Chromium-Modified Ultrathin CoFe LDH as High-Efficiency Electrode for Hydrogen Evolution Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CoFeCr LDH and CoFe LDH Electrodes
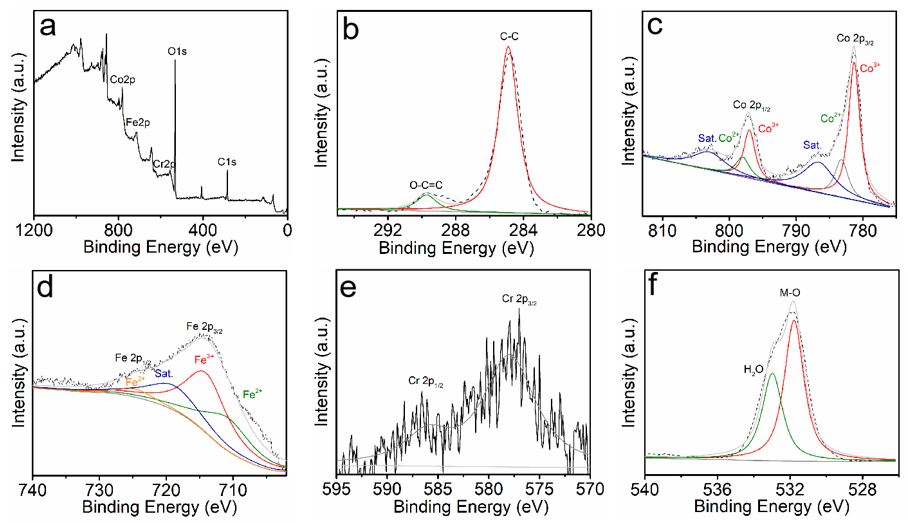
2.3. Characterizations of CoFeCr LDH and CoFe LDH Electrodes
2.4. Electrochemical Measurements
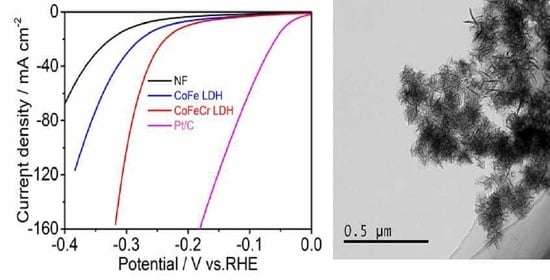
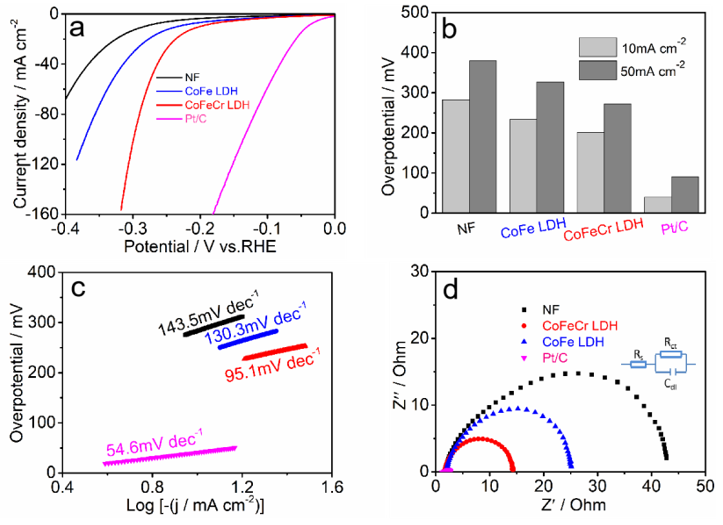
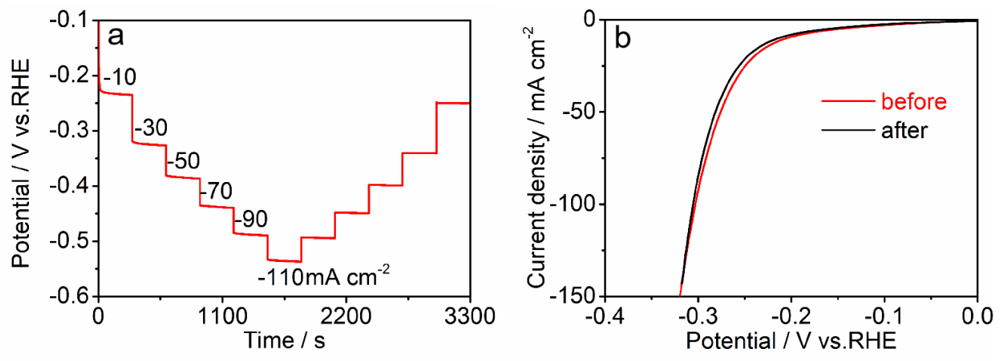
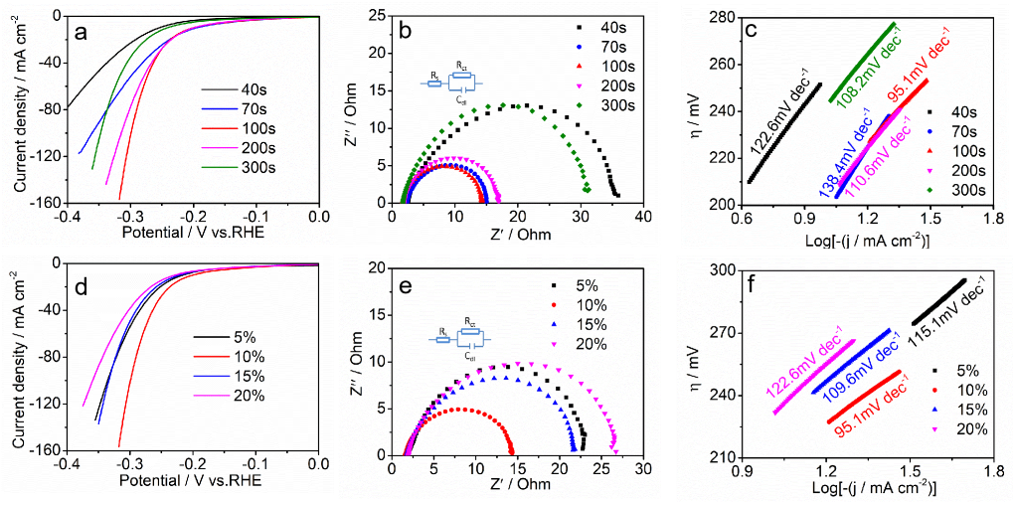
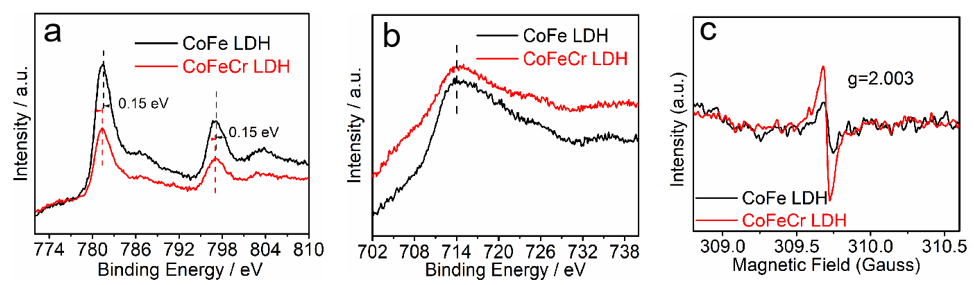
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nong, H.N.; Falling, L.J.; Bergmann, A.; Klingenhof, M.; Tran, H.P.; Spöri, C.; Mom, R.; Timoshenko, J.; Zichittella, G.; Knop-Gericke, A.; et al. Key role of chemistry versus bias in electrocatalytic oxygen evolution. Nature 2020, 587, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, Q.G.; Liu, W.; Li, Q.; Wang, Y.C.; Liu, B.; Zheng, L.R.; Wang, W.; Huang, L.; Chen, L.M.; et al. Boosting interfacial charge transfer for alkaline hydrogen evolution via rational interior Se modification. Nano Energy 2021, 81, 105641. [Google Scholar] [CrossRef]
- Tang, Y.J.; Zheng, H.; Wang, Y.; Zhang, W.; Zhou, K. Laser-induced annealing of metal-organic frameworks on conductive substrates for electrochemical water splitting. Adv. Funct. Mater. 2021, 31, 2102648. [Google Scholar] [CrossRef]
- Gao, L.K.; Cui, X.; Sewell, C.D.; Li, J.; Lin, Z.Q. Recent advances in activating surface reconstruction for the high-effiffifficiency oxygen evolution reaction. Chem. Soc. Rev. 2021, 50, 8428–8469. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.B.; Yu, L.; Xiao, X.; Zhang, F.H.; Song, S.W.; Chen, S.; Ren, Z.F. Recent advances in self-supported layered double hydroxides for oxygen evolution reaction. Research 2020, 2020, 3976278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Long, X.; Yang, S.H. Effects of metal combinations on the electrocatalytic properties of transition-metal-based layered double hydroxides for water oxidation: A perspective with insights. ACS Omega 2018, 3, 16529–16541. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Cao, Z.; Kozlov, S.M.; de Arquer, F.P.G.; Dinh, C.T.; Li, J.; Wang, Z.Y.; Zheng, X.L.; Zhang, L.S.; et al. High-valence metals improve oxygen evolution reaction performance by modulating 3d metal oxidation cycle energetics. Nat. Catal. 