Antibacterial Activity of Silver and Gold Particles Formed on Titania Thin Films
Abstract
:1. Introduction
2. Materials and Methods
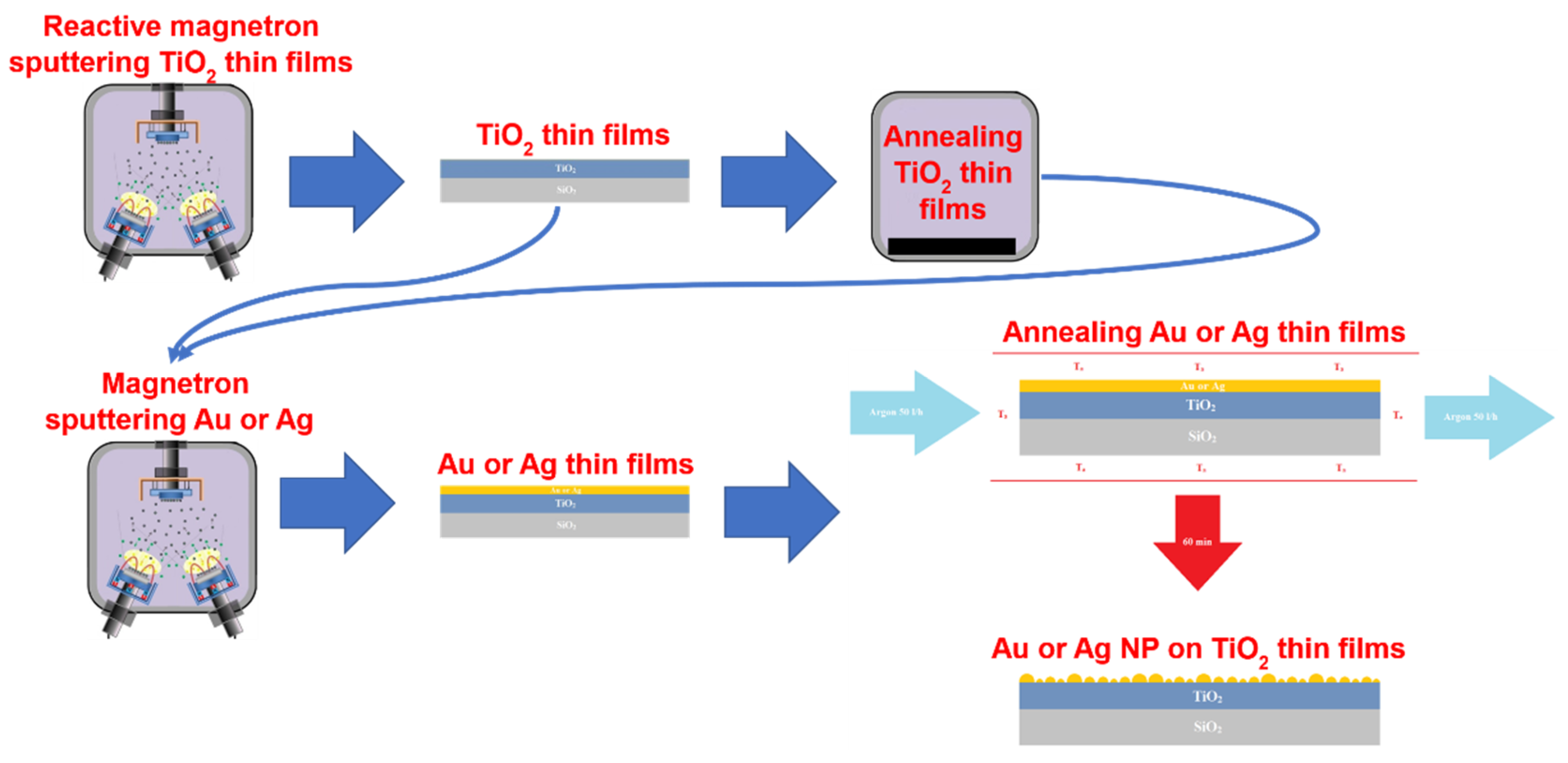
2.1. Formation and Investigation of NP/TiO2 Composites
2.2. Biofilm Formation Assay
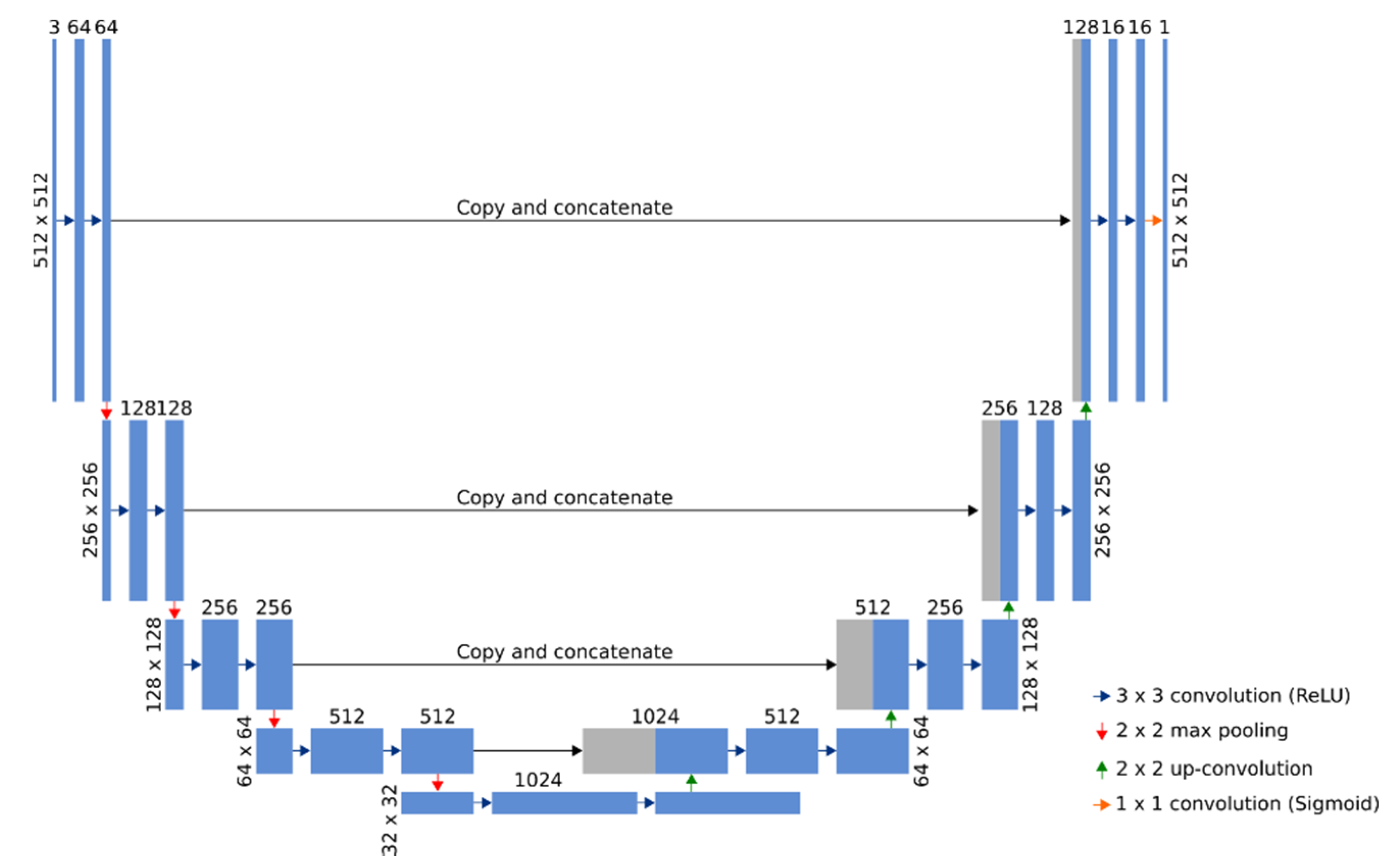
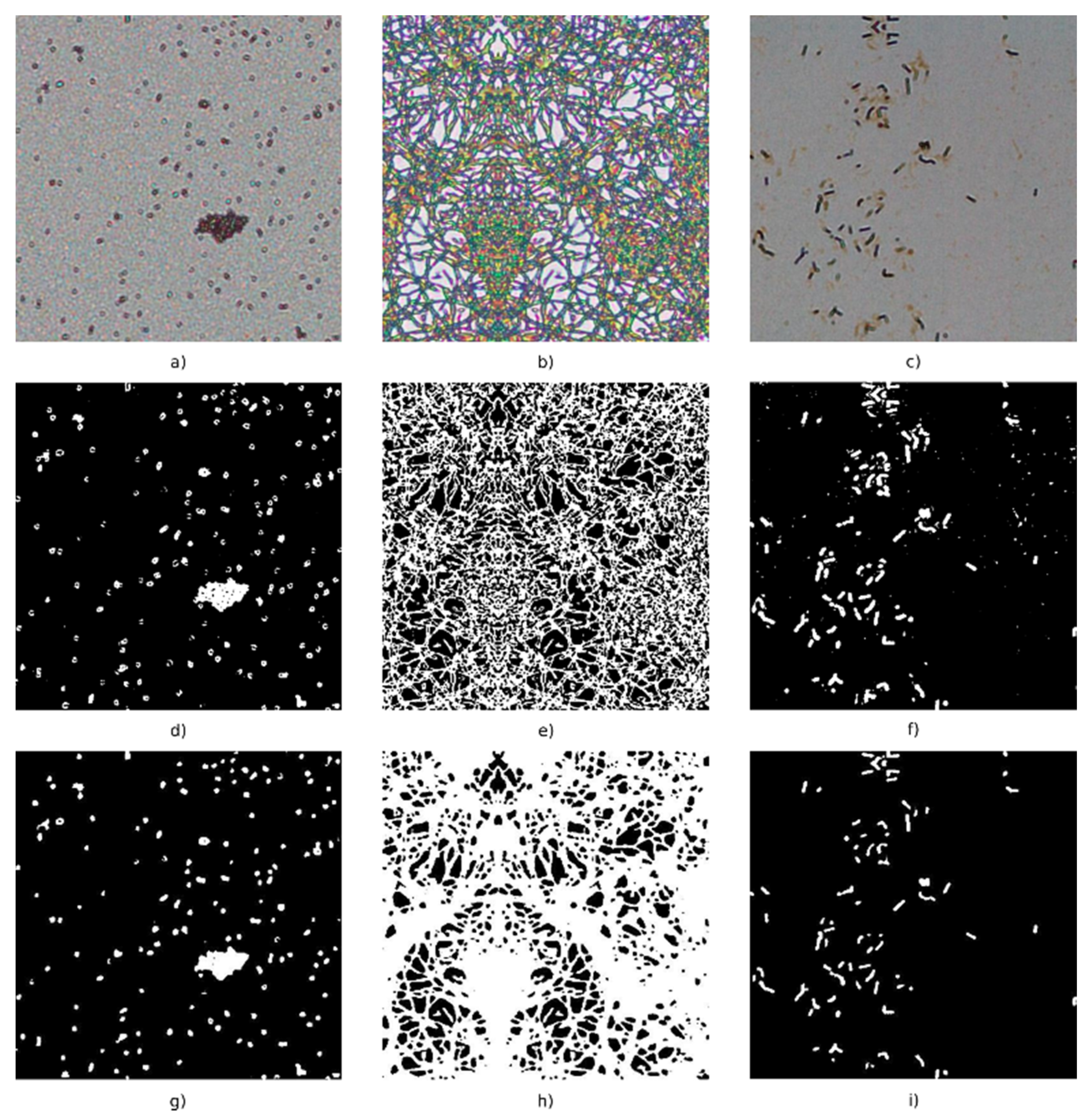
2.3. Observation of Bacteria in an Experimental Optical Microscope (OM) Images
3. Results
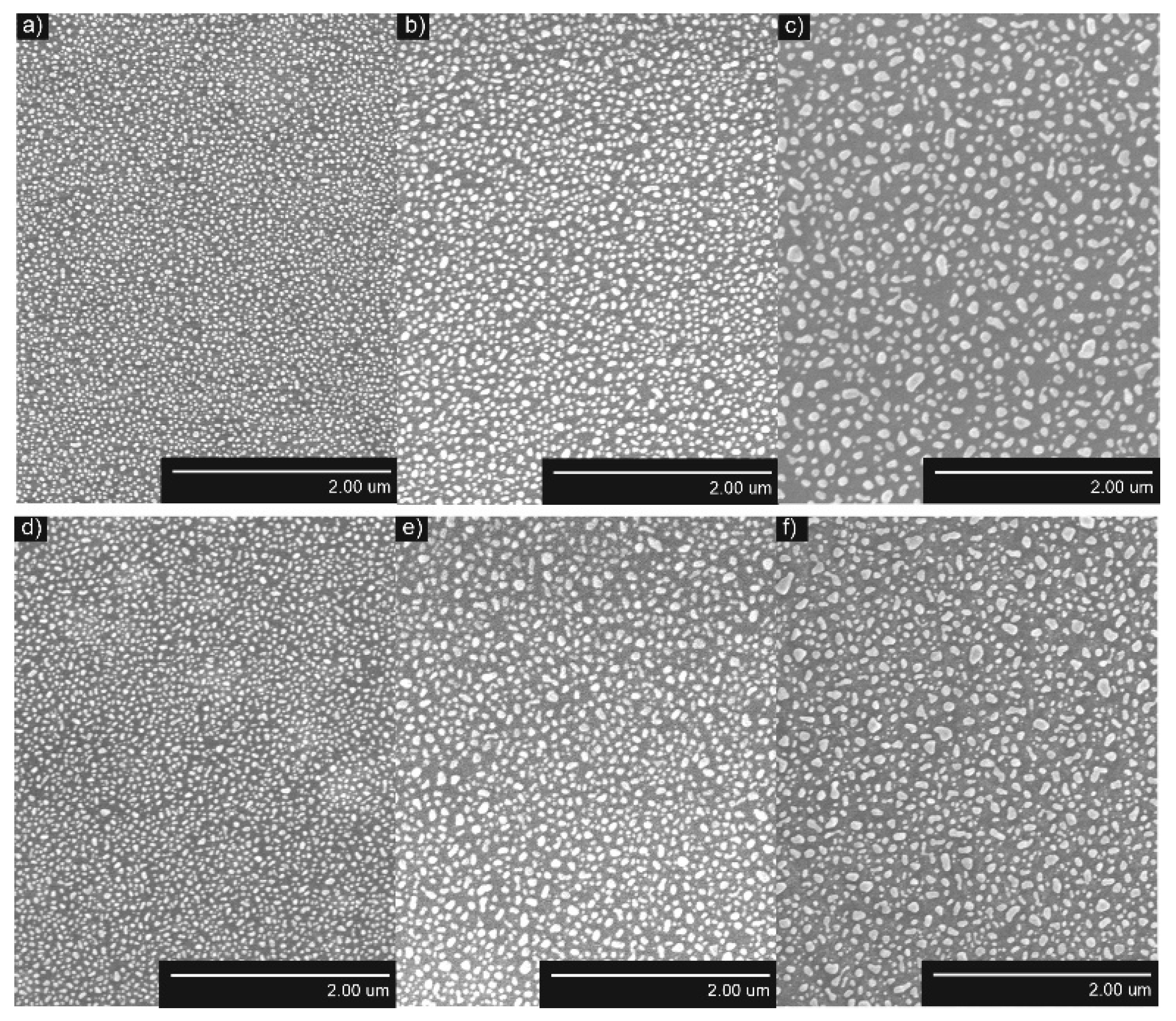
3.1. Surface Characterization
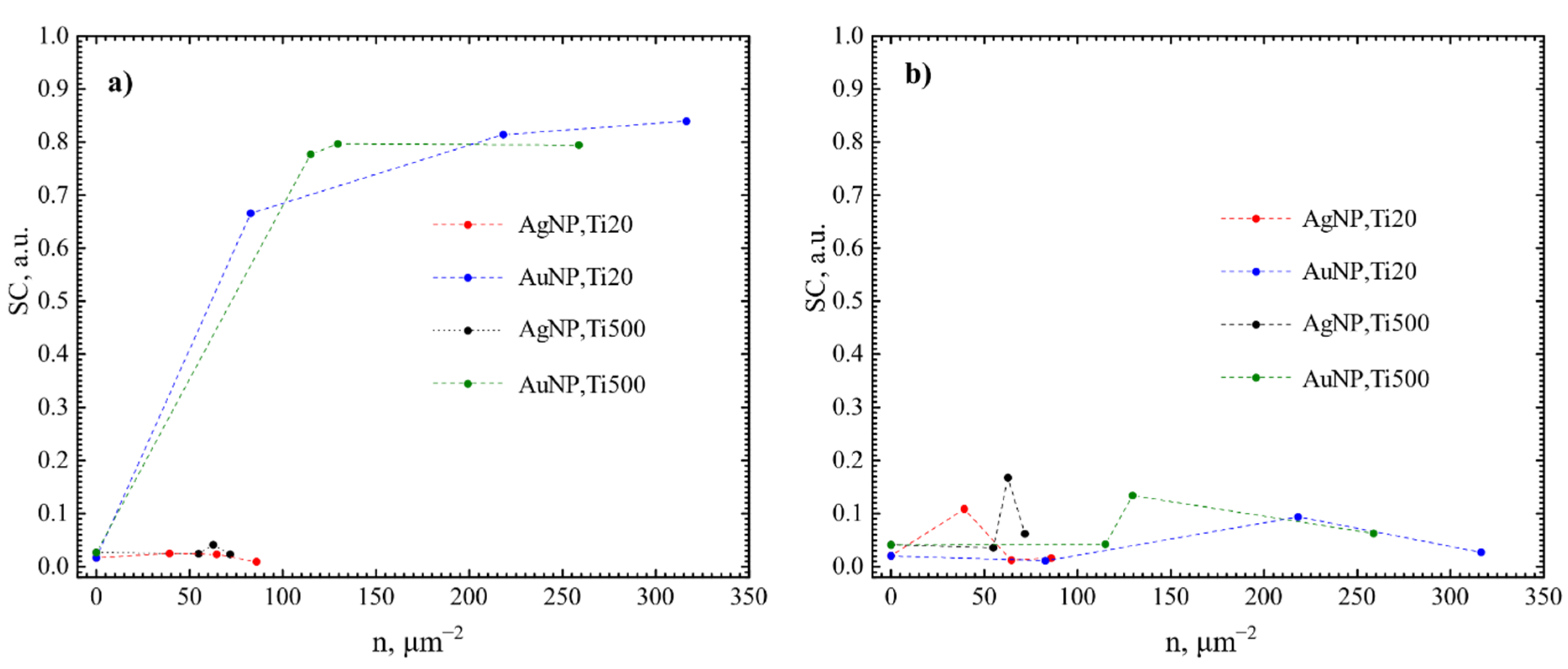
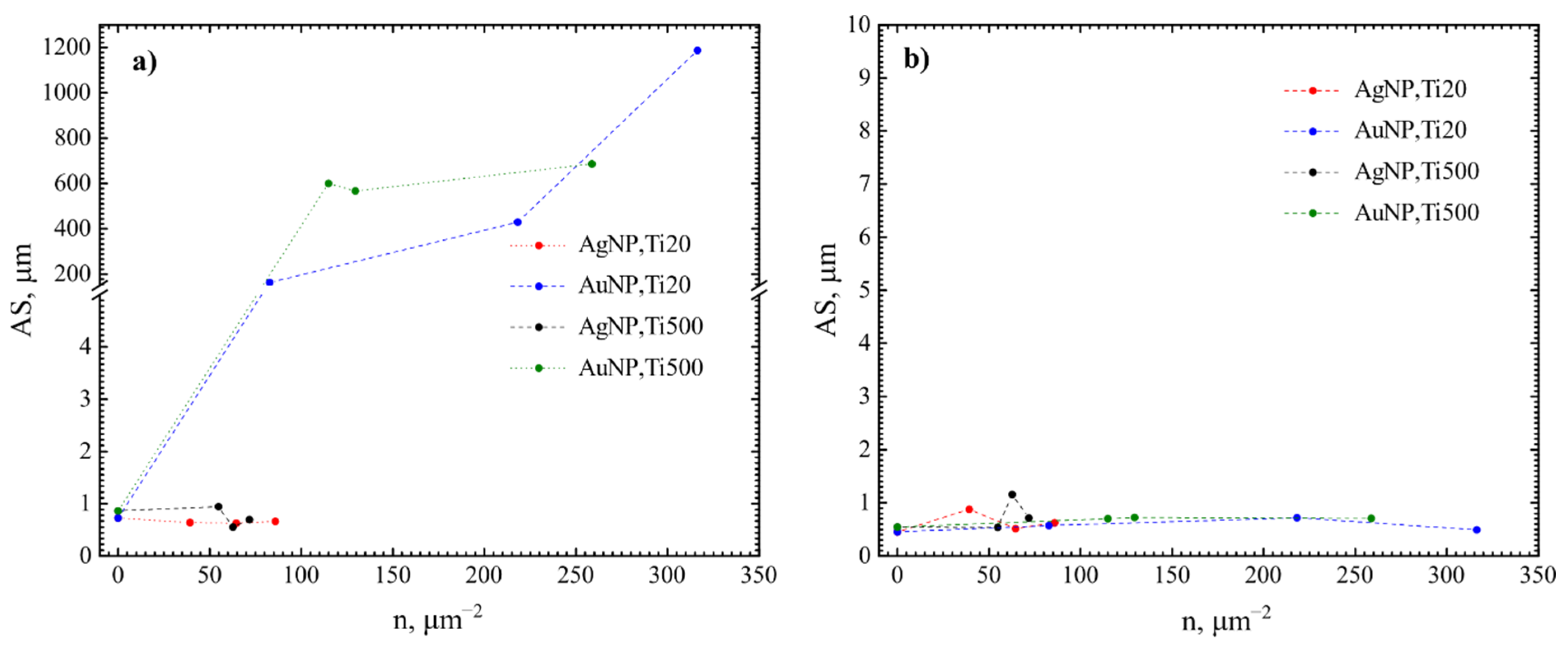
3.2. Antibacterial Effect
4. Discussion
4.1. Influence of the Underlying Substrate and Film Thickness on the NP Formation
4.2. Evaluation of the Antibacterial Activity of the Films Containing Either Au or Ag Nanoparticles
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tuson, H.H.; Weibel, D.B. Bacteria–surface interactions. Soft Matter 2013, 9, 4368–4380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Klein, M.I.; Falsetta, M.L.; Lu, B.; Delahunty, C.M.; Yates, J.R.; Heydorn, A.; Koo, H. The Exopolysaccharide Matrix Modulates the Interaction between 3D Architecture and Virulence of a Mixed-Species Oral Biofilm. PLoS Pathog. 2012, 8, e1002623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010 89 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Cheng, Y.; Wang, S.Y.; Borca-Tasciuc, D.A.; Worobo, R.W.; Moraru, C.I. Bacterial attachment and biofilm formation on surfaces are reduced by small-diameter nanoscale pores: How small is small enough? NPJ Biofilms Microbiomes 2015, 1, 1–9. [Google Scholar] [CrossRef]
- Bowen, W.H.; Koo, H. Biology of Streptococcus mutans-Derived Glucosyltransferases: Role in Extracellular Matrix Formation of Cariogenic Biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.W.; He, Y.; Ren, Y.; Zerdoum, A.; Libera, M.R.; Sharma, P.K.; van Winkelhoff, A.J.; Neut, D.; Stoodley, P.; van der Mei, H.C.; et al. Viscoelasticity of biofilms and their recalcitrance to mechanical and chemical challenges. FEMS Microbiol. Rev. 2015, 39, 234–245. [Google Scholar] [CrossRef] [Green Version]
- Kotronia, E.; Brown, H.; Papacosta, A.O.; Lennon, L.T.; Weyant, R.J.; Whincup, P.H.; Wannamethee, S.G.; Ramsay, S.E. Oral health and all-cause, cardiovascular disease, and respiratory mortality in older people in the UK and USA. Sci. Rep. 2021, 11, 16452. [Google Scholar] [CrossRef] [PubMed]
- Kotronia, E.; Wannamethee, S.G.; Papacosta, A.O.; Whincup, P.H.; Lennon, L.T.; Visser, M.; Kapila, Y.L.; Weyant, R.J.; Ramsay, S.E. Poor Oral Health and Inflammatory, Hemostatic, and Cardiac Biomarkers in Older Age: Results From Two Studies in the UK and USA. J. Gerontol. Ser. A 2021, 76, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.; Rasmussen, A.W.; Gudlavalleti, S.K.; Stephens, D.S.; Stojiljkovic, I. Biofilm formation by Neisseria meningitidis. Infect. Immun. 2004, 72, 6132–6138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashima, I.; Nakazawa, F. Interaction between Streptococcus spp. and Veillonella tobetsuensis in the early stages of oral biofilm formation. J. Bacteriol. 2015, 197, 2104–2111. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, J.; Eriksson, O.S.; Maudsdotter, L.; Palm, O.; Engman, J.; Sarkissian, T.; Aro, H.; Wallin, M.; Jonsson, A.B. Characterization of motility and piliation in pathogenic Neisseria Microbial biochemistry, physiology and metabolism. BMC Microbiol. 2015, 15, 92. [Google Scholar] [CrossRef] [Green Version]
