compounds. Surf. Interface Anal. 2004, 36, 1150–1163. [Google Scholar] [CrossRef]
- Hufnagel, M.; Schoch, S.; Wall, J.; Strauch, B.M.; Hartwig, A. Toxicity and Gene Expression Profiling of Copper- and Titanium-Based Nanoparticles Using Air-Liquid Interface Exposure. Chem Res. Toxicol. 2020, 33, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, M.; Neuberger, R.; Wall, J.; Link, M.; Friesen, A.; Hartwig, A. Impact of Differentiated Macrophage-Like Cells on the Transcriptional Toxicity Profile of CuO Nanoparticles in Co-Cultured Lung Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 5044. [Google Scholar] [CrossRef] [PubMed]
- Wall, J.; Seleci, D.A.; Schworm, F.; Neuberger, R.; Link, M.; Hufnagel, M.; Schumacher, P.; Schulz, F.; Heinrich, U.; Wohlleben, W.; et al. Comparison of Metal-Based Nanoparticles and Nanowires: Solubility, Reactivity, Bioavailability and Cellular Toxicity. Nanomaterials 2021, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Hininger, I.; Benaraba, R.; Osman, M.; Faure, H.; Marie Roussel, A.; Anderson, R.A. Safety of trivalent chromium complexes: No evidence for DNA damage in human HaCaT keratinocytes. Free Radic. Biol. Med. 2007, 42, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Barceloux, D.G. Chromium. J. Toxicol. Clin. Toxicol. 1999, 37, 173–194. [Google Scholar] [CrossRef]
- Shi, X.; Dalal, N.S. Chromium (V) and hydroxyl radical formation during the glutathione reductase-catalyzed reduction of chromium (VI). Biochem. Biophys. Res. Commun. 1989, 163, 627–634. [Google Scholar] [CrossRef]
- Stohs, S.J.; Bagchi, D.; Hassoun, E.; Bagchi, M. Oxidative mechanisms in the toxicity of chromium and cadmium ions. J. Environ. Pathol. Toxicol. Oncol. 2001, 20, 77–88. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, Q.; Yuan, Y.; Hai, R.; Zou, D. Bacterial community and molecular ecological network in response to Cr2O3 nanoparticles in activated sludge system. Chemosphere 2017, 188, 10–17. [Google Scholar] [CrossRef]
- Kumar, D.; Rajeshwari, A.; Jadon, P.S.; Chaudhuri, G.; Mukherjee, A.; Chandrasekaran, N.; Mukherjee, A. Cytogenetic studies of chromium (III) oxide nanoparticles on Allium cepa root tip cells. J. Environ. Sci. 2015, 38, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; He, Y.; Zhao, M.; Yu, T.; Qin, Y.; Lin, S. Differential effects of metal oxide nanoparticles on zebrafish embryos and developing larvae. Environ. Sci. Nano 2018, 5, 1200–1207. [Google Scholar] [CrossRef]
- Costa, C.H.D.; Perreault, F.; Oukarroum, A.; Melegari, S.P.; Popovic, R.; Matias, W.G. Effect of chromium oxide (III) nanoparticles on the production of reactive oxygen species and photosystem II activity in the green alga Chlamydomonas reinhardtii. Sci. Total Environ. 2016, 565, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Buxbaum, G.; Pfaff, G. Industrial Inorganic Pigments, 3rd ed.; Wiley: Weinheim, Germany, 2005. [Google Scholar] [CrossRef]
- De Loughery, Z.; Luczak, M.W.; Zhitkovich, A. Monitoring Cr Intermediates and Reactive Oxygen Species with Fluorescent Probes during Chromate Reduction. Chem. Res. Toxicol. 2014, 27, 843–851. [Google Scholar] [CrossRef]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef]
- Christmann, M.; Kaina, B. Epigenetic regulation of DNA repair genes and implications for tumor therapy. Mutat. Res. 2019, 780, 15–28. [Google Scholar] [CrossRef]
- Hu, G.; Li, P.; Cui, X.; Li, Y.; Zhang, J.; Zhai, X.; Yu, S.; Tang, S.; Zhao, Z.; Wang, J.; et al. Cr(VI)-induced methylation and down-regulation of DNA repair genes and its association with markers of genetic damage in workers and 16HBE cells. Environ. Pollut. 2018, 238, 833–843. [Google Scholar] [CrossRef]