2020, 3, 985–992. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.P.; Zhao, X.; Wang, Y.C.; Li, Q.; Wang, Q.C.; Tang, Y.G.; Lei, Y.P. Trimetallic oxyhydroxides as active sites for large-current-density alkaline oxygen evolution and overall water splitting. J. Mater.Sci. Technol. 2022, 110, 128–135. [Google Scholar] [CrossRef]
- Yang, C.M.; Zhou, L.H.; Wang, C.T.; Duan, W.; Zhang, L.; Zhang, F.C.; Zhang, J.J.; Zhen, Y.Z.; Gao, L.J.; Fu, F.; et al. Large-scale synthetic Mo@(2H-1T)-MoSe2 monolithic electrode for efficient hydrogen evolution in all pH scale ranges and seawater. Appl. Catal. B Environ. 2022, 304, 120993. [Google Scholar] [CrossRef]
- Yuan, S.; Peng, J.Y.; Cai, B.; Huang, Z.H.; Garcia-Esparza, A.T.; Sokaras, D.; Zhang, Y.R.; Giordano, L.; Akkiraju, K.; Zhu, Y.G.; et al. Tunable metal hydroxide-organic frameworks for catalysing oxygen evolution. Nat. Mater. 2022, 1–8. [Google Scholar] [CrossRef]
- Zhang, J.J.; Bao, W.W.; Li, M.Y.; Yang, C.M.; Zhang, N.N. Ultrafast formation of an FeOOH electrocatalyst on Ni for efficient alkaline water and urea oxidation. Chem. Commun. 2020, 56, 14713–14716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Zhao, T.J.; Wang, H.H.; Lin, Y.X.; Zhai, G.Y.; Jiang, Z.D.; Hirano, S.; Li, X.H.; Chen, J.S. Oriented arrays of Co3O4 nanoneedles for highly efficient electrocatalytic water oxidatio. Chem. Commun. 2019, 55, 3971–3974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Lv, L.B.; Zhao, T.J.; Lin, Y.X.; Yu, Q.Y.; Su, J.; Hirano, S.; Li, X.H.; Chen, J.S. Engineering the interfaces of super-absorbing graphene-based electrodes with gas and electrolyte to boost gas evolution/activation reactions. ChemSusChem 2018, 11, 2306–2309. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.M.; Liang, X.; Wu, X.Y.; Zhao, S.L.; Chen, J.; Wang, K.X.; Chen, J.S. Multistaged discharge constructing heterostructure with enhanced solid-solution behavior for long-life lithium-oxygen batteries. Nat. Commun 2019, 10, 5810. [Google Scholar] [CrossRef] [Green Version]