- Feng, G.; Cheng, Y.; Wang, S.Y.; Hsu, L.C.; Feliz, Y.; Borca-Tasciuc, D.A.; Worobo, R.W.; Moraru, C.I. Alumina surfaces with nanoscale topography reduce attachment and biofilm formation by Escherichia coli and Listeria spp. Biofouling 2014, 30, 1253–1268. [Google Scholar] [CrossRef]
- Mauclaire, L.; Brombacher, E.; Bünger, J.D.; Zinn, M. Factors controlling bacterial attachment and biofilm formation on medium-chain-length polyhydroxyalkanoates (mcl-PHAs). Colloids Surf. B Biointerfaces 2010, 76, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Vyas, V.; Patil, R.; Sharma, V.; Scopelliti, P.E.; Bongiorno, G.; Podestà, A.; Lenardi, C.; Gade, W.N.; Milani, P. Quantitative Characterization of the Influence of the Nanoscale Morphology of Nanostructured Surfaces on Bacterial Adhesion and Biofilm Formation. PLoS ONE 2011, 6, e25029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lüdecke, C.; Roth, M.; Yu, W.; Horn, U.; Bossert, J.; Jandt, K.D. Nanorough titanium surfaces reduce adhesion of Escherichia coli and Staphylococcus aureus via nano adhesion points. Colloids Surf. B Biointerfaces 2016, 145, 617–625. [Google Scholar] [CrossRef]
- Dewald, C.; Lüdecke, C.; Firkowska-Boden, I.; Roth, M.; Bossert, J.; Jandt, K.D. Gold nanoparticle contact point density controls microbial adhesion on gold surfaces. Colloids Surf. B Biointerfaces 2018, 163, 201–208. [Google Scholar] [CrossRef]
- Dauben, T.J.; Dewald, C.; Firkowska-Boden, I.; Helbing, C.; Peisker, H.; Roth, M.; Bossert, J.; Jandt, K.D. Quantifying the relationship between surfaces’ nano-contact point density and adhesion force of Candida albicans. Colloids Surf. B Biointerfaces 2020, 194, 111177. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Bollen, C.M.L.; Schotte, A.; Marechal, M.; van Steenberghe, D.; van der Mei, H.C.; Doornbusch, G.I.; Busscher, H.J.; Naert, I. An in vivo Study of the Influence of the Surface Roughness of Implants on the Microbiology of Supra- and Subgingival Plaque. J. Dent. Res. 1993, 72, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Größner-Schreiber, B.; Griepentrog, M.; Haustein, I.; Müller, W.D.; Lange, K.P.; Briedigkeit, H.; Göbel, U.B. Plaque formation on surface modified dental implants. Clin. Oral Implants Res. 2001, 12, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Pita, P.P.C.; Rodrigues, J.A.; Ota-Tsuzuki, C.; Miato, T.F.; Zenobio, E.G.; Giro, G.; Figueiredo, L.C.; Gonçalves, C.; Gehrke, S.A.; Cassoni, A.; et al. Oral streptococci biofilm formation on different implant surface topographies. Biomed Res. Int. 2015, 2015, 159625. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef] [Green Version]
- Page, K.; Palgrave, R.G.; Parkin, I.P.; Wilson, M.; Savin, S.L.P.; Chadwick, A.V. Titania and silver–titania composite films on glass—potent antimicrobial coatings. J. Mater. Chem. 2006, 17, 95–104. [Google Scholar] [CrossRef]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Kleszcz, K.; Hebda, M.; Kyzioł, A.; Krawiec, H.; Kyzioł, K. Towards prevention of biofilm formation: Ti6Al7Nb modified with nanocomposite layers of chitosan and Ag/Au nanoparticles. Appl. Surf. Sci. 2021, 557, 149795. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcus epidermidis—the “accidental” pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef] [Green Version]