- Rager, J.E.; Suh, M.; Chappell, G.A.; Thompson, C.M.; Proctor, D.M. Review of transcriptomic responses to hexavalent chromium exposure in lung cells supports a role of epigenetic mediators in carcinogenesis. Toxicol. Lett. 2019, 305, 40–50. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, J.; Humphries, B.; Kondo, K.; Jiang, Y.; Shi, X.; Yang, C. Upregulation of histone-lysine methyltransferases plays a causal role in hexavalent chromium-induced cancer stem cell-like property and cell transformation. Toxicol. Appl. Pharmacol. 2018, 342, 22–30. [Google Scholar] [CrossRef]
- Imai, K.; Yamamoto, H. Carcinogenesis and microsatellite instability: The interrelationship between genetics and epigenetics. Carcinogenesis 2008, 29, 673–680. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Kondo, K.; Hirose, T.; Nakagawa, H.; Tsuyuguchi, M.; Hashimoto, M.; Sano, T.; Ochiai, A.; Monden, Y. Microsatellite instability and protein expression of the DNA mismatch repair gene, hMLH1, of lung cancer in chromate-exposed workers. Mol. Carcinog 2005, 42, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhou, X.; Chen, H.; Li, Q.; Costa, M. Modulation of histone methylation and MLH1 gene silencing by hexavalent chromium. Toxicol. Appl. Pharmacol. 2009, 237, 258–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arita, A.; Costa, M. Epigenetics in metal carcinogenesis: Nickel, arsenic, chromium and cadmium. Metallomics 2009, 1, 222–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Codd, R.; Dillon, C.T.; Levina, A.; Lay, P.A. Studies on the genotoxicity of chromium: From the test tube to the cell. Coord. Chem. Rev. 2001, 216–217, 537–582. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, Toxicity and Oxidative Stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Shi, X. Gene expression profile in response to chromium-induced cell stress in A549 cells. Mol. Cell. Biochem. 2001, 222, 189–197. [Google Scholar] [CrossRef]
- Suzuki, Y.; Fukuda, K. Reduction of hexavalent chromium by ascorbic acid and glutathione with special reference to the rat lung. Arch. Toxicol. 1990, 64, 169–176. [Google Scholar] [CrossRef]
- Levina, A.; Zhang, L.; Lay, P.A. Structure and reactivity of a chromium(v) glutathione complex. Inorg. Chem. 2003, 42, 767–784. [Google Scholar] [CrossRef]
- Ryuno, H.; Naguro, I.; Kamiyama, M. ASK family and cancer. Adv. Biol. Regul. 2017, 66, 72–84. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schumacher, P.; Fischer, F.; Sann, J.; Walter, D.; Hartwig, A. Impact of Nano- and Micro-Sized Chromium(III) Particles on Cytotoxicity and Gene Expression Profiles Related to Genomic Stability in Human Keratinocytes and Alveolar Epithelial Cells. Nanomaterials 2022, 12, 1294. https://doi.org/10.3390/nano12081294
Schumacher P, Fischer F, Sann J, Walter D, Hartwig A. Impact of Nano- and Micro-Sized Chromium(III) Particles on Cytotoxicity and Gene Expression Profiles Related to Genomic Stability in Human Keratinocytes and Alveolar Epithelial Cells. Nanomaterials. 2022; 12(8):1294. https://doi.org/10.3390/nano12081294
Chicago/Turabian StyleSchumacher, Paul, Franziska Fischer, Joachim Sann, Dirk Walter, and Andrea Hartwig. 2022. "Impact of Nano- and Micro-Sized Chromium(III) Particles on Cytotoxicity and Gene Expression Profiles Related to Genomic Stability in Human Keratinocytes and Alveolar Epithelial Cells" Nanomaterials 12, no. 8: 1294. https://doi.org/10.3390/nano12081294
APA StyleSchumacher, P., Fischer, F., Sann, J., Walter, D., & Hartwig, A. (2022). Impact of Nano- and Micro-Sized Chromium(III) Particles on Cytotoxicity and Gene Expression Profiles Related to Genomic Stability in Human Keratinocytes and Alveolar Epithelial Cells. Nanomaterials, 12(8), 1294. https://doi.org/10.3390/nano12081294