- Fei, B.; Chen, Z.L.; Liu, J.X.; Xu, H.B.; Yan, X.X.; Qing, H.L.; Chen, M.; Wu, R.B. Ultrathinning nickel sulfide with modulated electron density for efficient water splitting. Adv. Energy Mater. 2020, 10, 2001963. [Google Scholar] [CrossRef]
- Sun, H.M.; Yan, Z.H.; Liu, F.M.; Xu, W.C.; Cheng, F.Y.; Chen, J. Self-supported transition-metal-based electrocatalysts for hydrogen and oxygen evolution. Adv. Mater. 2019, 32, 1806326. [Google Scholar] [CrossRef]
- Wu, L.B.; Yu, L.; Zhu, Q.C.; McElhenny, B.; Zhang, F.H.; Wu, C.Z.; Xing, X.X.; Bao, J.M.; Chen, S.; Ren, Z.F. Boron-modified cobalt iron layered double hydroxides for high efficiency seawater oxidation. Nano Energy 2021, 83, 105838. [Google Scholar] [CrossRef]
- Zhang, J.J.; Wang, H.H.; Zhao, T.J.; Zhang, K.X.; Wei, X.; Jiang, Z.D.; Hirano, S.; Li, X.H.; Chen, J.S. Oxygen vacancy engineering of Co3O4 nanocrystals through coupling with metal support for water oxidation. ChemSusChem 2017, 10, 2875–2879. [Google Scholar] [CrossRef]
- Yang, Y.B.; Cui, X.; Gao, D.; He, H.C.; Ou, Y.Q.; Zhou, M.; Lai, Q.X.; Wei, X.J.; Xiao, P.; Zhang, Y.H. Trimetallic CoFeCr hydroxide electrocatalysts synthesized at low temperature for accelerating water oxidation via tuning electronic structure of active sites. Sustain. Energy Fuels 2020, 4, 3647–3653. [Google Scholar] [CrossRef]
- Li, M.Y.; Zhang, J.J.; Li, X.; Bao, W.W.; Yang, C.M.; Jin, C.Q.; Li, M.; Wang, S.M.; Zhang, N.N. Tuning the electronic structures of self-supported vertically aligned CoFe LDH arrays integrated with Ni foam toward highly efficient electrocatalytic water oxidation. New J. Chem. 2021, 45, 13266–13270. [Google Scholar] [CrossRef]
- Yang, Y.; Dang, L.N.; Shearer, M.J.; Sheng, H.Y.; Li, W.J.; Chen, J.; Xiao, P.; Zhang, Y.H.; Hamers, R.J.; Jin, S. Highly active trimetallic NiFeCr layered double hydroxide electrocatalysts for oxygen evolution reaction. Adv. Energy Mater. 2018, 8, 1703189. [Google Scholar] [CrossRef]
- Fan, L.Z.; Zhang, P.L.; Zhang, B.B.; Daniel, Q.; Timmer, B.J.J.; Zhang, F.G.; Sun, L.C. A 3D core-shell NiFeCr catalyst on Cu nanoarray for water oxidation: Synergy between structural and electronic modulation. ACS Energy Lett. 2018, 3, 2865–2874. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Zhou, J.M.; Wei, H.L.; Dai, Y.F.; Li, S.J.; Shi, H.J.; Xu, G. Construction of hierarchical NiFe-LDH/FeCoS2/CFC composites as efficient bifunctional electrocatalysts for hydrogen and oxygen evolution reaction. J. Mater. Sci. 2020, 55, 16625–16640. [Google Scholar] [CrossRef]