- Svensson, S.; Forsberg, M.; Hulander, M.; Vazirisani, F.; Palmquist, A.; Lausmaa, J.; Thomsen, P.; Trobos, M. Role of nanostructured gold surfaces on monocyte activation and Staphylococcus epidermidis biofilm formation. Int. J. Nanomed. 2014, 9, 775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, C.; Han, C.; Wang, X.; Zheng, Y.; Li, Q.; Hu, X.; Sun, H. The progress of silver nanoparticles in the antibacterial mechanism, clinical application and cytotoxicity. Mol. Biol. Rep. 2012, 39, 9193–9201. [Google Scholar] [CrossRef] [PubMed]
- Swathy, J.R.; Sankar, M.U.; Chaudhary, A.; Aigal, S.; Anshup; Pradeep, T. Antimicrobial silver: An unprecedented anion effect. Sci. Rep. 2014, 4, 7161. [Google Scholar] [CrossRef] [Green Version]
- Shimabukuro, M. Antibacterial Property and Biocompatibility of Silver, Copper, and Zinc in Titanium Dioxide Layers Incorporated by One-Step Micro-Arc Oxidation: A Review. Antibiotics 2020, 9, 716. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Speziale, P.; Montanaro, L.; Costerton, J.W. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 2012, 33, 5967–5982. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhang, X.; Wu, H.; Tian, L.; Ma, Y.; Tang, B. Microstructure and antibacterial properties of Cu-doped TiO2 coating on titanium by micro-arc oxidation. Appl. Surf. Sci. 2014, 292, 944–947. [Google Scholar] [CrossRef]
- Qin, H.; Cao, H.; Zhao, Y.; Zhu, C.; Cheng, T.; Wang, Q.; Peng, X.; Cheng, M.; Wang, J.; Jin, G.; et al. In vitro and in vivo anti-biofilm effects of silver nanoparticles immobilized on titanium. Biomaterials 2014, 35, 9114–9125. [Google Scholar] [CrossRef]
- Huang, Y.; Zha, G.; Luo, Q.; Zhang, J.; Zhang, F.; Li, X.; Zhao, S.; Zhu, W.; Li, X. The construction of hierarchical structure on Ti substrate with superior osteogenic activity and intrinsic antibacterial capability. Sci. Rep. 2014, 4, 6172. [Google Scholar] [CrossRef] [Green Version]
- Narendrakumar, K.; Kulkarni, M.; Addison, O.; Mazare, A.; Junkar, I.; Schmuki, P.; Sammons, R.; Iglič, A. Adherence of oral streptococci to nanostructured titanium surfaces. Dent. Mater. 2015, 31, 1460–1468. [Google Scholar] [CrossRef]
- Yan, X.; He, B.; Liu, L.; Qu, G.; Shi, J.; Hu, L.; Jiang, G. Antibacterial mechanism of silver nanoparticles in Pseudomonas aeruginosa: Proteomics approach. Metallomics 2018, 10, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Saleh, T.A.; Wahab, A.; Khan, M.H.U.; Khan, D.; Khan, W.U.; Rahim, A.; Kamal, S.; Khan, F.U.; Fahad, S. Nanosilver: New ageless and versatile biomedical therapeutic scaffold. Int. J. Nanomed. 2018, 13, 733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Jun, B.H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mobed, A.; Hasanzadeh, M.; Seidi, F. Anti-bacterial activity of gold nanocomposites as a new nanomaterial weapon to combat photogenic agents: Recent advances and challenges. RSC Adv. 2021, 11, 34688–34698. [Google Scholar] [CrossRef]
- Ortiz-Benítez, E.A.; Velázquez-Guadarrama, N.; Durán Figueroa, N.V.; Quezada, H.; De Jesús Olivares-Trejo, J. Antibacterial mechanism of gold nanoparticles on Streptococcus pneumoniae. Metallomics 2019, 11, 1265–1276. [Google Scholar] [CrossRef]
- Joshi, A.S.; Singh, P.; Mijakovic, I. Interactions of Gold and Silver Nanoparticles with Bacterial Biofilms: Molecular Interactions behind Inhibition and Resistance. Int. J. Mol. Sci. 2020, 21, 7658. [Google Scholar] [CrossRef]
- Yue, W.; Wang, Z.; Yang, Y.; Chen, L.; Syed, A.; Wong, K.; Wang, X. Electron-beam lithography of gold nanostructures for surface-enhanced Raman scattering. J. Micromech. Microeng. 2012, 22, 125007. [Google Scholar] [CrossRef]
- Lopatynskyi, A.M.; Lytvyn, V.K.; Nazarenko, V.I.; Guo, L.J.; Lucas, B.D.; Chegel, V.I. Au nanostructure arrays for plasmonic applications: Annealed island films versus nanoimprint lithography. Nanoscale Res. Lett. 2015, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wiley, B.; Sun, Y.; Mayers, B.; Xia, Y. Shape-Controlled Synthesis of Metal Nanostructures: The Case of Silver. Chem.—Eur. J. 2005, 11, 454–463. [Google Scholar] [CrossRef]
- Niekiel, F.; Schweizer, P.; Kraschewski, S.M.; Butz, B.; Spiecker, E. The process of solid-state dewetting of Au thin films studied by in situ scanning transmission electron microscopy. Acta Mater. 2015, 90, 118–132. [Google Scholar] [CrossRef]
- Ye, J.; Zuev, D.; Makarov, S. Dewetting mechanisms and their exploitation for the large-scale fabrication of advanced nanophotonic systems. Int. Mater. Rev. 2018, 64, 439–477. [Google Scholar] [CrossRef]
- Altomare, M.; Nguyen, N.T.; Schmuki, P. Templated dewetting: Designing entirely self-organized platforms for photocatalysis. Chem. Sci. 2016, 7, 6865–6886. [Google Scholar] [CrossRef] [Green Version]
- Song, D.H.; Uhm, S.H.; Kim, S.E.; Kwon, J.S.; Han, J.G.; Kim, K.N. Synthesis of titanium oxide thin films containing antibacterial silver nanoparticles by a reactive magnetron co-sputtering system for application in biomedical implants. Mater. Res. Bull. 2012, 47, 2994–2998. [Google Scholar] [CrossRef]
- Jin, G.; Cao, H.; Qiao, Y.; Meng, F.; Zhu, H.; Liu, X. Osteogenic activity and antibacterial effect of zinc ion implanted titanium. Colloids Surf. B Biointerfaces 2014, 117, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.Y.; Aly, U.F.; Abd El-Baky, R.M.; Waly, N.G.F.M. Comparative Study of Antibacterial Effects of Titanium Dioxide Nanoparticles Alone and in Combination with Antibiotics on MDR Pseudomonas aeruginosa Strains. Int. J. Nanomed. 2020, 15, 3393–3404. [Google Scholar] [CrossRef]
- Soo, J.Z.; Chai, L.C.; Ang, B.C.; Ong, B.H. Enhancing the Antibacterial Performance of Titanium Dioxide Nanofibers by Coating with Silver Nanoparticles. ACS Appl. Nano Mater. 2020, 3, 5743–5751. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Ehsani, A.; Divband, B.; Alizadeh-Sani, M. Antimicrobial activity of Titanium dioxide and Zinc oxide nanoparticles supported in 4A zeolite and evaluation the morphological characteristic. Sci. Rep. 2019, 9, 17439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Liu, J.; Attarilar, S.; Wang, C.; Tamaddon, M.; Yang, C.; Xie, K.; Yao, J.; Wang, L.; Liu, C.; et al. Nano-Modified Titanium Implant Materials: A Way Toward Improved Antibacterial Properties. Front. Bioeng. Biotechnol. 2020, 8, 1314. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.V.; Christodoulides, M. Atypical, Yet Not Infrequent, Infections with Neisseria Species. Pathog. 2019, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Dinnebier, R.E.; Billinge, S.J.L. Chapter 1: Principles of Powder Diffraction. Powder Diffr. 2008, 1–19. [Google Scholar] [CrossRef]