- Rajeshkhanna, G.; Singh, T.I.; Kim, N.H.; Lee, J. Remarkable bifunctional oxygen and hydrogen evolution electrocatalytic activities with trace level Fe-doping in Ni- and Co-layered double hydroxides for overall water splitting. ACS Appl. Mater. Interfaces 2018, 10, 42453–42468. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.F.; Yang, S.; Zhang, B.; Yang, H.G. Defect-rich ultrathin cobalt-iron layered double hydroxide for electrochemical overall water splitting. ACS Appl. Mater. Interfaces 2016, 8, 34474–34481. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Wang, Z.L.; Xie, J.F.; Xu, L.; Lei, F.C.; Guan, M.L.; Zhao, Y.; Huang, Y.P.; Li, H.M. Ternary cobalt-molybdenum-vanadium layered double hydroxide nanosheet array as an efficient bifunctional electrocatalyst for overall water splitting. Chem. Commun. 2019, 55, 3521–3524. [Google Scholar] [CrossRef]
- Yang, C.M.; Wang, C.T.; Zhou, L.H.; Duan, W.; Song, Y.Y.; Zhang, F.C.; Zhen, Y.Z.; Zhang, J.J.; Bao, W.W.; Lu, Y.X.; et al. Refining d-band center in Ni0.85Se by Mo doping: A strategy for boosting hydrogen generation viacoupling electrocatalytic oxidation 5-hydroxymethylfurfural. Chem. Eng. J. 2021, 422, 130125. [Google Scholar] [CrossRef]
- Zhang, J.J.; Yang, C.M.; Jin, C.Q.; Bao, W.W.; Nan, R.H.; Hu, L.; Liu, G.; Zhang, N.N. Hierarchical iron molybdate nanostructure array for efficient water oxidation through optimizing electron density. Chem. Commun. 2021, 57, 3563–3566. [Google Scholar] [CrossRef]
- Zhang, J.J.; Ge, J.M.; Wang, H.H.; Wei, X.; Li, X.H.; Chen, J.S. Activating oxygen molecules over carbonyl-modified graphitic carbon nitride: Merging supramolecular oxidation with photocatalysis in a metal-free catalyst for oxidative coupling of amines into imine. ChemCatChem 2016, 8, 3441–3445. [Google Scholar] [CrossRef]