- Dosovitskiy, A.; Springenberg, J.T.; Riedmiller, M.; Brox, T. Discriminative Unsupervised Feature Learning with Convolutional Neural Networks. arXiv. 2014, arXiv:1406.6909. [Google Scholar] [CrossRef] [Green Version]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. Lect. Notes Comput. Sci. 2015, 9351, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Otoo, E.; Shoshani, A. Optimizing connected component labeling algorithms. Proc. SPIE Conf. Med. Imag. 2005, 5747, 1965–1976. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.; Zhao, Z.; Niu, M.; Mao, C.; Cao, D.; Cheng, D.; Feng, P.; Sun, Z. A facile and versatile method for preparation of colored TiO2 with enhanced solar-driven photocatalytic activity. Nanoscale 2014, 6, 10216–10223. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy (XPS) Database. Available online: https://srdata.nist.gov/xps/citation.aspx (accessed on 15 December 2021).
- Atuchin, V.V.; Kesler, V.G.; Pervukhina, N.V.; Zhang, Z. Ti 2p and O 1s core levels and chemical bonding in titanium-bearing oxides. J. Electron Spectros. Relat. Phenom. 2006, 152, 18–24. [Google Scholar] [CrossRef]
- Sylvestre, J.P.; Poulin, S.; Kabashin, A.V.; Sacher, E.; Meunier, M.; Luong, J.H.T. Surface chemistry of gold nanoparticles produced by laser ablation in aqueous media. J. Phys. Chem. B 2004, 108, 16864–16869. [Google Scholar] [CrossRef]
- Ferraria, A.M.; Carapeto, A.P.; Botelho Do Rego, A.M. X-ray photoelectron spectroscopy: Silver salts revisited. Vacuum 2012, 86, 1988–1991. [Google Scholar] [CrossRef]
- Ghidelli, M.; Mascaretti, L.; Bricchi, B.R.; Brognara, A.; Afifi, T.A.; Russo, V.; Casari, C.S.; Bassi, A.L. Light management in TiO2 thin films integrated with Au plasmonic nanoparticles. Semicond. Sci. Technol. 2020, 35, 035016. [Google Scholar] [CrossRef]
- Kang, M.; Park, S.G.; Jeong, K.H. Repeated Solid-state Dewetting of Thin Gold Films for Nanogap-rich Plasmonic Nanoislands. Sci. Rep. 2015, 5, 14790. [Google Scholar] [CrossRef]
- Abdolvand, A.; Hoffmann, M.; Wackerow, S.; Zolotovskaya, S.A. High performance SERS platforms via parametric optimization of the laser-assisted photodeposition of silver and gold nanoparticles. Opt. Mater. Express 2021, 11, 3079–3098. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2002, 107, 668–677. [Google Scholar] [CrossRef]
- Sriubas, M.; Kavaliūnas, V.; Bočkutė, K.; Palevičius, P.; Kaminskas, M.; Rinkevičius, Ž.; Ragulskis, M.; Laukaitis, G. Formation of Au nanostructures on the surfaces of annealed TiO2 thin films. Surf. Interfaces 2021, 25, 101239. [Google Scholar] [CrossRef]
- Li, W.; Thian, E.S.; Wang, M.; Wang, Z.; Ren, L. Surface Design for Antibacterial Materials: From Fundamentals to Advanced Strategies. Adv. Sci. 2021, 8, 2100368. [Google Scholar] [CrossRef]
- Thompson, C.V. Solid-State Dewetting of Thin Films. Annu. Rev. Mater. Sci. 2012, 42, 399–434. [Google Scholar] [CrossRef]
- Ding, S.; Wang, X.; Chen, D.J.; Wang, Q.Q.; Ricard, D.; Roussignol, P.; Flytzanis, C.; Liao, H.B.; Xiao, R.F.; Fu, J.S.; et al. Optical percolation and nonlinearity of sputtered Ag island films. Opt. Express 2006, 14, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Farzinpour, P.; Sundar, A.; Gilroy, K.D.; Eskin, E.Z.; Hughes, A.R.; Neretina, S. Altering the dewetting characteristics of ultrathin gold and silver films using a sacrificial antimony layer. Nanotechnology 2012, 23, 495604. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, M.G.; Malik, S.; Thigulla, S.; Mehta, B.R. Dependence of plasmonic properties of silver island films on nanoparticle size and substrate coverage. J. Nanomater. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Ginsburg, A.; Priel, M.; Barad, H.N.; Keller, D.A.; Borvick, E.; Rietwyk, K.; Kama, A.; Meir, S.; Anderson, A.Y.; Zaban, A. Solid state ITO|Au-NPs|TiO2 plasmonic based solar cells. Sol. Energy Mater. Sol. Cells 2018, 179, 254–259. [Google Scholar] [CrossRef]
- Kunwar, S.; Sui, M.; Zhang, Q.; Pandey, P.; Li, M.Y.; Lee, J. Various silver nanostructures on sapphire using plasmon self- assembly and dewetting of thin films. Nano-Micro Lett. 2017, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Hwang, C.S.H.; Ahn, M.S.; Lee, Y.; Chung, T.; Jeong, K.H. Ag/Au Alloyed Nanoislands for Wafer-Level Plasmonic Color Filter Arrays. Sci. Rep. 2019, 9, 9082. [Google Scholar] [CrossRef] [PubMed]
- Can Günendi, M.; Tanyeli, İ.; Akgüç, G.B.; Bek, A.; Turan, R.; Gülseren, O.; Atwater, H.A.; Polman, A.; Kelly, K.L.; Coronado, E.; et al. Understanding the plasmonic properties of dewetting formed Ag nanoparticles for large area solar cell applications. Opt. Express 2013, 21, 18344–18353. [Google Scholar] [CrossRef] [Green Version]
- Pandey, P.; Kunwar, S.; Shin, K.H.; Seo, M.K.; Yoon, J.; Hong, W.K.; Sohn, J.I. Plasmonic Core–Shell–Satellites with Abundant Electromagnetic Hotspots for Highly Sensitive and Reproducible SERS Detection. Int. J. Mol. Sci. 2021, 22, 12191. [Google Scholar] [CrossRef]
- Quan, J.; Zhang, J.; Qi, X.; Li, J.; Wang, N.; Zhu, Y. A study on the correlation between the dewetting temperature of Ag film and SERS intensity. Sci. Rep. 2017, 7, 14771. [Google Scholar] [CrossRef] [Green Version]
- Nsimama, P.D.; Herz, A.; Wang, D.; Schaaf, P. Influence of the substrate on the morphological evolution of gold thin films during solid-state dewetting. Appl. Surf. Sci. 2016, 388, 475–482. [Google Scholar] [CrossRef]
- Bakri, A.S.; Sahdan, M.Z.; Adriyanto, F. Effect of annealing temperature of titanium dioxide thin films on structural and electrical properties. In Proceedings of the International Conference on Engineering, Science and Nanotechnology 2016, Solo, Indonesia, 3–5 August 2017; Volume 1788, p. 30030. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Suo, X.; Zhang, J.; Han, B.; Li, P.; Xue, Y.; Shi, H. One-pot synthesis of Au/TiO2 heteronanostructure composites with SPR effect and its antibacterial activity. Mater. Lett. 2016, 162, 235–237. [Google Scholar] [CrossRef]
- Tiwari, A.; Shukla, A.; Tiwari, D.; Choi, S.S.; Shin, H.G.; Lee, S.M. Titanium Dioxide Nanomaterials and its Derivatives in the Remediation of Water: Past, Present and Future. Appl. Chem. Eng. 2019, 30, 261–279. [Google Scholar] [CrossRef]
- Yougbaré, S.; Mutalik, C.; Okoro, G.; Lin, I.H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Chang, C.C.; Kuo, T.R. Emerging Trends in Nanomaterials for Antibacterial Applications. Int. J. Nanomed. 2021, 16, 5831–5867. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Villa, A.; Dimitratos, N.; Chan-Thaw, C.E.; Hammond, C.; Veith, G.M.; Wang, D.; Manzoli, M.; Prati, L.; Hutchings, G.J. Characterisation of gold catalysts. Chem. Soc. Rev. 2016, 45, 4953–4994. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Yahia, L.; Sacher, E. Antimicrobial Properties of the Ag, Cu Nanoparticle System. Biology 2021, 10, 137. [Google Scholar] [CrossRef]