- Xiao, L.; Bao, W.W.; Zhang, J.J.; Yang, C.M.; Ai, T.T.; Li, Y.; Wei, X.L.; Jiang, P.; Kou, L.J. Interfacial interaction between NiMoP and NiFe-LDH to regulate the electronic structure toward high-efficiency electrocatalytic oxygen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 9230–9238. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Kong, H.; Kim, J.; Choi, S.; Ciucci, F.; Hao, Y.; Yang, S.H.; Shao, Z.P.; Lim, J. Non-precious-metal catalysts for alkaline water electrolysis: Operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 2020, 49, 9154–9196. [Google Scholar] [CrossRef]
- Li, S.; Chen, B.B.; Wang, Y.; Ye, M.Y.; van Aken, P.A.; Cheng, C.; Thomas, A. Oxygen-evolving catalytic atoms on metal carbides. Nat. Mater. 2021, 20, 1240–1247. [Google Scholar] [CrossRef]
- Zhang, J.J.; Su, H.; Wang, H.H.; Xue, Z.H.; Zhang, B.; Wei, X.; Li, X.H.; Hirano, S.I.; Chen, J.S. Constructing Ohmic contact in cobalt selenide/Ti dyadic electrode: The third aspect to promote the oxygen evolution reaction. Nano Energy 2017, 39, 321–327. [Google Scholar] [CrossRef]
- He, J.; Zhou, X.; Xu, P.; Sun, J.M. Promoting electrocatalytic water oxidation through tungsten-modulated oxygen vacancies on hierarchical FeNi-layered double hydroxide. Nano Energy 2021, 80, 105540. [Google Scholar] [CrossRef]
- Sun, Y.M.; Xue, Z.Q.; Liu, Q.L.; Jia, Y.L.; Li, Y.L.; Liu, K.; Lin, Y.Y.; Liu, M.; Li, G.Q.; Su, C.Y. Modulating electronic structure of metal-organic frameworks by introducing atomically dispersed Ru for efficient hydrogen evolution. Nat. Commun. 2021, 12, 1369. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Huang, J.; Cao, L.; Kajiyoshi, K.; Yang, D.; Feng, Y.; Kou, L.; Feng, L.L. Methanol-assisted synthesis of Ni3+-doped ultrathin NiZn-LDH nanomeshes for boosted alkaline water splitting. Dalton Trans. 2020, 49, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.C.; Zhang, W.; Li, J.G.; Li, Z.S.; Ao, X.; Xue, K.H.; Ostrikov, K.K.; Tang, J.; Wang, C.D. Rh-engineered ultrathin NiFe-LDH nanosheets enable highly-efficient overall water splitting and urea electrolysis. Appl. Catal. B Environ. 2021, 284, 119740. [Google Scholar] [CrossRef]
- Liu, S.J.; Zhu, J.; Sun, M.; Ma, Z.X.; Hu, K.; Nakajima, T.; Liu, X.H.; Schmuki, P.; Wang, L. Promoting the hydrogen evolution reaction through oxygen vacancies and phase transformation engineering on layered double hydroxide nanosheets. J. Mater. Chem. A 2020, 8, 2490–2497. [Google Scholar] [CrossRef]