- Sawant, S.N.; Selvaraj, V.; Prabhawathi, V.; Doble, M. Antibiofilm Properties of Silver and Gold Incorporated PU, PCLm, PC and PMMA Nanocomposites under Two Shear Conditions. PLoS ONE 2013, 8, e63311. [Google Scholar] [CrossRef] [Green Version]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.D.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.D.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic Insights into the Antimicrobial Actions of Metallic Nanoparticles and Their Implications for Multidrug Resistance. Int. J. Mol. Sci. 2019, 20, 2468. [Google Scholar] [CrossRef] [Green Version]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef]
- Cochis, A.; Azzimonti, B.; Della Valle, C.; Chiesa, R.; Arciola, C.R.; Rimondini, L. Biofilm formation on titanium implants counteracted by grafting gallium and silver ions. J. Biomed. Mater. Res. Part A 2015, 103, 1176–1187. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Gao, L.; Sun, Y.; Wang, J.; Qu, S.; Duan, K.; Weng, J.; Feng, B. Porous titanium scaffold surfaces modified with silver loaded gelatin microspheres and their antibacterial behavior. Surf. Coatings Technol. 2016, 286, 140–147. [Google Scholar] [CrossRef]
- Gouyau, J.; Duval, R.E.; Boudier, A.; Lamouroux, E.; Gouyau, J.; Duval, R.E.; Boudier, A.; Lamouroux, E.; Banti, C.N.; Rossos, A.K. Investigation of Nanoparticle Metallic Core Antibacterial Activity: Gold and Silver Nanoparticles against Escherichia coli and Staphylococcus aureus. Int. J. Mol. Sci 2021, 22, 1905. [Google Scholar] [CrossRef] [PubMed]
- Megrian, D.; Taib, N.; Witwinowski, J.; Beloin, C.; Gribaldo, S. One or two membranes? Diderm Firmicutes challenge the Gram-positive/Gram-negative divide. Mol. Microbiol. 2020, 113, 659–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gronow, S.; Welnitz, S.; Lapidus, A.; Nolan, M.; Ivanova, N.; del Rio, T.G.; Copeland, A.; Chen, F.; Tice, H.; Pitluck, S.; et al. Complete genome sequence of Veillonella parvula type strain (Te3 T). Stand. Genomic Sci. 2010, 2, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Brook, I. Anaerobic bacteria. Infect. Dis. 2010, 2, 1757–1776. [Google Scholar] [CrossRef]
- Van Putten, J.; Tønjum, T. Neisseria. Infect. Dis. 2010, 2, 1676–1689. [Google Scholar] [CrossRef]
- Choi, O.; Deng, K.K.; Kim, N.J.; Ross, L.; Surampalli, R.Y.; Hu, Z. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008, 42, 3066–3074. [Google Scholar] [CrossRef]
- Kędziora, A.; Speruda, M.; Krzyżewska, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskońska, G. Similarities and Differences between Silver Ions and Silver in Nanoforms as Antibacterial Agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Shareena Dasari, T.P.; Deng, H.; Yu, H. Antimicrobial Activity of Gold Nanoparticles and Ionic Gold. J. Environ. Sci. Health Part C 2015, 33, 286–327. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef] [Green Version]

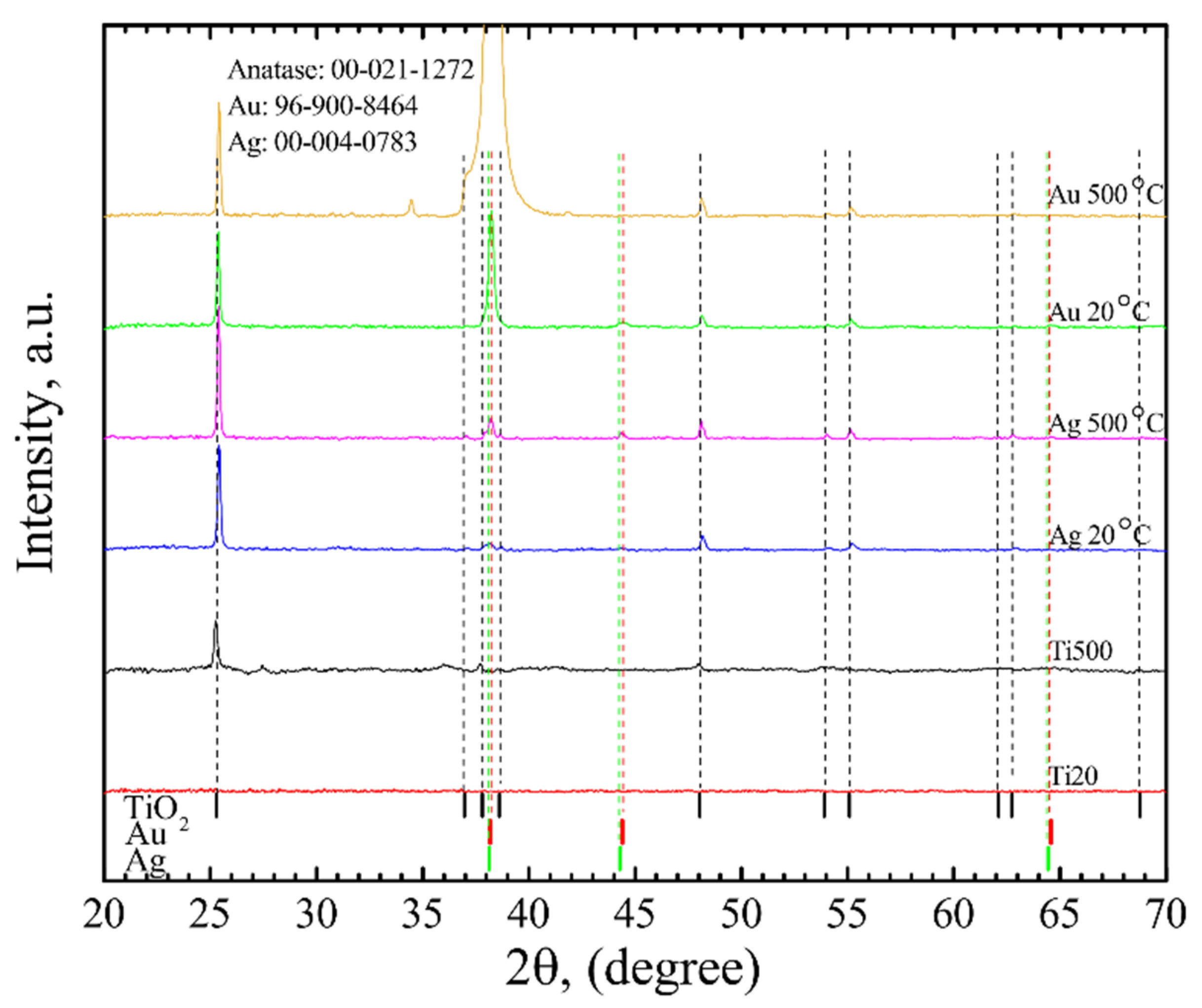
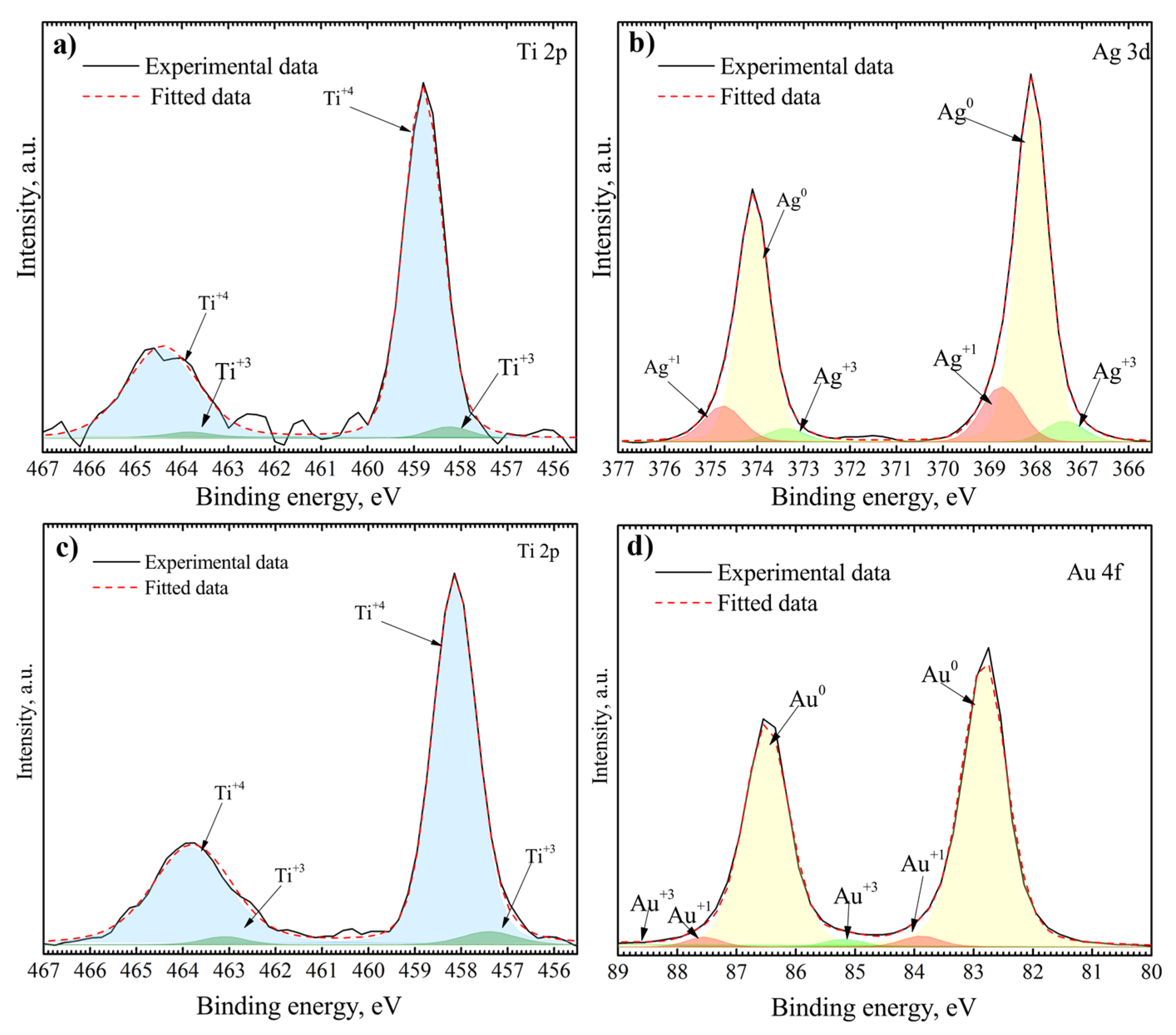
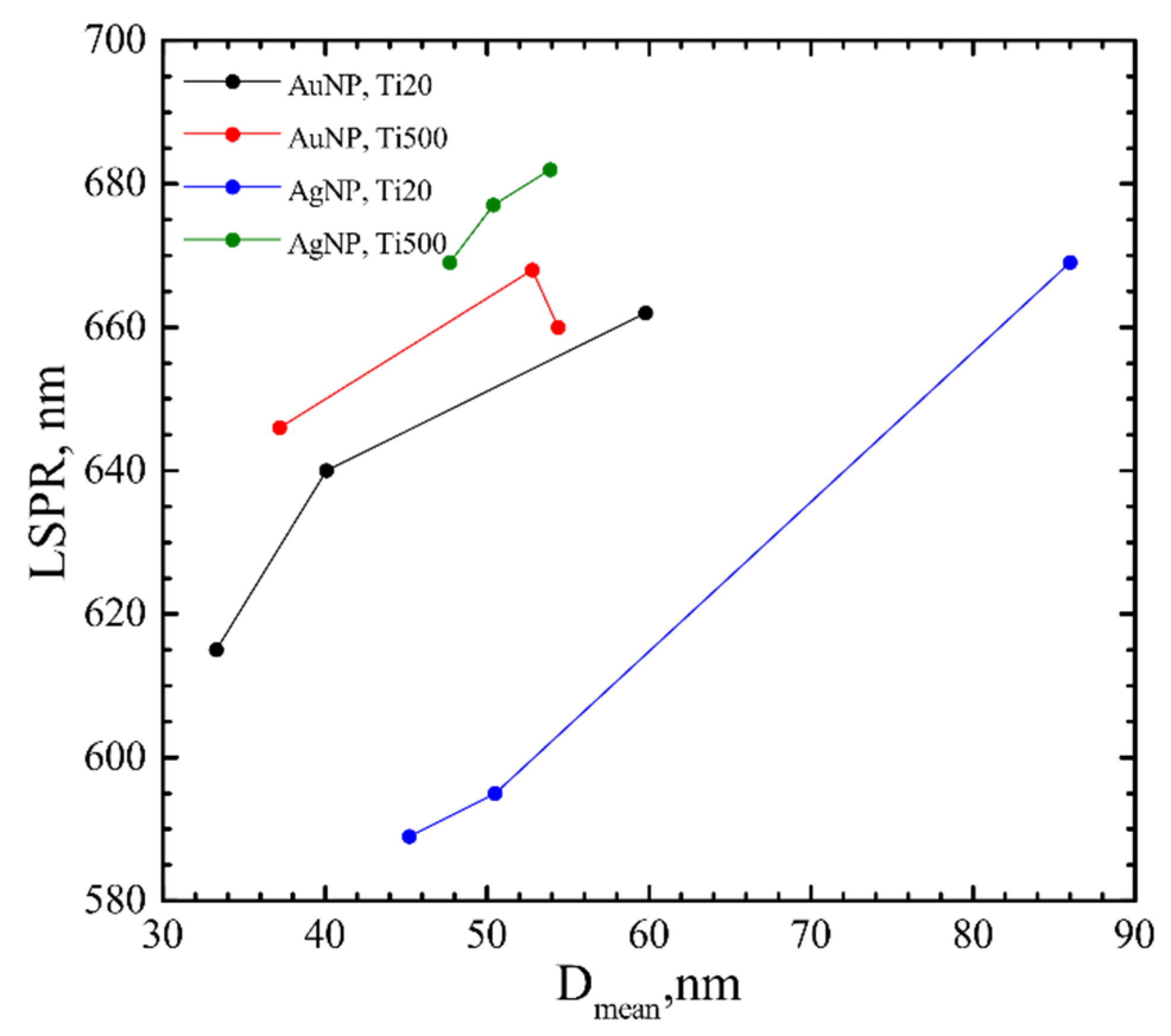
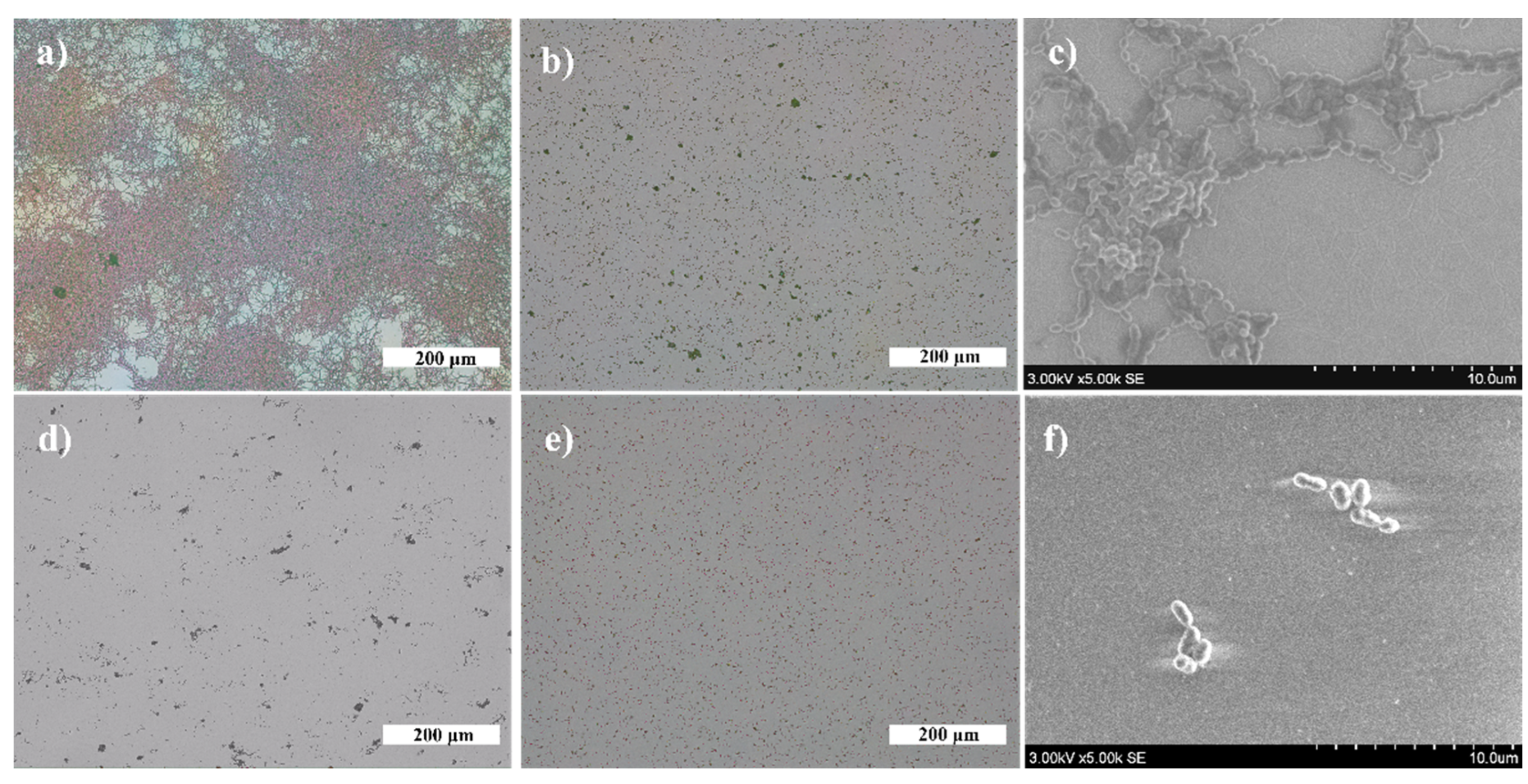


| h, nm | TTiO2, °C | AuNP | AgNP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SAR | nNP, μm−2 | Dmean, nm | NNDavg, nm | SAR | nNP, μm−2 | Dmean, nm | NNDavg, nm | ||
| 10 | 20 (Ti20) | 0.28 | 82.8 | 59.8 | 27.5 | 0.27 | 39.2 | 86.0 | 38.3 |
| 7.5 | 0.31 | 218.1 | 40.1 | 14.7 | 0.17 | 64.6 | 50.5 | 40.9 | |
| 5 | 0.31 | 316.4 | 33.3 | 12.0 | 0.17 | 85.8 | 45.2 | 31.4 | |
| 10 | 500 (Ti500) | 0.32 | 114.9 | 52.8 | 19.3 | 0.14 | 54.9 | 50.4 | 37.8 |
| 7.5 | 0.34 | 129.5 | 54.4 | 16.5 | 0.21 | 71.8 | 53.9 | 29.8 | |
| 5 | 0.31 | 258.8 | 37.2 | 13.7 | 0.15 | 62.8 | 47.7 | 33.7 | |
| TTiO2, °C | NP | nNP, μm−2 | N. sicca | V. parvula | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SC, % | nB, mm−2 | AS, µm | CV | SC, % | nB, mm−2 | AS, µm | CV | |||