- Xue, Y.Q.; Bai, X.J.; Xu, Y.Y.; Yan, Q.; Zhu, M.; Zhu, K.; Ye, K.; Yan, J.; Cao, D.X.; Wang, G.L. Vertically oriented Ni-doped MoS2 nanosheets supported on hollow carbon microtubes for enhanced hydrogen evolution reaction and water splitting. Compos. B Eng. 2021, 224, 109229. [Google Scholar] [CrossRef]
- Xue, Z.; Su, H.; Yu, Q.; Zhang, B.; Wang, H.; Li, X.; Chen, J. Janus Co/CoP nanoparticles as efficient Mott-Schottky electrocatalysts for overall water splitting in wide pH range. Adv. Energy Mater. 2017, 7, 1602355. [Google Scholar] [CrossRef]
- Karakaya, C.; Solati, N.; Savacı, U; Keleş, E.; Turan, S.; Ҫelebi, S.; Kaya, S. Mesoporous thin-film NiS2 as an idealized pre-electrocatalyst for a hydrogen evolution reaction. ACS Catal. 2020, 10, 15114–15122. [Google Scholar] [CrossRef]
- Luo, J.; Im, J.H.; Mayer, M.T.; Schreier, M.; Nazeeruddin, M.K.; Park, N.G.; Tilley, S.D.; Fan, H.J.; Grätzel, M. Water photolysis at 12.3% efficiency via perovskite photovoltaics and Earth-abundant catalysts. Science 2014, 345, 1593–1596. [Google Scholar] [CrossRef] [PubMed]
- Venkateshwaran, S.; Josline, M.J.; Kumar, S.M.S. Fine-tuning interlayer spacing in MoS2 forenriching 1T phase via alkylated ammonium ions for electrocatalytic hydrogen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 8377–8390. [Google Scholar] [CrossRef]
- Rajeshkhanna, G.; Kandula, S.; Shrestha, K.R.; Kim, N.H.; Lee, J.H. A new class of Zn1-xFex–oxyselenide and Zn1-xFex–LDH nanostructured material with remarkable bifunctional oxygen and hydrogen evolution electrocatalytic activities for overall water splitting. Small 2018, 14, 1803638. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Lohe, M.R.; Zhang, J.; Liu, S.; Zhuang, X.; Feng, X. Vertically oriented cobalt selenide/NiFe layered-double-hydroxide nanosheets supported on exfoliated graphene foil: an efficient 3D electrode for overall water splitting. Energy Environ. Sci. 2016, 9, 478–483. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, N.; Yao, Y.; Zhang, J.-W.; Pan, L.; Zhang, X.; Zou, J.-J. Electrocatalysts for hydrogen evolution in alkaline electrolytes:mechanisms, challenges, and prospective solutions. Adv. Sci. 2018, 5, 1700464. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Chen, D.; Liu, J.; Zhang, Z.; Shao, Z.; Ciucci, F. Water splitting with an enhanced bifunctional double perovskite. ACS Catal. 2017, 8, 364–371. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; Gao, G.; Chen, H.; Wang, B.; Zhou, J.; Soo, M.T.; Hong, M.; Yan, X.; Qian, G.; Zou, J.; Du, A.; Yao, X. A heterostructure coupling of exfoliated Ni-Fe hydroxide nanosheet and defective graphene as a bifunctional electrocatalyst for overall water splitting. Adv. Mater. 2017, 29, 1700017. [Google Scholar] [CrossRef]
- Wang, T.; Xu, M.; Li, F.; Li, Y.; Chen, W. Multimetal-based nitrogen doped carbon nanotubes bifunctional electrocatalysts for triiodide reduction and water-splitting synthesized from polyoxometalate-intercalated layered double hydroxide pyrolysis strategy. Appl. Catal. B Environ. 2021, 280, 119421. [Google Scholar] [CrossRef]
- Chen, G.-F.; Ma, T.Y.; Liu, Z.-Q.; Li, N.; Su, Y.-Z.; Davey, K.; Qiao, S.-Z. Efficient and stable bifunctional electrocatalysts Ni/NixMy (M = P, S) for overall water splitting. Adv. Funct. Mater. 2016, 26, 3314–3323. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.-J.; Li, M.-Y.; Li, X.; Bao, W.-W.; Jin, C.-Q.; Feng, X.-H.; Liu, G.; Yang, C.-M.; Zhang, N.-N. Chromium-Modified Ultrathin CoFe LDH as High-Efficiency Electrode for Hydrogen Evolution Reaction. Nanomaterials 2022, 12, 1227. https://doi.org/10.3390/nano12071227
Zhang J-J, Li M-Y, Li X, Bao W-W, Jin C-Q, Feng X-H, Liu G, Yang C-M, Zhang N-N. Chromium-Modified Ultrathin CoFe LDH as High-Efficiency Electrode for Hydrogen Evolution Reaction. Nanomaterials. 2022; 12(7):1227. https://doi.org/10.3390/nano12071227
Chicago/Turabian StyleZhang, Jun-Jun, Meng-Yang Li, Xiang Li, Wei-Wei Bao, Chang-Qing Jin, Xiao-Hua Feng, Ge Liu, Chun-Ming Yang, and Nan-Nan Zhang. 2022. "Chromium-Modified Ultrathin CoFe LDH as High-Efficiency Electrode for Hydrogen Evolution Reaction" Nanomaterials 12, no. 7: 1227. https://doi.org/10.3390/nano12071227
APA StyleZhang, J.-J., Li, M.-Y., Li, X., Bao, W.-W., Jin, C.-Q., Feng, X.-H., Liu, G., Yang, C.-M., & Zhang, N.-N. (2022). Chromium-Modified Ultrathin CoFe LDH as High-Efficiency Electrode for Hydrogen Evolution Reaction. Nanomaterials, 12(7), 1227. https://doi.org/10.3390/nano12071227