| 20 (Ti20) | Au | 0 | 1.7 | 23,417 | 0.7 | 2.2 | 2 | 42,686 | 0.5 | 0.9 |
| 82.8 | 66.6 | 8017 | 164.0 | 20.4 | 1.1 | 20,251 | 0.6 | 0.8 | ||
| 218.1 | 81.4 | 2375 | 429.2 | 11.8 | 9.4 | 126,620 | 0.7 | 1.5 | ||
| 316.4 | 83.4 | 810 | 1187.1 | 6.9 | 2.7 | 55,134 | 0.5 | 2.1 | ||
| 500 (Ti500) | 0 | 2.7 | 30,549 | 0.9 | 2.0 | 4.1 | 75,968 | 0.5 | 1.2 | |
| 114.9 | 77.7 | 1503 | 600.2 | 9.4 | 4.2 | 59,728 | 0.7 | 1.3 | ||
| 129.5 | 79.7 | 1789 | 567.3 | 10.2 | 13.4 | 190,816 | 0.7 | 4.6 | ||
| 258.8 | 79.4 | 1321 | 687.1 | 8.9 | 6.3 | 89,793 | 0.7 | 1.1 | ||
| 20 (Ti20) | Ag | 0 | 1.7 | 23,417 | 0.7 | 2.2 | 2 | 42,686 | 0.5 | 0.9 |
| 39.2 | 2.5 | 49,577 | 0.6 | 4.8 | 10.8 | 132,131 | 0.9 | 2.2 | ||
| 64.6 | 2.3 | 43,801 | 0.6 | 3.0 | 1.2 | 23,508 | 0.5 | 0.8 | ||
| 85.8 | 0.9 | 14,678 | 0.7 | 2.3 | 1.6 | 25,864 | 0.6 | 1.3 | ||
| 500 (Ti500) | 0 | 2.7 | 30,549 | 0.9 | 2.0 | 4.1 | 75,968 | 0.5 | 1.2 | |
| 54.9 | 2.4 | 24,874 | 1.0 | 1.2 | 3.6 | 66,667 | 0.5 | 1.4 | ||
| 62.8 | 4.1 | 88,324 | 0.6 | 3.0 | 16.7 | 141,514 | 1.2 | 2.4 | ||
| 71.8 | 2.3 | 45,523 | 0.7 | 2.5 | 6.2 | 87,496 | 0.7 | 1.5 | ||
| Optical glass | - | 88.5 | 159 | 347.0 | 1.4 | 5.5 | 96,138 | 0.6 | 0.2 | |
| AuNP | AgNP | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| h, nm | Ta, °C | Substrate | SAR | nNP, μm−2 | Dmean, nm | NNDavg, nm | Ref. | h, nm | Ta, °C | Substrate | SAR | nNP, μm−2 | Dmean, nm | NNDavg, nm | Ref. |
| 4 | 900 | Sapphire (0001) | - | 900 | 24 | 36 | [72] | 5 | 200 | Si | 0.41 | 67 | 93 | [73] | |
| 4 | 400 | TiO2 | 0.23 | - | 36 | - | [74] | 6 | 550 | Sapphire (0001) | - | 25 | - | [75] | |
| 5 | 500 | Quartz glass | 0.65 | - | 25 | - | [66] | 10 | 500 | SiO2 | - | 33 | - | [76] | |
| 7 | 400 | TiO2 | 0.21 | - | 74 | - | [74] | 10 | 550 | Sapphire (0001) | - | 50 | - | [75] | |
| 8 | 900 | Sapphire (0001) | - | 90 | 63 | 98 | [72] | 12 | 400 | SiO2 | - | 120 | - | [77] | |
| 10 | 500 | Quartz glass | 0.10 | - | 55 | - | [66] | 15 | 600 | SiO2 | - | 321 | - | [78] | |
| 12 | 900 | Sapphire (0001) | - | 50 | 100 | 150 | [72] | 20 | 400 | SiO2 | - | 180 | 140 | [79] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sriubas, M.; Bockute, K.; Palevicius, P.; Kaminskas, M.; Rinkevicius, Z.; Ragulskis, M.; Simonyte, S.; Ruzauskas, M.; Laukaitis, G. Antibacterial Activity of Silver and Gold Particles Formed on Titania Thin Films. Nanomaterials 2022, 12, 1190. https://doi.org/10.3390/nano12071190
Sriubas M, Bockute K, Palevicius P, Kaminskas M, Rinkevicius Z, Ragulskis M, Simonyte S, Ruzauskas M, Laukaitis G. Antibacterial Activity of Silver and Gold Particles Formed on Titania Thin Films. Nanomaterials. 2022; 12(7):1190. https://doi.org/10.3390/nano12071190
Chicago/Turabian StyleSriubas, Mantas, Kristina Bockute, Paulius Palevicius, Marius Kaminskas, Zilvinas Rinkevicius, Minvydas Ragulskis, Sandrita Simonyte, Modestas Ruzauskas, and Giedrius Laukaitis. 2022. "Antibacterial Activity of Silver and Gold Particles Formed on Titania Thin Films" Nanomaterials 12, no. 7: 1190. https://doi.org/10.3390/nano12071190
APA StyleSriubas, M., Bockute, K., Palevicius, P., Kaminskas, M., Rinkevicius, Z., Ragulskis, M., Simonyte, S., Ruzauskas, M., & Laukaitis, G. (2022). Antibacterial Activity of Silver and Gold Particles Formed on Titania Thin Films. Nanomaterials, 12(7), 1190. https://doi.org/10.3390/nano12071190








